Abstract
Enzyme linked immunosorbent assay (ELISA) has been widely used to measure antibody titers for evaluating the immunogenicity of a vaccine. However, there is as yet no generally accepted way of expressing the ELISA results in the case of experimental vaccines, since there is usually no uniform standard. Both end point and single dilution methods have significant disadvantages. In this paper, we obtained reproducible data with fewer dilutions of samples by addition of serially diluted standard serum to each ELISA plate. Since this ELISA method gives reliable antibody titer with less labor than other methods, it can strongly support vaccine development.
Keywords: Enzyme linked immunosorbent assay (ELISA), Antibody, Vaccine development
1. Introduction
The Enzyme linked immunosorbent assay (ELISA) was developed independently in 1971 by Engall and Perlmann [1] and by van Weemen and Schuurs [2] to avoid problems associated with radioimmunoassays. Since then ELISAs have been widely used for measurement of polyclonal and monoclonal antibodies in biological fluids or in culture media. Currently many commercial kits and automated systems are available to detect antigen-specific antibodies following vaccination [3,4] or to detect antibodies for diagnosing infectious diseases (extensively reviewed in [5]).
To evaluate the immunogenicity of experimental vaccine candidates, accurate measurement of antibody titers is one of the most important read-outs using antisera or other fluids from immunized experimental animals or human clinical trials. Many studies have been already done to improve the ELISA itself [6-9], so that the methodology of indirect ELISAs is well established. However, there is as yet no generally accepted way of expressing the ELISA results from such studies. Endpoint titer of a full dilution curve, e.g., the highest dilution that gives an optical density of 2 or 3 standard deviations above the negative control, is one of the most common ways to express these results. On the other hand, data obtained with samples tested at a single dilution are also commonly used, e.g., direct O.D. values, ratio of the O.D. of the test samples, etc. The relationship between the quantity of antibody and the O.D. is expressed by a hyperbolic curve function [10,11], and only in certain ranges can it be approximated as a straight line. Therefore there are potential problems with either method. If the relationship could be expressed by a linear function, an end point titer could be easily calculated using two data points. Since this is not the case, obtaining an end point titer requires serial dilution of all test samples, which is a time-consuming and labor-intensive process in the absence of automated equipment, and it requires more materials (e.g. antigen, plates, etc) compared with the single dilution method. Reducing the required amount of antigen for ELISA is desirable, especially in the case of an experimental vaccine where only a limited amount of antigen is available. In contrast, using single dilutions permits testing of large numbers of samples at the same time and since each sample is only tested at a single dilution, not at multiple dilutions, less material is required compared with the end point titer method. However, the data from portions of the curve outside of the region approximating a straight line are not relevant.
An additional problem relates to comparing data from different ELISA plates tested at the same time or different ELISA plates tested at different times. To facilitate such comparisons, it has been proposed that positive and negative controls be added to each ELISA plate [5,12], but there is no consensus on: 1) what are suitable positive and negative controls in the case of experimental vaccine antigens, since there is usually no uniform standard or, 2) the precision of the data when unknowns are tested with positive/negative controls. In this paper, three species of animals (mouse, rabbit and monkey) have been immunized with various malaria recombinant proteins: Plasmodium vivax sexual stage 25kDa (Pvs25), Plasmodium falciparum sexual stage 25kDa (Pfs25), and P. falciparum apical membrane antigen-1 (PfAMA1), all of which are leading malaria vaccine candidates. Sera from these animals were tested by indirect ELISA to detect the quantity of antibody in the sera. We show that addition of serially diluted standard serum to each ELISA plate can; 1) reduce the number of dilutions when compared with obtaining end point titers, 2) give reliable and reproducible data compared with the single dilution method and 3) reduce variation between the data obtained from different ELISA plates.
2. Materials and Methods
2.1 Antigen preparation
Clinical grade Pvs25 [13] and Pfs25 [14] were prepared as described previously. In brief, the parasite gene encoding Pvs25 was cloned into the plasmid YEpRPEU-3, transformed into Saccharomyces cerevisiae VK1 cells. The Pfs25 sequence was cloned into pPIC9K and transformed into Pichia pastoris. After fermentation, the protein products were purified with a Nickel nitrilo-triacetic acid Superflow column, and with a Phenyl Sepharose hydrophobic interaction chromatography column. A final purification/buffer-exchange step was performed by loading onto a Superdex 75 size-exclusion column pre-equilibrated with 152 mM NaCl, pH 7.2.
AMA1 was produced and purified as described previously [15]. In brief, the synthetic AMA1 gene sequence was subcloned into the pPIC9K vector and transformed into P. pastoris. After fermentation, Nickel nitrilo-triacetic acid Superflow column, G-25 column, anion-exchange column, butyl-Sepharose interaction column and Superdex 75 size-exclusion column were used for purification and buffer-exchange steps.
2.2 Immunization
Mouse and rhesus monkey studies were done in compliance with National Institutes of Health (NIH) guidelines and under the auspices of Animal Care and Use Committee-approved protocols and rabbit immunization was performed by Spring Valley Laboratories (Frederick, MD). BALB/c mice (6-12 weeks old), New Zealand White rabbits and rhesus monkeys (Macaca mulatta) were immunized with Pvs25, Pfs25 or AMA1 formulated with various adjuvants, mainly employing aluminum hydroxide (HCI Biosector, Frederikssund, Denmark) and Montanide ISA720 (SEPPIC, Paris) as adjuvants. Immunization and bleeding schedules for each study experiment are listed in Table 1.
