Abstract
The gastrin/CCK receptor (CCK2R) mediates the physiological functions of gastrin in the stomach, including stimulation of acid secretion and cellular proliferation and migration, but little is known about the factors that regulate its expression. We identified endogenous CCK2R expression in several cell lines and used luciferase promoter–reporter constructs to define the minimal promoter required for transcription in human gastric adenocarcinoma, AGS, and rat gastric mucosa, RGM1, cells. Consensus binding sites for SP1, C/EBP and GATA were essential for activity. Following serum withdrawal from RGM1 and AR42J cells, endogenous CCK2R mRNA abundance and the activity of a CCK2R promoter–reporter construct were significantly elevated. Transcription of CCK2R was also increased in AGS-GR and RGM1 cells by gastrin through mechanisms partly dependent upon protein kinase C (PKC) and mitogen/extracellular signal-regulated kinase (MEK). Gastrin significantly increased endogenous CCK2R expression in RGM1 cells, and CCK2R protein expression was elevated in the stomach of hypergastrinaemic animals. In mice with cryoulcers in the acid-secreting mucosa, CCK2R expression increased progressively in the regenerating mucosa adjacent to the ulcer repair margin, evident at 6 days postinjury and maximal at 13 days. De novo expression of CCK2R was observed in the submucosa beneath the repairing ulcer crater 6–9 days postinjury. Many of the cells in mucosa and submucosa that expressed CCK2R in response to cryoinjury were identified as myofibroblasts, since they coexpressed vimentin and smooth muscle α-actin but not desmin. The data suggest that increased CCK2R expression might influence the outcome of epithelial inflammation or injury and that the response may be mediated in part by myofibroblasts.
Amidated peptides of the gastrin–cholecystokinin (CCK) family exert their effects via two G-protein-coupled receptors, the CCK1 receptor, which has high affinity for CCK, and the CCK2 receptor (CCK2R), which has high affinity for both peptides (Noble et al. 1999; Dufresne et al. 2006). The CCK2R (formerly CCKBR) was originally defined in central nervous system (Innis & Snyder, 1980), where it is widely distributed, notably in cerebral cortex and striatum, and where CCK is likely to be the most important endogenous ligand. In the periphery, the CCK2R is located primarily on parietal cells (Kopin et al. 1992) and enterochromaffin-like (ECL) cells within the gastric epithelium, where it mediates the actions of gastrin (Dockray et al. 2005), but expression has also been reported on gastrointestinal smooth muscle cells (Reubi et al. 1997; de Weerth et al. 1997), pancreatic cells (Morisset, 2005; Cayrol et al. 2006) myenteric neurones (Mantyh et al. 1994) and cells of the immune system (Mezey & Palkovits, 1992; Iwata et al. 1996; Schmitz et al. 2001). The main established physiological function of gastrin is the regulation of gastric acid secretion by parietal cells, principally indirectly, through histamine release from ECL cells (Dockray et al. 2005). Increasing evidence suggests that direct activation of the parietal cell CCK2R may be more important for parietal cell maturation and for proliferation and migration of gastric epithelial cells than for acute stimulation of gastric acid secretion (Nagata et al. 1996; Koh et al. 1997; Friis-Hansen et al. 1998; Kirton et al. 2002; Dockray et al. 2005; Dimaline & Varro, 2007). The physiological significance of the CCK2R on peripheral cells other than ECL cells and parietal cells remains to be established.
There are a number of reports that in some clinical or experimental situations, expression of the CCK2R is augmented or induced in sites other than those seen in physiological circumstances. Thus, expression of the CCK2R was seen on proliferating cells in a hypergastrinaemic transgenic mouse that has gastric hyperproliferation (Nakajima et al. 2002) and in the regenerative zone at the margins of experimentally induced cryoulcers in rats (Schmassmann & Reubi, 2000). Upregulated CCK2R expression has also been described in the epithelium of Barrett's oesophagus compared with normal oesophageal epithelium (Haigh et al. 2003) and in intestinal epithelium of mice subjected to γ-irradiation (Ottewell et al. 2006). These data suggest that CCK2R expression may be upregulated during the hyperproliferation seen in response to inflammation and injury of gastrointestinal epithelia. Upregulation of the CCK2R has also been reported in response to its peripheral physiological ligand, gastrin (Takeuchi et al. 1979, 1980; Nakajima et al. 2002; Gunther et al. 2003). Several investigations have examined the association with disease of polymorphisms in the CCK2R promoter (Hamilton et al. 2001; Hattori et al. 2001), but little is generally known about mechanisms of transcription. In the present study, we used an in vivo model to examine expression of the CCK2R in response to gastric epithelial injury and in vitro models to explore expression in response to cellular stress and mechanisms of transcription.
Methods
Antibodies
Antibodies to CCK2R (SC33221) and β-actin were from Santa Cruz (Autogen Bioclear, Calne, UK). Affinity-purified CCK2R antibody no. 9491 was a gift from Dr G. V. Ohning (CURE Digestive Diseases Research Center, Los Angeles, CA, USA). Antibodies to vesicular monoamine transporter 2 (VMAT2), the gastric H+,K+-ATPase β-subunit, desmin, smooth muscle α-actin and vimentin were from Research Diagnostics (Flanders, NJ, USA). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was from AMS (Abingdon, UK).
Chemicals
The CCK2R antagonist L740,093, the PKC inhibitor Ro-32–0432 and the MEK inhibitor PD098059 were from Calbiochem, Beeston, UK. Unsulphated heptadecapeptide gastrin was from Bachem (St. Helens, UK).
Cell culture
Human gastric adenocarcinoma cell lines AGS and AGS-GR (stably transfected with the gastrin/CCK2R; Watson et al. 2001) were maintained in F12–Ham's medium supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin solution (Sigma, Poole, UK). The AGS-GR cells were maintained under selection by supplementing medium with puromycin (2 μg ml−1) every 4 weeks. Rat gastric mucosa RGM1 and rat exocrine pancreatic tumour AR42J cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM)–F12 supplemented with 10% fetal bovine serum and % penicillin–streptomycin. All cells were maintained in a humidified incubator at 37°C under 5% CO2–95% O2.
Reporter constructs
A 1.7 kb fragment of the CCK2R promoter was generated by polymerase chain reaction (PCR) from human genomic DNA. Forward and reverse primers spanned the region from −1765 to −52 bp upstream of the start of translation, and incorporated KpnI and SacI sites, respectively. The promoter fragment was cloned directly into pGEM-Teasy (Promega, Southampton, UK), then subcloned into the promoterless luciferase reporter vector pGL4.10 (Promega) between the KpnI and SacI sites to generate CCK2R-1700. A similar strategy was employed to generate a larger construct containing 4.5 kb of promoter. The 1.7 kb construct was used as a template to generate the deletional mutants CCK2R-928, CCK2R-284, CCK2R-196, CCK2R-130, CCK2R-103, CCK2R-71 and CCK2R-48. Transverse mutations of the CCK2R-196 construct were generated directly by PCR, incorporating appropriate base substitutions in the forward primer. Regions mutated were within consensus binding sites for C/EBPβ (CTGG to AGTT), SP1 (GCGG to TATT) and GATA (GATA to TCGC).
