Abstract
Aims
To investigate the effects of electronic prescribing (EP) on prescribing quality, as indicated by prescribing errors and pharmacists' clinical interventions, in a UK hospital.
Methods
Prescribing errors and pharmacists' interventions were recorded by the ward pharmacist during a 4 week period both pre- and post-EP, with a second check by the principal investigator. The percentage of new medication orders with a prescribing error and/or pharmacist's intervention was calculated for each study period.
Results
Following the introduction of EP, there was a significant reduction in both pharmacists' interventions and prescribing errors. Interventions reduced from 73 (3.0% of all medication orders) to 45 (1.9%) (95% confidence interval (CI) for the absolute reduction 0.2, 2.0%), and errors from 94 (3.8%) to 48 (2.0%) (95% CI 0.9, 2.7%). Ten EP-specific prescribing errors were identified. Only 52% of pharmacists' interventions related to a prescribing error pre-EP, and 60% post-EP; only 40% and 56% of prescribing errors resulted in an intervention pre- and post-EP, respectively.
Conclusions
EP improved the quality of prescribing by reducing both prescribing errors and pharmacists' clinical interventions. Prescribers and pharmacists need to be aware of new types of error with EP, so that they can best target their activities to reduce clinical risk. Pharmacists may need to change the way they work to complement, rather than duplicate, the benefits of EP.
What is already known about this subject
Electronic prescribing has been shown to reduce prescribing errors in US hospitals.
However we know little about its effect on prescribing quality, or its effectiveness in UK hospitals where systems for medication prescribing and supply are very different.
Hospital pharmacists already review prescriptions to both detect errors and improve prescription quality.
What this study adds
Electronic prescribing significantly increased prescribing quality in a UK hospital, as shown by fewer pharmacists' interventions and fewer prescribing errors.
However, some new types of error were introduced.
There was relatively little overlap between prescribing errors and pharmacists' interventions, signifying their different contributions to prescribing quality.
Electronic prescribing and pharmacists' interventions should be viewed as an integrated system.
Keywords: electronic prescribing, prescribing errors
Introduction
Delivering healthcare is not without risk [1]. There are an estimated 850 000 patient safety incidents per year in the UK alone [2] with 25 000 resulting deaths [3]. In the National Patient Safety Agency's first report on patient safety incidents [4], medication-related incidents accounted for 8.6% of reports in acute care settings. While an error can occur at any stage of the drug-use process [5, 6], prescribing errors are the most common type in the USA [7] and it is suspected that the situation is similar in the UK.
In UK hospitals, pharmacists have had a growing role in detecting prescribing errors and improving prescription quality since the late 1960s. As part of their routine duties, hospital pharmacists frequently make clinical interventions to improve patient care by rectifying prescribing errors and improving the quality of drug use [8–13]. Not all prescribing errors will result in a pharmacist's intervention (for example, the drug may have already been given and then discontinued, or the error rectified by another healthcare professional), and not all pharmacists' interventions result from prescribing errors (for example, the pharmacist may suggest changing a correctly prescribed drug to one that is in the hospital formulary).
Electronic prescribing (EP) has been shown to reduce prescribing errors in US hospitals [14–19]. However, systems of medication prescribing and supply are very different in the UK [20] and there have been few UK studies. Four UK studies have examined prescribing errors before and after the introduction of EP. However, one study suggesting a decrease in errors in inpatient medication orders was only published in abstract form [21]. Another, reporting no change in the pattern of prescribing errors, gives little information about the methods used [22]. A third paper reported a reduction in errors following the introduction of structured prescribing screens, but also gives little detail of the methods and definitions used [23]. The fourth study [24], reporting a reduction in prescribing errors, was based in critical care and its generalizability to other settings is unknown. There has been little work on the impact of EP on pharmacists' interventions; the only UK study, which reported an increase in interventions and a change in their nature, was published only in abstract form [25]. There have been no studies of the impact of EP on the whole process, including both prescribing errors and pharmacists' interventions.
An EP system was recently piloted and evaluated on a general surgical ward in our hospital. We previously published a summary of the key results [26]; we demonstrated significant reductions in the overall incidence of both prescribing errors and nonintravenous medication administration errors. We also showed a significant increase in confirmation of patient identity before medication administration, and increases in staff time spent on medication-related activities.
