Abstract
Purpose:
Hippocampal sclerosis (HS) is the most common cause of refractory temporal lobe epilepsy. Histopathologically, HS is characterized by neuron loss and gliosis. HS can be identified on MRI by signal increase on T2-weighted images and volume loss on T1-weighted volume images. The Periodically Rotated Overlapping Parallel Lines with Enhanced Reconstruction (“PROPELLER”) sequence has excellent contrast between grey and white matter and compensates for subjects moving during the scan. The aim of the current report was to explore PROPELLER image quality of the hippocampus compared to routine sequences.
Methods:
Routine sequences (T1 volume, T2-weighted, PD and FLAIR images) and PROPELLER images were acquired in four presurgical patients with HS using a GE 3T Excite HD scanner (General Electric). Resected tissue was stained with LFB, and for GFAP, NeuN and dynorphin immunohistochemistry. Hippocampal sections were compared with PROPELLER images.
Results:
PROPELLER images were T2-weighted and had superior tissue contrast compared to routine sequences. PROPELLER images showed the internal hippocampal structures and tissue changes associated with HS. This corresponded to changes seen on histopathological sections confirming that the sequence could distinguish between different strata and subfields of the hippocampus.
Discussion:
The PROPELLER sequence shows promise for detailed in vivo imaging of the hippocampus in patients who did not move overtly, negating the inevitable subtle movements during scans. More detailed in vivo studies of the hippocampal formation, investigating subtle abnormalities such as end folium sclerosis, and the neocortex are now possible and may increase the diagnostic yield of MRI.
Keywords: Epilepsy, Magnetic Resonance Imaging, PROPELLER, Hippocampal sclerosis, Histopathology
Introduction
Hippocampal sclerosis (HS) is the most common cause of refractory temporal lobe epilepsy. Histopathologically, HS is characterized by neuronal loss and gliosis. The neuron loss in classical HS is greatest in CA1 and the hilar region (including CA4) with accompanying gliosis (Bruton, 1988). In severe HS there can be almost total neuronal loss in all hippocampal subfields. There may also be dispersion of the dentate granule cell layer, making this appear broadened (Houser, 1990). In addition, sprouting of excitatory mossy fibers may be demonstrated using the Timms histochemical method (Sutula et al., 1989).
There are also milder forms of nerve cell loss, such as “end folium sclerosis” (Bruton, 1988) in which the cell loss appears to be restricted to the hilus. These cases are not readily identified on MRI, epilepsy onset is later and outcome after surgery is worse than in cases with classical HS (van Paesschen and Revesz, 1997). More recently a new classification system for HS has been proposed based on the pattern of nerve cell loss within the hippocampus seen histopathologically with different clinical phenotypes for the different groups (Blumcke et al., 2007). To date, these different patterns of HS have not been identified on in vivo MRI.
HS can readily be identified on MRI. The cardinal signs of HS on MRI are increased hippocampal signal on T2-weighted images and hippocampal volume loss on T1-weighted images (Jackson et al., 1990). In addition there may be decreased T1 signal intensity and disruption of the internal structure of the hippocampus (Jackson et al., 1993). The sequences used routinely to identify HS are T1-weighted volume acquisition, T2-weighted, proton density and fluid attenuation inversion recovery (FLAIR) sequences covering the whole brain (ILAE Commission on Neuroimaging, 1998).
The Periodically Rotated Overlapping Parallel Lines with Enhanced Reconstruction (“PROPELLER”) sequence is an effective means of compensating for head motion during MRI scans (Pipe, 1999). The mode of acquisition with PROPELLER allows it to intrinsically compensate for both translational and rotational head movements during the scan. Images are averaged and artifacts are reduced further since part of the acquired data can be rejected if correlation measures indicate through-plane motion (Pipe, 1999). Subtle movement can occur even in cooperative subjects. Reduction of motion artifacts has been shown using the PROPELLER sequence in both volunteers and patients (Forbes et al., 2001). The aim of the current report was to explore the PROPELLER image quality of the hippocampus compared to routine sequences, in patients with epilepsy and suspected HS.