Transfections
For luciferase reporter experiments, AGS, AGS-GR or RGM1 cells (2 × 106 per well) were plated into six-well plates, 24 h before transfection with either 1.0 μg CCK2R–luciferase constructs (AGS or RGM1) or empty vector using 3 μl Transfast (Promega) for 1 h. The AR42J cells (5 × 105) were plated into 12-well plates and transfected with 2.0 μg DNA per well using 1 μl Polymag (Oz Biosciences, Marseille, France); culture plates were incubated at room temperature for 25 min, placed on the magnetic plate for a further 20 min, then incubated overnight at 37°C with 2 ml full serum medium per well.
To take account of non-specific changes in luciferase activity, Renilla luciferase reporter vector (phRL-SV40; Promega) was included in all transfections and the ratio of firefly/Renilla luciferase activity calculated. Following transfection, cells were maintained in full medium for 18 h before stimulation with gastrin or serum withdrawal. Luciferase activity was determined using a Lumat LB9507 luminometer (Berthold, Redbourne, UK) and a dual luciferase assay system (Promega).
Extraction of RNA and PCR
Total RNA was extracted from cell lines using TRIzol (Invitrogen, Paisley, UK) according to the manufacturer's protocol. Residual contamination by genomic DNA was eliminated by incubation of 10–100 μg RNA with 1 U μg−1 RNA deoxyribonuclease 1 (Promega) in 10 mmol l−1 Tris-HCl, pH 8.3, 50 mmol l−1 KCl and 1.5 mmol l−1 MgCl2 for 30 min at 37°C. Samples of DNAse-treated total RNA (2 μg) from AGS-GR, AGS, RGM1 and AR42J cells were reverse transcribed using 20 U Avian myeloblastosis virus (AMV) reverse transcriptase (Promega). For each RNA sample, reverse transcription reactions were performed using 0.5 μg of oligonucleotide (dT) primer. The PCR amplification was performed on 1 μl first-strand cDNA using Taq DNA Polymerase (Promega). For quantitative PCR, samples of total RNA were treated for 30 min at 37°C with ribonuclease-free deoxyribonuclease (1 U (μg RNA)−1; Promega) and then extracted with acid phenol–chloroform and the aqueous phase precipitated. The RNA pellets were reconstituted in nuclease-free water, quantified, and 2 μg reverse transcribed using random hexamers. Real-time PCR was performed using TaqMan primer–probe sets for rat CCK2R and 18S rRNA (Eurogentec, Seraing, Belgium) on an ABI Prism 7700 sequence detection system (Applied Biosystems, Warrington, UK). Expression of CCK2R and 18S rRNA in each sample was separately determined using a standard curve method, and the CCK2R value normalized to 18S in the same sample.
Western blotting
Protein extracts from RGM1 and AR42J cells were prepared in lysis buffer (McCaig et al. 2006) containing 1% protease inhibitor cocktail set III and 1% phosphatase inhibitor cocktail set II (Calbiochem). Mice 10–12 weeks old were INS-GAS (Wang et al. 1996) or FVB, housed in polycarbonate-bottomed cages with a strict light cycle (lights on at 06.00 and off at 18.00 h) and fed on a commercial pellet diet with water ad libitum. Animals were killed by increasing CO2 concentrations followed by cervical dislocation. The stomach was removed and a full thickness section of the corpus homogenized in RIPA buffer (Upstate Biotechnology, Cambridge, UK), and Western blotting was performed as previously described (Khan et al. 2003) using a rabbit anti-CCK2R antibody (gift of Dr G. V. Ohning). Membranes were reprobed with a goat anti-actin antibody (Santa Cruz) or mouse anti-GAPDH antibody (AMS). Expression of CCK2R protein relative to actin or GAPDH in the same samples was estimated by densitometry (Multi-Analyst; Bio-Rad, Hemel Hempstead, UK).
Cryoinjury ulcer model in C57BL/6 mice
Animals used were 8- to 10-week-old female C57BL/6 mice, housed in polycarbonate-bottomed cages with a strict light cycle (lights on at 06.00 and off at 18.00 h) and fed on a commercial pellet diet with water ad libitum. Experimental protocols were approved by the institutional animal welfare committee. Mice were anaesthetized by isofluorane inhalation, and the abdomen was incised by a mid-line laparotomy. A cryoinjury was induced on the ventral serosal surface of the gastric corpus by applying, for 20 s, a 2 mm diameter stainless-steel probe cooled in liquid nitrogen, and the abdomen closed. After 2, 6, 9 or 13 days, the mice were killed by inhalation of increasing CO2 concentrations followed by cervical dislocation. Grossly visible damage to the mucosa was identified, and gastric tissue was immediately fixed in 4% paraformaldehyde for 1 h at room temperature before being transferred to 20% sucrose at 4°C overnight. Tissue was embedded in Cryo-M-Bed (Bright Instrument Co. Ltd, Huntingdon, UK) and frozen to −40°C in isopentane. Tissue was stored at −70°C prior to sectioning. Serial sections (10 μm) were cut (−30°C) using a cryostat and thaw-mounted onto poly-l-lysine-coated microscope slides prior to immnunohistochemical staining or in situ autoradiography. Sections were taken through the cryoulcers and adjacent margins. For comparison with normal gastric tissue, sections were taken from 0.5 cm away from the ulcer site and from the opposite, dorsal wall of the gastric corpus.
Immunohistochemistry
Immunohistochemistry was performed on frozen sections. Primary antibodies [polyclonal rabbit antihuman CCK2R (1:400, Santa Cruz); polyclonal guinea-pig antibovine vimentin (1:200, RDI); mouse monoclonal anti-smooth muscle α-actin (1:400); guinea-pig polyclonal anti-VMAT2 (1:400); mouse monoclonal anti-H+/K+-ATPase β subunit (1:1000); and rabbit polyclonal anti-desmin (1:400)] were incubated at 4°C overnight. Thereafter, the slides were washed by stirring in Tris-buffered saline containing 0.25% Triton X-100 three times for 10 min at room temperature. The CCK2R was visualized with fluorescein isothiocyanate-conjugated AffiniPure donkey antiserum raised against rabbit immunoglobulin G (1:200; Jackson ImmunoResearch, West Grove, PA, USA) and other antigens by Texas Red-conjugated AffiniPure antisera appropriate to the primary antiserum. Immunochemical staining was examined with a fluorescence microscope (Axioplan Universal, Carl Zeiss, Welwyn Garden City, UK).
In situ hybridization
Cryostat sections (10 μm) of paraformaldehyde-fixed stomach sections from control mice and mice with cryoulcers were thawed and acetylated in 0.25 m acetic anhydride and 0.1 m triethanolamide (10 min). Sections were dehydrated in 70–95% ethanol and stored in 95% ethanol at 4°C overnight or used immediately. Oligonucleotide probes complementary to bases 540–585, 887–932 and 2038–2083, relative to the start of translation, of the mouse CCK2R receptor (Eurogentec) were 3′ end-tailed with [35S]dATP (10 mCi ml−1; Amersham Biosciences, Little Chalfont, UK), purified using QIAquick nucleotide removal columns (Qiagen, Crawley, UK), and used at a concentration of 3000 c.p.m. μl−1 in hybridization buffer [50% formamide, 10% dextran sulphate, SSPE (1 × SSPE is composed of 3 m NaCl, 0.2 m NaH2PO4.2H2O and 0.2 m EDTA, pH 7.4), 0.2 mg ml−1 sheared salmon sperm DNA, 0.1 mg ml−1 poly A RNA, 5 × Denhardt's solution, and 20 mm dithiothreitol (DTT)]. Hybridization of each of the three oligonucleotides independently or of all three CCK2R oligonucleotides together (1:1:1) was performed overnight at 42°C; sections were washed in 1 × standard saline citrate (SSC) (150 mm NaCI, 15 mm sodium citrate, pH 7.0) at 55°C (30 min), 1 × SSC 22°C (30 min), 0.1 × SSC (30 s), and dehydrated through ethanol, air dried, dipped in autoradiographic emulsion (LM-1, Amersham Biosciences), and exposed for 3–4 weeks before development. Sections were developed, counterstained with Haematoxylin and Eosin, dehydrated, and mounted using DPX. Silver grains were visualized using an Axioplan Universal microscope, and images were processed using AxioVision 3.0 Imaging system (Zeiss) combined with dark-field and bright-field illumination. In control slides, 100-fold excess of the relevant unlabelled oligonucleotides were included in the hybridization.