The present paper describes the relationship between prescribing errors and pharmacists' interventions, the severity of the prescribing errors identified, whether they originated in prescription-writing or the prescribing decision, and at what stage of the patient's stay they occurred. Our aim was to investigate the effects of EP on prescribing quality, as indicated by different types of prescribing error and the number and types of pharmacists' clinical interventions. We also describe new types of prescribing error attributable to EP itself and make recommendations for how clinical pharmacists should work with similar electronic systems to maximize patient safety.
Methods
Setting and design
The study was approved by Riverside Research Ethics Committee. We studied a 28-bed general surgery ward in a London teaching hospital which admitted a wide range of patients, many with complex medical comorbidities. The ward received a pharmacy service typical of that in UK hospitals [20, 27], with a daily visit from the ward pharmacist on weekdays and a short visit on Saturdays, described in more detail elsewhere [26]. The pharmacists checked that each medication order was clear, legal and clinically appropriate, checked pre-admission drug histories, resolved any problems identified, initiated the supply of nonstock medication and provided discharge medication counselling wherever possible [26]. Pre-EP, medication orders were prescribed on paper drug charts. The EP system was a closed-loop system incorporating EP, ward-based automated dispensing, barcode patient identification and electronic medication administration records (ServeRx: MDG Medical, Israel, version 1 : 13) and is described in more detail elsewhere [26]. The prescribing of intravenous fluids and oral anticoagulants remained on paper post-EP, as did the prescribing of discharge medication (to take away; TTA) at the time of the study. The system included a drug dictionary and suggested default doses; no further decision support was enabled. When patients were transferred from other wards, their existing medication orders were transcribed onto the system by a doctor or pharmacist. The system went live in June 2003.
We used a before-and-after design, and collected data during two 4 week periods. The first was 3 months pre-EP (April 2003) and the second 6 months post-EP (November/December 2003). The same pharmacist provided ward pharmacy services to the study ward in both data collection periods. To obtain a denominator for calculating error and intervention rates, we counted the number of medication orders written for patients on the ward during each study period.
Counting the number of medication orders written
We recorded the numbers of once-only, regular, when required (PRN) and intravenous infusion medication orders written for each patient during their stay on the ward in each data collection period. The numbers of TTA items written during each study period were also recorded. These data were collected retrospectively from paper drug charts, TTAs, and EP records. Patients' health records were examined for this purpose either on the ward immediately after discharge or by retrieving them retrospectively from the health records department. The list of patients on the study ward during each data collection period was compiled from the ward's admissions book. We included TTAs, intravenous fluids and oral anticoagulants although these remained on paper charts post-EP.
Medication orders were classified according to whether they were written on admission, during the inpatient stay, or on re-writing paper charts/transcribing onto EP [28]. Occasionally medication orders prescribed as a single item on a paper drug chart were prescribed as two entries using EP (for example doses requiring more than one tablet strength). These were counted as two medication orders post-EP. Medication orders for dietary supplements, blood products, graduated compression hosiery, anaesthetic agents and patient-controlled analgesia were excluded. Where patients' health records could not be located, we extrapolated the total number of medication orders written based on the notes actually retrieved.
Prescribing errors and pharmacists' interventions
The ward pharmacist was asked to record any event that could be classified as a prescribing error, an intervention, or both, for inpatient medication orders and TTAs. A prescribing error was defined as a prescribing decision or prescription-writing process that results in an unintentional, significant: (i) reduction in the probability of treatment being timely and effective or (ii) increase in the risk of harm, when compared with generally accepted practice [29]. According to this definition, developed using consensus methods, prescribing without taking into account the patient's clinical status, failure to include essential information and errors in transcribing (from one prescription to another) are all considered prescribing errors [29]. Failures to adhere to standards such as prescribing guidelines or the drug's product licence, were not considered prescribing errors where these reflected accepted practice. A pharmacist's clinical intervention was defined as any proactive or reactive (in response to a question from another health care professional) activity undertaken to suggest changes in drug therapy or monitoring that involved contacting medical staff. Clarifications of minor ambiguities by pharmacists writing onto the drug chart or amending electronic records were excluded, unless they were also associated with a prescribing error or intervention.
