Abstract
Cardiac disease remains the major cause of death in thalassaemia major. This review deals with the mechanisms involved in heart failure development, the peculiar clinical presentation of congestive heart failure and provides guidelines for diagnosis and management of the acute phase of cardiac failure. It emphasizes the need for intensive medical – cardiac care and aggressive iron chelating management as, with such approaches, today, the patients outcomes can be favourable in the long term. It covers advances in the assessment of cardiac iron overload with the use of magnetic resonance imaging and makes recommendations for preventing the onset of cardiac problems by tailoring iron chelation therapy appropriate to the degree of cardiac iron loading found.
Keywords: thalassaemia major, cardiomyopathy, cardiac failure, transfusion therapy, iron chelation therapy
In beta thalassaemia major transfusions and iron chelation therapy have significantly improved the survival and reduced the morbidity (1, 2). In the 1960’s 80% of patients had died by the age of 16 (3) and now at least 80% survive beyond the age of 40 yrs (4). This improvement is unique, as no other formerly fatal genetic defect has shown such a benefit. However, heart complications still represent significant morbidity and remain the leading cause of mortality in transfusion dependent thalassaemia (TM) patients (2). Cardiac dysfunction with congestive cardiac failure (CCF), arrhythmias and ultimately, premature deaths continue to present. In some cases this was because of the difficulty in accepting the chelation treatment, which was cumbersome (5), but also occurred even in some patients who accepted the chelation therapy well (6, 7).
In this review, we present some aspects of the existing knowledge including our view, acquired of our 30 yrs experience in following the cardiac course of the disease in more than 1000 thalassaemic patients. Pathophysiology of the heart injury, clinical findings, diagnosis of CCF and the global strategies regarding therapeutic interventions for CCF in TM patients, as well as for prevention of its onset are herein presented.
Mechanisms of heart injury
Cardiac structure and function in TM are mainly affected by two competing factors: iron load and increased cardiac output (CO). The cardiac iron deposition results in a decrease of left ventricular function. The anaemia together with marrow expansion leads to volume overload and increased CO that then demands increased contractility adding additional stress to the heart. (Starling’s Law).
The cardiac iron load
Direct iron related injury
Iron overload results principally from the regular blood transfusions. Patients receive between 0.3 and 0.5 mg/kg/d of iron through transfusions. The average daily losses are less than 1 mg in males and 2 mg in females. There are no other physiological mechanisms for effecting body iron reduction. In addition to the transfused iron, TM patients absorb more iron than normal individuals. The mechanism of increased absorption is thought to be related to paradoxical Hepcidin suppression from the dyserythropoiesis (8–10). In the presence of excess iron Hepcidin should be elevated to inhibit iron absorption but the dyserythropoiesis overrides that effect. Among the different mechanisms in the cellular pathways of ferrous iron (Fe2+) membrane L-type calcium channels are significantly involved (11). L-type Ca2+ channels are high-capacity pathways for ferrous iron (Fe2+) uptake into cardiomyocytes in conditions of iron overload.
Iron is stored in cells, including myocytes, in the form of ferritin, haemosiderin and free iron. The latter is referred as the labile cellular iron (LCI) (12). There is a significant flux between the three forms, with haemosiderin being the least accessible. The LCI is thought to be the most accessible to chelation, but it is also the most toxic form as it stimulates the formation of free radicals. These result in peroxidative damage of membrane lipids and proteins provoking cellular injury. In the heart, this leads to impaired function of the mitochondrial respiratory chain and is clinically manifested by reduction of cardiac muscular contractility and CCF development (13). Furthermore, in the presence of increased intracellular ferrous iron, the ryanodine sensitive calcium channels of sarcoplasmic reticulum (SR), are inhibited. This modulates SR calcium release, resulting in further reduction of cardiac function and arrhythmia development. The new knowledge on calcium channels may offer new potential therapeutic interventions for cellular iron reduction and treatment of arrhythmia and cardiac dysfunction (11, 14, 15).
To date, at least 90 genes that control iron metabolism have been identified (16). Due to the possible gene variations the handling of iron in each individual is expected to be different. Similarly the action of iron chelators could be affected and act differently in the individual patient. It has been shown that TM patients who express the apo-lipoprotein E4 allele were at greater risk for left ventricular (LV) dysfunction. Apo E4 is less efficient at handling oxidative stress (17, 18) when compared to Apo E2 and Apo E3. Additionally the genetic variations of the GSTM1 enzyme (Glutathione S-Transferase M1) are associated with increased cardiac iron deposition in patients with TM (19). These concepts fit in well with the wide range of reported different clinical cardiac courses seen in TM patients who have followed similar life-time, well accepted treatment (6).
Knowledge derived by recent magnetic resonance imaging (MRI) studies which also assessed cardiac function, showed that all patients with reduced LV function had cardiac iron overload and in many cases this was severe (20–23). This strongly suggests that in addition to the damage caused by the accumulated iron, excessive iron in the myocytes results in greater amounts of LCI leading to free radical formation that overwhelms the antioxidant mechanisms and ultimately precipitates cardiac dysfunction. In the above MRI studies, despite heavy iron load, many TM patients maintained normal cardiac function, albeit perhaps temporarily, and this may be related to their different, intracellular iron metabolism, in particular their neutralisation of oxidants as discussed above.
Indirect iron related injury
Infections
Any significant infection may precipitate cardiac failure particularly in the presence of other underlying cardiac pathology. Immune competence in beta-thalassemia is impaired (24–27) and patients are more vulnerable to infections. Furthermore, siderophore bacteria, such as yersinia and klebsiella, rely on iron for multiplication and grow well in the microenvironment of TM patients (26, 27).
Iron overload is considered to be the main etiologic factor that can disturb the immune balance in favour of the growth of infectious organisms (25). This may also be affected by differences in the existing immunogenetic profile in TM (28) especially with respect to viral infections. Two severe cardiac complications, pericarditis and myocarditis, are linked to iron load induced viral infection susceptibility.
Pericarditis, frequently seen (50%) in TM patients with poor or no chelation in the past (3), is very rare today (5%), with the use of chelation therapy (6). Similarly, the reported myocarditis in TM patient with decreased LV function (29), seems most likely to be related to iron load. Even though there may be histological evidence of infections, as demonstrated by lymphocytic infiltration, recent evidence shows that LV failure only occurs in the presence of excessive iron (20–23). Viral myocarditis without iron in the heart may be rare and may follow similar outcomes to those of the normal population. Elevated plasma cardiac enzymes or troponine may be indicative of concomitant viral myocarditis.
Vascular involvement (afterload)
Systemic arterial involvement in TM, as observed recently through clinical, functional (30) and anatomical (31) studies, plays a role in the development of cardiac dysfunction by affecting heart afterload. Vascular involvement starts early in life and becomes obvious in the older patients (32). The anatomical component including elastic tissue abnormalities is expressed in arteries by thickening and disruption of the elastic laminae and adventitia, followed by calcium deposition. The injury is suggested to be mediated by the chronic haemolytic state along with the increased labile plasma iron (LPI). Erythrocyte membrane fragments, haem and free haemoglobin in addition to free iron, provoke a strong oxidative stress on endothelium (32). A component of the vascular dysfunction is due to the reduction of nitric oxide (NO). There are at least three mechanisms responsible for that effect related to haemolysis; a) Red cell destruction releases arginase which reduces arginine levels and its supplementation to the endothelium. b) Oxyhaemoglobin is transformed to methhaemoglobin after reacting with NO and converts it to inactive NO3 i.e. neutralising it and c) Oxidative stress inactivates endothelial cell enzymes and reduces formation of NO from the precursor arginine (33).
Similar mechanisms apply also to the pulmonary artery bed, where vascular contribution together with coexisting hypercoaguability is considered to be responsible for increased pulmonary artery resistance (34, 35).