Material and Methods
Scans were performed on a GE 3T Excite HD scanner. Four patients undergoing investigations for epilepsy surgery were scanned with the PROPELLER sequence using TR/TE 4000/114.8, FOV 22 × 22 cm, acquisition matrix 416 × 416 and slice thickness 2 mm (giving a voxel size of 0.53 × 0.53 × 2 mm). Scan time was 3 min and 20 s. To obtain coronal images perpendicular to the hippocampal long axis, scans were acquired with the head tilted forward since these scans could not be acquired obliquely. All patients also had routine scans, including T1-weighted volume sequence (TR/TE/TI 7/3/450, flip angle 20°, FOV 24 × 18 cm, acquisition matrix 256 × 256, slice thickness 1.1 mm, giving a voxel size of 0.94 × 0.70 × 1.1mm), T2-weighted and proton density (TE1/TE2/TR 30/80/2000 FOV 24 × 18 cm, acquisition matrix 256 × 192, slice thickness 5.0 mm, giving a voxel size of 0.94 × 0.94 × 5.0 mm) and FLAIR images (TE/TR/TI 144/11000/2250, FOV 24×24 cm, acquisition matrix 256 × 224, slice thickness 5.0 mm, giving a voxel size of 0.94 × 1.1 × 5.0 mm). T1-volume scan time was 7 min and 11 s, T2 scan time was 3 min and FLAIR scan time was 2 min and 57 s. All patients had HS diagnosed on routine scans. Clinical details are listed in Table 1.
Table 1.
Clinical data
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age at onset (years) | 7 | 10 | 1 | 7 |
| Age at operation (years) | 23 | 22 | 43 | 20 |
| Duration of epilepsy (years) | 16 | 12 | 42 | 13 |
| Precipitating factors for epilepsy | – | 1 FC | 1 prolonged FC | 1 prolonged FC |
| Seizure types | CPS: 4/month | CPS: 4/month | CPS: 2–12/month, SGTCS: 10/year | SPS: 15–20/month |
| Inter ictal EEG findings | L temp epileptiform discharges; occasionally bilat. theta activity | R temp epileptiform discharges; occasional brief gen spike wave episodes | Independent bitemp epileptiform discharges | L temp epileptiform discharges and theta activity |
| Ictal EEG findings | Evolving rhythmic theta activity L temp | R temp rhythmic theta activity | Bilat theta with temporal emphasis | L temp rhythmic theta activity |
| MRI findings | L HS | R HS | L HS | L HS |
| Outcome* | Seizure-free | Seizure-free | Seizure-free | Seizure-free |
FC, febrile convulsion; CPS, complex partial seizures; SGTCS, secondary generalized tonic–clonic seizure; SPS, simple partial seizures; L, left; R, right; temp, temporal; gen, generalized;
seizure outcome at most recent follow up, between 6 and 12 months after surgery.
The patients underwent a standard anterior temporal lobe resection. The hippocampal specimen was fixed for 1–3 days in 10% formalin and sectioned perpendicular to the long axis. Standard laboratory protocols were used to prepare resected tissue in sections stained for Nissl (cresyl violet/LFB), and immunolabeled for glial fibrillary acidic protein (GFAP; Dako, Cambridge, UK; polyclonal 1:1,500), neuronal nuclear antigen (NeuN; Chemicon, Temecula, CA, U.S.A.; monoclonal 1:2,000) and dynorphin (Serotec, Oxford, UK; polyclonal 1:100). Timms staining was carried out to confirm the presence of mossy fiber sprouting in each case. An experienced epilepsy neuropathologist (MT) made a qualitative tissue assessment for clinical purposes.
Results
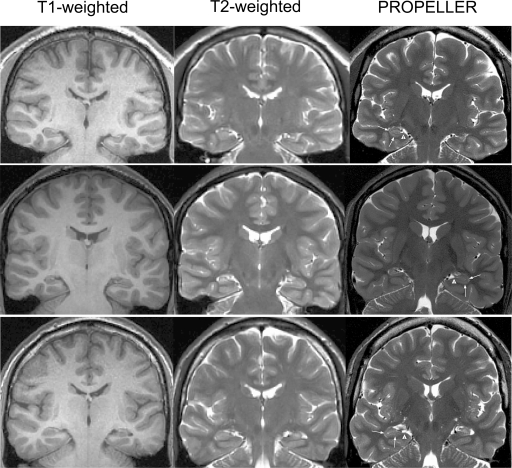
Three patients had left and one had a right temporal lobe resection. Classical HS (corresponding to Wyler grade 3) with variable degrees of granule cell dispersion was confirmed on routine histopathology in all cases. PROPELLER images gave superior contrast between grey and white matter compared to that seen on routine T1-volume and T2-weighted images (Fig. 1). There were no artifacts on PROPELLER images or routine sequences. HS was identified on all sequences, but PROPELLER images gave greater detail of hippocampal internal structures (Figs. 1–5).