The ward pharmacist was given training in data collection. Dispensary, on-call and weekend pharmacists were also given guidance notes and asked to record prescribing errors and interventions relating to the study ward. A senior clinical pharmacist (BDF) checked all medication orders weekly for prescribing errors overlooked either through nonidentification or nonrecording. Post-EP, this involved screening the ‘active’ medication orders, with ‘stopped’ medication orders checked as needed.
Prescribing errors were classified as resulting either from the process of medication order writing (e.g. missing information, or copying or selecting details incorrectly) or the prescribing decision (e.g. deciding on the choice of drug, formulation or dose) [28]. Errors and interventions were also classified according to the stage of patient stay and the stage of the prescribing process in which they occurred [28].
Scoring the severity of prescribing errors
As described previously [26], a panel of five judges assessed the potential clinical significance of each prescribing error, based on validated methods [30]. The panel comprised two senior clinical pharmacists, a care-of-the-elderly consultant, a clinical pharmacologist and a senior nurse. Each judge assigned a score from 0 (no harm) to 10 (death), and their mean score for each error was used as an index of severity. An error with a score of less than 3 was considered to be minor, a score from 3 to 7 moderate, and greater than 7 serious. Errors were presented in a random order so that judges were not aware which were identified pre- and post-EP.
Analysis
All data were transcribed into Excel and checked by a second investigator. The 95% confidence intervals (CI) were calculated for the absolute differences between proportions using the methods of Gardner & Altman [31].
Results
As reported previously [26], we retrieved 113 (88%) of 129 patients' health care records pre-EP and 126 (86%) of 147 post-EP. We estimated that 2450 medication orders were written for all patients in the pre-EP sample, and 2353 post-EP.
Pharmacists' interventions
Pharmacists' clinical interventions decreased from 73 (3.0% of all medication orders) pre-EP to 45 (1.9%) afterwards, an absolute reduction of 1.1% (95% CI 0.2%, 2.0%). The most common interventions both pre- and post-EP related to the need for drug therapy (either no drug prescribed where one was needed, or drugs prescribed where they were not indicated), and selection of dose. There were fewer interventions post-EP relating to selection of dose (24 and 14 pre- and post-EP, respectively) and specification of instructions for supply or administration (13 and 1 pre- and post-EP, respectively).
Prescribing errors
Prescribing errors decreased from 94 (3.8%) pre-EP to 48 (2.0%) post-EP. The absolute reduction of 1.8% was statistically significant (95% CI 0.9, 2.7%) [26]. As for pharmacists' interventions, the most common category of prescribing errors related to the need for drug therapy and selection of dose [26]. Figure 1 illustrates the relationship between errors and pharmacists' interventions. Only 52% of pharmacists' interventions related to a prescribing error pre-EP, and 60% post-EP. Similarly, only 40% and 56% of prescribing errors resulted in an intervention pre- and post-EP, respectively.
Figure 1.
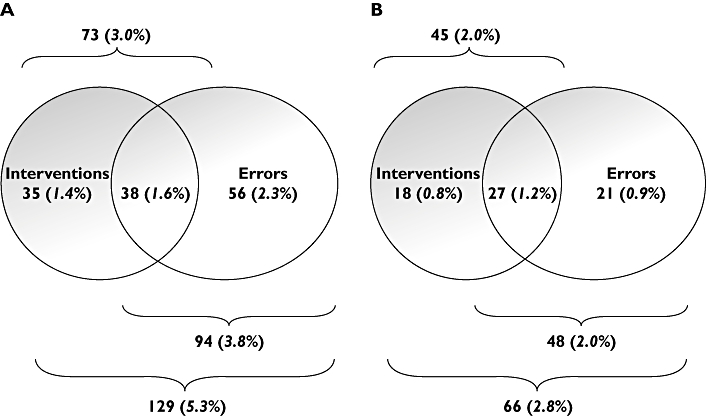
Prescribing errors and interventions identified pre- (A) and post-EP (B). Percentages are of all medication orders written
Both pre- and post EP, the majority of prescribing errors originated in the process of writing (or typing) medication orders rather than in the prescribing decision (Table 1). Pre-EP, many of these errors involved incomplete or otherwise ambiguous medication orders; these were generally eliminated by EP. The post-EP reduction in errors originating in medication order writing was statistically significant (95% CI for the absolute reduction 0.4, 2.0%), while that in errors originating in the prescribing decision was not (95% CI 0.0, 1.0%). A total of 32 errors post-EP related to the medication writing process of which 10 were considered to be caused by the use of EP itself (Table 2).
