Abstract
Recent studies have shown enhanced responsiveness to ozone in obese mice. Adiposity has not been examined as a possible modulator of ozone response in humans. We therefore examined the relationship between body mass index and the acute spirometric response to ozone (O3) exposure among 197 non-asthmatic young adults (aged 18-35) studied in our human exposure facility from 1992-1998. Each subject had been exposed to 0.42 ppm O3 for 1.5 h with intermittent exercise designed to produce a minute ventilation of 20 l/min / m2 body surface area (BSA). Spirometry (pulmonary function) was measured pre- and immediately post- exposure to determine acute ozone-induced changes. The decrement in forced expiratory volume in 1s (Δ FEV1) in % of baseline was significantly correlated with BMI, r = −0.16, p = 0.03 with a slightly stronger correlation in women (n=75), r = −0.22, p = 0.05, and no significant correlation in men. BMI had a greater range in women than in men in our study. In women greater ozone-induced decrements were seen in overweight (BMI > 25 kg/m2) than in normal weight (BMI 18.5 to 25 kg/m2), and in normal weight than in underweight (BMI < 18.5 kg/m2) for all spirometric variables considered (P trend ≤ 0.022). Although our population studied was predominantly normal weight, we found that higher body mass index may be a modest risk factor for adverse pulmonary effects associated with ozone exposure, especially for women.
Keywords: Body Mass Index, Ozone, Spirometry
Introduction
Recent studies in genetically obese mouse models suggest that obesity enhances various respiratory responses to ozone, including lung mechanics, airway hyper-responsiveness, and airway inflammation following exposure to ozone (O3) (Rivera-Sanchez et al., 2004, Shore and Fredberg, 2005). While O3 exposure provokes airway hyper-responsiveness (AHR) and airway inflammation in humans (Devlin et al., 1991, Seltzer et al., 1986), an important (and apparently independent) feature of the acute response to O3 is a reduction of forced expiratory volumes largely due to an inhibition of maximal inspiration (Bates et al., 1972). This response is relatively reproducible within an individual but varies widely among healthy nonsmokers. Many investigators (Hazucha et al., 2003, Horvath et al., 1981, Linn et al., 1986, McDonnell et al., 1993) have attempted to characterize the factors responsible for inter-individual variability in acute spirometric responses to O3. For example, we previously showed that spirometric responsiveness to ozone was dramatically reduced above age 35 (Hazucha et al., 2003). None of the previous studies assessed the effect of body mass index (BMI) on respiratory responses to ozone exposure. Given the recent murine data (Rivera-Sanchez et al., 2004, Shore and Fredberg, 2005, Shore et al., 2003), we re-analyzed our previous human acute O3 exposure data (Hazucha et al., 2003) to determine if BMI had an effect on spirometric responsiveness to O3 in young (18-35 years), healthy, non-smoking men and women.
Methods
The study protocol was approved by the School of Medicine Committee on the Protection of the Rights of Human Subjects of the University of North Carolina. The Office of Human Subjects Research at the National Institutes of Health also approved the current analysis. The subjects included in the current analysis were healthy, nonsmoking male and female volunteers from the local population between the ages of 18 and 35 yr (122M/75F) recruited during the years 1992 - 1998. Medical screening of subjects was as described previously (Hazucha et al., 2003). Potential subjects were excluded from the study if they had a history of allergic rhinitis, asthma, or chronic respiratory disease, heart disease, or considerable exposure to very dusty or polluted environments in the week prior to study. Subjects who qualified for the study were trained in correctly performing pulmonary function tests and underwent exercise on a treadmill to determine a load that would elicit a minute ventilation of 20 l/min/m2 body surface area (BSA). Height and weight were measured for each subject. BSA for these studies was determined by the equation: BSA = (weight in kg) 0.425 × (height in cm) 0.725 × 0.0072 (Wang et al., 1992). Body mass index (BMI) was determined for each subject as weight in kg/ (height in meters)2. Each subject then participated in one exposure session to 0.42 ppm O3 at normal temperature (22°C) and relative humidity (40%). Pre-exposure procedures for subjects and chamber description are as detailed previously (Glover et al., 1981, Hazucha et al., 2003). During the 90 min exposure to O3, subjects alternated 20 min of exercise at the load determined during the training session with 10 min of rest. The minute ventilation was monitored at minutes 3-5 and 17-19 during the first exercise period and at minutes 17-19 during the two subsequent exercise periods. At the same time the exercise load was adjusted if necessary to achieve the target ventilation. For a subset of the subjects (80 males/47 females) minute ventilation, tidal volume, and breathing frequency during these exercise sessions had been recorded and were available for the present analysis.