Arrhythmias
The iron induced cardiac toxicity is often complicated by arrhythmias such as extra atrial and ventricular beats, paroxysmal atrial tachycardia, flutter or fibrillation. Life threatening ventricular tachycardia is rare and often associated with reduced LV function. Short runs of non-specific ventricular tachycardia are quite common and are more common with elevated cardiac iron. Atrial arrhythmias occur more frequently. These are more clinically relevant and difficult to treat, but less specific for iron toxicity. Some of these arrhythmias can also be triggering factors for CCF or reduced cardiac function in TM patients without previous obvious LV dysfunction.
Endocrine abnormalities
Iron toxicity may also indirectly affect heart function by damaging other organs in varying degrees. The endocrine abnormalities hypothyroidism and diabetes mellitus can have a significant impact on cardiac function (36). Hypothyroidism can precipitate pericardial effusion, decreased LV function, bradycardia and increased peripheral vascular resistance. The onset of diabetes is often associated with the presentation of cardiac dysfunction. Chronic hyperglycaemia is an oxidative stress on many organs, particularly the heart. Hypocalcaemia associated with occult or overt hypoparathyroidism can precipitate heart dysfunction.
Medications
Vitamin C has been given to patients in order to enhance their iron excretion when they are on chelation therapy. There have been case reports of patients who developed sudden acute cardiac failure with a fatal outcome that had been precipitated by the administration of Vitamin C possibly by releasing free iron that is toxic (37).
Increased CO effect (preload)
Disease related increased CO, resulting in increased workload on the heart, contributes to the development of cardiac dysfunction in TM patients. In other chronic anemias, resting CO increases when Hb levels decline below 9 g/dL (38–41). TM patients, however, even those well transfused (mean pre transfusion Hb level > 9.5 g/dL) with excellent suppression of marrow activity and with mean Hb level between transfusions of 11.3 g/dL, still demonstrate some degree of high CO (Cardiac Index 4.3 ± 0.9 L/m2 in TM cf. 3.8 ± 0.8 P < .01 in normal individuals) (6). It is more obvious in cases were low Hb levels and tissue hypoxia stimulate compensatory reactions leading to development of peripheral shunts (42). Liver iron load or viral induced hepatic injury can also contribute, as cirrhosis can increase CO significantly (43). Furthermore, the presence of elastic fibre degeneration, affecting elastic lamina and adventitia, which render vessels more susceptible to dilatation by pulse pressure increase in the context of a hyperkinetic state also increases the total blood volume (31).
Summary of the mechanisms of heart injury
In TM, the impaired heart from iron overload, is obliged to maintain a high output through a rigid vascular bed that results from the abovementioned vascular damage and is therefore subjected to a continuous state of both volume and pressure overload rendering the LV more susceptible to decompensation. Similarly, in TM patients the coexistence of high CO state and gradually increasing pulmonary vascular resistance seems to lead to the development of pulmonary hypertension (PHT), which readily precipitates right ventricular (RV) failure (34). Infections, with a direct or indirect effect also have an impact on heart function. In well-treated TM patients, the inhibition of the above mechanisms, result in a considerable reduction of LV dysfunction incidence, vascular damage, PHT development and RV failure (44, 45).
Heart pathology
Iron is thought to saturate liver firstly, and then to accumulate in other organs. In the heart, it accumulates in all four chambers, papillary muscles and the electrical conduction system, including the sinoatrial and atrioventricular nodes (46). In the free wall of the left ventricle there is more iron concentrated in the epicardial layers than in the endocardial and middle third (47). From the epicardium, it encroaches upon the pericardium. Such iron deposition raises a possibility that pericarditis may also have an iron induced chemical inflammatory component and also may cause fibrosis of the pericardium with or without a history of viral pericarditis (Fig. 1). Histology has shown individual myocyte hypertrophy with multiple deposits of brown granular material within the cytoplasm of the myocytes (Fig. 2). These granules stain positive with Prussian blue, confirming heavy myocardial iron deposition. Interstitial macrophages containing iron are also present (48). Moreover, the study of cardiac biopsies from TM patients with light and electron microscopy, as well as with X-ray microanalysis has revealed the presence of disrupted myocytes showing loss of myofibers, dense nuclei, and a variable number of pleomorphic electron dense granules. These cytoplasmic granules or siderosomes consist of iron-containing particles as confirmed by X-ray microanalysis.
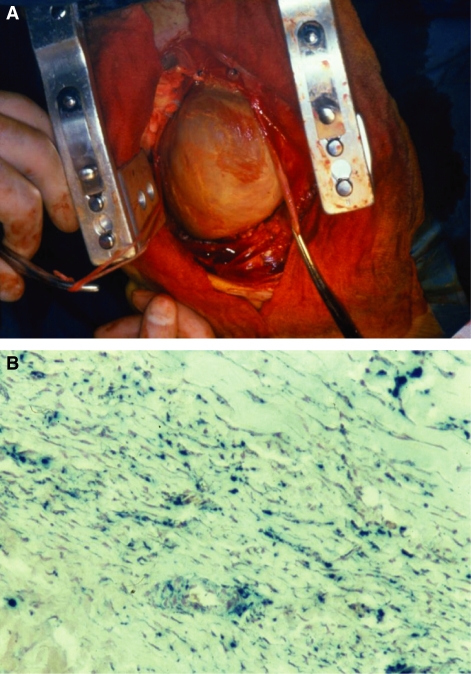
Figure 1.
(A) Operative field in a 27 yr old male thalassaemia patient with a history of recurrent pericarditis and effusive constrictive pericarditis at the time of surgery (B) with biopsy from the same patient demonstrating significant pericardial thickening with severe iron deposition and a small amount of muscle in the left hand corner which contains iron (Prussian Blue Stain).
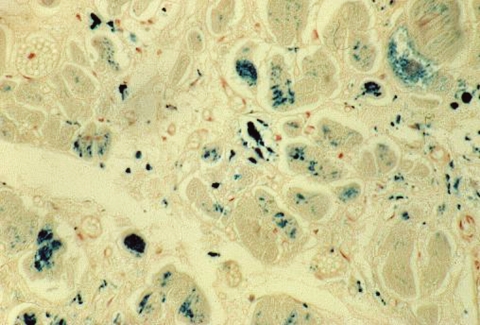
Figure 2.
Histological features from an autopsy from a 29 yr old male thalassaemia patient who died of congestive cardiac failure. Histology shows individual myocyte hypertrophy with multiple deposits of brown granular material within the cytoplasm of the myocytes. These granules stain positive with Prussian blue, confirming heavy myocardial iron deposition.
Clinical presentation of cardiac involvement in TM
Cardiac involvement includes heart failure, arrhythmias and pericarditis. The presentation of pericarditis is similar to that which occurs in the general population. This is also the case with arrhythmias.
Heart failure can present at any time after the age of 10 yrs but with optimal treatment, heart failure usually occurs in the third or fourth decade of life (6). The presentation can be abrupt, sometimes associated with an infection or with a slow relentless onset.
Although some patients can present with symptoms of left-sided heart failure including exertional dyspnoea, cough and fatigue, followed by râles and gallop rhythm on chest auscultation, it is worth noting that the majority presents with symptoms and signs of right ventricular dysfunction. The patients often present to an outpatient clinic with severe fatigability and abdominal pain, the latter due to liver distention. The patient may be lying on the examination couch without dyspnoea. These signs can easily be misinterpreted as not being symptoms of cardiac origin (49, 50). The clinical course in this young population, has often been associated with a gradual reduction in physical activity, which obscures and delays the presentation.
However, clinical examination with the patient in a correct position, will reveal a positive hepatojugular reflex with neck vein distention and a third and fourth heart sounds. In more severe cases, peripheral oedema and ascites may be found. This peculiar clinical appearance in TM patients should be kept in mind. It results from the thin iron loaded right ventricle decompensating earlier (51). Râles may be found in cases where there is also left sided heart failure.