Table 1.
Prescribing errors originating in the prescribing decision or in medication order writing
| Type of prescribing error (% of all medication orders written) | ||
|---|---|---|
| Origin of prescribing error | Pre-EP | Post-EP |
| Prescribing decision | 28 (1.1%) | 13 (0.6%) |
| Writing medication order | 66 (2.7%) | 35 (1.5%) |
| Total | 94 (3.8%) | 48 (2.0%) |
Table 2.
Prescribing errors originating in medication order writing post-EP, together with an indication of whether or not these were specific to EP; PRN = when required medication
| Description of error | Number of errors | Is this an EP specific error? |
|---|---|---|
| Prescription of PRN drugs to be given at an appropriate frequency (e.g. paracetamol 4 hourly), without specifying the maximum daily dose (e.g. four doses in 24 h) | 16 | No |
| Selection of incorrect product from menu | 5 | Yes |
| Omission of a drug | 3 | No |
| Prescription of PRN drugs to be given 1 hourly | 2 | Yes |
| Selection of the incorrect dosing frequency | 1 | Yes |
| Prescription of a drug for the wrong patient | 1 | Uncertain |
| Prescription of a drug to be given Mondays only when daily was intended | 1 | Yes |
| Prescription of an incorrect formulation | 1 | Yes |
| Prescription of the same drug twice | 1 | No |
| Failure to specify that amiodarone should be reduced to twice daily for 1 week and then once daily, following loading dose of 200 mg three times daily for 1 week | 1 | No |
In terms of the stage of the patient's stay, medication prescribed on admission was associated with the highest prescribing error rates both pre- and post-EP (Table 3). Reductions occurred at all stages post-EP, with the exception of writing the discharge prescription, for which EP was not yet enabled.
Table 3.
Number of prescribing errors originating in each stage of the patient's stay
| Stage of patient stay (% of all medication orders written) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| On admission | During stay | Rewriting chart/transcribing onto EP | Writing discharge prescription | Total | ||||||
| Origin of prescribing error | Post-EP | Pre-EP | Post-EP | Pre-EP | Post-EP | Pre-EP | Post-EP | Pre-EP | Post-EP | Pre-EP |
| Prescribing decision | 31 (1.6%) | 2 (0.5%) | 19 (1.6%) | 10 (0.8%) | 0 | 0 | 1 (0.3%) | 1 (0.6%) | 28 (1.1%) | 13 (0.6%) |
| Writing medication order | 23 (4.7%) | 9 (2.2%) | 29 (2.4%) | 17 (1.3%) | 13 (3.3%) | 9 (2.0%) | 1 (0.3%) | 0 | 66 (2.7%) | 35 (1.5%) |
| Total | 54 (6.3%) | 11 (2.7%) | 48 (4.0%) | 27 (2.0%) | 13 (3.3%) | 9 (2.0%) | 2 (0.6%) | 1 (0.6%) | 94 (3.8%) | 48 (2.0%) |
Severity of the prescribing errors identified
Eighteen (19%) and 9 (19%) errors were minor pre- and post-EP, respectively. The majority (73 (78%) and 33 (69%), respectively) were of moderate severity. Three (3%) severe errors were identified pre-EP; these related to medication orders for concentrated potassium, noradrenaline and fentanyl which were continued following a patient's transfer from the intensive care unit. Six (12%) severe errors were identified post-EP (Table 4); two were considered by author consensus to be specific to the default functions used by EP. As reported previously [26], there was no difference in mean severity scores pre- and post-EP (P = 0.24; unpaired t-test).
Table 4.
