Abstract
The characteristic pathological finding in carpal tunnel syndrome (CTS) is non-inflammatory fibrosis of the subsynovial connective tissue (SSCT), which lies between the flexor tendons and the visceral synovium (VS). How this fibrosis might affect tendon function is unknown. To better understand the normal function of the SSCT, the relative motion of the middle finger flexor digitorum superficialis (FDS III) tendon and VS was observed during finger flexion in patients with CTS and cadavers with a history of CTS and compared to normal cadavers.
A digital camcorder was used to monitor the gliding motion of the FDS III tendon and SSCT in eight patients with idiopathic CTS undergoing carpal tunnel surgery (CTR), in eight cadavers with an antemortem history of CTS and compared these with eight cadaver controls.
There were no significant differences noted in the total movement of the SSCT relative to the FDS III. However, the pattern of SSCT movement relative to the FDSIII in the CTS patients and cadavers with an antemortem history of CTS differed from the controls in one of two patterns, reflecting either increased SSCT adherence to FDS III or increased SSCT dissociation from FDS III. In CTS the gliding characteristics of the SSCT are qualitatively altered. These changes may be the result of increased fibrosis within the SSCT, which in some cases has ruptured, resulting in SSCT-tendon dissociation. Similar changes are also identified post mortem in the CTS patient.
Keywords: Carpal Tunnel Syndrome, Flexor Tendon, Subsynovial Connective Tissue, Tendon Motion, Fibrosis
INTRODUCTION
Carpal tunnel syndrome (CTS) is the most common peripheral nerve entrapment syndrome. It is often described as an occupational disease among persons who perform repetitive work with their hands (Abbas et al., 1998; Armstrong et al., 1984; Saleh et al., 2001; Stal et al., 1999; Szabo, 1998; Wu et al., 2003). The most commonly reported pathological finding is non-inflammatory fibrosis and thickening of the subsynovial connective tissue (SSCT) (Armstrong et al., 1984; Ettema et al., 2004; Kerr et al., 1992; Lluch, 1992; Nakamichi et al., 1998; Neal et al., 1987; Phalen, 1966). The etiology of these SSCT pathological changes is unknown.
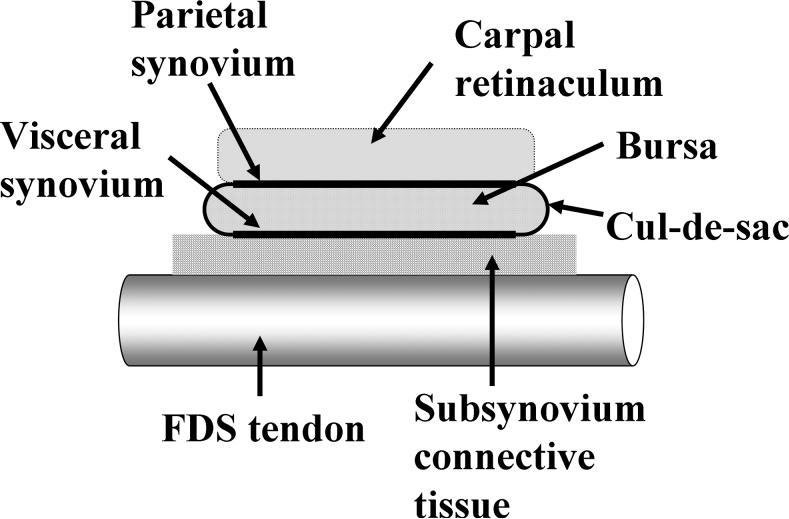
The SSCT is an important and unique structure. The SSCT surrounds the tendons and median nerve in the carpal tunnel, comprising all the tissue between the single cell layer visceral synovium (VS) and the tendons (Ettema et al., 2004; Ettema et al., 2006a; Guimberteau, 2001; Oh et al., 2004; Oh et al., 2005) (Fig. 1). Within the SSCT are layered bundles of collagen running parallel to the tendon. These layers are interconnected by smaller vertical fibers. By stretching and relaxing the SSCT during finger movement, the loose fibers between adjacent layers are stretched, and the fibrous bundles move layer by layer and are pulled by the interconnections, more or less as an arm would move within layers of sleeves (Ettema et al., 2006a). In this way, the lengthening propagates layer by layer until finally the VS moves (Ettema et al., 2006a).
