Abstract
Autism spectrum disorders (ASD) are a group of related neurodevelopmental syndromes with complex genetic etiology.1 We identified a de novo chromosome 7q inversion disrupting Autism susceptibility candidate 2 (AUTS2) and Contactin Associated Protein-Like 2 (CNTNAP2) in a child with cognitive and social delay. We focused our initial analysis on CNTNAP2 based on our demonstration of disruption of Contactin 4 (CNTN4) in a patient with ASD;2 the recent finding of rare homozygous mutations in CNTNAP2 leading to intractable seizures and autism;3 and in situ and biochemical analyses reported herein that confirm expression in relevant brain regions and demonstrate the presence of CNTNAP2 in the synaptic plasma membrane fraction of rat forebrain lysates. We comprehensively resequenced CNTNAP2 in 635 patients and 942 controls. Among patients, we identified a total of 27 nonsynonymous changes; 13 were rare and unique to patients and 8 of these were predicted to be deleterious by bioinformatic approaches and/or altered residues conserved across all species. One variant at a highly conserved position, I869T, was inherited by four affected children in three unrelated families, but was not found in 4010 control chromosomes (p = 0.014). Overall, this resequencing data demonstrated a modest nonsignificant increase in the burden of rare variants in cases versus controls. Nonethless, when viewed in light of two independent studies published in this issue of AJHG showing a relationship between ASD and common CNTNAP2 alleles,4,5 the cytogenetic and mutation screening data suggest that rare variants may also contribute to the pathophysiology of ASD, but place limits on the magnitude of this contribution.
Main Text
The clinical hallmarks of ASD (MIM 209850) are derangements in reciprocal social interaction, abnormal development of speech and language, and the presence of highly restricted interests and stereotyped behaviors.6 Fundamental impairment in some but not all of these domains defines a spectrum of conditions that includes Asperger syndrome and Pervasive Developmental Disorder Not Otherwise Specified (PDD-NOS). In the DSM-IV, rare developmental disorders including Rett Syndrome and Childhood Disintegrative Disorder6 are grouped in the same diagnostic category. A majority of patients with ASD have MR in addition to their social disability and up to one-third suffer from seizures.6 Individuals with ASD also show an increased burden of chromosomal abnormalities1 and de novo rare copy number variants.7
Despite multiple lines of evidence suggesting a complex genetic etiology, common ASD variants have been extremely difficult to identify.1 In addition, to date there has not been a convergence between the rare mutations identified in nonsyndromic autism, such as those in the Neuroligin gene family,8–13 and those genomic regions most strongly implicated by nonparametric linkage or common variant association studies. Difficulties in clarifying the genetic substrates of ASD likely reflect the combination of marked locus and allelic heterogeneity, the absence of reliable biological diagnostic markers, and the likelihood that any contributing common alleles will be found to carry quite small increments of risk, requiring very large sample sizes to definitively confirm their contributions.1
Because of high rates of overlap between ASD, MR, and chromosomal abnormalities, we have used molecular cytogenetic mapping of balanced rearrangements in children with social and cognitive delays as a means of identifying candidate genes that may harbor rare disease alleles. We evaluated a patient with MR and a de novo inversion of chromosome 7 (46,XY,inv(7)(q11.22;q35)) (Appendix A). Based on the Autism Diagnostic Interview-Revised (ADI-R), the index case met “broad spectrum” criteria according to the Autism Genetics Research Exchange (AGRE) phenotype algorithm (Appendix A) but did not meet the full criteria for ASD according to the combination of ADI-R and Autism Diagnostic Observations Scales (ADOS). We mapped the chromosomal rearrangement by using fluorescent in situ hybridization as described14 and found that the inversion breakpoints disrupted the genes AUTS2 at 7q11.22 and CNTNAP2 at 7q35 (Figure 1). AUTS2 maps to a 1.2 MB genomic region of 7q11.22; BAC RP11-709J20 spans the inversion and is within intron 5, placing the break between exons 5 and 6. CNTNAP2 maps to a 2.3 MB genomic region on 7q35; BAC RP11-1012D24 was found to span the inversion and includes coding exons 11 and 12, placing the break between exons 10 and 13. We further evaluated the patient by performing array-based competitive genomic hybridization with a chromosome 7-specific microarray containing approximately 385,000 probes with an average spacing of 400 base pairs (Nimblegen). No large-scale deletions or duplication were observed within several megabases of the breakpoints.
Figure 1.
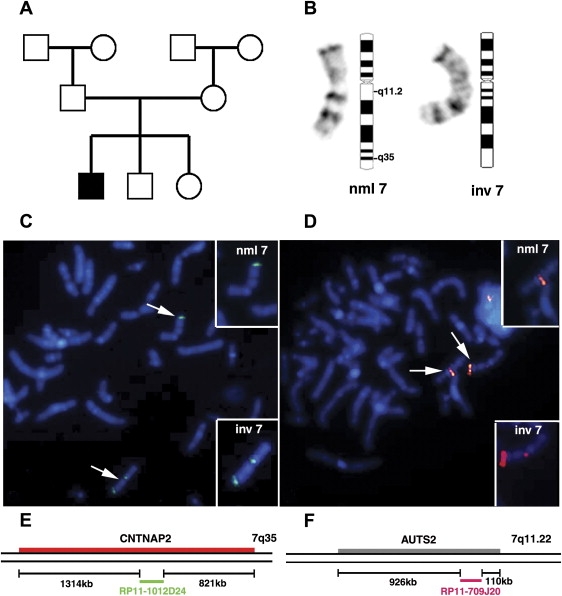
Mapping of a De Novo Inversion (inv(7)(q11.22;q35)) in a Child with Developmental Delay
(A) Pedigree of a family with an affected male child with developmental delay. The parents, grandparents, and two older siblings are not affected with a neurodevelopmental disorder.