Table1. Immunization and bleeding schedules.
| Animal | Immunogen | Route | Days of immunization | Days of bleed |
|---|---|---|---|---|
| Mouse | Pvs25 | I.P. | 0 and 28 | 0 and 42 |
| Mouse | Pfs25, Pvs25 | I.M., I.P. | 0 and 28 | 0 and 42 |
| Mouse | Pfs25, Pvs25 | I.P. | 0 and 28 | 0 and 42 |
| Mouse | AMA1 | I.M. | 0 and 28 | 0 and 42 |
| Rabbit | Pfs25 | I.M. | 0, 28 and 56 | 0, 42 and 70 |
| Monkey | Pvs25 | I.M. | 0, 28 and 181 | 0, 14, 28, 42, 90, 175, 195, 223, 269 and 365 |
| Monkey | AMA1 | I.M. | 0, 28 and 183 | 0, 14, 28, 42, 61, 90, 175, 197, 224, 270 and 365 |
2.3 Elisa
Four buffers (coating, blocking, dilution, washing and stopping buffer) were prepared before performing the ELISA. Coating buffer that contained 15mM sodium carbonate (Mallinckrodt, Phillipsburg, NJ) and 35mM sodium bicarbonate (Mallinckrodt) in distilled water (Advanced Biotechnologies, Inc., Columbia, MD) were stored at room temperature (RT). Blocking buffer that contained 5% w/v skim milk powder (Difco, Detroit, MI) in Tris Buffered Saline (TBS) (BioFluids, Camarillo, CA) was aliquoted and frozen at -20°C until needed. Dilution buffer containing 0.1% BSA (Sigma Chemical Co., St. Louis, MO) and 0.05% Tween-20 (Sigma) in TBS was kept at RT. Washing buffer containing 0.1% Tween-20 in TBS was maintained at RT. Stopping buffer contained 5N Sodium Hydroxide (J.T.Baker, Phillipsburg, NJ) in distilled water. Flat-bottom 96-well ELISA plates (Immunolon 4; Dynex Technology Inc., Chantilly, VA) were coated with 100 ng per well of test protein diluted with coating buffer. The plates were wrapped in plastic wrap and aluminum foil, stored at 4°C more than 12 hours and used within one week. To initiate the assay, plates were blocked with 200 μL/well of blocking buffer for 2 hour at RT. Independent triplicate dilutions of each test animal serum were prepared in dilution buffer. Diluted sera were added to antigen-coated wells (100 μL/well) and incubated for 2 hours at RT. After extensive washing with washing buffer using a plate washer, the plates were incubated with 100 μL/well of the appropriate secondary antibody conjugated with alkaline phosphatase for 2 hours at RT. For mouse serum, 0.1 μg/well of anti-Mouse IgG (H+L) antibody (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) was used. For rabbit serum, 0.1 μg/well of anti-Rabbit IgG (H+L) antibody (Kirkegaard & Perry Laboratories, Inc.), and for monkey serum, 0.05 μg/well of anti-Monkey IgG γ chain antibody (Rockland, Gilbertsville, PA) were used. After similar washing, the substrate (0.1 mg/well of p-nitrophenyl phosphate, Sigma 104 substrate; Sigma) diluted with coating buffer was added and the plates were incubated at RT for 20 minutes in the dark. Reactions were terminated by adding 25 μL/well of stopping buffer. Immediately after this addition, the absorbance at 405 nm was read using a Spectramax 340PC microplate reader (Molecular Devices Co., Sunnyvale, CA). A standard operating procedure for this assay can be obtained on request from the corresponding author.
2.4 Establishing a Standard ELISA Curve
Sera from animals selected for relatively high antibody titer were used to prepare a reference standard for each antigen and for each animal species. After the appropriate pool was made, all standard sera were diluted 1:100 with dilution buffer, aliquoted (200 μL of each) and maintained at -80°C until required. Antibody units were assigned to each standard serum as follows. First, one of the aliquots was diluted in two-fold steps from 1:1,000 to 1:526,000 with dilution buffer. These serially diluted sera were applied on an ELISA plate as primary antibodies. Two wells per set of serially diluted standard did not receive any samples or buffer at this step (blank wells), but the other ELISA steps (adding blocking buffer, incubating with secondary antibody, adding substrate and stopping buffer) were performed as those for other wells. These blank wells were used as the negative control wells (reciprocal number of dilution was assigned as 0). The relation between reciprocal number of the dilution and O.D. 405 was approximated by a 4-parameter hyperbolic curve (SOFTmax PRO ver.3; Molecular Devices Co., Sunnyvale, CA). If the R2 of that curve fit was less than 0.997, another standard serum aliquot was thawed and the entire procedure repeated. Based on the constants of the equation, antibody units were assigned to the standard as the reciprocal of the dilution giving an O.D. 405 = 1. Once the antibody units of a standard serum were determined, the number was used invariably for all samples tested by ELISA against that standard.
Thereafter a reference standard was used on each ELISA plate to make a standard curve. To do this, standard serum aliquots were freshly thawed and two independent serial dilutions (two-fold steps, 10 different dilutions) were prepared starting with a dilution of 20 antibody units. These were applied to the wells on the ELISA plate and 4 blank wells (assigned zero antibody units) were also designated. The OD values were then fitted to a 4 parameter standard curve (Antibody units = C[{(A-O.D. 405)/( O.D. 405-D)}˄(1/B)]) using SOFTmax PRO ver.3 software). The antibody units in test sera were then calculated from their O.D. 405 values using the parameters estimated from this standard curve.
2.5 Statistics
To test the correlation between two groups, Spearman rank correlation was used (UNISTAT 5.0, P-STAT Inc., Hopewell, NJ). Linear regression analysis was performed using UNISTAT 5.0 and curve fitting was performed using Sigma Plot (SPSS Inc., Chicago, IL). The R2 value of a standard curve was calculated by SOFTmax PRO ver.3.