Figure 1.
The structure of the sliding unit in the carpal tunnel region (Ettema et al., 2004). Copyright JBJS; used with permission.
Any pathological changes of the SSCT may alter the motion pattern between VS and tendon. For example, fibrosis of the SSCT might tether tendon motion, or limit the relative motion of adjacent tendons. Such changes, if present, might affect hand function, or even be a source of pain. To date, the role of the SSCT in conditions such as CTS has been speculated (Lluch, 1992), and pilot data has suggested that SSCT kinematics may be altered in patients with CTS (Ettema et al, 2007).
In this study, our primary objective was to investigate in greater detail the relative motion pattern between the SSCT and flexor tendon in hands from normal cadavers, cadavers with a diagnosis of CTS, and patients with CTS. Normal living controls were not used, because of ethical concerns regarding the need for surgical exposure, including release of the carpal tunnel, to perform the measurements. Thus, we also had a secondary objective, to compare the results obtained in patients with CTS and cadavers with CTS. If patients with CTS and cadavers with a history of CTS were to give similar results, then we think it would be reasonable to assume that normal hands and cadaver hands with no history of CTS would also be similar.
MATERIALS AND METHODS
This protocol was approved by our Institutional Review Board, and informed consent was obtained from each patient. We monitored the active gliding motion of the middle superficial flexor tendon (FDS III) and SSCT in eight patients with CTS undergoing carpal tunnel release surgery (CTR) and compared these with simulated active flexion in eight cadavers with an antemortem history of CTS and in eight cadaver controls without an antemortem history of CTS, using methods previously described (Ettema et al., 2007). These methods are briefly described below.
Patient Selection and Preparation During Surgery
Patients with specific etiologies or risk factors of carpal tunnel syndrome, such as diabetes, glucose intolerance, thyroid disease, rheumatoid arthritis, osteoarthrosis, degenerative joint disease, flexor tendinitis, gout, hemodialysis, obesity, sarcoidosis, amyloidosis, peripheral neuropathy, traumatic injuries to the ipsilateral arm, and wheelchair use, were excluded. Eight patients, five females and three males, met the inclusion criteria and agreed to participate. Surgery was performed on four left and four right hands. The dominant hand was involved in six patients. The mean age was 55 years (range 34−73). All patients had the diagnosis of idiopathic carpal tunnel syndrome confirmed by electrodiagnostic testing according to the standards established by the American Academy of Electrodiagnostic Medicine (AAEM, 1992; AAEM et al., 1993). There were four patients with moderate, two patients with moderate-severe and two patients with severe electrophysiological changes (Stevens, 1997).
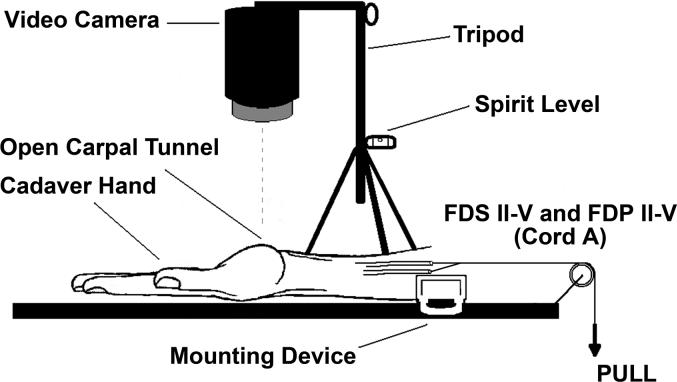
During carpal tunnel release surgery, a small window (approximately 3 mm diameter) was made in the VS and subsynovial connective tissue to expose the middle finger FDS tendon. With the fingers passively extended to the 0° position, a mark was made on the middle finger FDS tendon surface with a surgical marker (Skin Markers, Devon Industries, Inc, Buffalo, New York). The VS surface was marked at a level 5 mm proximal to the tendon mark. A third mark was made on the cut edge of the flexor retinaculum (the under surface of the retinaculum is covered by the parietal synovium) to serve as a reference point (Figure 2). The wrist was supported on the operating table in neutral position for testing. The definition of the neutral position was an angle between the radius and third metacarpal bone of 0 degrees with 0 degree ulnar/radial deviation. The patients were then asked to make a fist and subsequently to flex and extend the middle finger individually, with the other fingers blocked in extension by the surgeon, while a camcorder (Sony Digital 8® Camcorder DCR-TRV350, Sony Corporation, Japan) recorded the motion. The camcorder was set up perpendicular to the operating table, using a tripod with a spirit level. A millimeter ruler was included in the camcorder field, so that the data measured with the camcorder could be converted into a distance figure. The ruler was accurately placed in the sense of the movement. We have previously verified that this kinematic analysis is sufficient to accurately capture the displacement in comparison with a classical 3D analysis (Ettema et al, 2007).