(B) G-banded metaphase chromosomes and ideogram for normal (left) and inverted (right) chromosomes are presented.
(C and D) FISH mapping of q35 (C) and q11.22 (D) breakpoints. Images show the two BACs that span the breaks. The experimental probe is seen at the expected positions on the normal (nml) chromosomes 7q35 and 7q11.22, respectively. Two fluorescence signals are visible on the inverted (inv) chromosomes indicating that the probes span the break points. Photographs were taken with a 100× objective lens.
(E and F) Schematics showing the location of the spanning BACs relative to the disrupted genes.
(E) The edges of the BAC RP11-1012D24 are 1314 kb and 821 kb away from the centromeric and telomeric ends of CNTNAP2.
(F) The edges of the BAC RP11-709J20 are 926 kb and 110 kb away from the centromeric and telomeric ends of AUTS2.
Both genes, either alone or in combination, represent strong candidates for contributing to the etiology of the cognitive and social delays seen in the index case. AUTS2 encodes a predicted protein of unknown function that was originally identified through mapping of a chromosomal abnormality in a pair of twins with ASD.15 Additionally, three cases of MR and balanced translocations of AUTS2 have been reported recently.16 However, a copy number polymorphism in unaffected individuals has also been reported at the AUTS2 locus,17 suggesting that haploinsufficiency and structural rearrangements at this interval may be tolerated in some cases. We evaluated the expression of AUTS2 mRNA by RT-PCR in peripheral lymphoblasts from the patient as well as unaffected family members; the patient's expression levels were normal for exons 5′ to the break, but reduced by approximately 50% for exons distal to it (data not shown). Results of resequencing of this gene will be described in a separate paper.
CNTNAP2 is also a strong candidate for involvement in social and cognitive delay. It is a neuronal cell adhesion molecule known to interact with Contactin 2 (Cntn2), also known as TAG-1, at the juxtaparanodal region at the nodes of Ranvier, which are the regularly spaced gaps between the myelin-producing Schwann cells in the peripheral nervous system (PNS).18,19 Whereas previous investigations have largely focused on the role of CNTNAP2 in PNS development, a recent report demonstrated that a homozygous CNTNAP2 mutation in the Old Order Amish population results in intractable seizures, histologically confirmed cortical neuronal migration abnormalities, MR, and ASD.3 These data, along with our earlier identification of a cytogenetic disruption of CNTN4 in a child with MR and ASD,2 suggests the possible involvement of a Contactin-related pathway in these disorders.
As was the case with AUTS2, evidence from available reports of cytogenetic abnormalities involving CNTNAP2 has been inconsistent. In one instance, Tourette syndrome and developmental delay were identified in a family carrying a complex rearrangement disrupting CNTNAP2.20 More recently, carriers of a balanced t(7;15) translocation involving the coding region of CNTNAP2 were described as normal.21
Given the absence of expression of CNTNAP2 in peripheral lymphoblasts, we were not able to directly evaluate expression changes in our index case. However, our characterization of the de novo inversion in the only affected member of the pedigree, our previous findings with regard to CNTN4,2 and the strong evidence that rare homozygous mutations in CNTNAP2 cause ASD3 supported the hypothesis that this molecule plays a key role in central nervous system (CNS) development, and autism in particular, and led us to study the transcript and its protein product.
We examined the distribution of Cntnap2 mRNA in the mouse and human CNS by using in situ hybridization22 with digoxigenin-11-UTP RNA probes complementary to bases 3909 to 4890 of the mouse Cntnap2 cDNA (NM_025771) or to bases 1343 to 2496 of the human CNTNAP2 cDNA (NM_014141.3). We found widespread expression in embryonic and postnatal mouse brain including within the limbic system (Figures 2 and 3C), a neuroanatomical circuit implicated in social behavior. In human brain, we confirmed previous findings of CNTNAP2 mRNA in all cortical layers of the temporal lobe (Figure 3).
Figure 2.
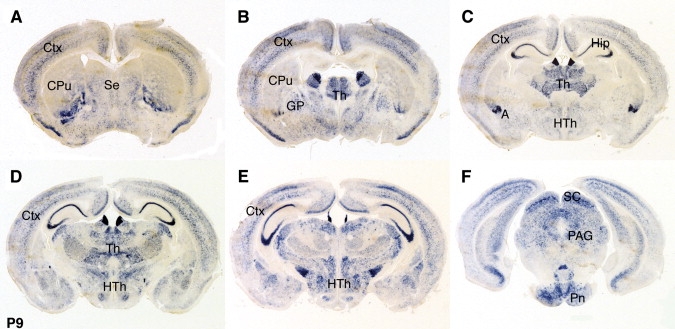
Expression of Cntnap2 mRNA in Postnatal Mouse Brain
Sections of P9 mouse brain were hybridized with a Cntnap2 antisense probe. We detected expression in the cortex (A–D), septum (A), basal ganglia (A and B), many thalamic (B–D) and hypothalamic (C–E) nuclei, with particularly high levels observed in the anterior nucleus and the habenula, part of the amygdala (C), the superior colliculus and the periaqueductal gray (F), pons, cerebellum, and medulla, again with particularly high levels seen in the inferior olive. All panels represent coronal sections and are shown in anterior to posterior order. Ctx, cortex; CPu, caudate putamen; Se, septum; GP, globus pallidus; Th, thalamus; Hip; hippocampal formation; A, amygdala; HTh, hypothalamus; SC, superior colliculus; PAG, periaqueductal gray; Pn, pontine nuclei.