Spirometric testing with the use of a dry seal spirometer (CPI model 220 or a Medical Graphics model 1085) was performed before and immediately after exposure (Hazucha et al., 2003). Because FEF25-75 can be affected by both changes in airway conductance as well as decreases in FVC associated with ozone exposure (Weinmann et al., 1995), we also report FEF25-75/FVC (i.e., normalized to FVC), which may reflect the changes in expiratory airway conductance. Changes in spirometric variables, e.g., FEV1, provoked by O3 exposure were analyzed as a decrement from the pre-exposure baseline, i.e. [(post-pre)/pre] expressed as a percent. SAS statistical programs (SAS Institute; Cary, NC) were used to calculate descriptive statistics, including Pearson correlation coefficients ( r ), for changes in spirometric variables, the primary variable of interest being FEV1, based on previous analyses (Folinsbee and Hazucha, 1989, Hazucha et al., 1989, Hazucha et al., 2003, Horvath et al., 1981, McDonnell et al., 1983), and to determine, by multivariate regression analysis, the effect of BMI and age on these changes in spirometric variables in males and females. All P-values presented are for two-tailed analysis.
Results
Table 1 summarizes the subject characteristics and baseline spirometric values. The range of BMI was greater in the women (15.7-33.4 kg/m2) than in the men (19.1-32.9 kg/m2). The mean decrements (as % change from baseline) in spirometric variables associated with ozone exposure for males and females are summarized in Table 2. As reported in our previous publication (Hazucha et al., 2003) there were no significant differences between males and females for ozone induced decrements in spirometric parameters (FEV1, FVC, FEF25-75, FEV1/FVC or FEF25-75/FVC).
Table 1.
Summary (mean and SD) of subject anthropometric and baseline spirometric data.
| Age | Ht (cm) |
Wt (kg) |
BMI (kg/m2) |
BSA m2 |
FVC liters |
FEV1 liters |
FEF25-75 L/min |
FEF25-75/ FVC (/min) |
|
|---|---|---|---|---|---|---|---|---|---|
| Males* N=122 |
24.9 3.9 |
181 6 |
79 11 |
24.2 3.1 |
1.99 0.15 |
5.34 0.81 |
4.35 0.61 |
4.48 1.22 |
0.85 0.26 |
| Females N=75 |
24.3 4.1 |
166 6 |
62 10 |
22.3 3.4 |
1.68 0.13 |
3.71 0.59 |
3.15 0.47 |
3.60 0.86 |
0.99 0.26 |
P< 0.001 for males vs. females for all variables other than age (NS).
Table 2.
Summary (Mean/SD) of ozone induced decrements (%) in spirometric variables.
| FEV1 | FVC | FEF25-75 | FEV1/FVC | FEF25-75/FVC | |
|---|---|---|---|---|---|
| Males | −15.9/11.1 | −10.5/8.5 | −25.2/17.2 | −6.3/6.3 | −16.9/15.5 |
| Females | −16.4/10.8 | −12.2/9.0 | −24.8/15.9 | −5.0/5.6 | −14.9/13.7 |
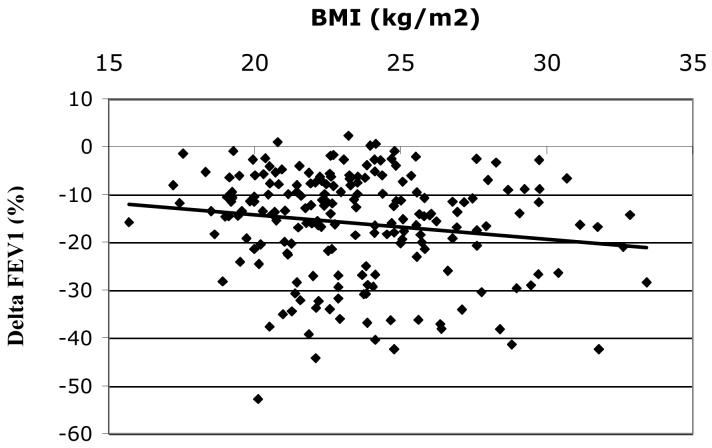
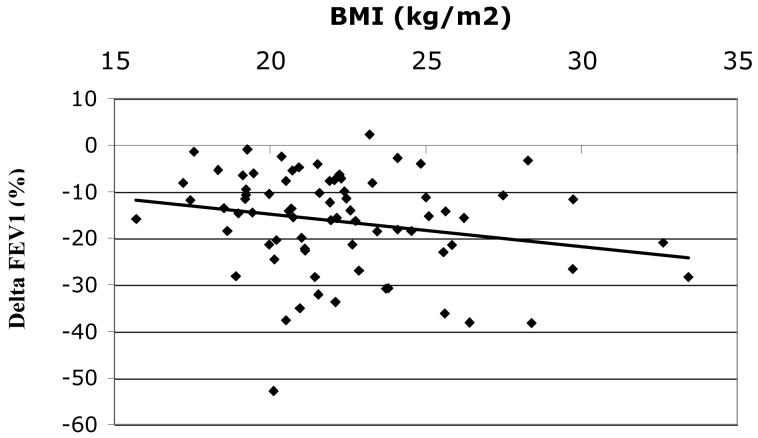
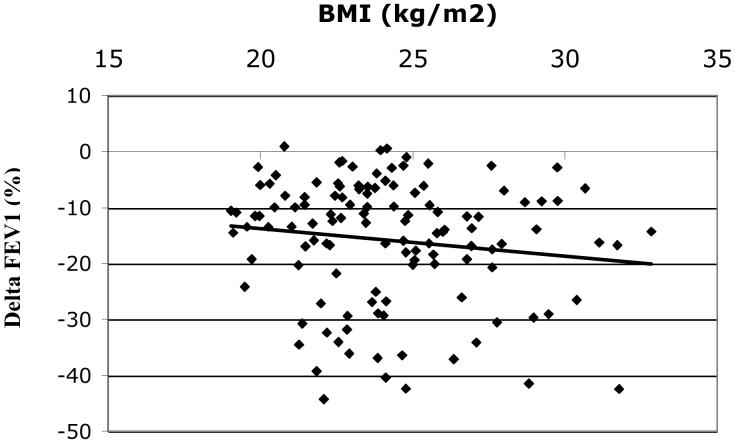
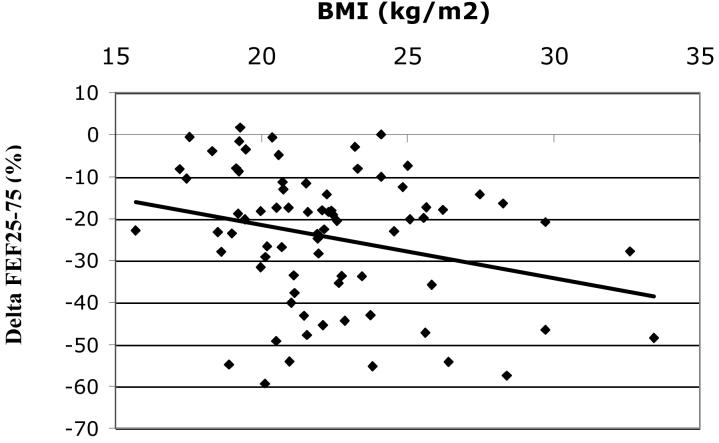
Among all subjects Δ FEV1 (mean+/−SD= − 16.1+/−11.0%) was inversely correlated with BMI, r = −0.16, p = 0.03, figure 1A. The inverse relationship between BMI and ΔFEV1appeared most evident in women (n=75) (mean ΔFEV1 = − 16.4+/−10.8%), r = −0.22, p = 0.05 (figure 1B) but was not statistically significant in men (n=122) (mean ΔFEV1 = − 15.9+/−11.1%), r = − 0.14, p = 0.13 (figure 1C), although the test of interaction was not statistically significant (p = 0.80). The effect of BMI on ozone responsiveness in women was most pronounced for ΔFEF25-75, figure 2, r = − 0.29, p < 0.05. Again, the correlation in men was lower and not statistically significant (r = − 0.12, p = 0.19) although there was no statistically significant interaction by gender (p = 0.39), i.e., the slopes of the relationship between ΔFEF25-75 and BMI for men and women were not significantly different.
