Abstract
Adenoviral vectors were used to deliver genes encoding a soluble interleukin 1 (IL-1)-type I receptor-IgG fusion protein and/or a soluble type I tumor necrosis factor α (TNFα) receptor-IgG fusion protein directly to the knees of rabbits with antigen-induced arthritis. When tested individually, knees receiving the soluble IL-1 receptor had significantly reduced cartilage matrix degradation and white blood cell infiltration into the joint space. Delivery of the soluble TNFα receptor was less effective, having only a moderate effect on white blood cell infiltration and no effect on cartilage breakdown. When both soluble receptors were used together, there was a greater inhibition of white blood cell infiltration and cartilage breakdown with a considerable reduction of synovitis. Interestingly, anti-arthritic effects were also seen in contralateral control knees receiving only a marker gene, suggesting that sustained local inhibition of disease activity in one joint may confer an anti-arthritic effect on other joints. These results suggest that local intra-articular gene transfer could be used to treat systemic polyarticular arthritides.
Rheumatoid arthritis (RA) is a systemic autoimmune disease primarily manifested by chronic, erosive inflammation of the joints. Although a variety of pharmacological agents are used in the management of the symptoms, no combination of these conventional medications has proven effective in halting disease progression. Gene therapy, however, offers potential as a novel means to treat RA. Although the precise etiology of RA remains unknown, it may be possible to interrupt the disease process by delivering genes encoding therapeutic proteins to the cells within the synovial lining of joints. The localized over-expression and secretion of these proteins should allow for a concentrated accumulation of the therapeutic agent at the site of disease, a major obstacle for existing drug delivery methods (1).
Initial efforts in our laboratory as well as others have focused on blocking the activity of proinflammatory cytokines such as interleukin 1 (IL-1) and tumor necrosis factor α (TNFα). These cytokines are considered to be principle mediators in RA (2, 3), driving such pathological effects as leukocytic infiltration, synovial hyperplasia and hypercellularity, synovial cell activation, cartilage breakdown, and inhibition of cartilage matrix synthesis. The ex vivo delivery of the gene encoding human IL-1 receptor antagonist (IL-1Ra) to the joints of animals with experimental arthritis was found to ameliorate the effects of antigen-induced arthritis (a.i.a) in the rabbit knee (4), bacterial cell-wall-induced arthritis in rats (5), cartilage degradation in a human–SCID mouse model (6), and osteoarthritis in dogs (7). IL-1Ra is a naturally occurring protein that binds to the type I IL-1 cell surface receptor, preventing its ability to interact with IL-1 (8). These experiments have formed the basis for the implementation of a clinical trial to assess the safety and efficacy of using an ex vivo gene transfer protocol in the treatment of arthritis (9).
Ex vivo gene delivery, while effective, is laborious and expensive and thus difficult to apply on a widespread scale. Other viral vector systems such as adenovirus, adeno-associated virus, and herpes simplex virus, which can infect a wide variety of nondividing cells, offer the potential to deliver genes directly to the synovium in situ (10–12). Indeed, adenovirus has been shown to readily infect human and rabbit synoviocytes in culture as well as rabbit synoviocytes in vivo (10, 13). Moreover, infection of the rabbit knee joint with an adenoviral vector encoding IL-1Ra has been shown to have a prophylactic effect from some of the pathologies induced by intraarticular injection of IL-1 (13).
In the present study we have tested the ability of local adenovirus-mediated gene delivery of type I soluble receptors for IL-1 and TNFα to protect the rabbit knee joint during the acute inflammatory phase of a.i.a. We find that simultaneous administration of both inhibitors is more therapeutic than administration of either alone, resulting in a reduction in joint leukocytosis, cartilage degradation, and synovitis. Interestingly, after delivery of the IL-1 receptor alone or in tandem with the TNFα receptor, anti-inflammatory effects were noted in the contralateral arthritic control knee. This result suggests that the anti-arthritic protective effects of local gene therapy may not be limited to the target joint, but can affect distal joints. Thus local intra-articular gene therapy may be applicable to the treatment of systemic polyarticular arthritis.
MATERIALS AND METHODS
Adenoviral Vectors.
Each recombinant adenoviral vector originates from replication-deficient type 5 adenovirus lacking E1 and E3 loci (14). cDNAs of the gene of interest were inserted in place of the E1 region, and expression is driven by the cytomegalovirus promoter. Ad.sTNF-RI-Ig encodes a fusion protein consisting of the extracellular domain of human 55-kDa TNFα receptor and the CH2 through CH3 domains of a mouse IgG1 heavy chain (15). Ad.sIL-1RI-Ig encodes the extracellular portion of the human type I IL-1 receptor fused to the mouse IgG1 heavy chain. Ad.lacZ encodes a bacterial β-galactosidase, and Ad.luciferase encodes firefly luciferase.