Figure 3.
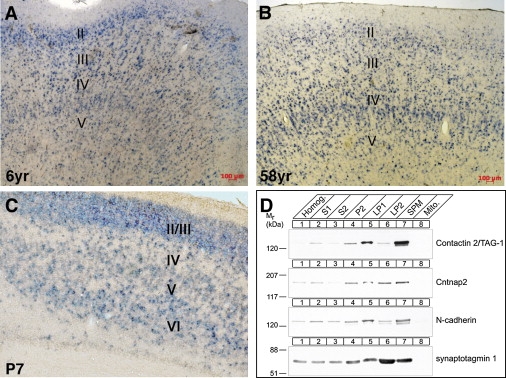
Expression and Biochemical Analyses of CNTNAP2/Cntnap2
(A–C) Cortical expression of CNTNAP2/Cntnap2. Sections of human temporal cortex at 6 and 58 years of age (A and B) and P7 mouse cortex (C) were hybridized with corresponding antisense riboprobes. Expression is detected in cortical layers II–V in the human temporal lobe (A and B) and II–VI in the mouse neocortex (C).
(D) Cofractionation of Cntn2/TAG-1 and Cntnap2 in synaptic plasma membranes. Rat forebrain homogenate (homog.) was subfractionated into postnuclear supernatant (S1), synaptosomal supernatant (S2), crude synaptosomes (P2), synaptosomal membranes (LP1), crude synaptic vesicles (LP2), synaptic plasma membranes (SPM), and mitochondria (mito.). The synaptic membrane protein N-cadherin and the synaptic vesicle protein synaptotagmin 1 served as markers for these respective fractions. Numbers on the left indicate positions of molecular weight markers. Protein concentrations were determined with the Pierce BCA assay and equal amounts of each fraction were analyzed. Monoclonal antibodies to Cntn2/TAG-1 (3.1C12, developed by Thomas Jessell, Columbia University) were obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa, to synaptotagmin 1 (41.1) from Synaptic Systems (Göttingen, Germany), and to N-cadherin from BD Biosciences (# 610920). Polyclonal antibodies to Cntnap2 were obtained from Sigma (# C 8737).
We also evaluated Cntnap2 protein and its putative binding partner, Cntn2/TAG-1, in subfractioned postnatal day 9 rat forebrain lysates.23,24 Both Cntnap2 and Cntn2/TAG-1 were present in the fraction containing synaptic plasma membranes, consistent with their forming a physical complex in this compartment (Figure 3D). These data localized CNTNAP2 and elements of a Contactin-related pathway with neuronal structures of marked interest with regard to autism,8,9,25–29 and prompted us to pursue an intensive genetic investigation of CNTNAP2 among individuals with ASD. All experiments were approved by the Yale University School of Medicine Institutional Animal Care and Use Committee.
Given our cytogenetic data and the recent findings of Strauss et al.,3 we elected to resequence all 24 coding exons of CNTNAP2 (Table 1) in 635 affected individuals and 942 uncharacterized controls. This approach was selected because it is robust in the face of allelic heterogeneity and has proven valuable in identifying rare causal mutations in idiopathic autism.8,9 Moreover, in other complex genetic disorders, heterozygote nonsynonymous variants found in genes contributing to rare recessive diseases have been shown to confer risks in the broader population.30
Table 1.
Primer Sequences for Mutation Screening of CNTNAP2
| Exon Numbera | Forward Primer | Reverse Primer | Product Size (bp) |
|---|---|---|---|
| 1 | CACACAGTGCAAGAGGCAATAC | GATGCACTTCGGAGTTGATACC | 420 |
| 2 | TTAACCAACACATACCAATCGTT | GATTTCTGGTGTCTGCCAACAT | 298 |
| 3 | GAAATAGAGCACTGCCAAGACC | CATTGGATAGAAATTACAGCCTGA | 481 |
| 4 | ACCATTGGATGACATTTGTGTT | GGTAGTTTATTGTCAGAGAAAGCAA | 355 |
| 5 | CATTTATTCTTTGCAGACACCTG | TTTAAAGAATTGAGCAACATGAACA | 368 |
| 6 | TATCCCAGGTTAACTCGAATGG | TCAGGTTTTTAAAATTGTCAGTGTC | 466 |
| 7 | ATTTTGGAGGCAGAATGCTATAA | TTTTGCCCAAACACAAATATGAT | 400 |
| 8 | AGGCTGTGCTTCAAAACTTGTA | GTAACACCAGCAAAACCAAACA | 458 |
| 9 | AAATCGTGATTTGTTGATTTTGG | TTTTTGTTTTGCTCAGTGGAATTA | 382 |
| 10 | GTAGTTGGATGTGATGGCTGTG | TGGTAATTTCCACCTTACCTGTTT | 399 |
| 11 | ATATATTGCCCAGACAGCTTGG | TTGGTTTTTCAGATTCGAGTGA | 318 |
| 12 | GGTTTGCTAGCATTGCAATATG | GAAACAAACCATTGGTGGAACT | 292 |
| 13 | AACACTGTTCTACACCAGCTCAG | TCTTAGCTTCATTCCCCAGAAA | 496 |
| 14 | TCAGAGTATTCCTGGGGAAGTG | TTTGTCAGTTGGGTTAGTTCCA | 391 |
| 15 | TGCTATGAGACCACCTATGGAA | AGTCTGATTGCAGGCATCTTCT | 390 |
| 16 | GAGGATTTGGTCCAATGTTGTT | GGCTTGTGTGTCCACCTCTAGT | 465 |
| 17 | ATTTTGCCATCGACCTTTGTAG | TGTGCAGGCTCTTAAAAATCAAC | 468 |
| 18 | CTATGCAGTGTCATCTCCTACCAC | TTGGAAAATTCCTACCTAAGTTGA | 488 |
| 19 | ACTTACTCAGATGCCCTTCCTG | TGGCAAGTTGTTTTCCTGATATT | 539 |
| 20 | GACATCAAGGGAGGGAGTAAAG | CTATCCCCTCAAAACAAAACCA | 667 |
| 21 | GGTGTTTTAGAGTCAGTGCTGATG | AGAACAACCACGTAACTTTCCTGT | 381 |
| 22 | TGCAGCCCTAAATCTTATCGAC | CCTGAGAACTCCGTACTCACAA | 560 |
| 23 | CTGTTGTGATTCTTGTGGGAGA | CAGCAAAATGAATAATGTAAAAACC | 367 |
| 24 | CTGACGGAGCTGTAGTGAAGTG | CACGGGTCTTTAGAACACCTCTA | 611 |
As defined by NM_014141.