Investigation findings for CCF
Chest X-ray, shows cardiomegaly but frequently there are no features of pulmonary congestion. Lung congestion and pleural effusion may be present as well as a prominent pulmonary artery in cases with coexisting PHT.
It is unusual for the electrocardiogram (ECG) to be normal. Wide QRS complex with low voltage, inverted T waves, non-specific ST-T changes, Left Ventricular Hypertrophy, prolonged A-V conduction and arrhythmias are frequently seen.
Doppler echocardiographic study usually shows biventricular dilatation and systolic and diastolic dysfunction. The variety, however, of the different abovementioned pathogenetic factors and their degree of contribution to the cardiac damage, including the different treatment regimes (lower transfusion schemes, inadequate chelation) lead TM patients, who present with CCF not always to show uniform cardiac injury. Restrictive cardiomyopathy-constrictive pericarditis or high cardiac state Doppler echocardiographic findings could present either alone or in combination. The development of significant PHT may accompany the CCF in almost all the above forms, contributing to the precipitation of right-sided heart failure. In cases with impaired LV function, thrombus formation in the apex of the heart may be present and can lead to the development of stroke (52) (Fig. 3).
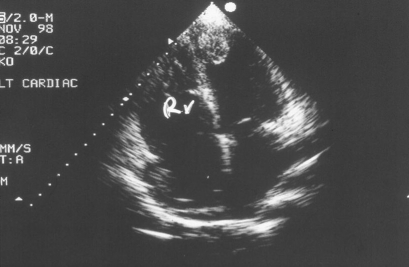
Figure 3.
A 30 yr old female thalassaemia patient 2-D four chamber view with the presence of an apical thrombus.
Therapeutic approach to TM patients with CCF
Thalassaemia patients with signs and symptoms of CCF should be hospitalised and closely monitored. Extensive laboratory tests should be performed and include:
arterial blood gas,
endocrine profile,
liver and renal function tests,
chest X-ray,
ECG and
Doppler echocardiographic study.
As stated above recent MRI studies have confirmed that almost all patients with decreased left ventricular function have severe iron load (20–23). In the acute failure patients the values of MRI measurements are only important to determine the degree of iron overload for future follow-up and can be postponed till after the patient has improved clinically.
Triggering factors for CCF development such as arrhythmias, blood volume overload after transfusion, infections, severe anaemia, should be identified and treated. In cases where Yersinia enterocolitica or Klebsiella pneumoniae infection is suspected (53), patients should be treated even before immunological or bacterial culture test results are available. If arrhythmias are present, the least negative inotropic antiarrhythmic agent, amiodarone should be infused intravenously (54). In general, implantable defibrillators are not recommended for the management of ventricular arrhythmias in TM and the essential intervention is intensification of chelation therapy. However, in rare case of sustained ventricular tachycardia, later in the clinical progress, an implantable defibrillator may be necessary.
Daily measurements of body weight, blood pressure and 24-hrs urine secretion are of paramount importance in these patients. Frequent monitoring of Hct, Hb, blood electrolytes, urea, creatinine, glucose, AST, ALT, uric acid, is also mandatory.
Chelation therapy
Combination of the two iron chelators (deferrioxamine and deferiprone) seems to maximise the efficacy producing additive and synergistic effects in iron excretion (55, 56). It seems that each of those two agents chelates iron from different pools and there is at least an additive effect when combined treatment is administered (57). Available evidence now suggests that combined therapy should be the treatment of choice for patients with established cardiac failure. We have reported two cases with severe CCF who reversed with intensive combination therapy (58, 59) and we have at least eight more patients with similar outcome. Two other studies show similar responses (56, 60). In a recent study with combined treatment, apart from significant reduction in ferritin, cardiac and liver iron and improvement in cardiac function, the absolute endothelial function was also improved (45). Furthermore, improvement with glucose tolerance with the use of combination therapy has been reported (61, 62) as well as anecdotal reports of improvement in other endocrine functions. Desferrioxamine should be administrated at a dose of 60–80 mg/kg/d intravenously and deferiprone at a dose of 75–100 mg/kg/d in three divided doses. If deferiprone is contraindicated, the patient should be managed with continuous desferrrioxamine infusions which usually requires the placement of an indwelling catheter (63). Continuous desferrioxamine infusions alone have been shown to improve cardiac function and salvage patients (64). It seems however, that the rate of removal of iron with such therapy is much slower than with combination therapy (45, 63).
Caution should be taken with the 24 h desferrioxamine infusion to avoid fluid overload especially when intravenous antibiotics and antiarrthymic agents are also indicated.
Transfusions
As CO may be close to normal when Hb level is ≥10 g/dL, patients’ Hb concentration should be kept above 10 g/dL by regular blood transfusions. The patients should only be transfused with one packed red cell unit no greater than 250 mL with diuretic treatment as well.
Endocrinopathies
If hypothyroidism is present, a titrated dose of hormone replacement should be carefully initiated. Diabetes mellitus should be regulated with caution. Although hypoparathyroidism is generally rare in the thalassaemic population, it is more often seen in patients with CCF and shows the typical ECG abnormalities of prolonged QT interval (36). Calcium is necessary for heart muscle cell contractility. CCF with the coexistence of low serum calcium levels is extremely resistant to conventional treatment (65). Serum calcium level should be corrected by intravenous calcium administration accompanied by oral vitamin D.
Conventional cardiac disease management
Diuretics, including loop diuretics (e.g. furosemide) and potassium-sparing agents (e.g. spironolactone), as well as angiotensin-converting enzyme (ACE)-inhibitors should be prescribed based on arterial blood pressure. In cases of persistent normal sinus tachycardia, small doses of Carvedilol may be given. Digoxin must be prescribed to patients with atrial fibrillation resistant to conversion. Diuretics must be prescribed with care for reasons stated in the following section.
Pulmonary hypertension
If PHT is present, then based on the recent observation of reduction of NO availability in haemolytic anaemia, sildenafil should be given in titrated dose, as a first choice. It has been shown to be effective in reducing pulmonary pressure by NO release (35).
Management of low CO state
Hypoalbuminemia is often present. The combination of excessive hepatic iron, hepatic viral infections and other recent infections with the stress of congestion leads to liver dysfunction, which, in turn, results in reduced albumin production. The low serum albumin level could be masked by diuretic administration. It is important to note that TM patients with CCF, after strong diuresis, in combination with hypoalbuminemia are at significant risk of developing reduced renal function or acute renal failure caused by further reduction in CO due to significant preload reduction in the presence of ventricular dysfunction. Thus, careful albumin administration with a gradual negative fluid balance by diuretics is necessary. In case of reduction of renal output, treatment with intravenous positive inotropic agents (e.g. dopamine-dobutamine) in a titrated dose alone and/or with haemodialysis should be considered. Though adrenal insufficiency seems to be rare in TM it is possible that the adrenal reserves are diminished. It may therefore be valuable in such cases in the ICU setting to give the patients stress dose steroids empirically. It can also profoundly improve their responses to inotropes.
The above treatment can yield very positive results, often within a short period of time – even before any significant reduction in the cardiac iron load would be expected. If the patient survives the acute phases of CCF, treatment should be continued. Patients’ general clinical condition, echocardiographic studies and MRI T2* measurements could guide subsequent treatment modifications. Until the ejection fraction approaches normal, transfusions should be given at 7–10 daily intervals with reduced amounts of packed cells avoiding volume overload. In particular, changing the intravenous desferrioxamine to daily subcutaneous infusions once the patient is stabilized, is the first step. This allows the patient to be discharged from hospital. It is essential to note that with appropriate intervention, particularly intensive chelation therapy the cardiac function can improve significantly within 6 months. In our own experience, we have seen that within 2–3 yrs of intensive chelation the heart iron can be significantly reduced and in some case rendered completely free of iron. Ultimately, heart function may revert to normal class I (NYHA) and eventually the patients can cease their cardiac medications.