3. Results
3.1 Comparison of ELISA antibody units derived from a single dilution versus full dilution curves
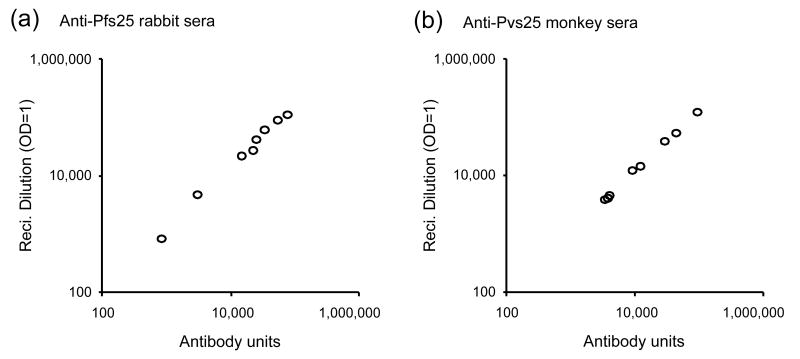
Eight anti-Pfs25 rabbit sera were serially diluted (five-fold steps) and tested by ELISA. Two numbers were generated for each test serum: 1) Using a single OD value which was closest to 1 in each set of diluted sample, the antibody units were calculated referring to a standard curve as described in Materials and Methods section, 2) Using all OD values from serially diluted sample, the reciprocal dilution giving an O.D. 405=1 was calculated based on a 4 -parameter fitted hyperbolic curve for each sample. As shown in Fig 1a, the antibody units calculated by a standard curve and the reciprocal dilution giving an O.D. of 1 calculated from full dilution curves are significantly correlated (Spearman Rank Correlation (SRC) = 1.0, p<0.0001). A similar comparison was performed with ten anti-Pvs25 monkey sera, which were four-fold serially diluted, and there is also significant correlation between the two data sets (SRC = 1.0, p<0.0001; Fig.1b). Thus to obtain the antibody units, a single dilution was enough if serially diluted standard serum was tested in the same ELISA plate.
Fig. 1.
Comparison of ELISA antibody units derived from a single versus multiple serial dilutions. Antibody units were calculated from O.D.405 of a single dilution referring to a standard curve and are plotted on the X-axis. The same sample was serially diluted to generate a complete dilution curve. From the dilution curve, the reciprocal of the dilution (Reci. Dilution) giving an O.D.405 = 1 was calculated (on the Y-axis). (a) Eight anti-Pfs25 rabbit sera were tested with Pfs25 coated plates. Spearman Rank Correlation (SRC) of two data sets is 1.0 (p<0.0001). (b) Ten anti-Pvs25 rhesus monkey sera were tested with Pvs25 coated plates. SRC of two data sets is 1.0 (p<0.0001).
3.2 Variability of antibody units with experimental conditions
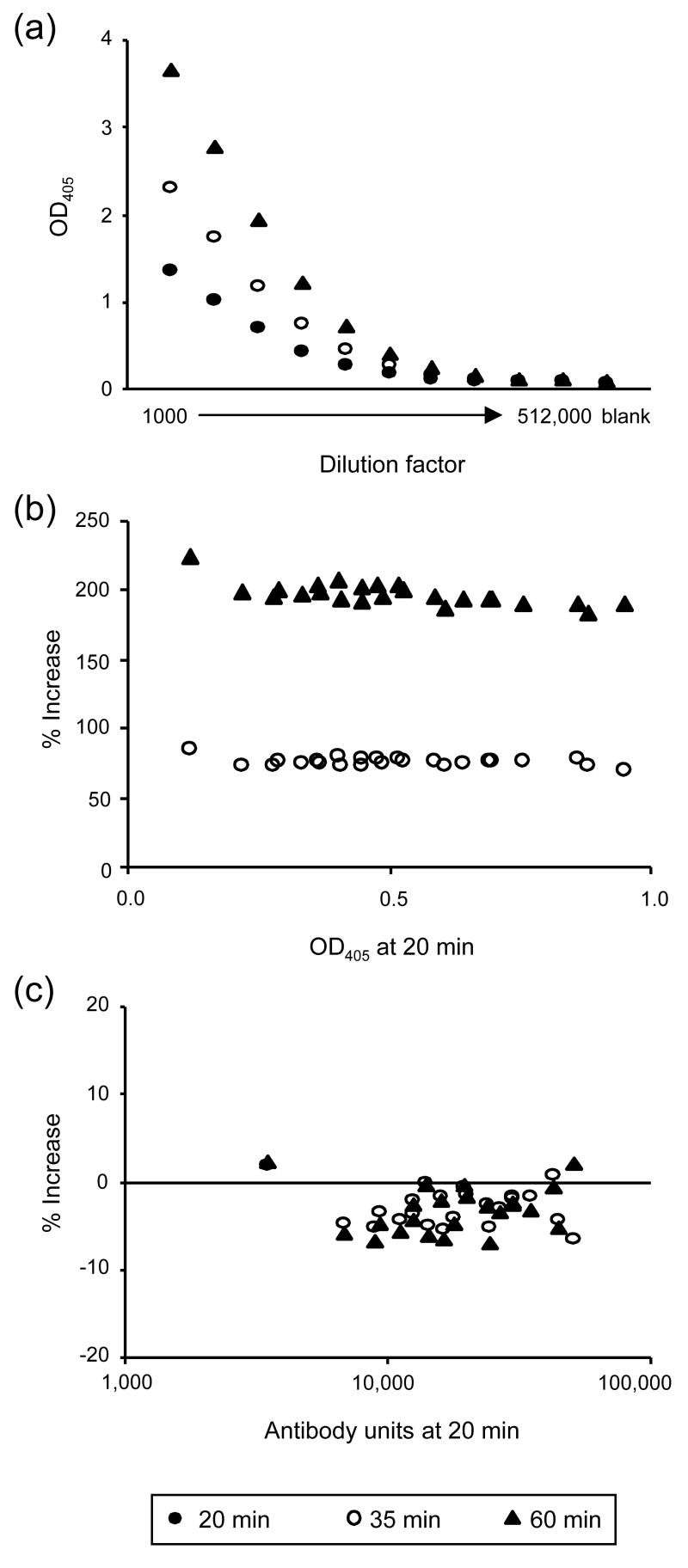
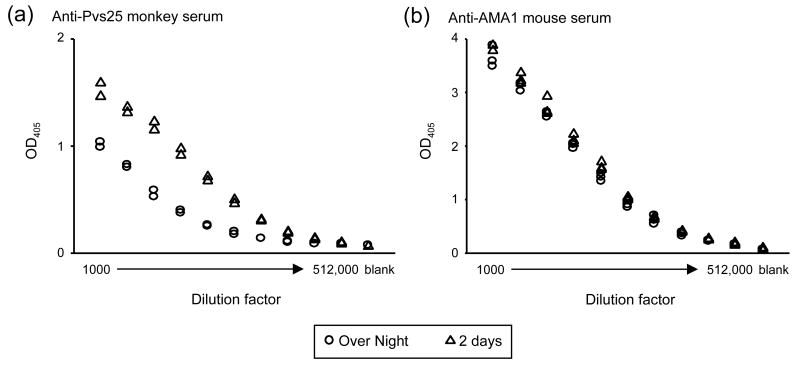
Second, the robustness of the method was tested by varying the experimental conditions. When the secondary alkaline phosphatase conjugate was incubated longer with substrate, the O.D. 405 increased with time for both a standard serum (Fig. 2a) and anti-AMA1 rhesus monkey sera (Fig. 2b) tested. When the O.D. values above background of the antisera were analyzed, there was no significant correlation between O.D. 405 at 20 min and % increase at 35 min (SRC = -0.137, p=0.2624). On the other hand, there was a negative correlation between O.D. 405 at 20 min and % increase at 60 min (SRC = -0.697, p<0.001). Despite the increase in O.D. 405, the antibody units calculated at the different times changed by less than 10%, regardless of the antibody units at 20 min (Fig. 2c). Comparable results were obtained with anti-Pfs25 rabbit sera (data not shown). O.D. 405 was also dependent on the time allowed