DNA was amplified with a standard polymerase chain reaction (PCR) over 35 cycles with a 56.7°C annealing temperature31 and analyzed with Sequencher (Genecodes) or PolyPhred software after dye terminating sequencing on one strand. Use of human subjects was approved and performed in accordance with the Yale University School of Medicine Human Investigation Committee. The Institutional Review Board at the University of Pennsylvania School of Medicine provides human subjects protection and oversight for AGRE.
We evaluated both cases and controls in the identical fashion in search of rare nonsynonymous, frame-shift, nonsense, and splice-site variants. Those changes that were found only in the case or the control group in the initial sequencing effort were further genotyped with Custom Taqman Genotyping assays (Applied Biosystems) in an additional control sample of 1073 unrelated white subjects. Variants with allele frequencies greater than 1/4000 in the combined control sample were excluded (data available on request). One variant, R283C, which was found once among the sequenced controls, failed further genotyping but was included in subsequent analyses. All rare nonsynonymous variants were examined for conservation across diverse species with a ClustalW alignment to the top full-length BLASTp hits of each species (Table 2; see Figure S1 available online). Additionally, substitutions were examined by the amino acid analysis programs PolyPhen and SIFT (protein submission option), with Q9UHC6 as the reference CNTNAP2 protein, to identify those predicted to be possibly or probably deleterious to protein function (Table 2).
Table 2.
Unique Nonsynonymous Variants Identified in ASD Cases and Controls
| Varianta | Race/Ethnicity | Predicted Deleteriousb | Conservedc |
|---|---|---|---|
| ASD (n = 635) | |||
| N407Sd | white | N | N |
| N418D | white-Hispanic | N | N |
| Y716C | white | N | N |
| G731Se,f | more than one (Asian)f | N | Y |
| I869Te | white | Y, S | Y |
| I869Td,e | white | Y, S | Y |
| I869Te | white | Y, S | Y |
| R906H | white | N | N |
| R1119He | white | Y, P&S | Y |
| D1129He | white-Hispanic | Y, P&S | Y |
| A1227T | white | N | N |
| I1253Te | white-Hispanic | Y, S | N |
| T1278Ie | white | Y, P&S | N |
| Controls (n = 942) | |||
| R114Q | white-Hispanic | N | N |
| T218Me | white | Y, P&S | Y |
| L226Me | white | Y, S | Y |
| R283Ce,g | white | Y, P&S | Y |
| S382Ne | white-Hispanic | Y, S | Y |
| E680Ke | white | Y, P&S | Y |
| P699Qe | white-Hispanic | N | Y |
| G779D | Asian | N | N |
| D1038N | white | N | N |
| V1102A | white | N | N |
| S1114G | white | N | N |
Amino acid changes found only in cases (top of table) or only in controls (bottom of table).
P, PolyPhen; S, SIFT.
Amino acids were considered conserved if all sequences were identical or only conserved substitutions were seen.
N407S/I869T were found in one proband on opposite chromosomes.
Variants predicted to be deleterious or conserved.
Parental DNA was sequenced and the suspect variant was determined to derive from the father who was Asian.
Variant failed genotyping.
The case group was comprised of affected children from 584 families that were obtained from the Autism Genetics Research Exchange (AGRE) and 51 affected children recruited at the Yale Child Study Center. Diagnoses included 96.7% autism, 2.0% broad spectrum, and 1.3% not quite autism (see AGRE diagnosis in Web Resources). Males accounted for 81.1% of the sample. The ethnic/racial composition of the group was 587 white (92.4%), 24 white-Hispanic (3.8%), 7 unknown (1.1%), 6 Asian (0.9%), 6 more than one race (0.9%), 3 black or African-American (0.5%), 1 Native Hawaiian or Pacific Islander-Hispanic (0.2%), and 1 more than one race-Hispanic (0.2%). The resequenced control group consisted of 942 individuals: 757 white (80.4%), 94 white-Hispanic (10%), and 91 Asian (9.6%). These individuals were not evaluated for developmental delay or autism and were drawn from studies of renal disease, myocardial infarction, or normal human variation panels.