High-titer suspensions of recombinant adenovirus were prepared by amplification in 293 cells by established methods. Titers were determined by optical density at 260 nm (OD260) and standard plaque assay (16, 17).
For adenoviral transduction in vitro, HIG-82 cells (18) were grown to confluence in 10-cm dishes in Ham’s F-12 medium supplemented with 10% fetal calf serum and 1% penicillin/streptomycin. The cells were washed with saline solution, and 7 × 107 plaque-forming units (pfu) of adenovirus suspended in 0.5 ml of saline was added. After incubation at 37°C for 2 hr, the medium was replaced. After 24 hr the medium was replaced with Neuman and Tytell serumless medium (GIBCO/BRL); 48 hr later the culture supernatant was removed and stored at −20°C.
Western Blot Analyses.
Tissue culture supernatants were separated and resolved by SDS/PAGE by standard methods (19) with minor modifications. Proteins were then transferred to nitrocellulose at 100 V for 1 hr at 4°C in 50 mM Tris⋅HCl, pH 8.3/200 mM glycine/1% SDS/20% (vol/vol) methanol. Membranes were blocked with 5% nonfat dried milk in 1× PBST (PBS with Tween 20: 140 mM NaCl/2 mM KCl/10 mM Na2HPO4/2 mM KH2PO4, pH 7.2/0.025% Tween 20) for 1 hr at room temperature. Peroxidase-labeled goat anti-mouse IgG whole molecule (Sigma) diluted 1:20,000 in 5% milk/1× PBST was used to probe the membranes for 1 hr at room temperature. After extensive washing with 1× PBST, proteins of interest were detected by enhanced chemiluminescence and exposure to film.
Experimental Arthritis and Biological Analyses.
To establish a.i.a., rabbits were first sensitized to ovalbumin by a series of two intradermal injections of 5 mg of ovalbumin emulsified in Freund’s complete adjuvant in the first injection and in Freund’s incomplete adjuvant in the second. Arthritis was initiated in both hind knees of rabbits by the intraarticular injection of 5 mg of ovalbumin dissolved in 0.5 ml of saline. Twenty-four hours after induction of a.i.a., 7 × 107 pfu of adenovirus encoding either the soluble TNFα and/or IL-1 receptors or β-galactosidase was suspended in 0.2 ml of saline and injected into the joint space of the knee through the patellar tendon.
To lavage rabbit knee joints, 1 ml of saline solution was injected into the joint space through the patellar tendon. After manipulation of the joint, the needle was reinserted and the fluid was aspirated. Leukocytes in recovered lavage fluids were counted by using a hemocytometer. Human TNFα receptor type I concentrations in conditioned media, lavage fluids, and blood sera were measured with ELISA kits from R & D Systems as directed by the supplier. Levels of sulfated glycosaminoglycans (GAGs) in lavage fluids were determined in a colorimetric dye-binding assay using 1,9-dimethylmethylene blue as previously described (20).
For histological analyses, tissues harvested from dissected knees were first fixed in 10% formalin for 24 hr. The fixed tissues were imbedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin.
Luciferase Assays.
To determine luciferase content in rabbit tissues, after sacrifice of the animals, tissues were dissected and stored at −80°C. Approximately 0.7 g of each tissue was mixed with 2 ml of 0.25 M Tris⋅HCl, pH 7.5, and the mixture was homogenized by hand with a tightly fitting Dounce homogenizer. The homogenate was collected, put through three freeze-thaw cycles, and centrifuged 15 min at low speed in a table-top clinical centrifuge. Luciferase activity in 100 μl of supernatant was measured in a luminometer as directed by using a luciferase assay system from Promega. For leukocytes, cells recovered from lavage fluid or blood were counted by using a hemocytometer. About 5 × 106 cells were then pelleted and resuspended in 200 μl of 0.25 M Tris⋅HCl, pH 7.5, and put through three freeze-thaw cycles. Lysates were cleared by centrifugation, and luciferase activity in 100 μl was quantitated as above.
RESULTS
Expression of TNFα and IL-1 Inhibitors After in Vitro Infection of Synoviocytes.