Figure 1.
Figure 1A. Δ FEV1 (%) vs. BMI for all subjects (n=197), r = − 0.16, p = 0.03.
Figure 1B. Δ FEV1(%) vs. BMI for females (n=75), r = − 0.22, p = 0.05.
Figure 1C. Δ FEV1(%) vs. BMI for males (n=122), r = − 0.14, p = 0.13 (NS).
Figure 2.
Δ FEF25-75(%) vs. BMI for females (n=75), r = −0.29, p = 0.01.
Table 3 provides regression results for the effect of age and BMI on the ozone-induced change in FEV1, FVC, FEF 25-75, FEV1/FVC, and FEF25-75/FVC for all subjects and by gender. BMI was inversely related to all parameters in both men and women with statistical significance for ΔFEV1, ΔFEF25-75 and ΔFEF25-75/FVC in women.
Table 3.
Relationship between age, body mass index and change in pulmonary function parameters after ozone exposure for all subjects and by gender. Beta is the coefficient for the associated predictor in the regression model and the square of the regression coefficient, R2, applies to the regression model that includes both predictors, age and BMI.
| All (n = 197) |
Males (n = 122) |
Females (n = 75) |
|||||
|---|---|---|---|---|---|---|---|
| Δ (%) | Predictor | Beta | p-value | Beta | p-value | Beta | p-value |
| FEV1 | Age | 0.406 | 0.039 | 0.164 | 0.54 | 0.718 | 0.016 |
| BMI | −0.580 | 0.014 | −0.533 | 0.11 | −0.716 | 0.044 | |
| R2 | 0.036 | 0.022 | 0.124 | ||||
| FVC | Age | 0.343 | 0.028 | 0.147 | 0.47 | 0.582 | 0.019 |
| BMI | −0.288 | 0.038 | −0.391 | 0.13 | −0.563 | 0.057 | |
| R2 | 0.041 | 0.021 | 0.116 | ||||
| FEF25-75a | Age | 0.375 | 0.212 | 0.083 | 0.84 | 0.724 | 0.098 |
| BMI | −0.952 | 0.008 | −0.693 | 0.19 | −1.336 | 0.012 | |
| R2 | 0.040 | 0.015 | 0.116 | ||||
| FEV1/FVC | Age | 0.103 | 0.350 | 0.063 | 0.68 | 0.169 | 0.29 |
| BMI | −0.259 | 0.050 | −0.211 | 0.27 | −0.224 | 0.25 | |
| R2 | 0.022 | 0.011 | 0.033 | ||||
| FEF25-75/FVCa | Age | 0.171 | 0.524 | 0.037 | 0.92 | 0.316 | 0.41 |
| BMI | −0.732 | 0.023 | −0.428 | 0.37 | −1.025 | 0.028 | |
| R2 | 0.027 | 0.007 | 0.074 | ||||
n = 121 for males; n = 74 for females
Table 4 provides the mean decrements in spirometric parameters according to body habitus categories used by the World Health Organization as well as the U.S. Centers for Disease Control; underweight (BMI < 18.5), normal weight (BMI = 18.5-24.9), and overweight/obese (BMI ≥ 25). In men the underweight category was not represented and we observed slight decreases in each parameter for the overweight/obese group that were not statistically significant in comparison with the normal weight group. In women, the ozone-induced decrements in pulmonary function increased across the three weight categories for FEV1, FVC, FEF25-75, and FEF25-75/FVC (P trend ≤ 0.022 for all four parameters). The ozone-induced decrement in FVC was significantly less in overweight males as compared to overweight females (p = 0.03). None of the other differences by gender were significant.