for antigen to coat the ELISA plates (Fig. 3). An anti-Pvs25 monkey serum and an anti-AMA1 mouse serum were serially diluted and tested on ELISA plates which were incubated either overnight or two days. The samples showed higher O.D. on plates coated 2 days than on plates coated overnight. Although the degree of increase varied with antigen and species of animal tested, an increase of the O.D. of the samples was generally observed with all antigens and animal species (data not shown). In case of the anti-Pvs25 monkey sera, the dilution giving an O.D. 405 of 1.0 on an overnight-coated plate was 7 times lower than that on a 2-day-coated plate (1034 and 6958 dilutions, respectively). To simulate using the curve to determine antibody units of an unknown sample, the antibody units of the 5th dilution (which was supposed to have 1.25 antibody units) were back calculated from the best-fit curve generated without the 5th dilution data point. The back calculated antibody units changed by less than 6% (1.214 and 1.286 ELISA on overnight-coated and 2-day-coated plates, respectively). Similar results were seen when the antibody units of other dilution points were back calculated from the best-fit curves (data not shown). Hence, the antibody units calculated using samples in which antibody units had been defined by running a standard on the same plate are more robust than the O.D. value itself.
Fig. 2.
Effect of varying substrate incubation time on O.D. 405 and on ELISA antibody units. Anti-AMA1 rhesus monkey sera were tested. After adding substrate to the ELISA plates, O.D. 405 of a standard and test samples were read at 20, 35 and 60 minutes without adding stopping buffer. (a) O.D. 405 of the serially diluted standard sera at the three time points; (b) % increase above background of O.D. 405 of each sample compared with the O.D. at 20 min; (c) % increase of antibody units of each samples compared with the antibody units at 20 min.
Fig. 3.
Effect of exposure time of coating antigen on ELISA plates. ELISA plates were coated with specific antigens overnight or for two days before washing. Aliquoted and frozen sera were freshly thawed just before applying to the coated ELISA plates. (a) Anti-Pvs25 rhesus monkey serum was tested with Pvs25 coated plates (2 plates for each condition). (b) Anti-AMA1 mouse serum was tested with AMA1 coated plates (3 plates for each).
3.3 Quality control of standard curves and tested samples
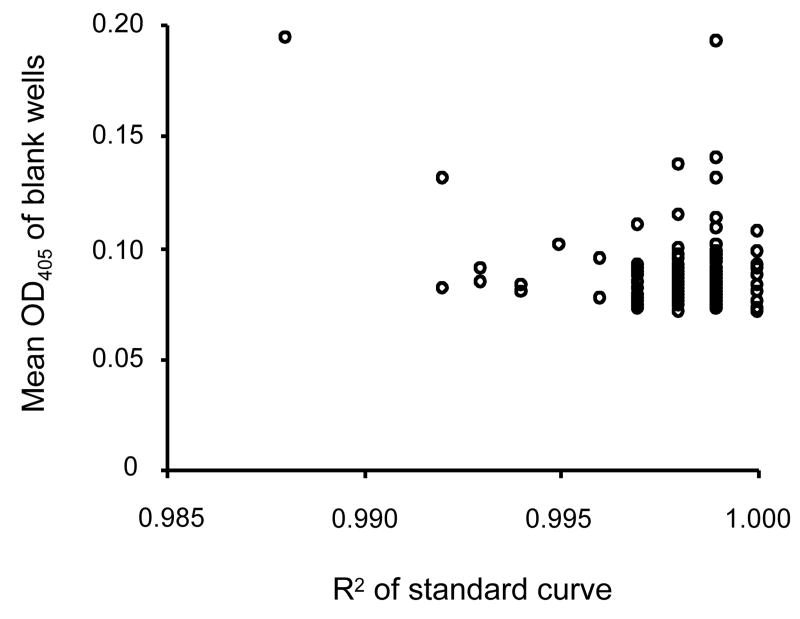
To determine the criteria for acceptable quality of an ELISA plate, data from 114 plates completed by two different operators using sera from all antigen/animal combinations listed in Table 1 were compiled (Fig.4). The proportion of the plates with standard curves having an R2 less than 0.994 was 4.4 %. The mean O.D. 405 of blank wells was 0.088 and the standard deviation was 0.019. The R2 of the standard curve and the O.D. 405 of blank wells were independent variables.