Statistical analyses
Data are presented as means ±s.e.m. Statistically significant differences (taken as P < 0.05) were determined by one-way ANOVA or Student's unpaired t test as appropriate.
Results
Endogenous expression of CCK2R in cell lines
Expression of endogenous CCK2R mRNA in cell lines was initially determined by conventional reverse transcriptase (RT)-PCR; AGS-GR cells permanently transfected with the human CCK2R were used as a positive control. Products of the anticipated size were detected in AGS-GR, RGM1 and AR42J cells. Subsequent analyses were performed using real time PCR (see below). We were unable to detect expression in AGS cells not permanently transfected with the receptor, or in IEC-6 cells derived from rat intestine.
Mechanism of basal CCK2R transcription
Transcription of the mouse CCK2R has been mapped to 199 bp upstream of the start of translation (Lay et al. 2000). For the human receptor, 185 bp of 5′ untranslated cDNA have been described (Herget et al. 1994), which agrees closely with the start of transcription annotated on the National Center for Biotechnology Information human genome database (193 bp upstream of the start of translation). The numbering of constructs used in this study assumes that transcription (+1) starts 193 bp upstream of translation.
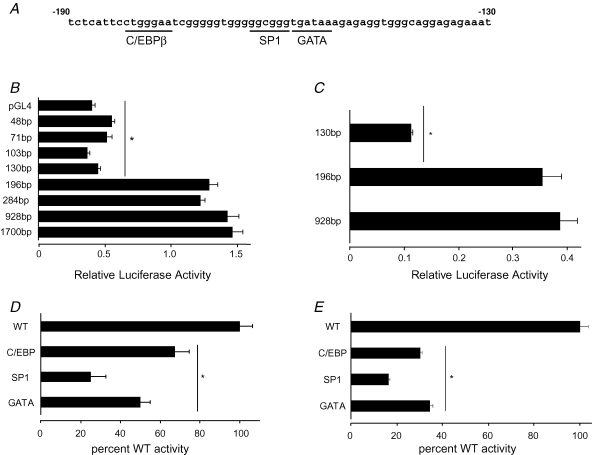
To determine the regions of the CCK2R promoter required to sustain basal levels of transcription, sequential deletions of the promoter were generated from 1700 to 48 bp. Truncation of the promoter from 1700 to 196 bp had little effect on basal transcription in AGS cells, but further shortening to 130 bp virtually abolished activity (Fig. 1). The region −196 to −130 contains consensus binding sites for the transcription factors C/EBPβ, SP1 and GATA, and to establish their importance we independently mutated each of the three sites in CCK2R-196. Mutation of each individual site significantly reduced basal activity, with maximal reduction of 74.8 ± 5.4%, compared with wild-type, seen in the SP1 mutant (Fig. 1). Similar data were obtained when basal transcription was analysed in RGM1 cells (Fig. 1).
Figure 1. Activity of CCK2R promoter–reporter constructs in gastric cell lines.
A, sequence of the human CCK2R promoter −130 to −190 region, showing consensus cis-regulatory elements. Numbering is relative to the start of transcription (+1), which is taken as 193 bp upstream of the start of translation (see main text). B, basal activity of CCK2R promoter–reporter constructs (size as indicated) 24 h after transfection in AGS cells. * Significantly reduced relative to 1700 bp construct (P < 0.001, n = 3, ANOVA). C, basal activity of CCK2R promoter–reporter constructs (size as indicated) 24 h after transfection in RGM1 cells. * Significantly reduced relative to 928 bp construct (P < 0.001, n = 3, ANOVA). D, basal activity of mutated 196 bp constructs 24 h after transfection into AGS cells. Mutations as indicated were made corresponding to the cis-regulatory elements underlined in A. Values are percentage of wild-type (WT) sequence activity (= 100%). * Significantly reduced compared with WT activity (P < 0.001, n = 3). E, basal activity of mutated 196 bp constructs 24 h after transfection into RGM1 cells. Mutations as described in D. Values are percentage of WT sequence activity (= 100%). * Significantly reduced compared with WT activity (P < 0.001, n = 3, ANOVA).
Expression of CCK2R in response to serum deprivation
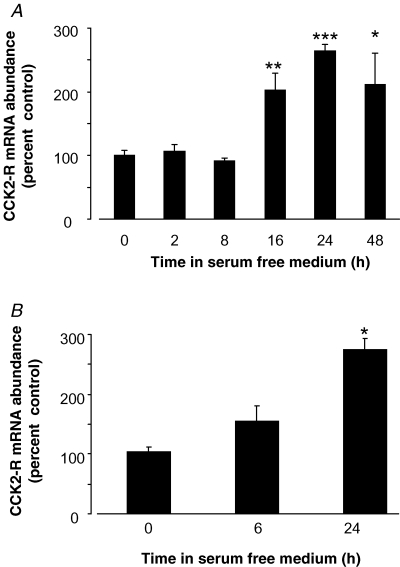
To establish whether endogenous CCK2R expression might be altered in response to changes in the cellular environment, we used real time PCR to determine expression following withdrawal of growth factors by serum starvation of the cells. When serum was withdrawn from cultured AR42J cells, CCK2R mRNA abundance was doubled after 16 h, increased 2.5-fold after 24 h, and was still elevated after 48 h (Fig. 2). This increase results at least in part from increased transcription, since expression of a 1700 bp promoter–reporter construct was increased in response to withdrawal of serum for 24 h (control 100 ± 10.0%; serum starvation, 184.8 ± 31.3%, P = 0.013, n = 3, Student's unpaired t test). Elevation of endogenous CCK2R mRNA abundance was also seen in RGM1 cells deprived of serum for 24 h (Fig. 2) and, again, expression of a promoter–reporter construct was significantly increased (control 100 ± 7.4%; serum starvation, 167.7 ± 25.1%, n = 3, P = 0.04, Student's unpaired t test).
Figure 2. Endogenous CCK2R mRNA abundance in response to serum withdrawal from cell lines.
A, endogenous CCK2R mRNA abundance in AR42J cells in response to serum withdrawal for times indicated. Results are normalized to 18S rRNA and are expressed as percentage of full serum value (= 100%). n = 4. *P = 0.016, **P = 0.013, ***P = 0.005 versus time 0, ANOVA. B, CCK2R mRNA abundance in RGM1 cells in response to serum withdrawal for times indicated. Results are normalized to 18S rRNA and are expressed as percentage of full serum value (= 100%). n = 3. *P = 0.0016 versus time 0, ANOVA.