Details of the severe errors identified post-EP, together with an indication of whether or not they were judged to be EP specific
| Description of error | Number of errors | Is this an EP specific error? |
|---|---|---|
| Co-prescription of two paracetamol-containing products | 2 | No |
| Selection of vancomycin 125 mg capsules when prescribing vancomycin 750 mg twice daily intravenously | 1 | Yes |
| Prescribing two tablets of Cocodamol to be taken every hour as required | 1 | Yes |
| Prescribing a prophylactic dose of enoxaparin when a full anticoagulation dose should have been used | 1 | No |
| Prescribing ceftazidime 1 g three times daily for a patient with very poor renal function who should have been prescribed 500 mg once daily | 1 | No |
Discussion
This is the first study of the simultaneous impact of EP on two aspects of prescribing quality: prescribing errors and pharmacists' clinical interventions. Pharmacists' interventions decreased significantly post-EP. The incidence of prescribing errors was also reduced; this was primarily due to a reduction in errors in medication order writing and occurred at all stages of the drug use process and throughout all stages of patient stay, with the exception of at discharge where EP was not used. As we suggest elsewhere [26], EP may prevent many of the more minor errors that pharmacists would have previously identified and corrected.
As far as we are aware, this is also the first study to explore the relationship between prescribing errors and pharmacists' clinical interventions. Only 52% of pharmacists' interventions related to a prescribing error pre-EP, and 60% post-EP. Similarly, only 40% and 44% of prescribing errors resulted in an intervention pre- and post-EP, respectively. This lack of overlap has implications for the interpretation of many previous studies where it has been assumed that the two are synonymous.
The EP system could be refined to reduce further the incidence of prescribing errors. First, a facility for changing the default frequency for PRN medication is required. This would avoid the current problem of drugs being unintentionally prescribed for one-hourly administration. Second, a facility for specifying the maximum daily dose for PRN medication would be useful. Third, some additional decision support is required to prevent the selection of an inappropriate dosage form for the required route (e.g. capsules for intravenous administration). Finally, some standard dosing schedules for complex, but standardized, dosing regimens are needed (such as the oral loading dose for amiodarone). Other errors could have been prevented by more advanced forms of decision support, by identifying therapeutic duplication and drug interactions, and by cross-checking of biochemistry or microbiology results.
As well as reducing the number of prescribing errors, EP decreased other types of intervention made by the ward pharmacist. This may be expected to decrease the pharmacist's time requirements. However, a related study shows that EP increased, rather than decreased, the time required by the ward pharmacist [32]. We suspect that many issues were discussed with medical staff in general terms and were therefore not classified and documented as interventions. There are also other potential reasons for the increase in time requirements [32].
The present study also highlighted new error types that were specific to EP. These mainly involved selection of the incorrect product dose or frequency from a menu, and inappropriate use or selection of default doses. While the system reduced straightforward errors in medication order writing, both prescribers and ward pharmacists need to be aware of these new types of error so that they can be identified and rectified, and so that system changes can be made to prevent them.
Until now, clinical pharmacists have provided the main source of support in improving the safety and quality of prescribing in the hospital setting. Our results show that an EP system reduced prescribing errors, although at the expense of an increase in staff time [26] and can be considered an additional approach to achieving these goals. However, some new types of error were introduced. Pharmacists need to understand the strengths and weaknesses of EP; they can then work to complement (rather than duplicate) its strengths, and to compensate for its weaknesses. New systems of working may be required.
Limitations
The method relied on pharmacists identifying and documenting errors and interventions. Previous work [28] suggests that there may be variability between pharmacists in the numbers of prescribing errors documented; for the present study we therefore included a quality assurance check of all medication orders by the principal investigator. This may account for the higher error rate identified (3.8%) using otherwise identical methods [28]. We included TTAs, oral anticoagulants and intravenous infusions in the study, although these remained on paper post-EP; this was because we wanted to include the whole system as it operated in practice.
There has been little work to date comparing the errors identified with paper-based and electronic prescribing. It may be more difficult for pharmacists to identify certain error types with EP. For example, discontinued prescriptions and the recent medication history are not as noticeable on an electronic system as they are on UK drug charts. Further work is needed to compare different methods of prescribing-error identification in different prescribing systems. It is not known whether this may have affected our results. The study also took place in one ward with one EP system; generalizability elsewhere and to other systems is unknown.
This study is also subject to the inherent limitations of before and after comparisons which cannot control for effects of time. Pre-EP data were collected in April, and post-EP data in November/December. Junior doctors start in August and change firms in February. Both data collection periods were therefore about 3 months after doctors started with new firms. However junior medical staff would have been slightly less experienced during the post-EP data collection period. The decline in prescribing errors cannot therefore be attributed to more experienced medical staff.