To determine levels of transgene expression in vitro, 7 × 107 pfu of an adenoviral vector encoding either the soluble type I TNFα receptor fused to the constant region of a murine IgG1 (Ad.sTNF-RI-Ig) (15) or the soluble type I IL-1 receptor fused to the same Ig domain (Ad.sIL-1RI-Ig) was used to transduce 3 × 106 HIG-82 cells, a lapine synovial fibroblast line (18). After 48 hr of culture in serumless medium, culture supernatants were collected and analyzed. ELISA measurements of the soluble TNFα receptor detected greater than 300 ng per 106 cells per 48 hr. Western-blot analyses using antibody specific for the murine IgG1 portion of the receptor fusion molecule demonstrated that proteins of the approximate predicted size of the IL-1 and TNFα receptor-Ig fusion proteins (IL-1 inhibitor and TNFα inhibitor, respectively) were produced by the transduced cells. The IL-1 inhibitor was found to be secreted at a level about 50% of that of the TNFα inhibitor (data not shown). The biological activity of the IL-1 and TNFα inhibitors was demonstrated by their ability to partially block the effects of constitutively expressed human IL-1β in the rabbit knee (S.C.G., C.H.E., and P.D.R., unpublished results) and TNFα induction of NF-κB, respectively (S. Makarov, personal communication).
Expression of TNFα and IL-1 Inhibitors After Intraarticular Injection of Recombinant Adenoviruses into the Rabbit Knee.
To test the ability of the inhibitor molecules to block the acute inflammatory effects of a.i.a. in the rabbit knee joint, a.i.a. was induced in both knees of 32 rabbits. Twenty-four hours after induction, approximately 7 × 107 pfu of Ad.sIL-1RI-Ig, Ad.sTNF-RI-Ig, or both adenoviral vectors was injected intraarticularly into the left knee of three sets of 8 rabbits. Approximately 7 × 107 pfu of adenovirus encoding bacterial β-galactosidase (Ad.lacZ) was injected into the right knee of all 32 rabbits. A control group of 8 rabbits received an equal volume injection of saline in the left knee. Three days after injection of the adenovirus, both knees of each rabbit were lavaged with 1 ml of saline solution. At 7 days after infection, the rabbits were sacrificed, and the knees were lavaged, dissected, and analyzed for effects of transgene expression. It should be noted that injections of Ad.sTNF-RI-Ig significantly exceeding 1 × 109 pfu proved inflammatory in naive rabbit joints, and they exacerbated the pathology in knees with a.i.a. This heightened inflammatory response was also accompanied by a loss of transgene expression in 3–7 days. Adenoviral doses of 7 × 107 pfu or less, however, produced no detectable leukocytic infiltrate into synovial fluid for up to 14 days after injection and maintained high levels of gene expression (data not shown).
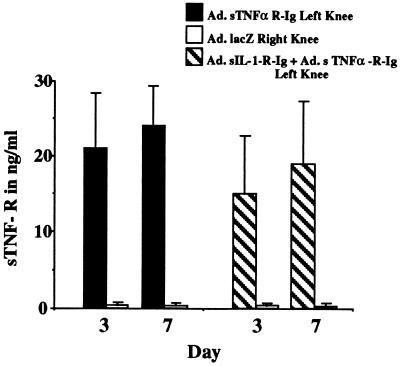
ELISA measurements of TNFα receptor levels in lavage fluids detected approximately 20 ng/ml of fluid at days 3 and 7 in knees receiving Ad.sTNF-RI-Ig alone. Significant levels of the receptor (>1 ng/ml) were not detected in contralateral joints receiving Ad.lacZ (Fig. 1). Similarly, in knees receiving a mixture of adenovirus encoding the TNFα and IL-1 inhibitors together, TNFα receptor expression of greater than 15 ng/ml of lavage fluid was observed at both day 3 and day 7. No significant expression was detected in the contralateral knee or in blood sera.
Figure 1.
In vivo expression of TNFα inhibitor after injection into rabbit knees with a.i.a. Twenty-four hours after induction of a.i.a. two groups of eight rabbits were injected in the left knee with either 7 × 107 pfu of Ad.sTNF-RI-Ig or 7 × 107 pfu of Ad.sTNF-RI-Ig and Ad.sIL-1-RI-Ig. Both groups were injected with 7 × 107 pfu of Ad.lacZ in the right knee. At days 3 and 7 after injection of virus the knees were lavaged and the recovered fluids were analyzed by ELISA for levels of human type I TNFα soluble receptor (sTNF-R).
Therapeutic Efficacy of IL-1 and TNFα Inhibitors in a Rabbit a.i.a. Model After Intraarticular Injection of Recombinant Adenoviral Vectors.