Fig. 4.
Quality control parameters for an ELISA standard curve. The R2 of a standard curve (fitted to a 4- parameter hyperbolic curve) is plotted on the X-axis and the arithmetic mean value of O.D.405 of 4 blank wells on the same plate is plotted on the Y-axis. The data (114 plates) from mice immunized with Pvs25, Pfs25 or AMA1, rabbits immunized with Pfs25, and monkeys immunized with Pvs25 or AMA1 are included in this figure.
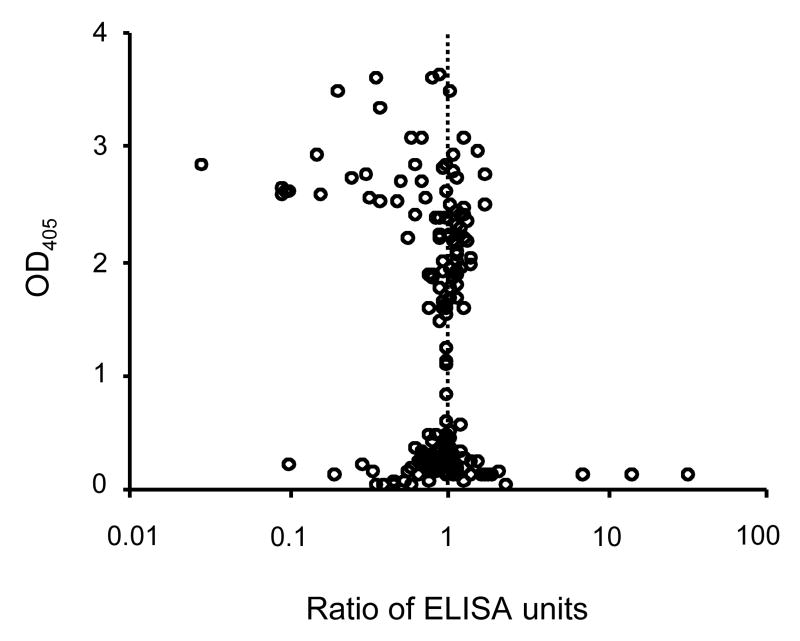
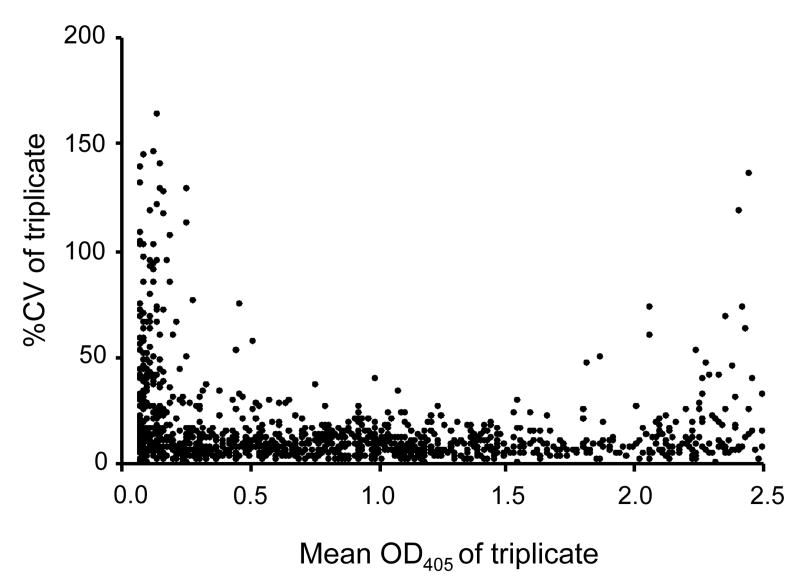
The quality of data obtained with test samples was examined using 2 sets of data. Seventy-eight samples (mouse Pfs25 or Pvs25, rabbit Pfs25 and monkey Pvs25 antisera) were serially diluted and antibody units for each dilution of each sample were calculated. The relative units were calculated by comparison with the antibody units of the sample that came from the dilution showing an O.D. 405 closest to 1.0 (Fig. 5). The figure shows that if the antibody units are calculated based on an O.D. 405 of less than 0.2 or of more than 2.5, the difference of the antibody units was very large when compared with the antibody units obtained from an O.D. 405 near 1.0. In contrast, the mean of the relative units of the 114 data sets in the range of O.D. 405 of 0.2 to 2.5 was 1.01 (95%CI, 0.97-1.04). The second set of data (1241 points, mouse Pvs25, Pfs25 or AMA1, rabbits Pfs25 and monkeys Pvs25 or AMA1 antisera) is shown in Fig. 6. Arithmetic mean O.D. 405 of triplicates of each sample is plotted on the X-axis and % coefficient of variation (%CV) of triplicates is plotted on the Y-axis. In the O.D. 405 range of 0.5 to 1.7, the %CV was generally less than 30%. However, the data with lower O.D. 405 values (<0.5) or higher O.D. 405 values (>1.7) both showed higher %CV.
Fig. 5.