In conclusion, EP reduced pharmacists' interventions and prescribing errors. Reductions in errors occurred on admission, during the patient stay and on transcribing. The reduction occurred in errors of prescription writing rather than the prescribing decision. However, although there was an overall benefit in prescription quality, new types of prescribing error occurred. Prescribers and pharmacists need to be aware of these new types of error so that they can best target their activities and the set-up of EP systems to reduce clinical risk, and so that pharmacists can work to complement, rather than duplicate, the benefits of EP.
Acknowledgments
The research was funded by MDG Medical and the Department of Health's Patient Safety Research Programme.
Competing interests: The research was partly funded by MDG Medical, the company who developed ServeRx, under an unrestricted grant. MDG Medical did not contribute to study design, data collection, analysis or interpretation of the data, nor to report writing or the decision to submit for publication. The authors' work was independent of MDG Medical.
References
- 1.Committee on Quality of Health Care in America Institute of Medicine. To err Is human: Building a safer health system. Washington, D.C.: National Academy Press; 2000. [PubMed] [Google Scholar]
- 2.Department of Health. An Organisation with a Memory. London: Department of Health; 2000. [Google Scholar]
- 3.Learning from Bristol. The report of the public inquiry into children's heart surgery at the Bristol Royal Infirmary 1984–95. Report of The Bristol Royal Infirmary Enquiry; 2001. [DOI] [PubMed] [Google Scholar]
- 4.National Patient Safety Agency. Building a memory: Preventing harm, reducing risks and improving patient safety. The first report of the National Reporting and Learning System and the Patient Safety Observatory. London: National Patient Safety Agency; 2005. [Google Scholar]
- 5.Ferner RE. Errors in prescribing and giving drugs. J Med Defence Union. 1992;8:60–3. [Google Scholar]
- 6.Leape LL, Bates DW, Cullen DJ, Cooper J, Demonaco HJ, Gallivan T, Hallisey R, Ives J, Laird N, Laffel G, Nemeskal AR, Petersen LA, Porter K, Servi D, Shea BF, Small SD, Sweitzer BJ, Thompson BT, Vander Vliet M. Systems analysis of adverse drug events. JAMA. 1995;274:35–43. [PubMed] [Google Scholar]
- 7.Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R, Vander Vliet M, Nemeskal R, Leape LL. Incidence of adverse drug events and potential adverse drug events: Implications for prevention. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 8.Boyko WL, Yurkowski PJ, Ivey MF, Armistead JA, Roberts BL. Pharmacist influence on economic and morbidity outcomes in a tertiary care teaching hospital. Am J Health-Syst Pharm. 1997;54:1591–5. doi: 10.1093/ajhp/54.14.1591. [DOI] [PubMed] [Google Scholar]
- 9.Barber ND, Batty R, Ridout DA. Predicting the rate of physician-accepted interventions by hospital pharmacists in the United Kingdom. Am J Health-Syst Pharm. 1997;54:397–405. doi: 10.1093/ajhp/54.4.397. [DOI] [PubMed] [Google Scholar]
- 10.Hawkey CJ, Hodgson S, Norman A, Daneshmend TK, Garner ST. Effect of reactive pharmacy intervention on quality of hospital prescribing. BMJ. 1990;300:986–90. doi: 10.1136/bmj.300.6730.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eadon H. Assessing the quality of ward pharmacists' interventions. Int J Pharmacy Prac. 1992;1:145–57. [Google Scholar]
- 12.Cousins D, Gerrett D, Luscombe D. Reliability and validity of hospital pharmacists' clinical intervention data. Am J Health-Systpharm. 1997;54:1596–603. doi: 10.1093/ajhp/54.14.1596. [DOI] [PubMed] [Google Scholar]
- 13.Beney J, Bero LA, Bond C. Expanding the roles of outpatient pharmacists: effects on health services utilisation, costs, and patient outcomes. Cochrane Database Syst Rev. 2000:13. doi: 10.1002/14651858.CD000336. CD000336. [DOI] [PubMed] [Google Scholar]
- 14.Schiff GD, Rucker TD. Computerized prescribing: building the electronic infrastructure for better medication usage. JAMA. 1998;279:1024–9. doi: 10.1001/jama.279.13.1024. [DOI] [PubMed] [Google Scholar]