Table 4.
Comparison of mean (SD) ozone-induced percentage decrements in spirometric parameters by standard body mass index categories for men and women*
|
Δ (%) |
Underweight BMI < 18.5 (n = 5) 0M/5F |
Normal Weight BMI = 18.5-24.9 (n = 135) 80M/55F |
Overweight/Obese BMI ≥ 25 (n = 57) 42M/15F |
Trend p-value |
|---|---|---|---|---|
|
FEV1 All Males Females |
−8.5 (5.6) −8.5 (5.6) |
−15.5 (11.2) −15.2 (11.6) −15.9 (10.8) |
−18.2 (10.2) −17.2 (10.0) −21.0 (10.8) |
0.028 0.171 0.012 |
|
FVC All Males Females |
−6.9 (4.5) −6.9 (4.5) |
−10.6 (8.9) −10.0 (8.9) −11.5 (9.0) |
−12.8 (8.2) −11.4 (7.6) −16.7 (8.6) |
0.073 0.184 0.017 |
|
FEF25-75 All Males Females |
−9.2 (8.5) −9.2 (8.5) |
−24.6 (17.0) −24.5 (18.1) −24.7 (15.5) |
−27.4 (15.6) −26.4 (15.4) −30.1 (16.5) |
0.009 0.276 0.005 |
|
FEV1/FVC All Males Females |
−1.5 (3.3) −1.5 (3.3) |
−5.7 (6.0) −6.0 (6.2) −5.2 (5.9) |
−6.3 (6.3) −6.6 (6.7) −5.5 (5.2) |
0.046 0.313 0.090 |
|
FEF25-75/FVC All Males Females |
−2.5 (7.2) −2.5 (7.2) |
−16.3 (14.6) −16.7 (15.5) −15.6 (13.4) |
−17.0 (15.4) −17.1 (15.7) −16.7 (15.0) |
0.018 0.460 0.022 |
Centers for Disease Control and World Health Organization categories for BMI. For normal weight, FEF25-75 and FEF25-75/FVC, n = 133 with 79M/54F
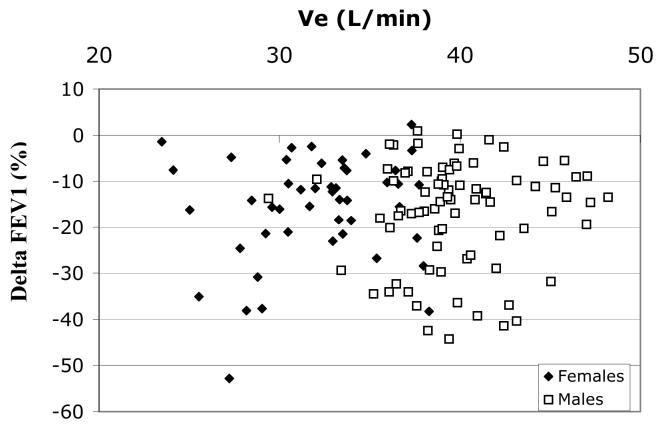
Because exercise ventilation was normalized to body surface area (BSA) in these studies we considered whether variable ozone dose to the lung might have affected our observations. BSA and BMI are both direct functions of subject weight and were correlated in our subject population (r = 0.65, p < 0.001). In a subset of 127 (80M/47F) subjects with recorded ventilation data during exercise periods of the ozone exposure protocol, the exercise minute ventilations (Ve) ranged from 23.5 to 48.2 liters/min and significantly correlated with BMI (r = 0.43, p < 0.001). However, there were no significant correlations between any of the ozone-induced decrements and minute ventilation (e.g., r = −0.01 and −0.03 for Ve vs. Δ FEV1 and ΔFEF25-75, respectively, for all subjects). Figure 3 shows no significant relationship between ΔFEV1 and Ve for both males and females. In fact, the slope of the regression for females in figure 3 is opposite to that of the ΔFEV1 vs. BMI relationship in females, figure 1B. Table 5 provides regression results for the effect of age, BMI, and Ve on the ozone-induced changes in FEV1, FVC, FEF 25-75, FEV1/FVC, and FEF25-75/FVC for this subset of subjects with Ve measurements. As we found in the larger data set, BMI was a significant predictor of ΔFEV1, Δ FVC , Δ FEF25-75 and ΔFEF25-75/FVC in women but not men. On the other hand, Ve was not a significant predictor of ozone-induced lung function decrements in either gender or the group as a whole. Consistent with figures 3 and 1B, the coefficient for Ve (i.e. beta) vs. is opposite in direction to that for BMI in the females for all pulmonary function decrements.