Impact of O.D. 405 on ELISA antibody units. Animal sera were serially diluted and tested by ELISA (78 samples, 261 data points). A relative unit for each dilution was calculated by comparison with the units that came from the dilution which gave an O.D. 405 closest to 1. The relative units of 1, as a definition, are not shown in this figure. Sera were collected from mice immunized with Pfs25 or Pvs25, rabbits immunized with Pfs25, and monkeys immunized with Pvs25.
Fig. 6.
Relationship of ELISA O.D. 405 to percent coefficient of variation (%CV) of triplicate wells. The arithmetic mean O.D.405 of triplicate wells tested by ELISA is plotted on the X-axis and the %CV of antibody units obtained from triplicate wells is plotted on the Y-axis. The data (1241 points) include mice immunized with Pvs25, Pfs25 or AMA1, rabbits immunized with Pfs25, and monkeys immunized with Pvs25 or AMA1.
Based on the data above, we determined the criteria for acceptable quality for ELISA plates as the following:
R2 of the standard curve should be equal to or greater than 0.994
The O.D. 405 of blank wells should be less than 0.15.
If the R2 of standard curve and blank values did not match these criteria, all data on the plates were discarded. If the ELISA plates passed the criteria, quality of individual test samples were checked by the criteria shown in Table 2.
Table 2. Data of test samples determined to be acceptable a).
| Sample dilution | O.D. 405 | %CV |
|---|---|---|
| ≤ 1: 500 | 0.25 ≤OD< 0.3 | <70% b) |
| 0.3 ≤OD< 0.4 | <50% b) | |
| 0.4 ≤OD< 0.5 | <40% b) | |
| 0.5 ≤OD< 1.6 | <30% b) | |
| > 1: 500 | 0.25 ≤OD< 1.6 | <30% |
First quality control of standard curve was tested for each ELISA plate, then the QC of unknown sample values were tested. Any data that did not match in the category above were not reliable.
The values of %CV were decided based on the mean plus 2SD of samples in the indicated O.D. range (Fig. 6).
3.4 Reproducibility of the ELISA data
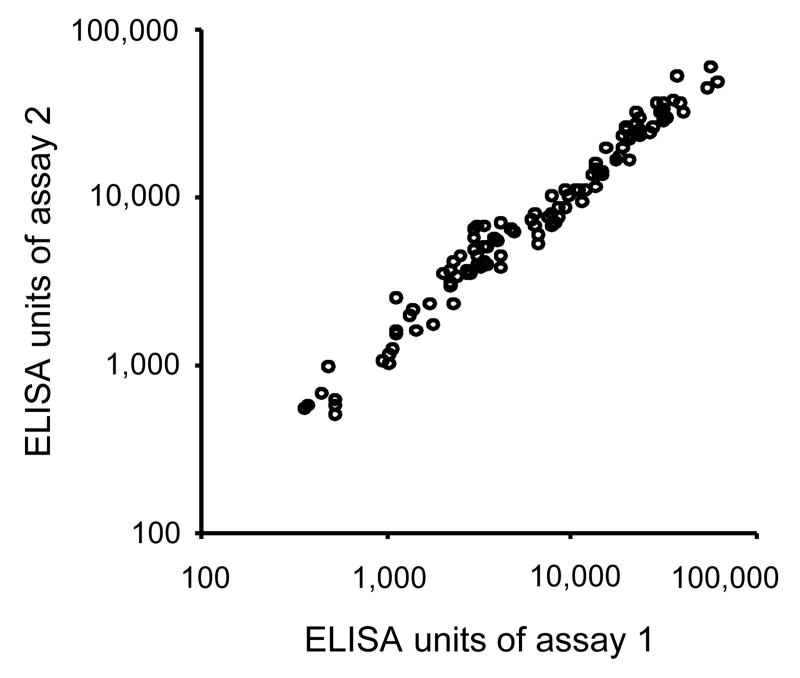
Following the criteria listed above, antibody units of a number of animal sera were tested by ELISA to evaluate the reproducibility of the data. Serum samples (n=112) were aliquoted and frozen at -80°C before use, and these samples were tested at an interval of 15 months (Fig. 7). The concordance between the two sets of the data is highly significant judged by the linear regression analysis (coefficient was 1.01; 95%CI, 0.97-1.06; p<0.001). The reproducibility of data obtained with 89 antisera by two different operators using the same standard was also high (coefficient was 0.84; 95%CI, 0.79-0.89; p<0.001, data not shown).
Fig. 7.
Reproducibility of ELISA antibody units. Anti-AMA1 rhesus monkey sera (112 samples) were tested by ELISA twice at an interval of 15 months. The sera were aliquoted and frozen at -80°C before use. The concordance between the two sets of the data is highly significant (coefficient was 1.01; 95%CI, 0.97-1.06; p<0.001).
Discussion
Results in this paper clearly show that using a standard serum, which is a pool of sera from immunized animals, to make a standard curve has two advantages on analyzing the quantity of antibody in experimental vaccines. First, we could reliably assess quantities of vaccine-induced antibodies with fewer different diluted samples compared with endpoint titration. Second, use of a standard serum allows comparison of data obtained with different ELISA plates on different days. Therefore by this method, more precise decisions can be made with less time and labor.
As Fig. 1 shows, antibody units calculated from a single dilution point (which was closest to O.D. 405 of 1 in each set of diluted sample) gave the same value as that calculated from a complete titration curve. Since the reliability of the data is less if the O.D. 405 of the test sample is too low or too high by our methods (Fig 5 and 6), we determined to use only the data within the range of O.D. 405 of 0.25 to 1.6 (Table 2) to calculate the antibody units of the samples. Therefore even by our methods, some of the samples need to be tested at more than one dilution to obtain reliable antibody units. However, a 10- (or 20-) fold dilution was enough to obtain reliable data by our method, because the range of O.D. 405 of 0.25 to 1.6 covered a large range of concentrations of antibodies (Fig 2a and 3), while two-fold dilutions are usually used for endpoint titrations.