Figure 2.
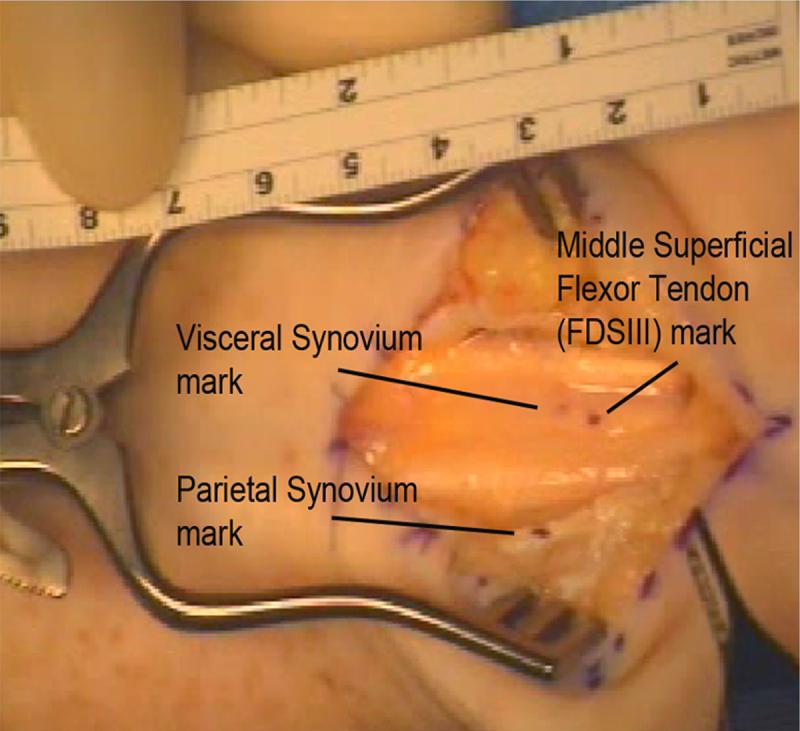
Markers on the middle superficial flexor tendon (FDS III), visceral synovium and parietal synovium in a patient hand during CTR surgery.
The data was digitized with the use of Analyze™ Software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN) to determine the motion characteristics of the three marks.
Cadaver Specimen with or without CTS Selection
A postmortem medical record review was performed on all donors to our institution's Willed Body Program. Eight fresh frozen cadavers with a recorded antemortem history of CTS (cadaver CTS) were identified. There were three left and five right affected hands from six females and two males with an average age of 77 years (range 58−92). In seven cases the affected hand was the dominant one. Five cadaver CTS specimens had a clinical diagnosis of carpal tunnel syndrome and no electrodiagnostic data was described. Three cadaver CTS specimens had a clinical diagnosis of CTS confirmed by electrodiagnostic testing according to the standards established by the American Academy of Electrodiagnostic Medicine (AAEM, 1992; AAEM et al., 1993). Table 1 includes the detailed medical record data for this experimental group. Eight fresh frozen cadavers were selected as the control group. They did not have an antemortem history of CTS recorded in the available medical records, nor evidence of any diseases associated with carpal tunnel syndrome. There were three females and five males with six right and two left hands. Hand dominance was noted in the medical records only in two right-hand dominant males, in the other 6 cadavers there was no information about hand dominance. The mean age was 86 years (range 78−98).
Table 1.