Figure 3.
Δ FEV1(%) vs. Minute ventilation (Ve) during exercise of ozone exposure for males (n=80) and females (n=47), r = −0.01 (NS) for all, r = −0.001 for males (NS), and r = 0.16 for females (NS).
Table 5.
Relationship between age, body mass index, minute ventilation (Ve) and change in pulmonary function parameters after ozone exposure for subset of subjects having Ve measures during exposure. Beta is the coefficient for the associated predictor in the regression model and the square of the regression coefficient, R2, applies to the regression model that includes both predictors, age and BMI.
| All (n = 127) |
Males (n = 80) |
Females (n = 47) |
|||||
|---|---|---|---|---|---|---|---|
| Δ (%) | Predictor | Beta | p-value | Beta | p-value | Beta | p-value |
| FEV1 | Age | 0.416 | 0.08 | 0.011 | 0.97 | 0.749 | 0.035 |
| BMI | −0.797 | 0.019 | −0.313 | 0.54 | −1.116 | 0.020 | |
| Ve | 0.181 | 0.38 | −0.222 | 0.60 | 0.704 | 0.11 | |
| R2 | 0.059 | 0.018 | 0.222 | ||||
| FVC | Age | 0.338 | 0.08 | 0.016 | 0.09 | 0.602 | 0.044 |
| BMI | −0.635 | 0.020 | −0.244 | 0.54 | −0.814 | 0.042 | |
| Ve | 0.192 | 0.24 | −0.267 | 0.42 | 0.518 | 0.17 | |
| R2 | 0.060 | 0.028 | 0.190 | ||||
| FEF25-75a | Age | 0.388 | 0.28 | −0.194 | 0.70 | 0.789 | 0.11 |
| BMI | −1.253 | 0.013 | −0.198 | 0.80 | −2.108 | 0.002 | |
| Ve | 0.218 | 0.48 | −0.429 | 0.51 | 1.061 | 0.09 | |
| R2 | 0.054 | 0.015 | 0.256 | ||||
| FEV1/FVC | Age | 0.120 | 0.36 | 0.020 | 0.92 | 0.194 | 0.29 |
| BMI | −0.241 | 0.19 | −0.102 | 0.72 | −0.426 | 0.09 | |
| Ve | 0.010 | 0.93 | 0.031 | 0.90 | 0.266 | 0.25 | |
| R2 | 0.021 | 0.002 | 0.103 | ||||
| FEF25- 75/FVCa |
Age | 0.155 | 0.63 | −0.198 | 0.67 | 0.345 | 0.43 |
| BMI | −0.823 | 0.07 | 0.042 | 0.95 | −1.645 | 0.007 | |
| Ve | 0.054 | 0.85 | −0.288 | 0.62 | 0.734 | 0.19 | |
| R2 | 0.030 | 0.006 | 0.173 | ||||
Discussion
Numerous studies (Folinsbee and Hazucha, 1989, Hazucha et al, 1989, Hazucha et al., 2003, Horvath et al, 1981, Linn et al, 1986, McDonnell et al, 1983, McDonnell et al, 1993, Passannante et al, 1998, Rivera-Sanchez et al, 2004, Weinmann et al, 1995) have established that ozone exposure of healthy individuals results in acute, but reversible impairment of pulmonary function, modestly increased airway resistance (McDonnell et al, 1983), and airways hyperreactivity (Seltzer et al, 1986). The primary mechanism associated with the spirometric response to ozone exposure is a reflex nociceptive inhibition of inspiration (Hazucha et al, 1989, Passannante et al, 1998) that likely originates from bronchial C fibers. However, other mechanisms including inflammation and airway hyperresponsiveness likely play some role (Passannante et al, 1998). A number of potential factors, including age, gender, race and antioxidant intake, have been evaluated as modifiers of responsiveness of humans to ozone (Hazucha et al., 2003, McDonnell et al, 1993). Recent data from mice (Rivera-Sanchez et al, 2004, Shore et al, 2003), suggest that obesity may also modulate the response to ozone. Genetically obese mice showed greater post-ozone airway hyperreactivity to methacholine and greater airway inflammation than wild type mice exposed under the same conditions (Shore et al, 2003). Obese mice also had significant changes in airway and parenchymal mechanics in response to ozone while wild-type mice did not (Rivera-Sanchez et al, 2004). We have found, for the first time in humans, that a respiratory response to ozone, in our study spirometry, may increase with greater adiposity, as measured by body mass index. It is notable that we observed this association in a population of predominantly normal weight individuals.