Although our ELISA method provides reliable antibody levels with fewer dilution steps, there is an assumption for this method that all test sera have the same shapes of dilution curves as the standard serum. The data shown in Fig. 5 (and unpublished our other ELISA data) supports this assumption. If each sample has a different dilution curve from the standard curve, the antibody units calculated from the O.D. 405 data at one dilution should be different from the units calculated at another dilution. However, the difference of antibody units between the two dilutions was less than 30 % if both of the data came from the range of O.D. 405 from 0.25 to 1.6. Therefore, if a standard serum pool was generated from animals immunized with same antigen, it is a reasonable assumption that unknown samples and the standard have almost the same dilution curves. On the other hand, if the samples are generated under different conditions (e.g., sera from subjects immunized with a protein antigen vs. immunized by DNA vaccination, or sera from vaccinees who live in non-endemic areas vs. sera from people who live in endemic areas, etc), it might be possible that different samples (and/or standard) have different shapes of dilution curves. However, if this happens, both the endpoint titer and single dilution methods have a problem. In the case of endpoint titer method, the result will change depending on the threshold of optical density used (e.g., the data set using 2 SD above the negative control as a threshold may be quite different from the data set using O.D. =0.5 as a threshold, etc) because the curves of the samples are not parallel, and it is difficult to judge which threshold is better. Similarly, with the single dilution method, the result will change depending on the dilution factor selected. Therefore, whichever method is used, it is difficult to interpret the result of ELISA if different samples have different shapes of dilution curves. For the ELISA method mentioned in this paper, there is a relatively easy way to check whether the dilution curve of a sample is similar to the curve of the standard. If a sample is tested at two or more dilutions, and the ELISA units determined from different dilutions are similar, as shown in Fig. 5, one can assume that the shapes of the curves are similar. If the ELISA units calculated from different dilutions are not concordant, additional assay(s) (and/or making a new standard using the same population of sera) is required.
There is another way that is commonly used to present the amount of antibody resulting from a single dilution, viz., all samples are tested at a single dilution and presented as the direct O.D. or a relative O.D. compared with a control. Although this approach allows a large number of samples to be measured in a short time, a disadvantage is that the data has a large quantitative uncertainty. Since the relationship between OD and antibody concentration is highly nonlinear (Fig. 2, 3 and previous paper [10,11]), a factor of two-fold difference in the result does not mean two-fold quantitative difference in the absolute amount of antibody. In addition to that, as shown in our results, the value of O.D. 405 and the shape of the hyperbolic curve could be changed by a number of factors (e.g., time of incubation, type of antigen, species of animal, etc). McLaren et.al. [8] had previously reviewed the factors that could alter the O.D. 405, including variables we have not tested in this paper. Therefore, if quantities of antibody, not rank order of the samples in a set, are considered, it is necessary to confirm that all data of O.D. 405 fall in the quasi-linear range for the particular ELISA plate.
Since the O.D. 405 values may change due to a number of factors when the data from different plates are compared, each plate should have an internal standard to adjust for the differences between plates. The O.D. 405 of blank wells can also change from plate to plate (Fig. 4), so that each plate needs to have a negative control. In addition to the negative control, each plate also should have a positive control. There are two major reasons to add a positive control in each ELISA plate in addition to a negative control: 1) The higher values of O.D. 405 were more sensitive than the lower values of O.D. 405 when the conditions of the visualization step (secondary alkaline phosphatase conjugate was incubated longer with substrate) changed (Fig 2 and 3, [6]); 2) changing the conditions of the secondary antibody incubation step (e.g., time and temperature of the incubation, different lot of secondary antibody) would likely have little impact on the O.D. of blank or negative control wells. Hence, either endpoint titer or data using a single dilution method should be done with negative and positive controls. Addition of a serially diluted standard serum in each ELISA plate can be a good control and it improves the reproducibility of the data obtained with different plates and different times (Fig 7).
To obtain reliable data, quality control (QC) of the data is important. Since all absorbance values of test sera on a plate were converted to units using the standard curve, QC of the standard is very important. We determined that the criteria for acceptable quality for ELISA plates is that the R2 of the standard curve should be equal to or greater than 0.994 (95.6% of plates tested in 114 plates) and O.D. 405 of the blank should be less than 0.15 (98.2%). Five percent rejection was used as the criteria for the R2, because changing the shape of the curve had a large impact on the antibody units. On the other hand, the criteria of O.D. of the blank are not so strict. To balance an appropriate level of rejection with the effort required to repeat the assays, we decided the level that approximately 95 % (108 in 114 plates, 94.7%) of the standards will be accepted. The QC criteria for the individual test samples were also determined. The acceptable range of the %CV shown in Table 2 was decided based on the data in Fig. 6. The criteria of %CV for QC at a 1:500 or less dilution was not strict as shown in Table 2 in the range of O.D. 405 < 0.5. The level of mean plus 2 standard error of %CV in each range of O.D. was used for the criteria at such dilution (in range of 0.25≤OD<0.3; %CV<70, 0.3≤OD<0.4; %CV<50, 0.4≤OD<0.5; %CV<40 was acceptable). We usually use 1:500 dilution as a minimal dilution to assess the antibody units, because the antibody units of minimal detection limit (O.D. 405 is 0.15) at 1:500 dilution is close to the level of the antibody in pre-immune animal sera (data not shown). Therefore, if the sample showed O.D. 405 <0.5 at 1:500 dilution (or less), it had very low antibody units, and generally it is not worthwhile to repeat the ELISA for samples with such low antibody titers in experimental vaccine trials in animals.