The cadavers with an antemortem diagnosis of CTS (cadaver CTS hands) and associated data recorded in available medical records.
| Cadaver CTS hand | Treatment | EMG (AAEM criteria) | CTS related conditions |
|---|---|---|---|
| 1 | CTR + biopsy | BMI>30, gout, glucose intolerance | |
| 2 | CTR + biopsy | BMI>30 | |
| 3 | CTR + neurolysis | BMI>30 | |
| 4 | CTR | idiopathic | |
| 5 | CTR | idiopathic | |
| 6 | Steroid injection | Mild | idiopathic |
| 7 | No treatment | Mild | idiopathic |
| 8 | No treatment | Mild | idiopathic |
CTR- carpal tunnel release; BMI- body mass index; EMG-electrodiagnostic testing; AAEM-American Academy of Electrodiagnostic Medicine
Cadaver Testing Methods
The cadaver upper extremities were amputated approximately 15 cm proximal to the wrist joint and thawed at room temperature immediately prior to testing. A longitudinal skin incision approximately 8 cm in length was made in the palm and distal forearm and the flexor retinaculum was transected to open the carpal tunnel. The FDS III tendon, VS, and flexor retinaculum were marked as the same procedure described in the clinical cases. The specimen was then fixed in a custom-made mounting device, holding the wrist in the neutral position, by clamping the proximal end of the radius and ulna.
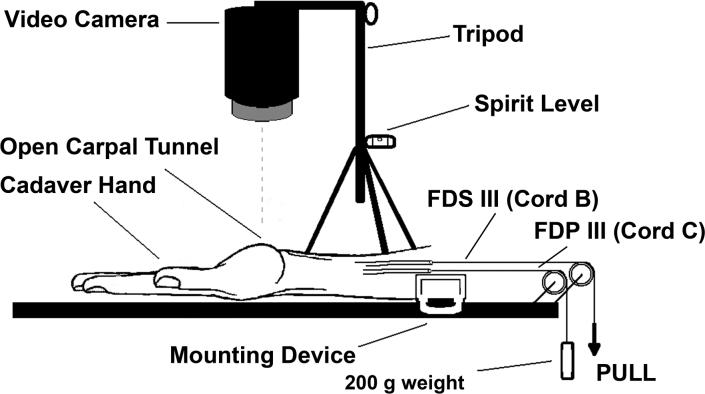
The four FDS and four FDP tendons were sutured together at the proximal end of the tendons in the maximum individual flexion position of the fingers and attached to a Dacron cord (cord A) for simultaneous finger motion (fist) simulation (Figure 3A). The middle finger FDS (cord B) and FDP tendons (cord C) were also separately sutured with a Dacron cord for isolated middle finger movement testing (Figure 3B). In this construct cord B, controlling the middle finger FDS tendon, was actively pulled by one of the investigators while cord C, controlling the middle finger FDP tendon, passed around a pulley with a 200g weight attached to the proximal end of the cord, to simulate physiological tension.
Figure 3A.
Setup to test full fist motion.
Figure 3B.
Setup to test isolated middle finger FDS motion.
Cord A was then actively pulled proximally by one investigator to maximum flexion of the fingers (simulated fist); while the motion of the three markers (from 0° extension to maximum individual flexion) was detected by anteroposterior recording with a digital camcorder. After cord A was cut and the second, fourth and fifth fingers were extended and fixed in 0° extension position, cord B was then actively pulled proximally to maximum flexion of the middle finger FDS tendon (simulated middle finger flexion with the other fingers blocked in extension), and the motion of the markers again recorded and analyzed as described in CTS patient procedure.
Statistical Methods
The relationship between the motion of the tendon and motion of the visceral synovium was estimated by the slope of the simple linear regression line. The slopes were calculated two ways. First, the slopes were calculated using the entire series of measurements of VS and tendon. Second, the presence or absence of an initial delay in the start of movement of the VS as compared to the start of tendon movement was noted for each subject. For subjects with a delay, the length of the delay was calculated and presented as the percentage of tendon movement in which the VS started to move. The slopes were then calculated a second time at the start of movement for each subject and these are represented in table 2. Due to skewed distribution of the slopes, non-parametric tests were used to compare the median slopes of the groups. Kruskal-Wallis or Wilcoxon rank sum tests were used accordingly, and the medians and ranges are reported, unless otherwise noted. The slopes were compared among the severities and between fist motion and isolated FDS III motion using a Kruskal-Wallis test. All analyses were conducted using SAS software (SAS institute Inc., Cary, NC).