We found some suggestive evidence for a greater effect of BMI on ozone response in women. When we classified subjects according to standard classifications of body weight, among women the spirometric response to ozone increased across the three categories of underweight, normal weight and overweight. One factor that could explain the clearer signal in women is that the range of BMI was greater in our women (15.7-33.4 kg/m2) than in the men (19.1-32.9 kg/m2); none of the men would be classified as underweight (BMI < 18.5) (table 4). We may therefore have had greater power to find an association among women than among men. However, even disregarding the five underweight women, we found a greater ozone response in the overweight and obese category (BMI > 25), compared with the normal weight group (BMI 18.5-24.9). This was seen for all parameters in both genders but attained statistical significance only for FVC in females. For FVC, this difference was significantly greater in women (P = 0.03). It is also possible that we observed a clearer relation between BMI and ozone response in women because BMI may be a better measure of adiposity in women than in men; for a given level of BMI, women have a higher percent body fat (Gallagher et al, 1996). To the extent that the percent body fat may play a role in modulating ozone response, we would have better ability to observe an effect in women then men based on BMI alone as an index of adiposity. Clearly in future studies of the possible effects of obesity on ozone response, multiple measures of adiposity, including waist circumference and percent body fat, would be desirable. Further, it is possible that the associations that we observed would be stronger with better measures of obesity in both men and women.
The rationale for normalizing ventilation (Ve) to body size was based on the desire to exercise individuals at similar relative work efforts and metabolic requirements. This commonly used normalization in ozone exposure protocols also presumes that larger individuals (based on height) will have larger lung volumes as well (Knudsen et al, 1983) and therefore inhaled ozone dose will be normalized to an individual's lung volume. Figure 3 illustrates that within this group of subjects there was no significant effect of Ve on Δ FEV1, despite the fact that there was a very large range of Ve. The trend was actually for the decrement in FEV1 to decrease with increasing Ve in the women, i.e. opposite slope to that of BMI vs. Δ FEV1, figure 1B. When we included Ve as a variable in the multiple regression of ozone-induced decrements, table 5, it was not a significant predictor of these decrements, while BMI still predicted the ozone effects in women, indicating that the effect of BMI is independent of any variability in Ve. These data indicate that our normalization of Ve to body surface area (BSA), and the resulting intersubject variability in Ve during exposure, had no effect on the variation in lung function decrements induced by exposure to a relatively high ozone concentration. The reason that intersubject variation in Ve has no observable effect on ozone induced decrements in lung function under our exposure conditions (90 minutes at 0.42 ppm ozone) may be because most subjects were at or near their maximum acute responsiveness (i.e. the plateau of their dose response curve for spirometric decrements) (McDonnell et al, 1983) and thus differences in dose by variable Ve had little or no further influence. On the other hand, we show that the intersubject variation in BMI slightly modified the intersubject variability in this maximum acute spirometric response.
The physiologic mechanisms responsible for the greater decline in spirometric volumes and flows after ozone exposure with increasing BMI are not clear. We considered the possibility that the decreased lung volumes with increased BMI may influence relative ozone dose to lung tissue. However, while it has been observed that the obese have lower lung volumes than normal weight individuals (King et al, 2005) there was no significant relationship between BMI and baseline FVC (i.e. an index of lung volume) in our data (for all subjects or for females alone, e.g. r = 0.01 for females). Obese humans are also known to breathe with relatively smaller tidal volumes than normal weight individuals (Sampson and Grassino, 1983), resulting in reduced stretching of lung tissue that might play a role in the increased responsiveness to ozone with BMI. However, in the subset of 127 (80M/47F) subjects with minute ventilation measurements, tidal volumes actually tended to increase with BMI, though not significantly [r = 0.13 in all subjects (P = 0.15) and r = 0.11 in females (P = 0.44)]. Finally, breathing frequency may also affect ozone dose to the lung by determining the residence time of ozone within the airways. But, again there was no relationship between BMI and breathing frequency among the subjects for whom ventilation data was recorded (r = 0.05, NS). Interestingly, spirometric responsiveness increased with higher breathing frequency during exercise in all subjects (r = − 0.24 for ΔFEV1 vs. frequency, P < 0.01), reflecting the fact that increased breathing frequency during exercise is another measure of increased ozone responsiveness (McDonnell et al, 1983).
A number of circulating hormones and other inflammatory factors (e.g. leptin, adiponectin, and plasminogen activator inhibitor) derived from adipose tissue (Nawrocki et al, 2004, Rajala and Scherer, 2003) might affect airway function and responsiveness to ozone. These hormones/factors have been shown to affect airway hyper-responsiveness and inflammation in animal models, either as pro- or anti-inflammatory modulators (Savov et al, 2003, Shore et al, 2003, Shore et al, 2005). If these adipocyte-derived factors play a role in the spirometric response we observed, then the fact that females have a considerably greater percent body fat for a given BMI than males (Gallagher et al, 1996) may explain our ability to observe a more significant relationship among women. Future studies incorporating markers of inflammation from adipose tissue would help address mechanistic questions.