Gastrin-induced CCK2R expression
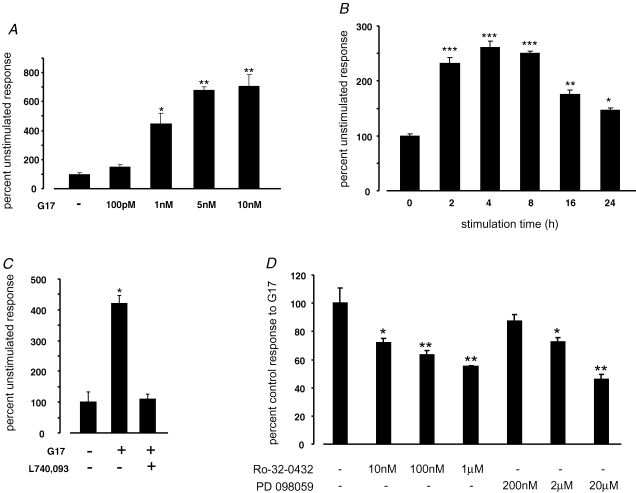
Gastrin has been reported to upregulate CCK2R expression in the gastric surface mucus cell line GSM06 (Nakajima et al. 2002) and in pancreatic AR42J cells (Gunther et al. 2003), in support of much earlier reports that CCK2R expression in vivo might be regulated by serum gastrin concentrations (Takeuchi et al. 1979, 1980). We used CCK2R promoter–reporter constructs to determine whether gastrin could induce CCK2R transcription in AGS-GR cells and in the untransformed cell line RGM1. In AGS-GR cells, gastrin increased CCK2R expression in a dose-dependent manner over the range 10−10 to 5 × 10−9m (Fig. 3). For 10−9m gastrin, the response peaked between 2 and 4 h, and could be prevented by the CCK2R antagonist L740,093 (Fig. 3). The response to gastrin was also reduced in a dose-dependent manner by the PKC inhibitor Ro-32–0432 and by the MEK inhibitor PD098059 (Fig. 3).
Figure 3. Gastrin-stimulated CCK2R expression in AGS-GR cells.
A, luciferase activity of a 4.5 kb CCK2R promoter–reporter construct in AGS-GR cells, 6 h after stimulation with G17 at the doses indicated. Results are expressed as a percentage of the unstimulated response. n = 3. *P = 0.0034, **P < 0.0001 versus unstimulated control cells, ANOVA. B, luciferase activity of a 4.5 kb CCK2R promoter–reporter construct in AGS-GR cells, after stimulation with G17 (1 nm) for the times indicated. Results are expressed as a percentage of the unstimulated response. n = 3. *P = 0.0116, **P = 0.0002, ***P < 0.0001 versus time 0, ANOVA. C, luciferase activity of a 4.5 kb CCK2R promoter–reporter construct in AGS-GR cells, 6 h after stimulation with G17 (1 nm) in the presence or absence of the CCK2R antagonist L740,093 (100 nm). Results are expressed as a percentage of the unstimulated response. n = 3. *P = 0.0003 versus unstimulated control cells, ANOVA. D, luciferase activity of a 4.5 kb CCK2R promoter–reporter construct in AGS-GR cells, 6 h after stimulation with G17 (1 nm) in the presence of the PKC inhibitor Ro-32–0432 or the MEK inhibitor PD 098059, at the doses indicated. Results are expressed as a percentage of the response to G17 in the absence of inhibitor (= 100%). n = 3. **P < 0.0025, *P < 0.025 versus control cells, ANOVA.
Reduction of the promoter–reporter construct from 4.5 to 1.7 kb significantly reduced the gastrin responsiveness (4.5 kb 100 ± 2.5%, 1.7kb 64.6 ± 3.9%, n = 3, P < 0.0001, ANOVA). Further reduction to 775 bp reduced the gastrin responsiveness to 50.8 ± 1.5% that of the 4.5 kb construct, but reduction from 775 to 196 bp had no additional effect. However, when the C/EBPβ and SP1 sites were mutated in the 196 bp construct, gastrin responsiveness was reduced to 45.5 ± 1.3 and 67.6 ± 1.5% of the wild-type construct (100 ± 3.2%), respectively (P < 0.002, ANOVA, n = 3). Mutations of the GATA site did not affect the response.
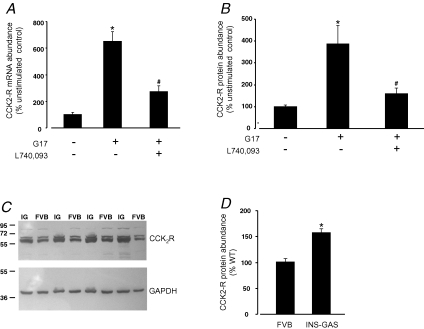
In RGM1 cells, G17 induced CCK2R expression at the level of transcription, mRNA abundance and protein abundance. Thus, expression of the 4.5 kb promoter–reporter construct was increased by G17 in a manner that was sensitive to the CCK2R antagonist L740,093 (control 100 ± 8.2%, G17 258 ± 6.0%, G17 + L740,093 148 ± 11.1%, n = 3). Endogenous CCK2R mRNA abundance was also significantly increased by gastrin in RGM1 cells cultured for 6 h in serum-free medium, and was reduced in the presence of L740,093 (Fig. 4). The CCK2R protein was also significantly increased when RGM1 cells were exposed to gastrin (Fig. 4).
Figure 4. Gastrin-stimulated CCK2R expression in RGM1 cells and in vivo.
A, endogenous CCK2R mRNA abundance in RGM1 cells. Cells were cultured for 6 h in serum-free medium in the presence or absence of G17 (1 nm) and the CCK2R antagonist L740,093 (100 nm). The abundance of CCK2R mRNA was determined by real time PCR and normalized to 18S rRNA. Data are expressed as a percentage of the unstimulated control cells. n = 3. *P < 0.0001 versus control cells; #P < 0.0001 versus G17 stimulated, ANOVA. B, CCK2R protein expression in RGM1 cells. Cells were cultured for 6 h in serum-free medium in the presence or absence of G17 (1 nm) and the CCK2R antagonist L740,093 (100 nm). The CCK2R protein was detected by Western blotting, and abundance estimated by densitometry and normalized to GAPDH. *P < 0.0001 versus control cells; #P < 0.0001 versus G17 stimulated, ANOVA. C, Western blot of CCK2R (upper panel) and GAPDH (lower panel) in gastric mucosa from INS-GAS (IG) and wild-type background (FVB) mice. Numbers to the left indicate size markers (in kDa) run in the same gel. D, CCK2R protein abundance in the gastric mucosa from INS-GAS mice and their wild-type background strain (FVB). Values, expressed as a percentage of the wild-type abundance, are normalized to GAPDH. *P = 0.0014, Student's unpaired t test, n = 8).
To determine whether the gastrin-stimulated CCK2R expression observed in cell lines might be relevant in vivo, we examined CCK2R expression by Western blotting in a transgenic mouse (INS-GAS, Wang et al. 1996) that expresses a gastrin transgene in pancreatic β cells and that is hypergastrinaemic. In INS-GAS mice there was a significant elevation of gastric CCK2R expression compared with wild-type control mice (Fig. 4).