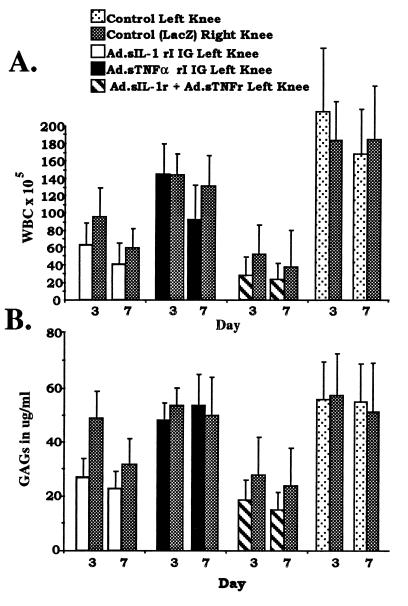
As a quantitative index of inflammation in the rabbit knees, leukocytes in recovered lavage fluids from each group of rabbits were counted and compared. As shown in Fig. 2A, in the control group of rabbits, injected with Ad.lacZ in the right knee and saline in the left knee, both knees were similarly inflamed, with mean levels of infiltrating leukocytes exceeding 2 × 107 per ml of fluid at days 3 and 7 after adenoviral infection. In the group of rabbits injected with Ad.sTNF-RI-Ig in the left knee and Ad.lacZ in the right, equally high numbers of infiltrating leukocytes were seen in both knees at day 3. By day 7, a modest decline in the mean leukocytic infiltration was observed in knees receiving the TNFα inhibitor. Knees injected with Ad.sIL-1RI-Ig showed a mean 65% reduction in infiltration over the control group of rabbits at day 3, which increased to about 80% by day 7. Interestingly, in this group of rabbits, the contralateral knees that were injected with Ad.lacZ also showed a reduction in infiltration at both day 3 and day 7 relative to the control group of rabbits. Rabbits injected in the left knee with both Ad.sIL-1RI-Ig and Ad.sTNF-RI-Ig vectors showed an 85–90% reduction in mean leukocytic infiltration at both day 3 and day 7 over the control group of rabbits, and the reduction was accompanied by an 80% reduction in the contralateral Ad.lacZ-injected knee.
Figure 2.
Leukocytic infiltration and GAG release in a.i.a. knees after intraarticular injection of Ad.sIL-1-RI-Ig and Ad.sTNF-RI-Ig. a.i.a. was induced in both knees of four groups of eight rabbits. Twenty-four hours after induction groups of rabbits received injection of adenoviral vectors as indicated. (A) At 3 and 7 days after adenoviral injection, knees of rabbits were lavaged. Numbers of infiltrating leukocytes (white blood cells; WBC) in synovial fluids for both right and left knees of each group are shown as indicated. (B) GAG levels in lavage fluids were determined by 1,9-dimethylmethylene blue assay and are shown for each group as indicated. Error bars designate ± one standard deviation. To determine whether differences observed among the groups of rabbits, left and right knees, and the days were significant, the appropriate F tests were performed. For A, differences among the groups, knees, and days, P values equal 0.0001, 0.0365, and 0.0008, respectively. For B, differences among the groups, knees, and days, P values equal 0.0001, 0.0149, and 0.0312, respectively.
To determine relative cartilage matrix degradation in the rabbit knees, GAGs released into synovial fluid as a result of proteoglycan breakdown were measured in recovered lavage fluids (20). The results of these assays, shown in Fig. 2B, correspond closely with the relative levels of leukocytic infiltration from Fig. 2A. The control group rabbits receiving injections of Ad.lacZ and saline in opposing knee joints had similarly high levels of GAGs in the lavage fluids of both knees at both day 3 and day 7. Rabbits injected with Ad.sTNF-RI-Ig and Ad.lacZ in opposite knees likewise had elevated GAGs in both knees at both time points. However, rabbits injected in one knee with Ad.IL-1 receptor showed a greater than 50% reduction in mean GAG levels at both day 3 and day 7 over the control group rabbits. By day 7, the contralateral knees, which had been injected with Ad.lacZ, also showed nearly a 40% reduction in GAG release. Rabbit knees that were injected with virus encoding both IL-1 and TNFα inhibitors had a 65% reduction in mean GAG level, while GAGs in opposing Ad.lacZ-injected knees were reduced >50%.
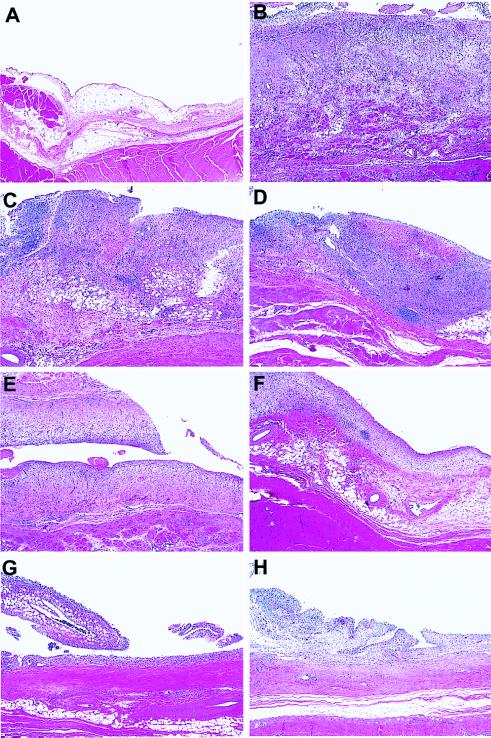
Histological Analyses.