Table 2.
The distributions of the slopes (i.e. the best fit linear regression line for the ratio of SSCT to tendon motion) for simultaneous (fist) and single digit movement. P values are from the Wilcoxon signed rank test for comparisons between median simultaneous and differential finger movement within each group. Mean, minimum and maximum data are included to give an idea of the skewness of the data.
| Slope from simultaneous (fist) movement | Slope from single digit movement | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Std | Median | Min | Max | Mean | Std | Median | Min | Max | p-value | |
|
2A. Overall Data |
||||||||||||
| All | 24 | 0.300 | 0.226 | 0.253 | 0.015 | 0.974 | 0.164 | 0.208 | 0.106 | 0.023 | 0.990 | <0.01 |
| Patient | 8 | 0.248 | 0.243 | 0.145 | 0.015 | 0.752 | 0.181 | 0.168 | 0.123 | 0.025 | 0.503 | 0.15 |
| Cadaver CTS | 8 | 0.300 | 0.295 | 0.227 | 0.076 | 0.974 | 0.183 | 0.328 | 0.065 | 0.023 | 0.990 | 0.08 |
| Cadaver Control | 8 | 0.353 | 0.125 | 0.329 | 0.207 | 0.585 | 0.127 | 0.061 | 0.120 | 0.057 | 0.233 | 0.01 |
| 2B. CTS Hands | ||||||||||||
| Patient “dissociated” | 6 | 0.128 | 0.066 | 0.137 | 0.015 | 0.218 | 0.153 | 0.175 | 0.098 | 0.025 | 0.503 | 0.56 |
| “adherent” | 2 | 0.611 | 0.199 | 0.611 | 0.470 | 0.752 | 0.267 | 0.158 | 0.267 | 0.155 | 0.379 | ** |
| Cadaver “dissociated” | 4 | 0.109 | 0.038 | 0.101 | 0.076 | 0.158 | 0.056 | 0.039 | 0.048 | 0.023 | 0.107 | 0.38 |
| CTS “adherent” | 1 | 0.974 | * | 0.974 | 0.974 | 0.974 | 0.990 | * | 0.990 | 0.990 | 0.990 | ** |
Not applicable
N too small to compare
RESULTS
Simultaneous Digit Motion (Fist Motion)
The mean total excursion of the FDS III tendons while making a fist was similar among all three groups: 26.1±4.9 mm for the patients, 25.2±3.4 mm for the cadaver CTS hands and 24.4±5.1 mm for the cadaver control hands. The mean total excursion of the VS was somewhat reduced in the CTS affected hands, but not significantly so: 6.9±7.0 mm for the patients, 7.8±7.2 mm for the cadaver CTS hands and 9.0±4.2 mm for the cadaver control hands. The minimum and maximum movement of the VS layer as a percentage of the individual FDS III tendon excursion at the point of full flexion of the middle finger while making a fist was between 1.1%-75.9% for the patients and 7.9%-96.0 % for the cadaver CTS hands, while it ranged between 24.6%-52.4 % for the cadaver control hands. As demonstrated in Figures 4 and 5, the distribution of relative motions in the control hands appeared to follow a normal distribution pattern, while that of the CTS affected hands followed a bimodal distribution pattern. This qualitative difference in distribution was an unanticipated finding. The differences, while qualitatively appearing to be different, were not significantly different on the statistical tests we employed.
Figure 4.