- 15.Overhage JM, Tierney WM, Zhou XH, McDonald CJ. A randomized trial of ‘corollary orders’ to prevent errors of omission. J Am Med Inform Assoc. 1997;4:364–75. doi: 10.1136/jamia.1997.0040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bates DW, Leape LL, Cullen DJ, Laird N, Petersen LA, Teich JM, Burdick E, Hickey M, Kleefield S, Shea B, Vander Vliet M, Seger DL. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280:1311–6. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- 17.Bates DW, Teich JM, Lee J, Seger D, Kuperman GJ, Ma'Luf N, Boyle D, Leape L. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc. 1999;6:313–21. doi: 10.1136/jamia.1999.00660313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potts AL, Barr FE, Gregory DF, Wright L, Patel NR. Computerized physician order entry and medication errors in a pediatric critical care unit. Pediatrics. 2004;113:59–63. doi: 10.1542/peds.113.1.59. [DOI] [PubMed] [Google Scholar]
- 19.Chertow GM, Lee J, Kuperman GJ, Burdick E, Horsky J, Seger DL, Lee R, Mekala A, Song J, Komaroff AL, Bates DW. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286:2839–44. doi: 10.1001/jama.286.22.2839. [DOI] [PubMed] [Google Scholar]
- 20.Stebbing C, Jacklin A, Barber B, Bates DW. A comparison of the US and UK inpatient medication systems. Eur J Hospl Pharm Practice. 2006;12:36–40. [Google Scholar]
- 21.Fowlie F, Bennie M, Jardine G, Bicknell S, Toner D, Caldwell M. Evaluation of an electronic prescribing and administration system in a British hospital. Pharmaceut J. 2000;265(Suppl):R16. [Google Scholar]
- 22.Almond M, Gordon K, Kent JR, Jones BW, Nice SW, Dhillon S. The effect of the controlled entry of electronic prescribing and medicines administration on the quality of prescribing, safety and success of administration on an acute medical ward. Br J Healthcare Comput Infor Manage. 2002;19:41–6. [Google Scholar]
- 23.Farrar K, Caldwell N, Robertson J, Roberts W, Power B, Slee A. Use of structured paediatric-prescribing screens to reduce the risk of medication errors in children. Br J Healthcare Comput Infor Manage. 2003;20:25–7. [Google Scholar]
- 24.Shulman R, Singer M, Goldstone J, Bellingan G. Medication errors: a prospective cohort study of hand-written and computerised physician order entry in the intensive care unit. Crit Care. 2005;9:R516–21. doi: 10.1186/cc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marriott J, Curtis C, Carruthers T, Feeley G, Langley C, Tounge R, Wilson K. The influence of electronic prescribing on pharmacist clinical intervention reporting. Int J Pharmacy Prac. 2004;12:R44. [Google Scholar]
- 26.Franklin BD, O'Grady K, Donyai P, Jacklin A, Barber N. The impact of a closed-loop electronic prescribing and automated dispensing systems on prescribing errors, administration errors and staff time: a before and after study. Qual Saf Health Care. 2007. in press. [DOI] [PMC free article] [PubMed]
- 27.Brock T, Franklin BD. Differences in pharmacy terminology and practice between the United Kingdom and the United States. Am J Health Syst Pharm. 2007;64:1541–6. doi: 10.2146/ajhp060444. [DOI] [PubMed] [Google Scholar]
- 28.Dean B, Schachter M, Vincent C, Barber N. Prescribing errors in hospital inpatients: their incidence and clinical significance. Qual Saf Health Care. 2002;11:340–4. doi: 10.1136/qhc.11.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dean B, Barber N, Schachter M. What is a prescribing error? Qual Health Care. 2000;9:232–7. doi: 10.1136/qhc.9.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dean BS, Barber ND. A validated, reliable method for scoring the severity of medication errors. Am J Health Syst Pharm. 1999;56:57–62. doi: 10.1093/ajhp/56.1.57. [DOI] [PubMed] [Google Scholar]
- 31.Gardner MJ, Altman DG. London: BMJ Publications; 1989. Statistics with confidence – Confidence intervals and statistical guidelines. [Google Scholar]
- 32.Franklin BD, O'Grady K, Donyai P, Jacklin A, Barber N. The impact of a closed loop electronic prescribing and automated dispensing system on the ward pharmacist's time and activities. Int J Pharm Practice. 2007;15:133–9. [Google Scholar]