We found a total of 27 nonsynonymous variants among 635 cases, 13 of which had an allele frequency of less than 1/4000 (Figure 4; Table 2). Of these 13 rare variants, 8 were predicted to be deleterious or were found at regions conserved across all species examined (Figure S1; Figure 4A). In four cases, these potentially deleterious alleles were identified in pedigrees with more than one affected individual and three of these showed segregation with ASD in the affected first-degree relatives (Figure 4B). Among the 942 controls, 35 nonsynonymous variants were identified; 11 of these were rare and 6 were predicted to be deleterious or were conserved across all species (Figure S1; Table 2).
Figure 4.
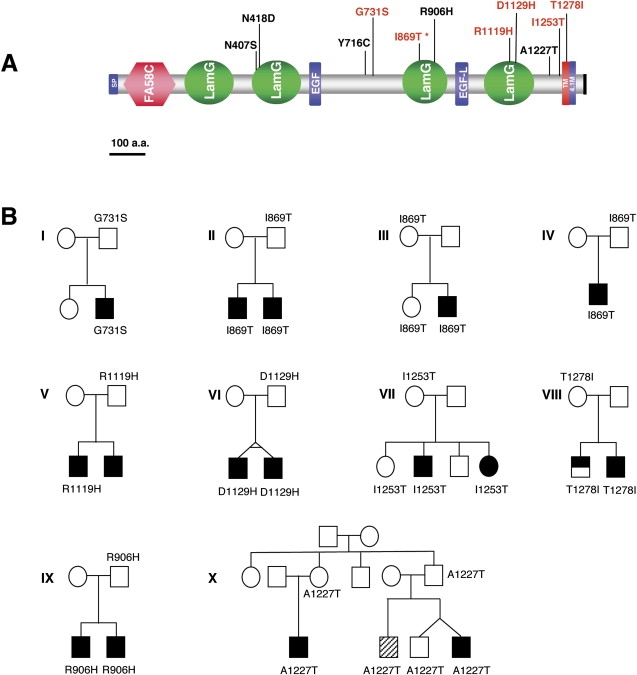
Sequencing of CNTNAP2 Identifies Rare Unique Nonsynonymous Variants
(A) Diagram of the CNTNAP2 protein highlighting the location of unique predicted deleterious variants (modified from SMART). The locations of patient variants are indicated. Variants in red are predicted to be deleterious or at conserved sites. Asterisk indicates variant was identified in three independent families; SP, signal peptide; FA58C, coagulation factor 5/8 C-terminal domain; LamG, Laminin G domain; EGF and EFG-L, epidermal growth factor-like domains; TM, transmembrane domain; 4.1M, putative band 4.1 homologs' binding motif; black vertical bar, C-terminal type II PDZ binding sequence. Figure is to scale.
(B) Pedigrees for all families with variants predicted to be deleterious at conserved sites (I to XIII) or which all affected relatives carry the identified variant (IX–X). The individuals carrying the suspect allele are noted and are heterozygous. The brothers inheriting the D1129H variant are monozygotic twins. Affected status was calculated with the AGRE diagnosis algorithm, which is based on ADI-R scores. Blackened symbols represent an autism diagnosis, half-filled symbols indicate a not-quite-autism (NQA) diagnosis, and crosshatched individuals have a broad spectrum diagnosis.
Although the rates of all unique and predicted deleterious/conserved variants were, respectively, 1.75- and 2-fold higher in cases compared to controls, neither met a statistical threshold for an association of increased mutation burden with ASD (Fisher exact test p = 0.21, OR 1.76 95% CI: 0.80-3.87; p = 0.27, OR 1.98 95% CI: 0.72-5.49).
One highly conserved variant, I869T, which was predicted to be deleterious by SIFT, was identified in four affected individuals from three unrelated families with autism but was not present in 4010 control chromosomes, supporting an association for this substitution (Fisher exact test; p = 0.014). In each family, the variant was inherited from an apparently unaffected parent. Its absence among several thousand control chromosomes, its conservation across species, and its segregation with affected status among first-degree relatives (Figure 4B) all suggest that this variant warrants further attention. However, in light of the low observed allele frequency, it is not possible to rule out false positive results because of cryptic population stratification, despite the fact that the variant was found only among white families, the ethnic group that also represented the vast majority of controls.
When viewed in the context of two independent studies demonstrating linkage and/or association of common SNPs near CNTNAP2 with ASD,4,5 our results both lend support to these findings and demonstrate the bounds of the potential contribution of rare variants in this transcript. Our confirmation of the expression of CNTNAP2 in brain regions considered relevant in ASD as well as the demonstration of CNTNAP2 protein and its binding partner in the synaptic membrane support the biological plausibility of these findings, particularly given the identification of ASD-related mutations in other synaptic proteins including Neuroligin 3, Neuroligin 4 X-linked, SHANK3, and Neurexin 1.8,9,28,29 The finding of a disrupted CNTNAP2 transcript resulting from a de novo chromosomal abnormality, the identification of multiple, rare, highly conserved variants in the case group that were not present in controls, and the association of I869T with ASD all suggest that some rare variants that disrupt protein function may contribute to disease risk. However, the absence of a statistically significant burden of rare or highly conserved mutations in cases versus controls suggests that the risk for an ASD diagnosis resulting from rare heterozygote coding changes is not likely to be large, because our sample was well powered to identify an odds ratio of 3.5 or greater (log-additive case-control design, QUANTO v.1.1).