Induction of CCK2R expression in gastric epithelium following cryoulceration
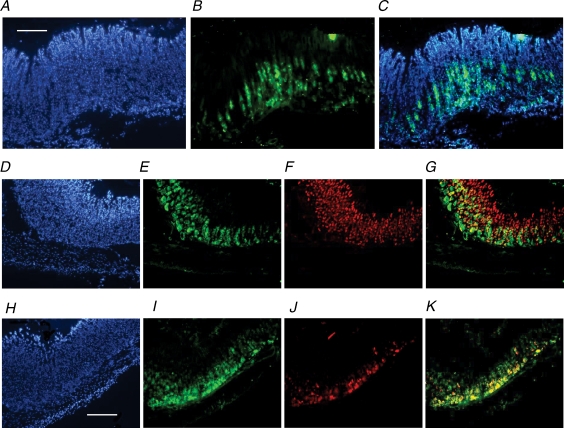
In normal undamaged mucosa from the dorsal surface of the gastric corpus, CCK2R-like immunoreactivity was confined predominantly to epithelial cells in the lower two-thirds of the gastric glands, consistent with localization in parietal cells and ECL cells and compatible with previously published data (Kulaksiz et al. 2000; Schmassmann & Reubi, 2000; Khan et al. 2003). Dual staining with antisera against the β subunit of the gastric H+,K+-ATPase or VMAT2 identified the CCK2R-expressing cells as parietal cells and ECL cells, respectively (Fig. 5). Parietal cells in the deeper regions of the gland generally expressed the CCK2R, whereas those in the more superficial regions frequently did not (Fig. 5).
Figure 5. Expression of CCK2R in normal corpus.
A–C, CCK2R immunoreactivity in section of full-thickness gastric corpus. A, 4′,6-diamidino-2-phenylindole (DAPI); B, CCK2R (fluorescein isothiocyanate; FITC); and C, overlay. D–G, CCK2R and H+,K+-ATPase β subunit immunoreactivity in gastric corpus. D, DAPI; E, CCK2R (FITC); F, H+,K+-ATPase β subunit (Texas Red); and G, CCK2R and H+,K+-ATPase β subunit overlay. H–K, CCK2R and VMAT2 immunoreactivity in gastric corpus. H, DAPI; I, CCK2R (FITC); J, VMAT2 (Texas Red); and K, CCK2R and VMAT2 overlay. Sections are representative of samples obtained from four individual animals. For A–C, scale bar respresents 250 μm; for D–K, scale bar represents 200 μm.
Two days after application of a cryoprobe to the serosal surface of the gastric corpus, single cryoulcers approximately 2 mm diameter were macroscopically visible in the mucosa, corresponding to the site of cryoprobe application. Progressive repair of the ulcer crater occurred at 6, 9 and 13 days postinjury, and by 13 days the mucosal ulceration was barely discernible macroscopically. There was evidence of expansion of the submucosal layer 6 days after injury (Fig. 6).
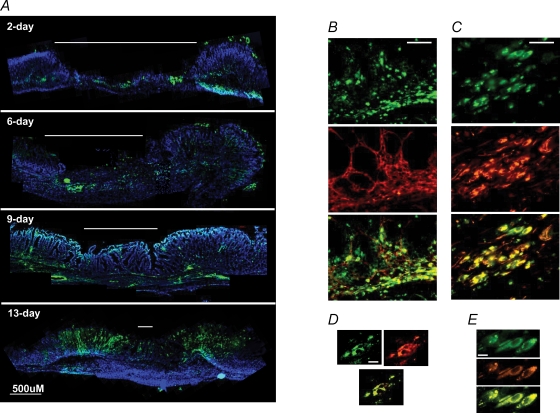
Figure 6. Expression of CCK2R in gastric corpus following cryoulceration.
A, CCK2R immunoreactivity and DAPI staining in full-thickness corpus 2, 6, 9 and 13 days following cryoulcer generation. Note intense epithelial staining at ulcer repair margins at 13 days, and de novo staining in the submucosa beneath the ulcer crater (indicated by white line) at 9 days. Scale bar represents 500 μm. Sections are representative from four individual animals at each time point. B, CCK2R and smooth muscle α-actin immunoreactivity in gastric corpus epithelium at the repair margin of a 9 day ulcer. Upper panel, CCK2R (FITC); centre panel, smooth muscle α-actin (Texas Red); and lower panel, overlay. C, CCK2R and vimentin immunoreactivity in gastric corpus submucosa beneath the repairing crater of a 9 day ulcer. Upper panel, CCK2R (FITC); centre panel, vimentin (Texas Red); and lower panel, overlay. D, deconvolved images of gastric corpus epithelial cells at the repair margin of a 9 day ulcer. Upper left panel, CCK2R (FITC); upper right panel, smooth muscle α-actin (Texas Red); and lower panel, overlay. E, deconvolved images of gastric corpus submucosal cells beneath the repairing crater of a 9 day ulcer. Upper panel, CCK2R (FITC); centre panel, vimentin (Texas Red); and lower panel, overlay. For B and C, scale bar represents 50 μm; for D and E, scale bar respresents 10 μm.
Two days following cryoulcer generation, CCK2R-like immunoreactivity at the ulcer margins remained similar to that seen in non-damaged areas of stomach, consistent with a previous report concerning CCK2R expression at a single time point of 2 days after cryoulcer generation (Khan et al. 2003). However, 6, 9 and 13 days following cryoulcer generation there was a progressive increase in CCK2R immunoreactivity in regenerating mucosa adjacent to the ulcer crater, maximal after 13 days (Fig. 6).
Induction of CCK2R expression in gastric submucosal layers following cryoulceration
In addition to the increased CCK2R expression in the regenerative epithelium seen in response to cryoinjury, there was also a time-dependent increase in CCK2R immunoreactivity in cells within the submucosa. Expression of CCK2R on submucosal cells was not discernible after 2 days, was maximal at 6–9 days and reduced by 13 days, when the ulcer was mostly healed, at least at the macroscopic level. No CCK2R immunoreactivity was observed in the submucosa of tissue away from the ulcer, at any time point (Fig. 6). Epithelial or submucosal CCK2R expression was not seen when primary antibody was omitted from the reactions (data not shown). Moreover, the pattern of CCK2R expression on cells within the mucosa and submucosa was confirmed by in situ hybridization (Fig. 7).
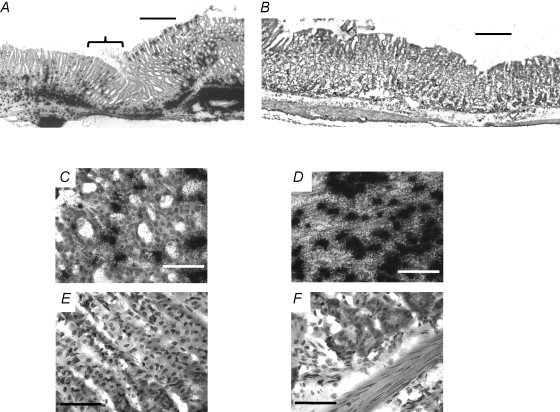
Figure 7. In situ hybridization of the CCK2R in gastric corpus.