Histological analyses of tissue recovered from the knees of each group of rabbits is shown in Fig. 3. Compared with tissue recovered from normal naive rabbits (Fig. 3A), sections from the Ad.lacZ/saline group showed a severe synovitis typical of that seen with a.i.a. (Fig. 3B) (21). The synovium was dramatically thickened, highly fibrous, and hypercellular, with increased numbers of synovial cells and infiltrating mononuclear leukocytes. A small population of polymorphonuclear leukocytes was also present. Treatment of joints with Ad.sTNF-RI-Ig alone had little observable effect on the severity of synovitis in either the treated (Fig. 3C) or the contralateral (Fig. 3D) joint. Synovial sections from this group of rabbits were largely indistinguishable from those of the a.i.a. knees from the Ad.lacZ/saline group. Although somewhat variable among the rabbits within the group, knees receiving intraarticular injection of Ad.sIL-1RI-Ig (Fig. 3E) showed a limited but distinct reduction in synovitis. The general pathology was the same as the Ad.lacZ/saline group, but the severity was observably reduced. Opposing contralateral joints also had a detectable reduction in synovitis (Fig. 3F). Knees of rabbits receiving both the Ad.sIL-1RI-Ig and Ad.sTNF-RI-Ig vectors together showed a marked reduction in synovial pathology (Fig. 3G). Contralateral knees within this group also showed a significant reduction in synovitis (Fig. 3H).
Figure 3.
Histological analyses of synovial tissue recovered from rabbit knees. a.i.a. was induced in both knees of four groups of eight rabbits. Twenty-four hours after induction all rabbits were injected in the right knee with 7 × 107 pfu of Ad.lacZ. In the left knee, groups of 8 rabbits were injected with 7 × 107 pfu of Ad.sTNF-RI-Ig, 7 × 107 pfu of Ad.sIL-1-RI-Ig, 7 × 107 pfu each of Ad.sTNF-RI-Ig and Ad.sIL-1-RI-Ig, or saline. Seven days after virus injection synovial tissues from each group were harvested, sectioned, and stained with hematoxylin and eosin. (×40.) (A) Synovium from a normal naive rabbit knee. (B) Synovium recovered from rabbit knee receiving saline after a.i.a. induction. Relative to A, the tissue is dramatically thickened, fibrous, and hypercellular, with infiltrating leukocytes and synovial cells. (C and D) Synovial tissue recovered from rabbit knees injected with Ad.sTNF-RI-Ig in the left (C) and Ad.lacZ in the right (D). (E and F) Tissue recovered from rabbit knees injected with Ad.sIL-1-RI-Ig (E) in the left and Ad.lacZ in the right (F). (G and H) Synovium from rabbits injected with both Ad.sTNF-RI-Ig and Ad.sIL-1-RI-Ig in the left knee and Ad.lacZ in the right.
Distribution of Transgene Expression After Intraarticular Injection of an Adenoviral Vector.
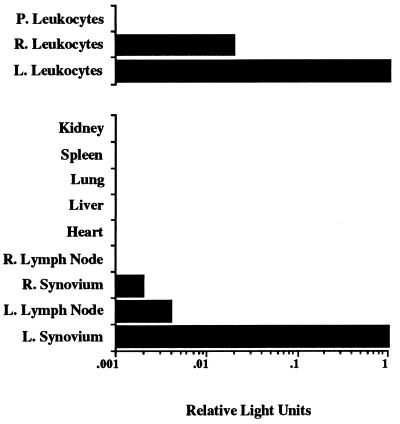
The results of the experiments described above suggested that direct intraarticular delivery of adenoviral vectors encoding IL-1 and TNFα inhibitors could have an anti-inflammatory effect that was not limited to the site of virus injection. One possible explanation for this observation is that sufficient levels of inhibitor protein were present in the contralateral joint to confer a protective effect. However, significant levels (>1 ng/ml) of TNFα inhibitor molecules were not detected in sera or lavage fluids from contralateral joints. Alternatively, adenoviral particles or virally transduced cells could be migrating from the joint of injection to the opposite knee or other organs, thereby causing a systemic anti-inflammatory effect. To test this possibility, an adenoviral vector encoding the firefly luciferase reporter gene (22, 23) (Ad.luciferase) was utilized. As in experiments described above, a.i.a. was induced in both knees of two rabbits. Twenty-four hours after induction, 1.5 × 109 pfu of the Ad.luciferase virus was injected into one knee of each rabbit, whereas the contralateral knee received 7 × 107 pfu of Ad.lacZ. At 7 days after injection, the rabbits were bled and sacrificed, the joints were lavaged, and the joint capsules of both knees were harvested along with regional lymphoid tissue, heart, liver, lung, spleen, and kidney. Recovered tissues and leukocytes were then analyzed for the presence of intracellular luciferase activity. A low level of luciferase activity was observable in lymphoid tissue obtained near the site of injection and in synovial tissue of the contralateral knee joint relative to knees receiving the Ad.luciferase vector (Fig. 4). Analysis of similar numbers of leukocytes obtained from both knee joints and peripheral blood showed luciferase activity in leukocytes obtained from the injected knee and a lower level in the contralateral knee. No appreciable activity was detected in circulating leukocytes. These results demonstrate that a population of transduced leukocytes can migrate to the opposing inflamed knee joint, suggesting a possible mechanism for the observed contralateral effect. However, if and how the adenovirally transduced trafficking cells confer anti-inflammatory effects at distal joints is still unclear.