Given the recent spate of whole-genome association studies in complex disorders demonstrating that common disease alleles may confer much smaller risks than previously anticipated,32,33 it is plausible that alleles conferring risks lower than 3.5 might still be subject to considerable negative selection, particularly in the case of ASD given its early onset and pathognomonic impairment in social interaction. A much larger case-control mutation burden analysis would be needed to answer this important question. Moreover, if investigations of other complex disorders serve as a model,30 evaluation of the relationship between heterozygote coding variants and quantitative traits may prove more informative than relying on categorical diagnoses. Again, based on the low frequency of highly conserved nonsynonymous changes in CNTNAP2, a markedly larger sample would be required to support such an analysis. Of course, it is also possible that as a deeper understanding of the mechanism by which homozygous mutations lead to neuronal migration abnormalities and ASD is attained, the ability to better distinguish truly deleterious heterozygote variants from neutral substitutions will increase the power of the type of case-control mutation burden analyses presented here.
At present, data from two common variant studies,4,5 parametric linkage analysis in an isolated population,3 as well as the molecular cytogenetic, expression, biochemical, and resequencing studies presented here suggest that further study of both common and rare variation in CNTNAP2 is needed and may lead to a better understanding of the genetic etiology and molecular mechanisms of autism and related neurodevelopmental disorders.
Acknowledgments
We are grateful to John Spertus, Ali Gharavi, and Isabel Beerman for the contribution of control samples. This project was supported by K23 RR16118-04 (M.W.S.), the Lawrence Family (to M.W.S.), the Shephard Foundation (to M.W.S.), NIDA grant R01 DA018928 (T.B.), NIMH grant R01 MH 64547 (D.H.G.), the UCLA Center for Autism Research and Treatment (D.H.G.), and the Cure Autism Now Foundation (AGRE and D.H.G.). Most importantly, we would like to thank the patients and families who participated in this study and the AGRE Consortium (see below) for resource oversight.
AGRE Consortium Scientific Steering Committee: D.H.G., M.D., Ph.D. (UCLA, Los Angeles, CA); Maja Bucan, Ph.D. (University of Pennsylvania, Philadelphia, PA); W. Ted Brown, M.D., Ph.D., F.A.C.M.G. (N.Y.S. Institute for Basic Research in Developmental Disabilities, Staten Island, NY); Rita M. Cantor, Ph.D. (UCLA School of Medicine, Los Angeles, CA); John N. Constantino, M.D. (Washington University School of Medicine, St. Louis, MO); T. Conrad Gilliam, Ph.D. (University of Chicago, Chicago, IL); Martha Herbert, M.D., Ph.D. (Harvard Medical School, Boston, MA); Clara Lajonchere, Ph.D. (Cure Autism Now, Los Angeles, CA); David H. Ledbetter, Ph.D. (Emory University, Atlanta, GA); Christa Lese-Martin, Ph.D. (Emory University, Atlanta, GA); Janet Miller, J.D., Ph.D. (Cure Autism Now, Los Angeles, CA); Stanley F. Nelson, M.D. (UCLA School of Medicine, Los Angeles, CA); Gerard D. Schellenberg, Ph.D. (University of Washington, Seattle, WA); Carol A. Samango-Sprouse, Ed.D. (George Washington University, Washington, DC); Sarah Spence, M.D., Ph.D. (UCLA, Los Angeles, CA); M.W.S., M.D., Ph.D. (Yale University, New Haven, CT); and Rudolph E. Tanzi, Ph.D. (Massachusetts General Hospital, Boston, MA).
Supplemental Data
Appendix A
Clinical Description of the (46,XY,inv(7)(q11.22;q35)) Patient
The patient is a 4.5-year-old male who was born at 38 weeks of gestation to his 33-year-old G3P3 mother by Caesarian section because of breech position. Birth weight was 3.3 kg. His neonatal course and infancy were complicated by poor feeding and severe gastresophageal reflux (confirmed by KUB/UGI at 2.5 months) in the context of global hypotonia. This eventually led to PEG tube placement at 6 months of age. Weight at 7 weeks was 4.4 kg (10th–25th percentile). Genetic evaluation and testing at 3 months of age, in addition to a karyotype, included a normal FISH study for the Prader-Willi locus (SNRPN probe, 15q11.2), performed because of significant hypotonia. Antiviral antibody titers for toxoplasma, herpes simplex, and cytomegalovirus were negative at 2.5 months. Rubella IgG was 1.1 (at lower limit of immune range). Serum glucose and electrolytes were normal, with bicarbonate of 21 mEq/L and anion gap of 11. Urinalysis was normal, with no ketones. Lactic acid, at 3 months of age, was 1.4 (range 0.5–2.2) and ammonia was 63 (range 28–80). Creatine kinase level was 106 (normal range 0–200 IU/L). Hepatic transaminase values were within normal limits. Plasma amino acid and acylcarnitine analyses, and urine acylglycine and organic acid profiles, were normal. Transferrin isoelectric focusing to rule out carbohydrate-deficient glycoprotein syndromes was normal, as was plasma 7 dehydrocholesterol determination, to rule out Smith-Lemli-Opitz Syndrome. Cerebrospinal fluid amino acids, lactate, and pyruvate were normal. Ophthalmological evaluation at 3.5 months was initiated for a history of visual inattention during early infancy. Electro-retinogram and Preferential Looking Test of Visual Acuity were normal for age. Echocardiogram was normal at 7 months of age. Brain MRI at 2.5 months showed delayed myelination (lack of myelin within the anterior limb of the internal capsule, but normal myelination within the perirolandic white matter and posterior limbs of the internal capsules). In addition, there was a prominent subarachnoid space bifrontally with prominent ventricular system consistent with hypotrophy of the frontal and temporal lobes. EEG was normal.