Figure 1.
T1-weighted volume (left column), T2-weighted (middle) and PROPELLER (right) MRI scans at equivalent planes through the hippocampus from three patients. The PROPELLER scans had superior contrast between grey and white matter and within the hippocampal formation. HS can be seen in all patients (left HS, i.e., right on MR images, top and bottom rows; right HS, i.e., left on MR images, middle row). Arrowheads indicate the normal band of myelinated tissue in the superficial aspect of the parahippocampal gyrus extending into the hippocampus proper. Arrows indicate the normal grey matter signal of the subiculum, CA1 and the “C”-shaped contours of the dentate gyrus with intervening white matter strata (strata moleculare, lacunosum and radiatum).
Figure 5.
Zoomed PROPELLER image of the left temporal lobe (a) with corresponding histopathological sections for patient 4. All hippocampal subfields are available in the histopathological sections. The myelinated tissue in the strata moleculare, lacunosum, and radiatum is clearly seen on the LFB stained section (b, red arrow indicates the corresponding area of low signal on the PROPELLER images). Sections immunolabeled for GFAP (c) show the abrupt transition to gliosis in CA1 (black arrow). This corresponds to signal increase on PROPELLER images (white arrow). The neuronal loss can be seen on sections immunolabeled for NeuN (d, black arrow) with good preservation of nerve cells in subiculum and some nerve cells also preserved in CA3. Section immunolabeled for dynorphin shows mossy fiber sprouting (e).
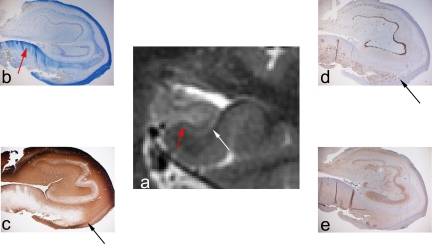
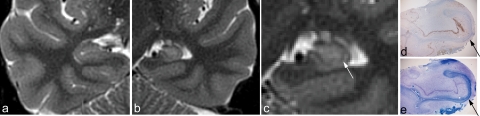
Figure 3.
Zoomed PROPELLER images of right (a) and left (c) temporal lobe in patient 2 with right HS. The right temporal lobe has been magnified (b) for the comparison with matched histopathological sections (d, e). NeuN immunhistochemistry confirmed neuronal loss and atrophy (d). The myelinated tissue in the intervening white matter strata (strata moleculare, lacunosum, and radiatum) is clearly seen on the LFB-stained section (e, red arrow) and is indicated by a red arrowhead in the unaffected, normal hippocampus (c). It is less conspicuous on the sclerotic side in this case (b, red arrow). The black arrow and arrowhead indicate the hilus on the sclerotic and normal side respectively.
On both the affected and unaffected sides, PROPELLER images showed the normal band of myelinated tissue (seen as a band of low signal) in the superficial aspect of the parahippocampal gyrus extending into the hippocampus proper (Figs. 1–5). Further, PROPELLER images showed the normal grey matter signal of the subiculum, CA1 and the “C”-shaped contours of the dentate gyrus. The intervening white matter strata (strata moleculare, lacunosum, and radiatum) appeared clearly delineated as a low contrast band. The nature of this area with lower signal seen on PROPELLER images was confirmed as myelinated tissue on the corresponding histopathological sections (Figs. 2–5). On the sclerotic side, and comparing this directly with the resected tissue, the subiculum was seen to retain approximately normal size and signal with an abrupt transition to atrophy and signal increase in CA1 (Figs. 2–5). This signal increase and atrophy also involved the hilar area. Neuronal loss and gliosis was confirmed in these regions on histopathological sections (Figs. 2–5). Mossy fiber sprouting and dispersion of the dentate granule cell layer were not appreciable on PROPELLER images of the sclerotic side.