Figure 4.
Expression of luciferase activity in various tissues after intraarticular injection of Ad.luciferase into a.i.a. knees. Twenty-four hours after induction of a.i.a. in both knees of two rabbits, 1.5 × 109 pfu of Ad.luciferase was injected into the left knee joint (L.), and 7 × 107 pfu of Ad.lacZ was injected into the right knee joint (R.). Seven days after injection of adenovirus, the rabbits were sacrificed. Blood was collected, knees were lavaged, and synovium, heart, liver, lung, spleen, and kidney tissues were harvested. Luciferase activities in lysates from tissues and leukocytes from lavages of right (R.) and left (L.) knees and blood (peripheral [P.] leukocytes) were quantitated and are expressed relative to luciferase activity found in synovium and leukocytes from the left knee.
DISCUSSION
IL-1 and TNFα are considered to be the principle inflammatory cytokines in the pathogenesis in RA (2, 3) and are thus natural targets for therapy in this disease. Currently there is much interest in the use of biological agents that block the activity of these cytokines (24). These agents include such molecules as cytokine-specific antibodies (25), soluble receptor molecules (26, 27), and receptor antagonists such as IL-1Ra (8). In vitro, soluble receptors for TNFα and IL-1 can bind directly with their respective ligand and preclude that cytokine from interacting with cellular receptors. When delivered systemically by infusion or intravenous injection to animal models with experimental RA, each soluble receptor was found to prevent the onset of arthritic pathology (24). Recently a clinical trial using subcutaneous injection of a soluble TNFα receptor has shown therapeutic efficacy in humans; however, twice-weekly injections were required, and arthritic symptoms rapidly returned upon cessation of treatment (28).
The results of experiments described here demonstrate that intraarticular adenoviral gene delivery of soluble receptor molecules for IL-1 and TNFα can substantially block an acute inflammatory and erosive response in a.i.a in the rabbit knee. Delivery of the IL-1 soluble receptor gene alone was found to be considerably more effective at inhibiting synovial fluid leukocytosis and cartilage matrix degradation than the TNFα inhibitor. Neither inhibitor had a significant effect on synovitis. However, simultaneous gene delivery of both inhibitors was the most effective, resulting in a net reduction in leukocytosis, cartilage matrix degradation, and synovitis. Surprisingly, these anti-inflammatory responses were also apparent in contralateral knees that received adenovirus containing the lacZ gene, suggesting that local intraarticular gene therapy may have distal or systemic anti-arthritic effects. We are unaware of any other study reporting an anti-arthritic effect by in vivo gene therapy.
Although the anti-arthritic properties of IL-1 and TNFα antagonists have been extensively studied, the therapeutic efficacy of simultaneous inhibition of IL-1 and TNFα has not previously been evaluated. The enhanced therapeutic effects observed after injection of both Ad.sIL-1RI-Ig and Ad.sTNF-RI-Ig suggest that strategies directed at blocking the activity of both TNFα and IL-1 may be considerably more effective in the treatment of RA than targeting either cytokine individually. This result correlates with earlier observations by Henderson and Pettipher (29) that IL-1 and TNFα may work synergistically in the pathology of RA. The coinjection of both cytokines into the joints of rabbits was found to stimulate significantly greater leukocytic infiltration into the synovial fluid than when either was administered alone. This observation is consistent with RA being a disorder driven by the imbalance of a cytokine network and implies that therapies which target the activities of multiple inflammatory effectors would be the most beneficial.