Conclusions on CCF in TM
Treatment of iron induced cardiomyopathy requires close follow-up and significant effort until the patient is stabilised. If the patient survives the acute phase, the potential reversibility of heart injury by heart iron removal promises an outcome better than that seen in the past in such situations and may be better than that seen with other causes of cardiomyopathy with equivalent clinical severity in the general population (29). However as Hippocrates stated, it is better to prevent than to cure. Today the great challenge in TM patients is to achieve even better results in preventing heart injury.
Prevention of heart disease
The nowadays accepted treatment has two main components: transfusion and chelation therapy. Until recently the latter was only available as parenteral desferrioxamine.
Hb levels
The main cause of increased CO is low Hb levels, marrow expansion and their consequences. Transfusion therapy should reduce the CO, however many patients on the recommended transfusion regimes (between 9.5 and 10 g/dL) still have an increased CO (6). In general high CO states are well tolerated even with low Hb levels and in older patients (41). However coupled with other factors, especially that of transfusion iron overload, it increases the risk of cardiac impairment.
It is uncertain how the situation in which there are good transfusion levels but there is still increased CO, could or should be rectified. Even though an older study showed that after 5 months at higher pretransfusion Hb levels an increased red cell consumption (66) did not result, higher transfusion levels did not necessarily convey any particular patient benefit on bone marrow expansion and increased blood volume (67). Thus, higher pretransfusion Hb levels (mean approx. 10.5 g/dL) may be desirable and long term may reduce the CO and marrow expansion. However, the target should be to reduce iron rather than to be too concerned about the marginally elevated CO. In contrast, in patients who have low pretransfusion Hb levels, their transfusions should be increased in either frequency or volume. If the low Hb levels persist despite adequate transfusions and the red cell consumption is elevated, splenectomy should be considered (68).
Iron load
The suggested iron chelation regimes available till recently i.e. with desferrioxamine at 30–40 mg/kg body weight per infusion, 8–10 hrs per infusion 5–7 days per week, improved survival and reduced morbidity (69). However, careful iron balance studies have shown that only 52% of patients on the most commonly used 5 day per week regime will be in negative iron balance (55). Although desferrioxamine enters the liver rapidly, it enters all other cells very slowly. These, accompanied by varying compliance and other factors mentioned in the section on mechanisms of iron induced heart injury, result in continuing presentation of cardiac dysfunction and premature cardiac deaths.
Predictive factors of heart injury from iron
For many years, prediction of potential heart iron injury in TM patient was considered necessary in order to assess the efficacy of the treatment regimes, particularly the chelation therapy and to propose any modification.
Ferritin levels
The traditional biochemical parameter, serum ferritin, was relied on universally.
Ferritin levels seen in iron load states mainly represent a component that has leaked out of cells. It was shown that ferritin levels had an increasing linear relationship to the number of transfusions that patients received (70). With chelation therapy, ferritin levels most often showed significant reduction. Analysed as single measurements or as mean measurements, they had been regarded as reasonable indicators of iron load and prognosis. There have been a number of studies that relate the risk of death from cardiac disease to ferritin levels that have been maintained by patients (71). In general, it was considered that once chelation was commenced, ferritin levels should be maintained below 1500 ng/L. Even in a recent Italian study, deaths in TM were related to higher ferritin levels at the time of death (2). Overall, persistently high levels of ferritin are associated with poor outcomes and efforts should be consistently made to maintain them low. However cardiac deaths still occurred in patients with satisfactory ferritin levels. In two recent studies, one which assessed most recent (23) and one of which assessed highest, lowest, mean 5 yr and most recent ferritin (22), there was a statistically significant relationship of ferritin to MRI assessed cardiac iron but no predictive value between the two indices.
The limitations of ferritin levels in predicting iron load and toxicity, include that it is an acute phase reactant and is reduced in the presence of low ascorbic acid (common in TM patients) (72). Additionally, free iron as LPI or LCI could affect cells membranes and therefore result in a different relationship between tissue iron load and ferritin levels. It has been shown that high levels of ferritin may be present even before the tissue iron storage is excessive. This may be caused by leakage across the cell membranes because of the iron toxicity (73). In contrast, intensified chelation therapy in heavily iron loaded patients, may rapidly reduce serum ferritin because the cell membranes are stabilised, while tissue iron, especially in the heart, remains elevated (74). These hypotheses may explain some of the limitations.
Liver iron concentrations
In previous post mortem studies, the liver iron quantity per mg wet weight was approximately tenfold that of the heart (48) supporting the concept that the rate of accumulation in the liver is greater than in the heart and that heart is much more sensitive to iron loading. The excessive hepatic iron load may relate to the iron initially loading mostly in reticulo-endothelial cells and because the majority (90%) of the body’s R-E system is in the liver (75). Histological findings in heavily loaded livers however, show excessive deposition in parenchymal cells as well.
Liver iron concentrations (LIC) were subsequently regarded as the gold standard for determining the total body iron load (76) and as a better indicator of risks than ferritin. Until recently, the LIC was given great significance with respect to the risk of cardiac disease and it was recommended that levels >12 mg/g dry weight were associated with cardiac death (77). However, one study has shown that LIC (by biopsy) was not related to cardiac dysfunction as assessed by stress multiple-gated acquisition/gated pooled cardiac scan (MUGA) (78), and another study for LIC estimated by SQUID and Echocardiography findings showed similar lack of relationship (A. Piga personal communications). These findings have been elucidated by recent MRI studies. One did not show statistical significance between LIC and cardiac iron and the others did, while there was no predictive value between them (20–23). Therefore, using LIC as a predictor of cardiac mortality can be misleading. Caution should be exercised, particularly in patients with satisfactory ferritin levels and LIC as these give a false sense of security and it is not realised that patients with such levels may have excessive cardiac iron and need intensification of chelation therapy (63). Irrespective of this, major efforts should be made to maintain low LIC’s because high levels are potentially dangerous and are associated with other morbidities such as increased risk of siderophore bacterial infections, cirrhosis and hepatoma.
With respect to the discrepancy between LIC and cardiac iron, new knowledge may assist in the understanding of this. The rates of iron accumulation seem to be different in the liver and the heart (21), while the MRI studies have shown that when chelation therapy is intensified, the rate of iron clearance between the two organs seems to be significantly different and the liver responds faster (74). Furthermore, there is also a difference with respect to the individual chelator’s access to different iron pools (79).
Echo studies
In TM patients, heart remodelling and function is affected by many factors as discussed above. Therefore, structural and functional parameters that can be assessed by Doppler Echo in normal individuals have less predictive value in TM with respect to the risk of the development of cardiac dysfunction. In particularly in patients with high output state, LV ejection fraction is expected to be higher than in normal subjects. Thus, for TM patients, it has been recommended that a normal left ventricular ejection fraction (LVEF) should be above 60% (80) and the degree of CO increase should be taken into account when assessing EF in each individual patient (81).
In general, by knowledge derived mainly from hemochromatosis, it is believed that cardiac diastolic factors in TM are affected earlier than the systolic ones. TM patients with normal systolic function have been shown to have impaired diastolic Doppler indices with restrictive filling pattern (82). These latter signs were questioned as to their predictive value and have been attributed solely to increased CO (83). Consistently, in a recent study, standard Doppler left ventricular filling pattern and pulsed Doppler tissue imaging parameters in TM were similar to those seen in conditions of increased preload (84). In contrast, in a 5 yr follow-up study which assessed left ventricular diastolic filling variables by echocardiography, it was found that these were important predictors of the cardiac outcome in TM patients (81, 85). Similarly in a recent study that followed TM patients over a 10 yr period, under regular constant transfusion-chelation treatment, some of the diastolic and systolic indices were able to predict the potential for cardiac risk (81).