We have applied this ELISA method for our phase 1 human trials to evaluate several vaccine candidates [16-18]. Although this ELISA method is less labor intensive when it is compared with an endpoint titer method, for phase 2 (or 3) trials, we are trying to improve this method to increase the number of samples that can be tested. For example, we have begun to evaluate machines that dispense liquid into a 96-well plate, instead of doing all ELISA steps manually.
Although using this ELISA method gives more reliable values for antibody levels than endpoint titer or data using a single dilution method without negative/positive controls, there are three disadvantages of this approach. First, fewer samples can be tested in a plate, because 24 in 96 wells are used to make a standard curve. The second is that standard serum needs be prepared for each antigen and for each species of animal. However, the starting dilution of standard serum is usually at a 1:1000 dilution or more, and about 600 μL of diluted standard per plate are required to make two sets of serially diluted standard serum. That means only 0.6 μL (or less) of original standard serum per plate are required. Finally, the constants of a hyperbolic fitted curve need to be calculated so that the absorbance of the samples can be converted to units. However, software is available to do that automatically or semi-automatically. Thus, these disadvantages are relatively small when the accuracy of the antibody data by this method is taken into account. Therefore, our ELISA method mentioned in this paper can be an extremely useful tool to evaluate the immunogenicity of experimental vaccine candidates and strongly support development of novel vaccines.
Acknowledgments
This study was funded by the Intramural Program of NIAID, National Institutes of Health and in part by the Malaria Vaccine Initiative of the Bill and Melinda Gates Foundation.
We thank Lynn Lambert, Brian Keegan, Josh Reece and Cheryl Kothe for skillfully conducting the animal studies described.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engvall E, Perlman P. Enzyme-linked immunosorbent assay (ELISA) Quantitative assay of immunoglobulin G Immunochemistry. 1971;8(9):871–4. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 2.Van Weemen BK, Schuurs AH. Immunoassay using antigen-enzyme conjugates. FEBS Lett. 1971;15(3):232–236. doi: 10.1016/0014-5793(71)80319-8. [DOI] [PubMed] [Google Scholar]
- 3.Gheesling LL, Carlone GM, Pais LB, Holder PF, Maslanka SE, Plikaytis BD, et al. Multicenter comparison of Neisseria meningitidis serogroup C anti-capsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. J Clin Microbiol. 1994;32(6):1475–82. doi: 10.1128/jcm.32.6.1475-1482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Player VA, White D. Comparison of an ELISA system for the quantification of hepatitis B antibody with an automated and a semi-automated system. J Virol Methods. 1993;45(1):67–72. doi: 10.1016/0166-0934(93)90140-m. [DOI] [PubMed] [Google Scholar]
- 5.Voller A, Bartlett A, Bidwell DE. Enzyme immunoassays with special reference to ELISA techniques. J Clin Pathol. 1978;31(6):507–20. doi: 10.1136/jcp.31.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macy E, Kemeny M, Saxon A. Enhanced ELISA: how to measure less than 10 picograms of a specific protein (immunoglobulin) in less than 8 hours. FASEB J. 1988;2(14):3003–9. doi: 10.1096/fasebj.2.14.3263291. [DOI] [PubMed] [Google Scholar]
- 7.Jitsukawa T, Nakajima S, Sugawara I, Watanabe H. Increased coating efficiency of antigens and preservation of original antigenic structure after coating in ELISA. J Immunol Methods. 1989;116(2):251–7. doi: 10.1016/0022-1759(89)90211-1. [DOI] [PubMed] [Google Scholar]
- 8.McLaren ML, Lillywhite JE, Au AC. Indirect enzyme linked immunosorbent assay (ELISA): practical aspects of standardization and quality control. Med Lab Sci. 1981;38(3):245–51. [PubMed] [Google Scholar]
- 9.Ravindranath MH, Ravindranath RM, Morton DL, Graves MC. Factors affecting the fine specificity and sensitivity of serum antiganglioside antibodies in ELISA. J Immunol Methods. 1994;169(2):257–72. doi: 10.1016/0022-1759(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 10.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay, ELISA. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972;109(1):129–35. [PubMed] [Google Scholar]
- 11.Plested JS, Coull PA, Gidney MA. ELISA. Methods Mol Med. 2003;71:243–61. doi: 10.1385/1-59259-321-6:243. [DOI] [PubMed] [Google Scholar]
- 12.Engvall E. Quantitative enzyme immunoassay (ELISA) in microbiology. Med Biol. 1977;55(4):193–200. [PubMed] [Google Scholar]
- 13.Miles AP, Zhang Y, Saul A, Stowers AW. Large-scale purification and characterization of malaria vaccine candidate antigen Pvs25H for use in clinical trials. Protein Expr Purif. 2002;25(1):87–96. doi: 10.1006/prep.2001.1613. [DOI] [PubMed] [Google Scholar]
- 14.Zou L, Miles AP, Wang J, Stowers AW. Expression of malaria transmission-blocking vaccine antigen Pfs25 in Pichia pastoris for use in human clinical trials. Vaccine. 2003;21(15):1650–7. doi: 10.1016/s0264-410x(02)00701-6. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy MC, Wang J, Zhang Y, Miles AP, Chitsaz F, Saul A, et al. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun. 2002;70(12):6948–60. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malkin EM, Durbin AP, Diemert DJ, Sattabongkot J, Wu Y, Miura K, et al. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine. 2005;23(24):3131–8. doi: 10.1016/j.vaccine.2004.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malkin EM, Diemert DJ, McArthur JH, Perreault JR, Miles AP, Giersing BK, et al. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73(6):3677–85. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malkin E, Long CA, Stowers AW, Zou L, Singh S, Macdonald NJ, et al. Phase 1 study of two merozoite surface protein 1 (MSP1(42)) vaccines for Plasmodium falciparum malaria. PLoS clinical trials. 2007;2(4):e12. doi: 10.1371/journal.pctr.0020012. [DOI] [PMC free article] [PubMed] [Google Scholar]