The experiments described here suggest that intraarticular gene delivery of the IL-1 and TNFα inhibitors has systemic anti-inflammatory effects, or at least therapeutic effects that extend to the contralateral knee joint. If this observed contralateral joint effect was due to therapeutic levels of inhibitor molecules leaking from the joint space to the circulation, it would seem that they should be detectable in the serum or in lavage fluids recovered from the contralateral joint. However, significant levels of sTNFα receptor were not detected outside the injected joint. In contrast, cell trafficking studies conducted here using the Ad.luciferase vector indicate that a percentage of leukocytes transduced by adenovirus in one joint migrate to the adjacent lymph node and opposing inflamed joint. This observation would indicate that even though appreciable levels of soluble TNFα receptor were not be detected in animal fluids by ELISA, a population of leukocytes capable of expressing the inhibitor genes does indeed migrate from the site of transduction and travel via either the circulatory or the lymphatic system. However, whether these cells contribute to the contralateral therapeutic effect is still unclear. The bilateral symmetry of the response suggests that a neural component may also play a role. Certain neurogenic factors such as substance P or corticotropin-releasing hormone are known to modulate articular inflammation (30–32). Recently, a similar contralateral joint effect has been observed by Makarov et al. (33), who reported that intraarticular delivery of oligodeoxynucleotides containing decoy binding sequences for the transcription factor NF-κB into the knees of rats with experimental arthritis appears to down-regulate inflammation in the knee receiving the oligonucleotides and in uninjected contralateral knees as well.
The results of these experiments clearly demonstrate that adenoviral vectors can be used to efficiently deliver potentially therapeutic genes directly to intraarticular tissues, and that subsequent expression of these genes can occur at levels sufficient to produce beneficial effects in an animal model of arthritis. In principle, the use of adenovirus for direct delivery of therapeutic genes to diseased joints appears promising; however, the current generation of adenoviral vectors is unsuitable for use in the treatment of joint disease in humans (34, 35). Altering the properties of the adenovirus to increase infectivity of the vector may allow for high levels of transgene expression with administration of fewer adenoviral particles (36). Furthermore, reducing the antigenicity of the adenovirally infected cell may also allow extended transgene expression (14). Because both these type of approaches are currently being studied, there is optimism that adenoviral vectors may be applicable for future use in gene therapy for arthritis and other joint diseases.
One criticism of the local delivery approach to gene therapy for arthritis is that RA is a systemic disorder and that treatment of multiple joints might be impractical. However, our results demonstrate that local gene delivery of anti-inflammatory agents can have distal effects, suggesting that it may be necessary to treat only a limited number of sites to down-regulate or halt the disease progression throughout the body. Certainly further investigation into the generality of this apparent systemic anti-inflammatory effect and its biological basis is in order.
Acknowledgments
We are grateful to Dr. David M. Nickerson for his assistance with the statistical analyses. This work was supported in part by Grants DK44935 and AR62225 from the National Institutes of Health, a grant from the Western Pennsylvania Chapter of the Arthritis Foundation, and a National Research Service Award fellowship from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to S.C.G.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: a.i.a., antigen-induced arthritis; GAG, glycosaminoglycan; IL-1, interleukin 1; IL-1Ra, IL-1 receptor antagonist; pfu, plaque-forming units; RA, rheumatoid arthritis; TNFα, tumor necrosis factor α; sTNF-R, soluble tumor necrosis factor receptor.
References
- 1.Kang R, Ghivizzani S C, Herndon J H, Robbins P D, Evans C H. Biochem Soc Transact. 1997;25:533–537. doi: 10.1042/bst0250533. [DOI] [PubMed] [Google Scholar]
- 2.Arend W P, Dayer J M. Arthritis Rheum. 1995;38:151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- 3.Feldmann M, Elliot M J, Woody J N, Maini R N. Adv Immunol. 1997;64:283–350. doi: 10.1016/s0065-2776(08)60891-3. [DOI] [PubMed] [Google Scholar]
- 4.Otani K, Nita I, Macaulay W, Georgescu H I, Robbins P D, Evans C H. J Immunol. 1996;156:3558–3562. [PubMed] [Google Scholar]