Clinical genetic evaluation at 3.5 years revealed a past medical history significant for reflux in the first year of life, three previous episodes of pneumonia, hypotonia, tight heel cords, strabismus repair, and left inguinal hernia repair. He had pressure-equalizing tubes inserted into both ears for recurrent otitis media with conductive hearing loss. Family history was significant for two normally developing older siblings, and no history of cognitive or motor delays in an extended 3-generation pedigree. On physical examination, height was 100.2 cm (75th–90th percentile), weight was 14.7 kg (25th–50th percentile), and occipitofrontal head circumference was 49.4 cm (25th–50th percentile). Facies were essentially nondysmorphic except for surgically corrected strabismus and downslanting palpebral fissures. Distinctive physical findings included mild bilateral 5th digit clinodactyly, 2–3 toe syndactyly (not Y-shaped), genu and pes valgus, persistent fetal pads on toes, tight Achille's tendons, and prominent scrotal raphe. Measurements of ocular distances, hands, feet, inter-nipple distance, and stretched penile length were within normal limits. No genetic syndrome was recognizable by his clinical geneticist (T.M.M.).
Developmentally, the patient did not smile socially until after 3 months, crawled at 13.5 months, walked and said his first word at 24 months, and began constructing 2-word phrases at 3 years of age. The Bayley Scales of Infant Development showed that the child was in the “significantly delayed” range. On the Vineland-II, a parent report instrument, the patient had the following standard scores (the mean for each test is 100 with a standard deviation of 15): communication, 67; daily living skills, 77; socialization, 77; motor, 64; and adaptive behavior composite, 68. Tests of fine motor skills with the Peabody Developmental Motor Scales-2 (PDMS-2) placed him 2 SD below the mean.
The patient was evaluated with the ADI-R and ADOS at the Yale Child Study Center at 49 months of age. On ADI-R, the parents reported an age at first word of 30 month and at first phrase of 48 months, which differs slightly from the documented medical history. Additionally, the parents reported that the patient had a “history of attacks that might be epileptic.” These, as noted, were followed up by a pediatrician with an EEG, which was normal. The patient met ADI-R scoring criteria for social (10), behavior (4), and age of onset (4). The patient did not meet cutoffs on the communication domains: verbal (0) or nonverbal (3). Based on the ADI-R algorithm used by AGRE repository (from which the mutation screening sample was derived), the patient would be classified as “Broad Spectrum.” However, the patient did not meet the ADOS criteria for a diagnosis of ASD.
Web Resources
The URLs for data presented herein are as follows:
Autism Genetic Research Exchange, http://agre.org/agrecatalog/algorithm.cfm
EMBL-EBI ClustalW, http://www.ebi.ac.uk/clustalw/
Ensembl Genome Browser, http://www.ensembl.org/
National Center for Biotechnology Information, http://www.ncbi.nih.gov/Genomes/
Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim
PolyPhen, http://genetics.bwh.harvard.edu/pph/
Primer3, http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi
QUANTO, http://hydra.usc.edu/gxe/
Stat Pages, Fisher Exact Test, http://statpages.org/ctab2x2.html
UCSC Genome Browser, http://www.genome.ucsc.edu/
References
- 1.Gupta A.R., State M.W. Recent advances in the genetics of autism. Biol. Psychiatry. 2007;61:429–437. doi: 10.1016/j.biopsych.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez T., Morgan T., Davis N., Klin A., Morris A., Farhi A., Lifton R.P., State M.W. Disruption of contactin 4 (CNTN4) results in developmental delay and other features of 3p deletion syndrome. Am. J. Hum. Genet. 2004;74:1286–1293. doi: 10.1086/421474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strauss K.A., Puffenberger E.G., Huentelman M.J., Gottlieb S., Dobrin S.E., Parod J.M., Stephan D.A., Morton D.H. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N. Engl. J. Med. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- 4.Alarcón M., Abrahams B.S., Stone J.L., Duvall J.A., Perederiy J.V., Bomar J.M., Sebat J., Wigler M., Martin C.L., Ledbetter D.H. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am. J. Hum. Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arking D.E., Cutler D.J., Brune C.W., Teslovich T.M., West K., Ikeda M., Rea A., Guy M., Lin S., Cook E.H., Jr., Chakravarti A. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am. J. Hum. Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuchman R., Rapin I. Epilepsy in autism. Lancet Neurol. 2002;1:352–358. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 7.Sebat J., Lakshmi B., Malhotra D., Troge J., Lese-Martin C., Walsh T., Yamrom B., Yoon S., Krasnitz A., Kendall J. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamain S., Quach H., Betancur C., Rastam M., Colineaux C., Gillberg I.C., Soderstrom H., Giros B., Leboyer M., Gillberg C. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laumonnier F., Bonnet-Brilhault F., Gomot M., Blanc R., David A., Moizard M.P., Raynaud M., Ronce N., Lemonnier E., Calvas P. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am. J. Hum. Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent J.B., Kolozsvari D., Roberts W.S., Bolton P.F., Gurling H.M., Scherer S.W. Mutation screening of X-chromosomal neuroligin genes: no mutations in 196 autism probands. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2004;129:82–84. doi: 10.1002/ajmg.b.30069. [DOI] [PubMed] [Google Scholar]