A, CCK2R expression in mucosa and submucosa 9 days following cryoulcer generation. Bracket indicates residual ulcer crater. B, CCK2R expression in mucosa and submucosa 9 days following cryoulceration, 0.5 cm away from the ulcer site. C, regenerative mucosa, adjacent to cryoulcer. D, submucosa below residual ulcer crater. E, normal mucosa, 0.5 cm away from site of ulcer. F, normal submucosa, 0.5 cm away from site of ulcer. For A and B, scale bar respresents 300 μm; for D–F, scale bar represents 50 μm. Hybridizations were performed using a mixture (1:1:1) of the three oligonucleotides described in the Methods.
Identification of CCK2R expression in myofibroblasts
In order to determine which cell types express the CCK2R within the mucosa and submucosa during the repair process, dual immunohistochemistry was performed. Previous reports have suggested that the CCK2R may be expressed on smooth muscle or smooth muscle-like cell types in stomach (Reubi et al. 1997). Consequently, 9 day sections were dual stained with CCK2R antibody together with antibodies to cell markers of smooth muscle, fibroblasts and myofibroblasts. The CCK2R immunopositive cells colocalized with vimentin and with smooth muscle α-actin (Fig. 6). No desmin immunoreactivity was observed in CCK2R-expressing cells. Taken together, these data suggest novel expression of the CCK2R on gastric myofibroblasts in mucosa and submucosa.
Discussion
The present study identified endogenous expression of the CCK2R in both transformed and untransformed cell lines derived from the gastrointestinal tract. When cells were stressed by withdrawal of serum, CCK2R expression was upregulated, at least in part through transcriptional activation. The expression of CCK2R in cell lines was also upregulated by gastrin at close to physiological concentrations, and the receptor was upregulated in vivo in transgenic mice that were hypergastrinaemic. In normal gastric mucosa of C57BL/6 mice, CCK2R expression was restricted primarily to the epithelium, on parietal and ECL cells. One to 2 weeks following cryoulceration of the mucosa, there was intense expression of CCK2R at the repair margins of the ulcer and in the submucosa beneath it, in cells that were identified as myofibroblasts.
Numerous studies employing a variety of approaches indicate that in the normal acid-secreting mucosa, the CCK2R is expressed primarily on ECL and parietal cells (Kopin et al. 1992; Prinz et al. 1993; Kulaksiz et al. 2000; Dockray et al. 2005), and this expression pattern was confirmed in the present study. The CCK2R plays a major role in the physiological regulation of gastric acid secretion, which is now believed to occur primarily through gastrin-stimulated histamine release from the ECL cell (Dockray et al. 2005). The importance for acute regulation of acid secretion of the parietal cell CCK2R is therefore open to question. Evidence is accumulating to suggest instead that the CCK2R may be crucial for parietal cell maturation and perhaps migration, as well as for the release of a number of paracrine mediators (Koh et al. 1997; Friis-Hansen et al. 1998; Kirton et al. 2002; Dimaline & Varro, 2007). Studies on rabbit gastric glands provided evidence for heterogeneity of the parietal cell population, with the acid secretory capacity of cells near to the base of the gland appearing to be lower than that of those from the mid- or upper regions (Karam et al. 1997). Interestingly, in the present study, colocalization of the CCK2R with the H+,K+-ATPase β subunit was markedly greater in the base region of the gland, with much lower CCK2R expression in parietal cells towards the mid-glandular region. It seems plausible that this reflects a lesser role for the CCK2R in the more active acid secretory population of parietal cells and raises the possibility that it might also be involved in regulating non-acid secretory functions of the more deeply situated parietal cell population.
The factors that regulate expression of the CCK2R are not well known. Expression of the CCK2R is well documented in the pancreatic cancer cell line AR42J (Seva et al. 1994) and has been reported in the untransformed rat gastric mucosa-derived cell line RGM1 (Cui et al. 2006). In the present study, we were able to detect endogenous CCK2R expression at the level of both mRNA and protein in AR42J and RGM1 cells. Using luciferase–reporter constructs we showed that a 196 bp fragment of the proximal promoter was sufficient to support basal CCK2R expression in RGM1 cells, and that consensus binding sites for SP1, GATA and C/EBPβ were required for this expression. Similar data were obtained using AGS-GR cells. Putative binding sites for these transcription factors were also identified in the proximal promoters of the rat and mouse genes using the transcription element search system (TESS, http://www.cbil.upenn.edu/tess), suggesting that they are likely to be important determinants of CCK2R expression.
Several studies have sought associations between a CT repeat polymorphism (conserved as CA repeats in the rat and mouse promoters) that lies between 250 and 200 bp upstream of the start of transcription and panic disorder or schizophrenia (Kennedy et al. 1999; Hamilton et al. 2001; Hattori et al. 2001). No clear association was found and, in the present study, truncation of this region had little or no effect on basal transcription in RGM1 or AGS cells. Increases in CCK2R expression were seen in RGM1 and AGS cells in response to deprivation of growth factors by serum withdrawal for more than about 8 h. The response was at least in part due to changes in transcription, since expression of reporter constructs containing 1.7 kb of the CCK2R promoter was significantly elevated in similar circumstances. The data are consistent with the idea that expression of the CCK2R may respond to changes in the microenvironment, such as the alteration of growth factor profiles that might occur during responses to inflammation or injury in vivo.
Studies from a number of years ago that correlated serum gastrin concentrations with the capacity of gastric mucosal membrane preparations to bind radiolabelled G17 led to the suggestion that gastrin might stimulate the production of its own receptor (Takeuchi et al. 1979, 1980). These findings remained unconfirmed for many years, although support for the idea came from recent studies using transgenic hypergastrinaemic mice and a pit cell precursor line, which suggested that gastrin induced expression of the CCK2R in pit cell precursors (Nakajima et al. 2002). Transient expression of the CCK2R in response to a relatively high concentration of G17 (10 nm) has also been reported in a PCR-based study using AR42J cells (Gunther et al. 2003).
In the present study, we showed that gastrin increased transcription of CCK2R promoter–reporter constructs both in AGS-GR cells that are permanently transfected with the CCK2R and in untransformed RGM1 cells that endogenously express it. Transcription was significantly increased in a dose-dependent manner from 100 pm to 10 nm G17, and increases in transcription were detectable within 2 h of stimulation. The response could be completely prevented by a CCK2R antagonist, and was reduced in a dose-dependent manner by inhibitors of PKC and MEK to a minimum of around 50% of control values. Previous studies have shown that a number of CCK2R-mediated increases in gene transcription are effected, at least in part, through PKC- and MEK-dependent pathways (Hocker et al. 1997; Watson et al. 2001; Raychowdhury et al. 2002; Khan et al. 2003), and it seems this is also the case for gastrin-stimulated CCK2R expression. As with basal expression, the response was not dependent on the CT polymorphic region, but did depend upon intact C/EBPβ and SP1 sites in the proximal promoter. In RGM1 cells, endogenous CCK2R mRNA abundance and protein levels were elevated around fivefold in response to 1 nm G17, and the response was in both cases significantly attenuated by a CCK2R antagonist.
To further address the relevance of these findings in vivo, we determined expression of the CCK2R in the stomach of a mouse model that has altered expression of the gastrin gene with consequential effects on circulating gastrin concentrations. The INS-GAS mouse model expresses a gastrin transgene in pancreatic β cells and is hypergastrinaemic (Wang et al. 1996). In these animals, a 50% increase in gastric CCK2R expression was seen compared with wild-type FVB control mice. These data are consistent with the notion that peripheral CCK2R expression may be chronically regulated by gastrin and provide support for earlier reports linking expression of a functional gastrin receptor with circulating hormone concentrations (Takeuchi et al. 1979, 1980).