- 5.Makarov S S, Olsen J C, Johnston W N, Anderle S K, Brown R R, Baldwin A S, Jr, Haskill J S, Schwab J H. Proc Natl Acad Sci USA. 1996;93:402–406. doi: 10.1073/pnas.93.1.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullerladner U, Roberts C R, Franklin B N, Gay R E, Robbins P D, Evans C H, Gay S. J Immunol. 1997;158:3492–3498. [PubMed] [Google Scholar]
- 7.Pelletier J-P, Caron J P, Evans C H, Robbins P D, Georgescu H I, Jovanovic D, Fernandes J C, Martel-Pelletier J. Arthritis Rheum. 1997;40:1012–1019. doi: 10.1002/art.1780400604. [DOI] [PubMed] [Google Scholar]
- 8.Arend W P. Adv Immunol. 1993;54:167–227. doi: 10.1016/s0065-2776(08)60535-0. [DOI] [PubMed] [Google Scholar]
- 9.Evans C H, Robbins P D, Ghivizzani S C, Herndon J H, Kang R. Hum Gene Ther. 1996;7:1261–1280. doi: 10.1089/hum.1996.7.10-1261. [DOI] [PubMed] [Google Scholar]
- 10.Nita I, Ghivizzani S C, Galea-Lauri J, Bandara G, Georgescu H I, Robbins P D, Evans C H. Arthritis Rheum. 1996;39:820–828. doi: 10.1002/art.1780390515. [DOI] [PubMed] [Google Scholar]
- 11.Roessler B J, Allen E D, Wilson J M, Hartman J W, Davidson B L. J Clin Invest. 1993;92:1085–1092. doi: 10.1172/JCI116614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glorioso J C, Krisky D, Marconi P, Oligino T, Ghivizzani S C, Robbins P D, Schmidt M C, Goins W F, Evans C H. Adv Drug Delivery Rev. 1997;27:41–57. doi: 10.1016/s0169-409x(97)00021-5. [DOI] [PubMed] [Google Scholar]
- 13.Roessler B J, Hartman J W, Vallance D K, Latta J M, Janich S L, Davidson B L. Hum Gene Ther. 1995;6:307–316. doi: 10.1089/hum.1995.6.3-307. [DOI] [PubMed] [Google Scholar]
- 14.Yeh P, Perricaudet M. FASEB J. 1997;11:615–623. doi: 10.1096/fasebj.11.8.9240963. [DOI] [PubMed] [Google Scholar]
- 15.Kolls J K, Peppel K, Silva M, Beutler B. Proc Natl Acad Sci USA. 1994;91:215–219. doi: 10.1073/pnas.91.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham F L, Van der Eb A J. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 17.Mittereder N, March K L, Trapnell B C. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgescu H I, Mendelow D, Evans C H. In Vitro. 1988;24:1015–1022. doi: 10.1007/BF02620875. [DOI] [PubMed] [Google Scholar]
- 19.Harlow E, Lane D. Immunoblotting. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. pp. 471–510. [Google Scholar]
- 20.Farndale R W, Buttle D J, Barrett J J. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 21.Edwards J C W, Read N, Trefty B, Coulstock J, Henderson B. Br J Exp Path. 1988;69:739–748. [PMC free article] [PubMed] [Google Scholar]
- 22.de Wet J R, Wood K V, DeLuca M, Helinski D R. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ow D W, Wood K V, DeLuca M, de Wet J R, Helinski D R, Howell S H. Science. 1986;234:856–859. doi: 10.1126/science.234.4778.856. [DOI] [PubMed] [Google Scholar]
- 24.Moreland L W, Heck L W, Koopman W J. Arthritis Rheum. 1997;40:397–409. doi: 10.1002/art.1780400302. [DOI] [PubMed] [Google Scholar]
- 25.Elliott M J, Maini R N, Feldmann M, Kalden J R, Antoni C, Smolen J S, Leeb B, Breedveld F C, Macfarlane J D, Biji H. Lancet. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 26.Arend W P, Malyak M, Smith M F, Jr, Whisenand T D, Slack J L, Sims J E. J Immunol. 1994;153:4766–4774. [PubMed] [Google Scholar]
- 27.Moreland L W, Margolies G R, Heck L W, Saway P A, Blosch C, Hanna R, Koopman W J. J Rheumatol. 1996;23:1849–1855. [PubMed] [Google Scholar]
- 28.Moreland L W, Baumgartner S W, Schiff M H, Tindall E A, Fleischmann R M, Weaver A L, Ettlinger R E, Cohen S, Koopman W J, Mohler K, Widner M B, Blosch C M. N Engl J Med. 1997;337:141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 29.Henderson B, Pettipher E R. Clin Exp Immunol. 1989;75:306–310. [PMC free article] [PubMed] [Google Scholar]
- 30.Garrett N E, Mapp P I, Cruwys S C, Kidd B L, Blake D R. Ann Rheum Dis. 1992;51:1014–1018. doi: 10.1136/ard.51.8.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorgensen C, Sany J. Clin Exp Rheumatol. 1994;12:435–441. [PubMed] [Google Scholar]
- 32.Scott D T, Lam F Y, Ferrell W R. Gen Pharmacol. 1994;25:1285–1296. doi: 10.1016/0306-3623(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 33.Makarov S S, Baldwin A S. Arthritis Rheum. 1996;39:S121. (abstr.). [Google Scholar]
- 34.Sawchuk S, Boivin G, Duwel L, Ball W, Bove K, Trapnell B, Hirsch R. Hum Gene Ther. 1996;7:499–506. doi: 10.1089/hum.1996.7.4-499. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Nunes F A, Berensci K, Furth E E, Bonczol E, Wilson J M. Proc Natl Acad Sci. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wickham T J, Roelvink P W, Brough D E, Kovesdi I. Nat Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]