Doppler Echo and tissue Doppler studies can only identify the damage rather than delineating the cause, so limitation exists also in predicting iron load by Doppler Echo measurements (86). Structural and functional (systolic or diastolic indices) relationship to the amount of iron in the heart assessed by cardiac magnetic resonance imaging (CMR) was found, but these did not completely differentiate with specificity and selectivity the patients at risk for iron load (87).
Therefore, echo techniques can select a number of patients who are at risk but they do not identify a large percentage of those. Doppler Echo remains an important monitoring tool for TM patients, particularly if the cardiologist is familiar with the underlying mechanisms of the cardiac disease and should continue to be used. It is able to perform assessments that cannot be done with MRI such as pulmonary artery pressures, valvular reflux and diastolic parameters. Doppler Echo remains particularly valuable in circumstances in which MRI is not available.
Radionucleide cardiac scans (MUGA)
Resting or combined Resting-Stress radionuclear studies have been used for assessing left ventricular LV function in TM (88, 89). Estimation of systolic function was more accurate than that assessed by echocardiography, especially during exercise stress, when an inadequate response to stress, indicative of subclinical cardiac dysfunction could be revealed. MUGA has been shown to have good application especially for allowing appropriate treatment modification with good clinical outcome (4). It is however time consuming, should not be performed frequently as it involves radionucleide injection and has almost the same limitations as echo regarding the pathophysiology of the measured injury.
BNP levels
B-type naturetic protein (BNP) was considered useful in predicting the risk of developing cardiac disease. (Studies). Recent data has demonstrated that in TM patients there is no predictive value with that measurement for heart iron load (23). BNP was only elevated in patients who had overt cardiac dysfunction and the levels did not reflect the severity of heart failure (90).
Cardiac magnetic resonance imaging
As there was no particular examination giving a real indication of cardiac risk, the ability to determine cardiac iron was therefore crucial. Cardiac biopsy is invasive and inaccurate (91, 92), therefore the ability to assess cardiac iron non-invasively, reproducibly and accurately, was imperative. CMR has offered that capability and has revolutionized the approach to management of TM. A number of studies have demonstrated the value of CMR in indirect assessment of cardiac iron overload (T2*) and function parameters (20, 93–96). Many other centres are instituting either the same or similar MRI techniques. The results appear to be comparable using different machines and in different countries (97). They are reproducible and robust, provided the T2* method is used and the area measured is the intraventricular septum (98). The classification of patients is that those with T2* > 25 ms are regarded as not having cardiac iron (22), though some centres suggest >20 ms (20, 23). Both from our experience and that of other centres, it seems that even though T2* of the heart between 20 and 25 ms may be a grey zone with respect to iron load, patients with such levels or more are thought not to have increased risk of developing CCF. Those with T2* between 10 and 20 ms have mild to moderate cardiac iron load and those <10 ms are considered to have heavy cardiac iron load.
Advantages
The major benefit from the use of MRI is that it allows the comparison of iron load to heart function. It is clear that cardiac damage is related to the amount of cardiac iron. MRI provides the ability to determine the predictive value of ferritin, LIC, echo and BNP with respect to cardiac iron. It can also assess the impact of the patients’ compliance to therapy and their red cell consumption on cardiac iron. Furthermore with the confidence in the studies, we can make appropriate treatment modifications, for each individual patient based on the assessed cardiac iron load. Finally it allows monitoring of the effect of the treatment modifications.
Disadvantages
The disadvantages of CMR are that it is expensive, time consuming, performed within a claustrophobic environment, and cannot be used in patients with cardiac pacemakers, defibrillators or implantable pumps in the chest. Indwelling portacaths with titanium ports or stents do not interfere with the study. Also, it is not universally available.
Heart iron MRI measurements are limited to the deposited forms of ferritin and haemosiderin that comprises the majority of the cellular iron but it cannot measure the toxic LCI. Despite this, given the fact that a continuous interaction between the three forms of iron exists, MRI measurement remains the best method for estimating potential iron toxicity.
Chelation treatment for prevention of iron induced heart disease
Chelation treatment today should be guided by MRI findings, if the technique is available. We are in a transient phase of knowledge with the availability of MRI and new chelating agents. Important questions with respect to best management to avoid iron induced cardiac disease remain to be elucidated. Optimal management may be clarified from results of different trials and current ongoing follow up studies from many subgroups of patients using different regimes.
In the presence of excess cardiac and or hepatic iron, treatment strategies include increase of the dose and/or frequency of desferrioxamine, switch to oral chelators (deferiprone or deferasirox) or to the combination of deferiprone with desferrioxamine, provided there are no contraindications to their use (45). With respect to hepatic iron removal, the efficacy of the two oral chelators is at least equal to the standard doses of desferrioxamine (99–101). Recent and ongoing studies have demonstrated that deferiprone, a small molecule that permeates all tissues, is more efficient in removing cardiac iron and improving cardiac function than desferrioxamine (99, 100). Some preliminary clinical and laboratory observations with deferasirox are encouraging with respect to removal of cardiac iron (102, 103). As yet, there are no studies with combinations of deferasirox and desferrioxamine so this therapeutic regime cannot be recommended at this stage. Renal dysfunction is rare in TM, however, it is important to note that adequate renal function is essential for the elimination of the chelators. Chelator adjustment will be necessary. If the patient has renal failure, it is essential that aggressive haemodialysis is instituted.
According to the current knowledge and based on the CMR findings, the suggested chelation regimes are as follows:
Acceptable cardiac iron
For patients with T2* greater than 20 ms, the therapeutic strategy should be continuation of monotherapy with either desferrioxamine or either of the available oral chelators (deferiprone and desferasirox) with regular follow-up. For patients convenience, desferrioxamine administration may be converted to either of the two oral chelators.
Mild to moderate cardiac iron loading
T2* values between 10 and 20 ms are considered to reflect a mild to moderately iron loaded myocardium. Bearing in mind that the patients may be at risk of developing cardiac problems under stress such as infections, clearing myocardial tissue from iron seems to be a rational target. Therefore, combined treatment for these patients should not be a priori excluded. Patients have presented with LV dysfunction at levels of T2* of 15 ms, without any precipitating factors (22). Therefore, if T2* is ≤15 ms, combination chelation therapy is recommended (45). However, questions still exist, regarding the frequency and the amount of desferrioxamine administration that is appropriate in a combined regimen. A dose of 35–40 mg/kg/d three-four times weekly combined with deferiprone at a dose of 75 mg/kg/d seems to be reasonable. In patients with T2* 15–20 ms, monotherapy with either deferiprone or deferasirox together with careful follow up are available options (99, 100). Patients treated up to the time of the MRI with desferrioxamine in this category and who availed themselves of that treatment satisfactorily, should not be on monotherapy with that compound, as deferrioxamine was inadequate at preventing the iron accumulation in the heart and may indicate some type of resistance to its efficacy within that patient.
Heavy cardiac iron load
Patients with T2* <10 ms are considered to have severe iron overload and this category includes most patients with reduced LV function. Even those patients with normal ejection fraction in this category are considered to be at very high risk of developing cardiac dysfunction. Thus all patients in this category have a strong indication for combined chelation treatment. The doses of the two medications should be similar to those described for patients with CCF but with the desferrioxamine being given as a subcutaneous infusion.
Any treatment modification should be followed by close monitoring. Should any serious adverse effect present as a consequence of the administration of a particular chelator, appropriate guidelines as to its continued use should be followed.
If treatment has ultimately modified the MRI patient’s classification then, it may be adjusted as discussed above according to the changes in MRI values.