- 11.Gauthier J., Bonnel A., St-Onge J., Karemera L., Laurent S., Mottron L., Fombonne E., Joober R., Rouleau G.A. NLGN3/NLGN4 gene mutations are not responsible for autism in the Quebec population. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2005;132:74–75. doi: 10.1002/ajmg.b.30066. [DOI] [PubMed] [Google Scholar]
- 12.Ylisaukko-oja T., Rehnstrom K., Auranen M., Vanhala R., Alen R., Kempas E., Ellonen P., Turunen J.A., Makkonen I., Riikonen R. Analysis of four neuroligin genes as candidates for autism. Eur. J. Hum. Genet. 2005;13:1285–1292. doi: 10.1038/sj.ejhg.5201474. [DOI] [PubMed] [Google Scholar]
- 13.Blasi F., Bacchelli E., Pesaresi G., Carone S., Bailey A.J., Maestrini E. Absence of coding mutations in the X-linked genes neuroligin 3 and neuroligin 4 in individuals with autism from the IMGSAC collection. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2006;141:220–221. doi: 10.1002/ajmg.b.30287. [DOI] [PubMed] [Google Scholar]
- 14.Dracopoli N.C., Haines J.L., Korf B.R., Morton C.C., Seidman C.E., Seidman J.G., Smith De R. John Wiley & Sons; Hoboken, NJ: 2005. Current Protocols in Human Genetics. [Google Scholar]
- 15.Sultana R., Yu C.E., Yu J., Munson J., Chen D., Hua W., Estes A., Cortes F., de la Barra F., Yu D. Identification of a novel gene on chromosome 7q11.2 interrupted by a translocation breakpoint in a pair of autistic twins. Genomics. 2002;80:129–134. doi: 10.1006/geno.2002.6810. [DOI] [PubMed] [Google Scholar]
- 16.Kalscheuer V.M., FitzPatrick D., Tommerup N., Bugge M., Niebuhr E., Neumann L.M., Tzschach A., Shoichet S.A., Menzel C., Erdogan F. Mutations in autism susceptibility candidate 2 (AUTS2) in patients with mental retardation. Hum. Genet. 2007;121:501–509. doi: 10.1007/s00439-006-0284-0. [DOI] [PubMed] [Google Scholar]
- 17.Redon R., Ishikawa S., Fitch K.R., Feuk L., Perry G.H., Andrews T.D., Fiegler H., Shapero M.H., Carson A.R., Chen W. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traka M., Goutebroze L., Denisenko N., Bessa M., Nifli A., Havaki S., Iwakura Y., Fukamauchi F., Watanabe K., Soliven B. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J. Cell Biol. 2003;162:1161–1172. doi: 10.1083/jcb.200305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poliak S., Salomon D., Elhanany H., Sabanay H., Kiernan B., Pevny L., Stewart C.L., Xu X., Chiu S.Y., Shrager P. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J. Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verkerk A.J., Mathews C.A., Joosse M., Eussen B.H., Heutink P., Oostra B.A. CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics. 2003;82:1–9. doi: 10.1016/s0888-7543(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 21.Belloso J.M., Bache I., Guitart M., Caballin M.R., Halgren C., Kirchhoff M., Ropers H.H., Tommerup N., Tumer Z. Disruption of the CNTNAP2 gene in a t(7;15) translocation family without symptoms of Gilles de la Tourette syndrome. Eur. J. Hum. Genet. 2007;15:711–713. doi: 10.1038/sj.ejhg.5201824. [DOI] [PubMed] [Google Scholar]
- 22.Grove E.A., Tole S., Limon J., Yip L., Ragsdale C.W. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- 23.Jones D.H., Matus A.I. Isolation of synaptic plasma membrane from brain by combined flotation-sedimentation density gradient centrifugation. Biochim. Biophys. Acta. 1974;356:276–287. doi: 10.1016/0005-2736(74)90268-5. [DOI] [PubMed] [Google Scholar]
- 24.Biederer T., Sara Y., Mozhayeva M., Atasoy D., Liu X., Kavalali E.T., Sudhof T.C. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 25.Zoghbi H.Y. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
- 26.Talebizadeh Z., Bittel D.C., Veatch O.J., Butler M.G., Takahashi T.N., Miles J.H. Do known mutations in neuroligin genes (NLGN3 and NLGN4) cause autism? J. Autism Dev. Disord. 2004;34:735–736. doi: 10.1007/s10803-004-5295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craig A.M., Kang Y. Neurexin-neuroligin signaling in synapse development. Curr. Opin. Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durand C.M., Betancur C., Boeckers T.M., Bockmann J., Chaste P., Fauchereau F., Nygren G., Rastam M., Gillberg I.C., Anckarsater H. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat. Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szatmari P., Paterson A.D., Zwaigenbaum L., Roberts W., Brian J., Liu X.Q., Vincent J.B., Skaug J.L., Thompson A.P., Senman L. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat. Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen J.C., Kiss R.S., Pertsemlidis A., Marcel Y.L., McPherson R., Hobbs H.H. Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science. 2004;305:869–872. doi: 10.1126/science.1099870. [DOI] [PubMed] [Google Scholar]
- 31.Abelson J.F., Kwan K.Y., O'Roak B.J., Baek D.Y., Stillman A.A., Morgan T.M., Mathews C.A., Pauls D.L., Rasin M.-R., Gunel M. Sequence variants in SLITRK1 are associated with Tourette's Syndrome. Science. 2005;310:317–320. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- 32.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altshuler D., Daly M. Guilt beyond a reasonable doubt. Nat. Genet. 2007;39:813–815. doi: 10.1038/ng0707-813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