Ozone-induced spirometric decrements are vagally mediated through nociceptive sensory neurons (Beckett et al, 1985, Passannante et al, 1998). Thus, the question arises as to whether modulation of vagal responses might vary with BMI. For example, it may be that circulating catecholamines such as epinephrine can interfere with or inhibit these vagal reflexes. There is some evidence for this in the cardiovascular system where increased alpha-adrenergic agonists appear to interfere with the baroreceptor control of blood pressure and heart rate (Airaksinen et al, 2001). Reims et al (2004) recently reported that overweight men had lower plasma epinephrine levels. Unfortunately, there have been no studies designed to assess a possible role of sympathetic modulation on ozone-induced spirometric responses.
Our study has a number of important limitations. We did not have measures of systemic or airway inflammation, nor of airway hyperresponsiveness. However, in addition to greater increase in airway responsiveness and inflammation (Shore et al, 2003), the obese mice also had greater changes in airway and parenchymal mechanics in response to ozone than wild-type mice (Rivera-Sanchez et al, 2004). Also, we had only one measure of adiposity (BMI) and are lacking measures of central obesity that may be more relevant to the mouse models. Further, as these subjects were not selected with the study of BMI in mind, we had a paucity of obese individuals. While there was no exclusion based on subject weight, there may have been some recruitment bias based on a subject's ability to perform the treadmill exercise during the exposures.
A major strength of this study is that we were able to examine a measure of adiposity in relation to acute spirometric response to ozone in a substantial number of healthy individuals. We found, for the first time, that body mass index was positively related to greater acute spirometric response to controlled ozone exposure. Other studies have recently shown important particle deposition/responsiveness relationships with BMI, with few or no obese subjects in their analysis: e.g. 1) Bennett and Zeman (2004) showed greater fine particle deposition in children with increasing BMI, rather than obesity, and 2) Alexis and Peden (2006) showed greater responsiveness to inhaled endotoxin in asthmatics with increasing BMI, with no truly obese subjects in their population. Our findings add to this small body of data for variation in air pollution effects based on variation in BMI rather than frank obesity. Future studies with targeted selection of obese and lower weight subjects and additional adiposity and outcome measures would better elucidate the relationship between obesity and ozone response in humans.
Acknowledgments
Supported by USEPA Cooperative Agreement CR824915 and CR829522 and in part by the Division of Intramural Research, NIEHS, NIH, DHHS
Footnotes
Publisher's Disclaimer: DISCLAIMER: Although the research described in this article has been funded wholly or in part by the United States Environmental Protection Agency through cooperative agreement CR829522 with the Center for Environmental Medicine, Asthma, and Lung Biology at the University of North Carolina at Chapel Hill, it has not been subjected to the Agency's required peer and policy review, and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- Airaksinen KE, Huikuri HV, Huhti L, Kuusela TA, Tahvanainen KU, Tulppo M, Makikallio T, Eckberg DL. Effects of noradrenaline on human vagal baroreflexes. Ann Med. 2001;33(3):193–200. doi: 10.3109/07853890109002077. [DOI] [PubMed] [Google Scholar]
- Alexis NE, Peden DB. Inflammatory response of the airway to inhaled endotoxin correlates with body mass index in atopic patients with asthma but not in normal volunteers. J Allergy Clin Immunol. 2006;117(5):1185–6. doi: 10.1016/j.jaci.2005.12.1305. [DOI] [PubMed] [Google Scholar]
- Bates DV, Bell GM, Burnham CD, Hazucha M, Mantha J, Pengelly LD, Silverman F. Short term effects of ozone on the lung. J Appl Physiol. 1972;32:176–181. doi: 10.1152/jappl.1972.32.2.176. [DOI] [PubMed] [Google Scholar]
- Beckett WS, McDonnell WF, Horstman DH, House DE. Role of the parasympathetic nervous system in acute lung response to ozone. J Appl Physiol. 1985;59:1879–1885. doi: 10.1152/jappl.1985.59.6.1879. [DOI] [PubMed] [Google Scholar]
- Bennett WD, Zeman KL. Effect of body size on breathing pattern and fine particle deposition in children. J Appl Physiol. 2004;97:821–826. doi: 10.1152/japplphysiol.01403.2003. [DOI] [PubMed] [Google Scholar]
- Devlin RB, McDonnell WF, Mann R, Becker S, House DE, Schreinemachers D, Koren HS. Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am J Respir Cell Mol Biol. 1991;4:72–81. doi: 10.1165/ajrcmb/4.1.72. [DOI] [PubMed] [Google Scholar]