Aside from its primary physiological expression in ECL and parietal cells, a number of recent studies have reported increased or de novo expression of the CCK2R in other unspecified gastrointestinal cell types in some experimental or clinical circumstances. For example, expression was demonstrated by autoradiography in the margins of cryoulcers induced in rats within 5 days of injury, and ulcer repair was enhanced by induction of hypergastrinaemia (Schmassmann & Reubi, 2000). The cell type in which the CCK2R was induced was not identified, but they were presumed to be relatively undifferentiated cells derived from progenitor cells. Induction of CCK2R expression has been described in proliferating cells in a transgenic mouse with a gastric hyperproliferative phenotype (Nakajima et al. 2002). Expression of a functional CCK2R was also increased in the epithelium of Barrett's metaplasia, compared with unaffected individuals (Haigh et al. 2003), and in the intestinal epithelium of mice following γ-radiation-induced injury (Ottewell et al. 2006). Taken together, these data suggest that upregulation of the CCK2R occurs in a range of hyperproliferative conditions that are likely to include inflammation or injury to the gastrointestinal epithelium.
In a previous study, we reported that 2 days after cryoulceration of mouse gastric corpus mucosa, the intensity and distribution of CCK2R expression was largely unaltered from that seen in uninjured tissue (Khan et al. 2003), and these findings were confirmed in the present study. However, during the subsequent repair of the injury, there was a progressive increase in CCK2R expression in the mucosa at the ulcer margins that was maximal after 13 days. This time course of CCK2R expression is consistent with that previously reported in rat gastric mucosa in response to cryoinjury (Schmassmann & Reubi, 2000). It seems plausible to suppose that a proportion of the postinjury CCK2R-expressing cells are likely to be relatively undifferentiated, as suggested previously (Schmassmann & Reubi, 2000).
To further explore their identity, we performed colocalization studies with markers of smooth muscle-like cells. A substantial proportion of the cells that expressed the CCK2R, 9 days after cryoinjury, also expressed vimentin and smooth muscle α-actin but not desmin, which strongly suggests they are likely to be myofibroblasts. Myofibroblasts are a key mesenchymal cell type. In gastric glands, they are in close association with the neck or isthmus region and are thought to be important determinants of the stem cell niche, e.g. by secretion of growth factors and cytokines (Spradling et al. 2001; Leedham et al. 2006; McCaig et al. 2006; Dimaline & Varro, 2007). Whilst it might not, therefore, be surprising to see increased myofibroblast numbers in regions undergoing rapid regeneration of gastric glands, they have not hitherto been reported to express the CCK2R. Cells expressing vimentin were sparse in uninjured mucosa and showed little or no colocalization with CCK2R.
In addition to the increased mucosal postinjury expression of the CCK2R at the ulcer margins, intense areas of expression were seen in the submucosa beneath and adjacent to the ulcer that were maximal at 6–9 days and had largely disappeared by 13 days. A significant proportion of these CCK2R-expressing submucosal cells were again identified as myofibroblasts on the basis of coexpression of vimentin and smooth muscle α-actin. It is well established that myofibroblasts have important functions in the response to inflammation and injury in the gastrointestinal tract and elsewhere (Powell et al. 1999; Jackson et al. 2000; Dimaline & Varro, 2007). The origin of myofibroblasts generated in response to injury is to some extent dependent upon the nature of the pathology and the organ system affected. Commonly, they differentiate from fibroblasts, although transdifferentiation from epithelial cells or other mesenchymal cell types is possible (Hinz et al. 2007). The relationship between the myofibroblasts seen in submucosa and mucosa in the present study is unclear. The earlier appearance in submucosa may be a consequence of the nature of cryoulceration, where the initial site of the injury is the serosal surface of the stomach. It seems possible that some of the myofibroblasts appearing later in the mucosa may have migrated from the submucosa.
The mechanisms that lead to de novo expression of the CCK2R by myofibroblasts in response to ulceration of the gastric mucosa presently remain unclear. The functional consequences of such expression also remain to be established. In a recent report (Varro et al. 2007), gastrin was demonstrated to stimulate myofibroblast proliferation indirectly via release of matrix metalloproteinase 7 from epithelial cells. The present data suggest that in response to inflammation or injury, gastrin might act directly on myofibroblasts following induction of CCK2R expression, to further enhance epithelial–mesenchymal interactions.
Acknowledgments
The authors are grateful to Professor Timothy Wang (Columbia University) for supplying INS-GAS transgenic mice. This work was supported by grant number 072501/Z/03 from the Wellcome Trust.
References
- Cayrol C. Cholecystokinin-2 receptor modulates cell adhesion through β1-integrin in human pancreatic cancer cells. Oncogene. 2006;25:4421–4428. doi: 10.1038/sj.onc.1209484. [DOI] [PubMed] [Google Scholar]
- Cui G. Gastrin-induced apoptosis contributes to carcinogenesis in the stomach. Lab Invest. 2006;86:1037–1051. doi: 10.1038/labinvest.3700462. [DOI] [PubMed] [Google Scholar]
- de Weerth A. Characterization of CCK receptors in stomach smooth muscle: evidence for two subtypes. Biochim Biophys Acta. 1997;1327:213–221. doi: 10.1016/s0005-2736(97)00060-6. [DOI] [PubMed] [Google Scholar]
- Dimaline R. Attack and defence in the gastric epithelium – a delicate balance. Exp Physiol. 2007;92:591–601. doi: 10.1113/expphysiol.2006.036483. [DOI] [PubMed] [Google Scholar]
- Dockray G. Gastrin: old hormone, new functions. Pflugers Arch. 2005;449:344–355. doi: 10.1007/s00424-004-1347-5. [DOI] [PubMed] [Google Scholar]
- Dufresne M. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–847. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- Friis-Hansen L. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 1998;274:G561–G568. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- Gunther R. Transient agonist-induced regulation of the cholecystokinin-A and cholecystokinin-B receptor mRNA levels in rat pancreatic acinar AR42J cells. Pancreatology. 2003;3:47–54. doi: 10.1159/000069142. [DOI] [PubMed] [Google Scholar]
- Haigh CR. Gastrin induces proliferation in Barrett's metaplasia through activation of the CCK2 receptor. Gastroenterology. 2003;124:615–625. doi: 10.1053/gast.2003.50091. [DOI] [PubMed] [Google Scholar]
- Hamilton SP. No association or linkage between polymorphisms in the genes encoding cholecystokinin and the cholecystokinin B receptor and panic disorder. Mol Psychiatry. 2001;6:59–65. doi: 10.1038/sj.mp.4000788. [DOI] [PubMed] [Google Scholar]