Guidelines if MRI is not available
In countries where MRI is not available, then all the patients’ traditional parameters need to be analysed, (ferritins, liver iron concentrations) as well as ECG and echocardiogram, taking into account the above discussed limitations. These may serve as a guide to treatment. Furthermore, according to knowledge from MRI studies in countries where follow up of patients occurs, up to 65% of patients have cardiac iron load. In Sardinia, 13% had severe cardiac iron overload (23). In our study 48% of patients have T2* < 15 ms (22). In countries were patients compliance to treatment is inadequate, there was poor availability of chelation and/or the follow up was not well organised, the percentage of cardiac iron loaded patients is likely to be higher. Therefore, for patients who have never had optimal care, it is very likely the patients will have cardiac iron load and intensive chelation is the treatment of choice. In patients who have been poorly chelated, the risk of chelation toxicity is minimal and would only be likely to occur after prolonged therapy, however, it is important to be vigilant for such complications. MRI is more necessary for those patients who have had good chelation therapy but who are at risk of chelation inadequacy with respect to the heart and for those who have had treatment modification in order to follow the efficacy of the changed chelation regime.
Conclusions on prevention of heart disease
This formerly catastrophic genetic defect has been revolutionized with the availability of adequate chelation therapy and more recently with other important advances.
It remains important, practically, to aim to maintain low LIC’s and ferritin levels, particularly as the latter are easily accessible and assessable. Similarly, echocardiography should remain a routine tool as it does have some predictive value and can also be used to monitor patients in whom intensification of chelation therapy has been instituted.
Cardiac magnetic resonance imaging can be particularly helpful in identifying all TM patients at risk of developing heart disease by assessing the cardiac iron load. Chelation therapy can be tailored to remove the excess heart iron. Attention to patient’s continuous compliance with adequate chelation is mandatory.
The definite ability to know and reduce cardiac iron as well as improvement in cardiac function that has been reported, should certainly lead to even further significant reduction in cardiac mortality and morbidity.
References
- 1.Modell B. Survival in beta-thalassemia major in the UK: data from the UK Thalassemia Register. Lancet. 2000;355:2051–2. doi: 10.1016/S0140-6736(00)02357-6. [DOI] [PubMed] [Google Scholar]
- 2.Borgna-Pignatti C, et al. Survival and complications in patients with thalassemia major treated with transfusion and desferrioxamine. Haematologica. 2004;89:1187–93. [PubMed] [Google Scholar]
- 3.Engle MA. Late cardiac complications of chronic, refractory anemia with hemochromatosis. Circulation. 1964;30:698–705. doi: 10.1161/01.cir.30.5.698. [DOI] [PubMed] [Google Scholar]
- 4.Davis BA. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004;104:263–9. doi: 10.1182/blood-2003-08-2841. [DOI] [PubMed] [Google Scholar]
- 5.Caro JJ. Impact of thalassemia major on patients and their families acta. Haematologica. 2002;107:150–7. doi: 10.1159/000057633. [DOI] [PubMed] [Google Scholar]
- 6.Aessopos A. Cardiac status in well- treated patients with thalassemia major. Eur J Haematol. 2004;73:359–66. doi: 10.1111/j.1600-0609.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 7.Olivieri NF. Survival in medically treated patients with homozygous beta-thalassemia. NEJM. 1994;331:574–8. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- 8.Nemeth E. Hepcidin and iron-loading anemias. Haematologica. 2006;91:727–32. [PubMed] [Google Scholar]
- 9.Papanicolaou G. Hepcidin in iron overload disorders. Blood. 2005;105:4103–5. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardenghi S, et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109:5027–35. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oudit GY. Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J Mol Med. 2006;84:349–64. doi: 10.1007/s00109-005-0029-x. Epub 2006 Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito BP. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood. 2003;102:2670–7. doi: 10.1182/blood-2003-03-0807. [DOI] [PubMed] [Google Scholar]
- 13.Glickstein H. Action of chelators in iron-loaded cardiac cells: accessibility to intracellular labile iron and functional consequences. Blood. 2006;108:3195–203. doi: 10.1182/blood-2006-05-020867. [DOI] [PubMed] [Google Scholar]
- 14.Kim E. Iron(II) is a modulator of ryanodine-sensitive calcium channels of cardiac muscle sarcoplasmic reticulum. Toxicol Appl Pharmacol. 1995;130:57–66. doi: 10.1006/taap.1995.1008. [DOI] [PubMed] [Google Scholar]
- 15.Scoote M. The cardiac ryanodine receptor (calcium release channel): emerging role in heart failure and arrhythmiapathogenesis. Cardiovasc Res. 2002;56:359–72. doi: 10.1016/s0008-6363(02)00574-6. [DOI] [PubMed] [Google Scholar]
- 16.Trinder D. Molecular pathogenesis of iron overload. Gut. 2002;51:290–5. doi: 10.1136/gut.51.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Economou-Petersen E, et al. Apolipoprotein E epsilon4 allele as a genetic risk factor for left ventricular failure in homozygous beta-thalassemia. Blood. 1998;92:3455–9. [PubMed] [Google Scholar]
- 18.Ferrara M. Role of apolipoprotein E (APOE) polymorphism on left cardiac failure in homozygous beta thalassaemic patients. Br J Haematol. 2001;114:959–60. doi: 10.1046/j.1365-2141.2001.03006-6.x. [DOI] [PubMed] [Google Scholar]
- 19.Kang-Hsi W. Glutathione S-Transferase M1 Gene Polymorphisms are associated with cardiac iron deposition in patient’s with -thalassemia major. Hemoglobin. 2006;30:251–6. doi: 10.1080/03630260600642575. [DOI] [PubMed] [Google Scholar]
- 20.Anderson LJ, et al. Cardiovascular T2* magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171, 9. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 21.Wood JC. Myocardial iron loading in transfusion dependent thalassemia and sickle-cell disease. Blood. 2003;103:1934–6. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- 22.Aessopos A. Cardiac magnetic resonance imaging R2* assessments and analysis of historical parameters in patients with transfusion-dependent thalassemia. Haematologica. 2007;92:131–2. doi: 10.3324/haematol.10455. [DOI] [PubMed] [Google Scholar]
- 23.Tanner MA. Myocardial iron loading in patients with thalassemia major on desferrioxamine chelation. J Cardiovasc Magn Reson. 2006;8:543–7. doi: 10.1080/10976640600698155. [DOI] [PubMed] [Google Scholar]
- 24.Farmakis D. Pathogenetic aspects of immune deficiency associated with beta-thalassemia. Med Sci Monit. 2003;9:RA19–22. [PubMed] [Google Scholar]
- 25.Walker EM. Effects of iron overload on the immune system. Ann Clin Lab Sci. 2000;30:354–65. [PubMed] [Google Scholar]
- 26.Lesic B. Comparison of the effects of deferiprone versus desferrioxamine on growth and virulence of yersinia enterocolitica. Antimicrob Agents Chemother. 2002;46:1741–5. doi: 10.1128/AAC.46.6.1741-1745.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khimji PL. Microbial iron-chelators and their action on Klebsiella infections in the skin of guinea-pigs. Br J Exp Pathol. 1978;59:137–47. [PMC free article] [PubMed] [Google Scholar]