- Folinsbee LJ, Hazucha MJ. Persistence of ozone-induced changes in lung function and airway responsiveness. In: Schneider T, Grant L, editors. Atmospheric Ozone Research and Its Policy Implications. Elsevier; Amsterdam: 1989. pp. 483–492. [Google Scholar]
- Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidem. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- Glover DE, Berntsen JH, Crider WL, Strong AA. Design and performance of a system to control concentrations of common gaseous air pollutants within environmental laboratories used for human exposure studies. J Environ Sci Health B. 1981;A16:501–522. [Google Scholar]
- Hazucha MJ, Bates DV, Bromberg PA. Mechanism of action of ozone on the human lung. J Appl Physiol. 1989;67:1535–1541. doi: 10.1152/jappl.1989.67.4.1535. [DOI] [PubMed] [Google Scholar]
- Hazucha MJ, Folinsbee LJ, Bromberg PA. Distribution and reproducibility of spirometric response by gender and age. J Appl Physiol. 2003;95:1917–1925. doi: 10.1152/japplphysiol.00490.2003. [DOI] [PubMed] [Google Scholar]
- Horvath SM, Gliner JA, Folinsbee LJ. Adaptation to ozone: duration of effect. Am Rev Respir Dis. 1981;123:496–499. doi: 10.1164/arrd.1981.123.5.496. [DOI] [PubMed] [Google Scholar]
- King GG, Brown NJ, Diba C, Thorpe CW, Munoz P, Marks GB, Toelle B, Ng K, Berend N, Salome CM. The effects of body weight on airway calibre. Eur Respir J. 2005;25(5):896–901. doi: 10.1183/09031936.05.00104504. [DOI] [PubMed] [Google Scholar]
- Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- Linn WS, Avol EL, Shamoo DA, Spier CE, Valencia LM, Venet TG, Fischer DA, Hackney JD. A dose-response study of healthy, heavily exercising men exposed to ozone at concentrations near the ambient air quality standard. Toxicol Ind Health. 1986;2:99–112. doi: 10.1177/074823378600200105. [DOI] [PubMed] [Google Scholar]
- McDonnell WF, Horstman DH, Hazucha MJ, Seal E, Jr, Haak ED, Salaam SA, House DE. Pulmonary effects of ozone exposure during exercise: dose-response characteristics. J Appl Physiol. 1983;54:1345–1352. doi: 10.1152/jappl.1983.54.5.1345. [DOI] [PubMed] [Google Scholar]
- McDonnell WF, Muller KE, Bromberg PA, Shy CM. Predictors of individual differences in acute response to ozone. Am Rev Respir Dis. 1993;147:818–825. doi: 10.1164/ajrccm/147.4.818. [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Scherer PE. The delicate balance between fat and muscle: adipokines in metabolic disease and musculoskeletal inflammation. Curr Opin Pharmacol. 2004;4:281–9. doi: 10.1016/j.coph.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Passannante AN, Hazucha MJ, Bromberg PA, Seal E, Folinsbee L, Koch G. Nociceptive mechanisms modulate ozone-induced human lung function decrements. J Appl Physiol. 1998;85:1863–1870. doi: 10.1152/jappl.1998.85.5.1863. [DOI] [PubMed] [Google Scholar]
- Rajala MW, Scherer PE. Minireview: the adipocyte—at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–73. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- Reims HM, Fossum E, Høieggen A, Moan A, Eide I, Kjeldsen SE. Adrenal medullary overactivity in lean, borderline hypertensive young men. Am J Hypertens. 2004;17:611. doi: 10.1016/j.amjhyper.2004.03.676. [DOI] [PubMed] [Google Scholar]
- Rivera-Sanchez YM, Johnston RA, Schwartzman IN, Valone J, Silverman ES, Fredberg JJ, Shore SA. Differential effects of ozone on airway and tissue mechanics in obese mice. J Appl Physiol. 2004;96:2200–2206. doi: 10.1152/japplphysiol.00960.2003. [DOI] [PubMed] [Google Scholar]
- Sampson MG, Grassino AE. Load compensation in obese patients during quiet tidal breathing. J Appl Physiol. 1983;55:1269–1276. doi: 10.1152/jappl.1983.55.4.1269. [DOI] [PubMed] [Google Scholar]
- Savov JD, Brass DM, Berman KG, McElvania E, Schwartz DA. Fibrinolysis in LPS-induced chronic airway disease. Am J Physiol Lung Cell Mol Physiol. 2003;285:L940–8. doi: 10.1152/ajplung.00102.2003. [DOI] [PubMed] [Google Scholar]
- Seltzer J, Bigby BG, Stulbarg M, Holtzman MJ, Nadel JA, Ueki IF, Leikauf GD, Goetzl EJ, Boushey HA. O3-induced change in bronchial reactivity to methacholine and airway inflammation in humans. J Appl Physiol. 1986;60:1321–1326. doi: 10.1152/jappl.1986.60.4.1321. [DOI] [PubMed] [Google Scholar]
- Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol. 2005;115:925–7. doi: 10.1016/j.jaci.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol. 2003;95:938–945. doi: 10.1152/japplphysiol.00336.2003. [DOI] [PubMed] [Google Scholar]
- Shore SA, Schwartzman IM, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–9. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Moss J, Thisted R. Predictors of body surface area. J Clin Anesth. 1992;4(1):4–10. doi: 10.1016/0952-8180(92)90111-d. [DOI] [PubMed] [Google Scholar]
- Weinmann GG, Weidenbach-Gerbase M, Foster WM, Zacur H, Frank R. Evidence for ozone-induced small-airway dysfunction: lack of menstrual-cycle and gender effects. Am J Respir Crit Care Med. 1995;152:988–996. doi: 10.1164/ajrccm.152.3.7663815. [DOI] [PubMed] [Google Scholar]