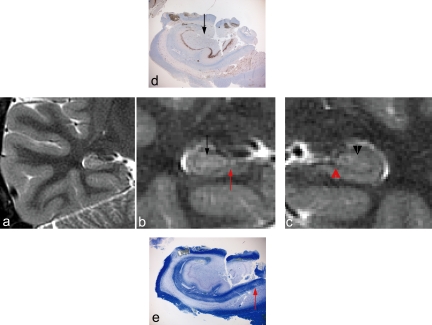
Figure 2.
Zoomed PROPELLER image of right (a) and left (b) temporal lobe in patient 1 with left HS. The left temporal lobe has been magnified (c) for comparison with corresponding histopathological sections. Subiculum appears normal on both PROPELLER images and histopathological sections (d; LFB, e; GFAP and f; NeuN immunohistochemistry). There is an abrupt transition to atrophy and signal increase in CA1 (red arrow). This signal increase and atrophy involved all hippocampal subfields, including the hilus. Gliosis (seen best with GFAP) and neuronal loss (seen best with NeuN) were confirmed in these regions on histopathological sections. Mossy fiber sprouting was confirmed on dynorphin immunohistochemistry (g). This was not appreciable in PROPELLER images.
Discussion
Hippocampal sclerosis has been identified reliably in vivo by MRI for more than 15 years, but routine scans (T1-weighted volume and T2-weighted scans) do not clearly show the internal structures of the hippocampus. Ex vivo 9.4T scans have shown detailed images of formalin-fixed cerebral tissue, including the hippocampus (Fatterpekar et al., 2002). Such scanning requires very high field strength and long scan times (e.g., 14 h) and is hence not feasible in patients. PROPELLER images have the advantage of being rapid, taking approximately half the time needed for the T1-volume scan, but still providing greater anatomical detail. The sequence used for this study did, however, not cover the whole brain and could not be prescribed in an oblique coronal plane, so patients had to tilt their heads forward during the scan, which precluded some other patients from having this sequence.
There were no overt head movements in the patients in the current study. Subtle head movements during MRI scans can occur even in motivated control subject (Forbes et al., 2001). Previous results from studies of the PROPELLER sequence show improved contrast and image quality in volunteers or patients asked to keep still during the scan on both 1.5 and 3T scanners (Forbes et al., 2001; Wintersperger et al., 2006). Improved image quality has also been shown in volunteers asked to move during the scan (Forbes et al., 2001) as well as patients who were unable to keep still (Forbes et al., 2003; Wintersperger et al., 2006). This suggests that the PROPELLER sequence could be used to study brain structures in detail even in patients more prone to movement, for example patients with learning difficulties or neurodegenerative disorders including Alzheimer's disease.
A previous study comparing PROPELLER to a single-shot Fast Spin Echo sequence noted more susceptibility artifacts, particularly metallic, on PROPELLER images (Forbes et al., 2003). None of our patients had any metallic implants and no artifacts were seen with either image sequence used.
PROPELLER images showed a good correlation with the histopathological sections, confirming that this MRI sequence could distinguish between different strata and subfields of the hippocampus. There was some discrepancy in the details of the morphology of internal hippocampal structures between in vivo MRI and histopathological sections. This is expected (Eriksson et al., 2005) and is due to tissue distortions after resection when the hippocampus is no longer supported by surrounding cerebral tissue. The main features, however, were clearly identifiable and comparable between in vivo PROPELLER MRI and histopathology. The severe gliosis seen on GFAP immunohistochemistry was mirrored by a signal increase on PROPELLER images in the same hippocampal areas. Both histopathological sections and PROPELLER images showed the abrupt transition between the relatively normal-appearing subiculum and the severely atrophic and gliotic CA1. The band of tissue with low signal stretching from the parahippocampal gyrus into the hippocampus was also confirmed to be myelinated tissue on histopathological sections (LFB), corresponding to strata lacunosum and radiatum (most clearly seen in Fig. 4).
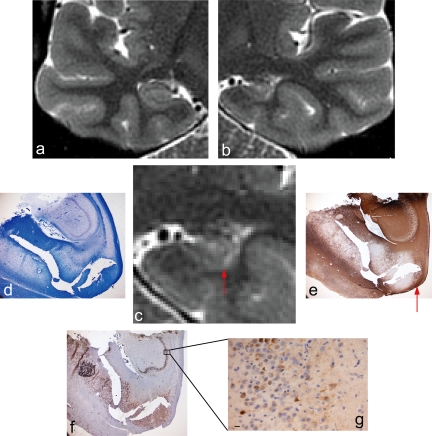
Figure 4.