- 28.Kremastinos DT. Association of heart failure in homozygous beta-thalassemia with the major histocompatibility complex. Circulation. 1999;100:2074–8. doi: 10.1161/01.cir.100.20.2074. [DOI] [PubMed] [Google Scholar]
- 29.Kremastinos DT. Heart failure in beta thalassemia: a 5-year follow-up study. Am J Med. 2001;111:349–54. doi: 10.1016/s0002-9343(01)00879-8. [DOI] [PubMed] [Google Scholar]
- 30.Cheung YF. Arterial stiffness and endothelial function in patients with beta-thalassemia major. Circulation. 2002;106:2561–6. doi: 10.1161/01.cir.0000037225.92759.a7. [DOI] [PubMed] [Google Scholar]
- 31.Tsomi K. Arterial elastorrhexis in beta-thalassaemia intermedia, sickle cell thalassaemia and hereditary spherocytosis. Eur J Haematol. 2001;67:135–41. doi: 10.1034/j.1600-0609.2001.5790349.x. [DOI] [PubMed] [Google Scholar]
- 32.Aessopos A. Elastic tissue abnormalities resembling pseudoxanthoma elasticum in beta thalassemia and the sickling syndromes. Blood. 2002;99:30–5. doi: 10.1182/blood.v99.1.30. Review. [DOI] [PubMed] [Google Scholar]
- 33.Kato GJ, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–85. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aessopos A. Pulmonary hypertension in beta-thalassemia. Ann N Y Acad Sci. 2005;1054:342–9. doi: 10.1196/annals.1345.041. Review. [DOI] [PubMed] [Google Scholar]
- 35.Derchi G. Efficacy and safety of sildenafil in the treatment of severe pulmonary hypertension in patients with hemoglobinopathies. Haematologica. 2005;90:452–8. [PubMed] [Google Scholar]
- 36.Tsironi M. Hypocalcemic heart failure in thalassemic patients. Int J Hematol. 2006;83:314–7. doi: 10.1532/IJH97.E0532. [DOI] [PubMed] [Google Scholar]
- 37.Nienhuis AW. Vitamin C and iron. N Engl J Med. 1981;304:170–1. doi: 10.1056/NEJM198101153040311. [DOI] [PubMed] [Google Scholar]
- 38.Braunwald E. In: Heart Disease. 5. Saunders WB, editor. Philadelphia: 1997. [Google Scholar]
- 39.Varat MA. Cardiovascular effect of anemia. Am Heart J. 1972;83:415. doi: 10.1016/0002-8703(72)90445-0. [DOI] [PubMed] [Google Scholar]
- 40.Asimacopoulos PJ. Pernicious anemia manifesting as angina pectoris. South Med J. 1994;87:671. doi: 10.1097/00007611-199406000-00020. [DOI] [PubMed] [Google Scholar]
- 41.Aessopos A. Cardiovascular adaptation to chronic anemia in the elderly: an echocardiographic study. Clin Invest Med. 2004;27:265–73. [PubMed] [Google Scholar]
- 42.Aessopos A. Cardiovascular effects of splenomegaly and splenectomy in beta-thalassemia. Ann Hematol. 2005;84:353–7. doi: 10.1007/s00277-004-1002-4. [DOI] [PubMed] [Google Scholar]
- 43.Murray JF. Circulatory changes in chronic liver diseases. Am J Med. 1958;24:358–67. doi: 10.1016/0002-9343(58)90322-x. [DOI] [PubMed] [Google Scholar]
- 44.Aessopos A. Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest. 2005;127:1523–30. doi: 10.1378/chest.127.5.1523. [DOI] [PubMed] [Google Scholar]
- 45.Tanner MA, et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with desferrioxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–84. doi: 10.1161/CIRCULATIONAHA.106.648790. Epub 2007 Mar 19. [DOI] [PubMed] [Google Scholar]
- 46.Buja LM. Iron in the heart. Etiology and clinical significance. Am J Med. 1971;51:209–21. doi: 10.1016/0002-9343(71)90240-3. [DOI] [PubMed] [Google Scholar]
- 47.Sanyal SK. Fatal “iron heart” in an adolescent: biochemical and ultrastructural aspects of the heart. Pediatrics. 1975;55:336–41. [PubMed] [Google Scholar]
- 48.Modell B. The Clinical Approach to Thalassaemia. New York: Grune and Stratton; 1984. pp. 165–9. [Google Scholar]
- 49.Forni GL. Typical manifestation of acute congestive heart failure in patients with thalassemia major causing diagnostic delay in the emergency room. The European Journal of Heart Failure. 2003;5:607–8. doi: 10.1016/s1388-9842(03)00102-8. [DOI] [PubMed] [Google Scholar]
- 50.Hahalis G. Right ventricular diastolic function in beta-thalassemia major: echocardiographic and clinical correlates. Am Heart J. 2001;141:428–34. doi: 10.1067/mhj.2001.113077. [DOI] [PubMed] [Google Scholar]
- 51.Hahalis G. Right ventricular cardiomyopathy in beta-thalassaemia major. Eur Heart J. 2002;23:147–56. doi: 10.1053/euhj.2001.2709. [DOI] [PubMed] [Google Scholar]
- 52.Aessopos A. Pseudoxanthoma elasticum lesions and cardiac complications as contributing factors for strokes in beta-thalassemia patients. Stroke. 1997;28:2421–4. doi: 10.1161/01.str.28.12.2421. [DOI] [PubMed] [Google Scholar]
- 53.Venti S. Infections and thalassaemia. Lancet Infect Dis. 2006;6:226–33. doi: 10.1016/S1473-3099(06)70437-6. [DOI] [PubMed] [Google Scholar]
- 54.Sermsappasuk P. Kinetic analysis of myocardial uptake and negative inotropic effect of amiodarone in rat heart. Eur J Pharm Sci. 2006;28:243–8. doi: 10.1016/j.ejps.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Grady RW. Optimizing chelation therapy: Combining deferiprone and desferrioxamine.; 42nd Annual Meeting of the American Society of Hematology; Dec 2000; San Fransisco. Abstract. [Google Scholar]
- 56.Origa R. Combined therapy with deferiprone and desferrioxamine in thalassemia major. Haematologica. 2005;90:1309–14. [PubMed] [Google Scholar]
- 57.Kontoghiorghes GJ. Future chelation monotherapy and combination therapy strategies in thalassemia and other conditions. comparison of deferiprone, desferrioxamine, ICL670, GT56-252, L1NAll and starch desferrioxamine polymers. Hemoglobin. 2006;3:329–47. doi: 10.1080/03630260600642674. [DOI] [PubMed] [Google Scholar]
- 58.Tsironi M. Reversal of heart failure in thalassemia major by combined chelation therapy: a case report. Eur J Haematol. 2005;74:84–5. doi: 10.1111/j.1600-0609.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- 59.Tsironi M. Transfusional hemosiderosis and combined chelation therapy in sickle thalassemia. Eur J Haematol. 2005;75:355–8. doi: 10.1111/j.1600-0609.2005.00528.x. [DOI] [PubMed] [Google Scholar]
- 60.Wu K-H. Combined therapy with deferiprone and desferrioxamine successfully regresses heart failure in patients with β-thalassaemia major. Ann Haematol. 2004;83:471–3. doi: 10.1007/s00277-003-0820-0. [DOI] [PubMed] [Google Scholar]
- 61.Farmaki K. Effect of enhanced iron chelation therapy on glucose metabolism in patients with beta-thalassaemia major. Br J Haematol. 2006;134:438–44. doi: 10.1111/j.1365-2141.2006.06203.x. [DOI] [PubMed] [Google Scholar]
- 62.Christoforidis A. Combined chelation therapy improves glucose metabolism in patients with beta-thalassaemia major. Br J Haematol. 2006;135:271–2. doi: 10.1111/j.1365-2141.2006.06296.x. [DOI] [PubMed] [Google Scholar]
- 63.Anderson LJ. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassemia. Lancet. 2002;360:516–20. doi: 10.1016/s0140-6736(02)09740-4. [DOI] [PubMed] [Google Scholar]
- 64.Davis BA. Long-term outcome of continuous 24-hour desferrioxamine infusion via indwelling intravenous catheters in high-risk beta-thalassemia. Blood. 2000;95:1229–36. [PubMed] [Google Scholar]
- 65.Chopra D. Insensitivity to digoxin associated with hypocalcemia. N Engl J Med. 1977;296:917–8. doi: 10.1056/NEJM197704212961607. [DOI] [PubMed] [Google Scholar]
- 66.Masera G. Evaluation of the supertransfusion regimen in homozygous beta-thalassaemia children. Br J Haemat. 1982;52:111–3. doi: 10.1111/j.1365-2141.1982.tb03867.x. [DOI] [PubMed] [Google Scholar]