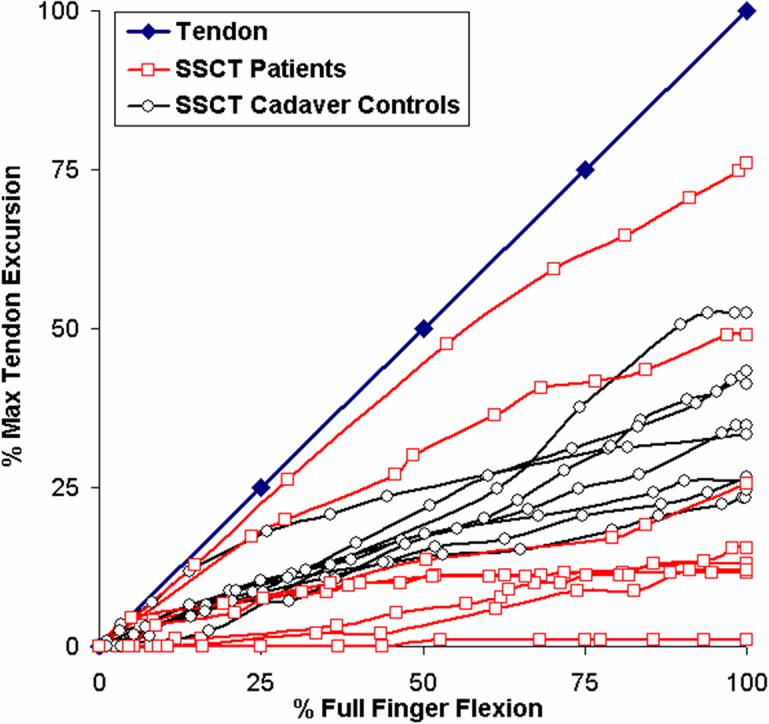
Movement of the tendon (blue) and SSCT of the eight patients (red) versus the eight cadaver controls (black) as a percentage of each maximum tendon movement. Six out of the eight patients show a decreased displacement as compared to the controls. In two patients the SSCT moves en bloc with the tendon.
Figure 5.
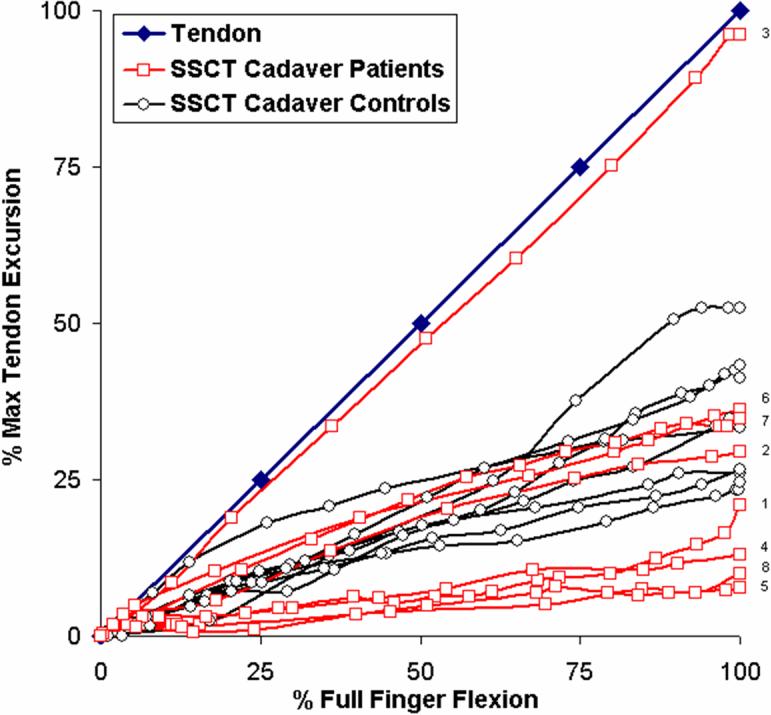
Movement of the tendon (blue) and SSCT of the eight cadaver CTS hands (red) versus the eight cadaver control hands (black) as a percentage of each maximum tendon movement. Four out of the eight cadaver CTS hands show a decreased displacement as compared to the controls. Three cadaver patients moved similar to the controls. Number 1−8 represent individual specimens.
As a result of the bimodal distribution in the CTS affected hands, the median slope did not adequately characterize the results in these hands, which are also displayed graphically in figures 4 and 5. In contrast, the median slope in the cadaver control hands did adequately reflect the generally normal (i.e., unimodal) distribution of the control hands. As shown in Table 2A, while the means and medians are quite similar for the cadaver control hands, the means and medians differ in the CTS-affected hands. Thus, while the motion patterns of the affected and unaffected hands appeared to be different qualitatively, as shown in Figures 4 and 5, they were not significantly different quantitatively, and there was no significant difference between the median slopes of the patients and cadaver CTS hands.