Zoomed PROPELLER image of right (a) and left (b) temporal lobe in patient 3 with left HS. The left temporal lobe has been magnified (c) for comparison with corresponding histopathological sections (d, e). Only a small amount of hippocampal subfields CA1-3 were available in the histopathological sections. The myelinated tissue in the strata moleculare, lacunosum, and radiatum is clearly seen on the LFB-stained section (e) and can also be appreciated on the section immunolabeled for NeuN (d). Arrows indicate the corresponding areas on the PROPELLER image of the sclerotic hippocampus (c) and histopathological sections (d, e).
In summary, the PROPELLER sequence provided fast imaging with excellent contrast in patients who did not move overtly during the scan, negating the inevitable subtle movements in even the most cooperative subjects. More detailed in vivo studies of the hippocampal formation, looking for changes including more subtle abnormalities such as end folium sclerosis or different types of HS, and the neocortex are now possible and may not only increase the diagnostic yield of MRI in epilepsy but also in conditions such as neurodegenerative disorders, including Alzheimer's disease.
Acknowledgments
We acknowledge the financial support of the Wellcome Trust project grant number 066185 and we are grateful to the Big Lottery Fund, Wolfson Trust, and National Society for Epilepsy for supporting the 3T MRI scanner at NSE. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. This study was carried out as part of the UCL-UCLH Comprehensive Biomedical Research Centre.
Conflicts of Interest
We have no conflicts of interest to declare in relation to this study.
References
- Blumcke I. A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathologica. 2007;113:235–244. doi: 10.1007/s00401-006-0187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton CJ. The neuropathology of temporal lobe epilepsy. Oxford: Oxford University Press; 1988. [Google Scholar]
- Eriksson SH. Reliable registration of preoperative MRI with histopathology after temporal lobe resections. Epilepsia. 2005;46:1646–1653. doi: 10.1111/j.1528-1167.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- Fatterpekar GM. Cytoarchitecture of the human cerebral cortex: MR microscopy of excised specimens at 9.4 Tesla. Am J Neuroradiol. 2002;23:1313–1321. [PMC free article] [PubMed] [Google Scholar]
- Forbes KPN. PROPELLER MRI: clinical testing of a novel technique for quantification and compensation of head motion. J Magn Reson Imaging. 2001;14:215–222. doi: 10.1002/jmri.1176. [DOI] [PubMed] [Google Scholar]
- Forbes KP. Brain imaging in the unsedated pediatric patient: comparison of periodically rotated overlapping parallel lines with enhanced reconstruction and single-shot fast spin-echo sequences. Am J Neuroradiol. 2003;24:794–798. [PMC free article] [PubMed] [Google Scholar]
- Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res. 1990;535:195–204. doi: 10.1016/0006-8993(90)91601-c. [DOI] [PubMed] [Google Scholar]
- ILAE Commission on Neuroimaging Guidelines for neuroimaging evaluation of patients with uncontrolled epilepsy considered for surgery. Epilepsia. 1998;39:1375–1376. doi: 10.1111/j.1528-1157.1998.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Jackson GD. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869–1875. doi: 10.1212/wnl.40.12.1869. [DOI] [PubMed] [Google Scholar]
- Jackson GD. Optimizing the diagnosis of hippocampal sclerosis using MR imaging. Am J Neuroradiol. 1993;14:753–762. [PMC free article] [PubMed] [Google Scholar]
- Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn Reson Med. 1999;42:963–969. doi: 10.1002/(sici)1522-2594(199911)42:5<963::aid-mrm17>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Sutula T. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- van Paesschen W. In: Hippocampal sclerosis Neuropathology of epilepsy. Scaravilli F, editor. Singapore: World Scientific; 1997. pp. 505–578. [Google Scholar]
- Wintersperger BJ. Brain magnetic resonance imaging at 3 Tesla using BLADE compared with standard rectilinear data sampling. Invest Radiol. 2006;41:586–592. doi: 10.1097/01.rli.0000223742.35655.24. [DOI] [PubMed] [Google Scholar]