- Hattori E. Association studies of the CT repeat polymorphism in the 5′ upstream region of the cholecystokinin B receptor gene with panic disorder and schizophrenia in Japanese subjects. Am J Med Genet. 2001;105:779–782. doi: 10.1002/ajmg.10043. [DOI] [PubMed] [Google Scholar]
- Herget T. Cholecystokinin stimulates Ca2+ mobilization and clonal growth in small cell lung cancer through CCKA and CCKB/gastrin receptors. Ann N Y Acad Sci. 1994;713:283–297. doi: 10.1111/j.1749-6632.1994.tb44076.x. [DOI] [PubMed] [Google Scholar]
- Hinz B. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocker M. Gastrin and phorbol 12-myristate 13-acetate regulate the human histidine decarboxylase promoter through Raf-dependent activation of extracellular signal-regulated kinase-related signaling pathways in gastric cancer cells. J Biol Chem. 1997;272:27015–27024. doi: 10.1074/jbc.272.43.27015. [DOI] [PubMed] [Google Scholar]
- Innis RB. Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci U S A. 1980;77:6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N. Autocrine loop through cholecystokinin-B/gastrin receptors involved in growth of human leukemia cells. Blood. 1996;88:2683–2689. [PubMed] [Google Scholar]
- Jackson LM. Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut. 2000;47:762–770. doi: 10.1136/gut.47.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam SM. Functional heterogeneity of parietal cells along the pit-gland axis. Am J Physiol Gastrointest Liver Physiol. 1997;272:G161–G171. doi: 10.1152/ajpgi.1997.272.1.G161. [DOI] [PubMed] [Google Scholar]
- Kennedy JL. Investigation of cholecystokinin system genes in panic disorder. Mol Psychiatry. 1999;4:284–285. doi: 10.1038/sj.mp.4000507. [DOI] [PubMed] [Google Scholar]
- Khan ZE. Transcriptional regulation of the human trefoil factor, TFF1, by gastrin. Gastroenterology. 2003;125:510–521. doi: 10.1016/s0016-5085(03)00908-9. [DOI] [PubMed] [Google Scholar]
- Kirton CM. Regulation of parietal cell migration by gastrin in the mouse. Am J Physiol Gastrointest Liver Physiol. 2002;283:G787–G793. doi: 10.1152/ajpgi.00538.2001. [DOI] [PubMed] [Google Scholar]
- Koh TJ. Gastrin deficiency results in altered gastric differentiation and decreased colonic proliferation in mice. Gastroenterology. 1997;113:1015–1025. doi: 10.1016/s0016-5085(97)70199-9. [DOI] [PubMed] [Google Scholar]
- Kopin AS. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc Natl Acad Sci U S A. 1992;89:3605–3609. doi: 10.1073/pnas.89.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaksiz H. Expression and cell-specific localization of the cholecystokinin B/gastrin receptor in the human stomach. Cell Tissue Res. 2000;299:289–298. doi: 10.1007/s004419900114. [DOI] [PubMed] [Google Scholar]
- Lay JM. Structure and developmental expression of the mouse CCK-B receptor gene. Biochem Biophys Res Commun. 2000;272:837–842. doi: 10.1006/bbrc.2000.2875. [DOI] [PubMed] [Google Scholar]
- Leedham SJ. The stomach periglandular fibroblast sheath: all present and correct. Gut. 2006;55:295–296. [PMC free article] [PubMed] [Google Scholar]
- McCaig C. The role of matrix metalloproteinase-7 in redefining the gastric microenvironment in response to Helicobacter pylori. Gastroenterology. 2006;130:1754–1763. doi: 10.1053/j.gastro.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Mantyh CR. Localization of cholecystokinin A and cholecystokinin B/gastrin receptors in the canine upper gastrointestinal tract. Gastroenterology. 1994;107:1019–1030. doi: 10.1016/0016-5085(94)90226-7. [DOI] [PubMed] [Google Scholar]
- Mezey E. Localization of targets for anti-ulcer drugs in cells of the immune system. Science. 1992;258:1662–1665. doi: 10.1126/science.1333642. [DOI] [PubMed] [Google Scholar]
- Morisset J. The gastrointestinal cholecystokinin receptors in health and diseases. Rocz Akad Med Bialymst. 2005;50:21–36. [PubMed] [Google Scholar]
- Nagata A. G protein-coupled cholecystokinin-B/gastrin receptors are responsible for physiological cell growth of the stomach mucosa in vivo. Proc Natl Acad Sci U S A. 1996;93:11825–11830. doi: 10.1073/pnas.93.21.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T. Gastrin stimulates the growth of gastric pit cell precursors by inducing its own receptors. Am J Physiol Gastrointest Liver Physiol. 2002;282:G359–G366. doi: 10.1152/ajpgi.00117.2001. [DOI] [PubMed] [Google Scholar]
- Noble F. International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol Rev. 1999;51:745–781. [PubMed] [Google Scholar]
- Ottewell PD. Gastrin increases murine intestinal crypt regeneration following injury. Gastroenterology. 2006;130:1169–1180. doi: 10.1053/j.gastro.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Powell DW. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol Cell Physiol. 1999;277:C1–C19. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- Prinz C. Histamine secretion from rat enterochromaffinlike cells. Gastroenterology. 1993;105:449–461. doi: 10.1016/0016-5085(93)90719-s. [DOI] [PubMed] [Google Scholar]
- Raychowdhury R. Interaction of early growth response protein 1 (Egr-1), specificity protein 1 (Sp1), and cyclic adenosine 3′5′-monophosphate response element binding protein (CREB) at a proximal response element is critical for gastrin-dependent activation of the chromogranin A promoter. Mol Endocrinol. 2002;16:2802–2818. doi: 10.1210/me.2001-0292. [DOI] [PubMed] [Google Scholar]
- Reubi JC. Localization of cholecystokinin A and cholecystokinin B-gastrin receptors in the human stomach. Gastroenterology. 1997;112:1197–1205. doi: 10.1016/s0016-5085(97)70131-8. [DOI] [PubMed] [Google Scholar]
- Schmassmann A. Cholecystokinin-B/gastrin receptors enhance wound healing in the rat gastric mucosa. J Clin Invest. 2000;106:1021–1029. doi: 10.1172/JCI8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz F. Identification of CCK-B/gastrin receptor splice variants in human peripheral blood mononuclear cells. Regul Pept. 2001;101:25–33. doi: 10.1016/s0167-0115(01)00281-6. [DOI] [PubMed] [Google Scholar]
- Seva C. Growth-promoting effects of glycine-extended progastrin. Science. 1994;265:410–412. doi: 10.1126/science.8023165. [DOI] [PubMed] [Google Scholar]
- Spradling A. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Takeuchi K. Mucosal gastrin receptor. I. Assay standardization and fulfillment of receptor criteria. Am J Physiol Endocrinol Metab. 1979;237:E284–E294. doi: 10.1152/ajpendo.1979.237.3.E284. [DOI] [PubMed] [Google Scholar]
- Takeuchi K. Mucosal gastrin receptor. III. Regulation by gastrin. Am J Physiol Gastrointest Liver Physiol. 1980;238:G135–G140. doi: 10.1152/ajpgi.1980.238.2.G135. [DOI] [PubMed] [Google Scholar]
- Varro A. Increased gastric expression of MMP-7 in hypergastrinemia and significance for epithelial-mesenchymal signaling. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1133–G1140. doi: 10.1152/ajpgi.00526.2006. [DOI] [PubMed] [Google Scholar]
- Wang TC. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996;98:1918–1929. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson F. Transcriptional activation of the rat vesicular monoamine transporter 2 promoter in gastric epithelial cells: regulation by gastrin. J Biol Chem. 2001;276:7661–7671. doi: 10.1074/jbc.M006697200. [DOI] [PubMed] [Google Scholar]