- 67.Cazzola M. Barella S. Relationship between transfusion regimen and suppression of erythropoiesis in beta-thalassaemia major. Br J Haematol. 1995;89:473–8. doi: 10.1111/j.1365-2141.1995.tb08351.x. [DOI] [PubMed] [Google Scholar]
- 68.Pinna AD. Indications and results for splenectomy for beta thalassemia in two hundred and twenty-one pediatric patients. Surg Gynecol Obstetric. 1988;167:109–13. [PubMed] [Google Scholar]
- 69.Cunningham MJ. Complications of beta-thalassemia major in North America. Blood. 2004;104:34–9. doi: 10.1182/blood-2003-09-3167. [DOI] [PubMed] [Google Scholar]
- 70.Kattamis C. Iron Overload and Chelation in Thalassemia. Bern: Hans Huber Publishers; 1987. [Google Scholar]
- 71.Telfer PT. Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major. Br J Haematol. 2000;110:971–7. doi: 10.1046/j.1365-2141.2000.02298.x. [DOI] [PubMed] [Google Scholar]
- 72.Chapman RW. Effect of ascorbic acid deficiency on serum ferritin concentration in patients with beta-thalassaemia major and iron overload. J Clin Pathol. 1982;35:487–91. doi: 10.1136/jcp.35.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cazzola M. Internal distribution of excess iron and sources of serum ferritin in patients with thalassemia. Scand J Haematol. 1983;30:289–96. doi: 10.1111/j.1600-0609.1983.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 74.Anderson LJ. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous deseferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haemat. 2004;127:348–55. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 75.Bircher J. Rodes – Editors Oxford Textbook of Clinical Hepatology. 2. Vol. 2. New York: Oxford Univ Press; 1999. 1862. [Google Scholar]
- 76.Angelucci E. Hepatic iron concentration and total body iron stores in thalassaemia major. N Engl J Med. 2000;343:327–31. doi: 10.1056/NEJM200008033430503. [DOI] [PubMed] [Google Scholar]
- 77.Olivieri NF. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–61. [PubMed] [Google Scholar]
- 78.Berdoukas V. Lack of correlation between iron overload cardiac dysfunction and needle liver biopsy iron concentration. Haematologica. 2005;90:685–6. [PubMed] [Google Scholar]
- 79.Cabantchik ZI. A fluorescence assay for assessing chelation of intracellular iron in a membrane model system and in mammalian cells. Annal Bioch. 1996;233:221–7. doi: 10.1006/abio.1996.0032. [DOI] [PubMed] [Google Scholar]
- 80.Pepe A. Multislice multiecho T2* cardiovascular magnetic resonance for detection of the heterogeneous distribution of myocardial iron overload. J Magn Reson Imaging. 2006;23:662–8. doi: 10.1002/jmri.20566. [DOI] [PubMed] [Google Scholar]
- 81.Aessopos A, et al. Predictive echo-Doppler indices of left ventricular impairment in B-thalassemic patients. Ann Hematol. 2007;86:429–34. doi: 10.1007/s00277-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 82.Spirito P. Restrictive diastolic abnormalities identified by Doppler echocardiography in patients with thalassemia major. Circulation. 1990;82:88–94. doi: 10.1161/01.cir.82.1.88. [DOI] [PubMed] [Google Scholar]
- 83.Kremastinos DT. Left ventricular diastolic Doppler characteristics in beta-thalassemia major. Circulation. 1993;88:1127–35. doi: 10.1161/01.cir.88.3.1127. [DOI] [PubMed] [Google Scholar]
- 84.Iarussi D. Pulsed Doppler tissue imaging and myocardial function in thalassemia major. Heart Vessels. 2003;18:1–6. doi: 10.1007/s003800300000. [DOI] [PubMed] [Google Scholar]
- 85.Hou JW. Prognostic significance of left ventricular diastolic indexes in beta-thalassemia major. Arch Pediatr Adolesc Med. 1994;148:862–6. doi: 10.1001/archpedi.1994.02170080092018. [DOI] [PubMed] [Google Scholar]
- 86.Vogel M. Tissue Doppler echocardiography in patients with thalassaemia detects early myocardial dysfunction related to myocardial iron overload. Eur Heart J. 2003;24:113–9. doi: 10.1016/s0195-668x(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 87.Aessopos A. Correlation of echocardiography parameters with cardiac magnetic resonance imaging in transfusion-dependent thalassaemia major. Eur J Haematol. 2007;78:58–65. doi: 10.1111/j.1600-0609.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 88.Leon MB. Detection of early cardiac dysfunction in patients with severe beta-thalassemia and chronic iron overload. N Engl J Med. 1979;301:1143. doi: 10.1056/NEJM197911223012103. [DOI] [PubMed] [Google Scholar]
- 89.Freeman AP. Early left ventricular dysfunction and chelation therapy in thalassaemia major. Ann Intern Med. 1983;99:450–4. doi: 10.7326/0003-4819-99-4-450. [DOI] [PubMed] [Google Scholar]
- 90.Aessopos A. Plasma B-type natriuretic peptide concentration in beta-thalassaemia patients. Eur J Heart Fail. 2007;9:537–41. doi: 10.1016/j.ejheart.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 91.Barosi G. Myocardial iron grading by Endomyocardial biopsy. A clinico-pathologic study on iron overloaded patients. Eur J Haematol. 1989;42:382–8. doi: 10.1111/j.1600-0609.1989.tb01229.x. [DOI] [PubMed] [Google Scholar]
- 92.Fitchett DH. Cardiac involvement in secondary haemochromatosis: a catheter biopsy study and analysis of myocardium. Cardiovasc Res. 1980;14:719–24. doi: 10.1093/cvr/14.12.719. [DOI] [PubMed] [Google Scholar]
- 93.Wood JC, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle-cell disease patients. Blood. 2005;106:1460–5. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Voskaridou E. Magnetic resonance imaging in the evaluation of iron overload in patients with beta thalassemia and sickle cell disease. BJH. 2004;126:736–42. doi: 10.1111/j.1365-2141.2004.05104.x. [DOI] [PubMed] [Google Scholar]
- 95.Ooi GC. Magnetic resonance screening of iron status in transfusion-dependent β-thalassemia patients. BJH. 2004;124:385–90. doi: 10.1046/j.1365-2141.2003.04772.x. [DOI] [PubMed] [Google Scholar]
- 96.Olivieri NF, et al. First prospective randomised trial of the iron chelators Deferiprone (L1) and Desferrioxamine. Blood. 1995;86:249a. [Google Scholar]
- 97.Westwood MA. Intercentre reproducibility of magnetic resonance T2* measurements of myocardial iron in thalassaemi. Int J Cardiovasc Imaging (US) 2005;21:531–8. doi: 10.1007/s10554-005-0651-2. [DOI] [PubMed] [Google Scholar]
- 98.Ghugre NR. Improved R2* measurements in myocardial iron overload. J Magn Reson Imaging. 2006;23:9–16. doi: 10.1002/jmri.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pennell DJ, et al. Randomized controlled trial of deferiprone or desferrioxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107:3738–44. doi: 10.1182/blood-2005-07-2948. [DOI] [PubMed] [Google Scholar]
- 100.Peng CT. Safety monitoring of cardiac and hepatic systems in beta-thalassemia patients with chelating treatment in Taiwan. Eur J Haematol. 2003;70:392–7. doi: 10.1034/j.1600-0609.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 101.Cappellini MD, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107:3455–62. doi: 10.1182/blood-2005-08-3430. Epub 2005 Dec 13. [DOI] [PubMed] [Google Scholar]
- 102.Eleftheriou P. Response of Myocardial T2* to oral deferasirox montherapy for 1 year in 29 patients with transfusion-dependent anaemias; A subgroup analysis; European Haematology Association Annual General Meeting; 2006. Abstract 0999. [Google Scholar]
- 103.Wood JC. Deferasirox and deferiprone remove cardiac iron in the iron-overloaded gerbil. Transl Res. 2006;148:272–80. doi: 10.1016/j.trsl.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]