Because the bimodal distribution of the CTS affected hands made direct comparison with the normally-distributed control motions difficult, we divided the data into two groups (Table 2B). The slopes that clustered near the slope of tendon motion were considered “adherent”, in that the SSCT in these hands moved at a velocity similar to that of the tendon. The slopes that clustered away from the tendon were defined as being “dissociated”, as the SSCT velocity was slower and more independent of tendon motion. As shown in Table 2B, the slopes of the six patients who exhibited a pattern of dissociation had a significantly lower median slope than the eight cadaver controls (p<0.01). The four cadaver hands with an antemortem CTS history and a pattern of dissociation showed a significantly lower median slope than the eight cadaver controls (p<0.01).
The slopes of the two patients and the slope of the one cadaver CTS hand with an antemortem CTS history were observed to be higher than the median of the eight controls, but due to small numbers, a formal statistical comparison was not performed.
Simultaneous Versus Single Digit Flexion
In isolated FDS III tendon motion experiment, there were no significant difference in total FDS tendon and VS excursion among three groups. As with the fist movement, again we noted a different pattern of distribution of slopes in the CTS affected hands for the FDS III isolated motion, although somewhat less pronounced, as shown in figure 6 and Tables 2A and B. We also compared the motion of the SSCT as a percentage of the FDS tendon of the middle finger between making a fist and isolated flexion of the middle finger in all 24 hands (Table 2). The median slope of fist movement was significantly higher than the slope of isolated middle finger motion (p<0.01).
Figure 6.
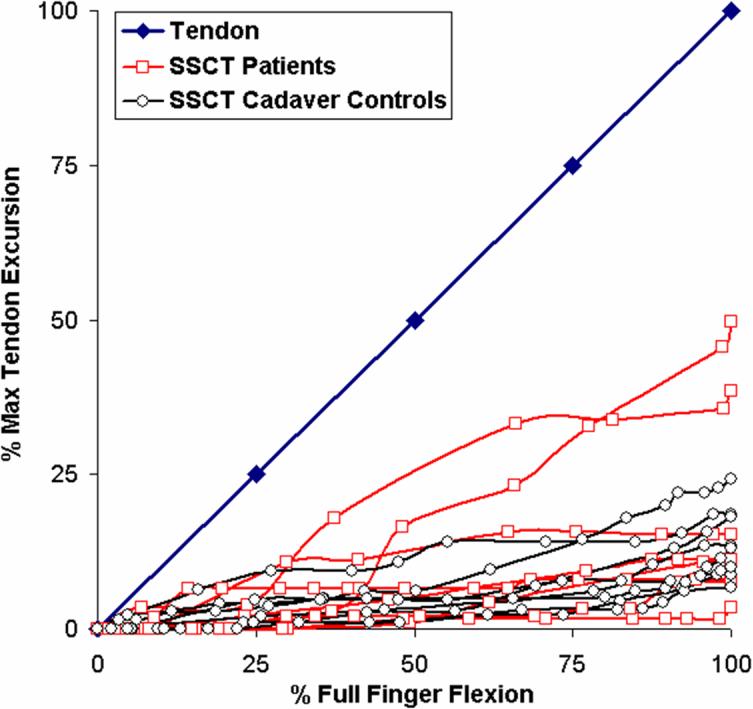
Movement of the tendon (blue) and SSCT of eight patients and eight controls during single digit (differential) motion as a percentage of each maximum tendon movement.
Severity of CTS and Delayed SSCT Movement
Due to small sample size of the patients and cadaver CTS group, the median slope for different severities of CTS was not significantly different. Five patients (63%) and two cadaver control hands (25%) showed a pronounced initial delay of movement of the VS. In the five patients, the tendon moved a mean of 5.9±5.2 mm (23% of total tendon movement) before the VS started to move. In the two controls, the delay was 1.4 mm (5% of total tendon movement) and 1.7 mm (9% of total tendon movement). All other patients and cadavers did not show a delay, and the tendon and VS started to move at the same time.
DISCUSSION
This study had two main objectives: to investigate the relative motion of the SSCT in hands with and without CTS, and to validate the use of cadaver hands as controls for living subjects. We believe that both objectives have been achieved. We have shown that the motion pattern differs in hands with and without CTS, and that these differences are similar in both living subjects with CTS and in hands from cadavers with a history of CTS, even though the cadaver hands in some cases represented postoperative rather than intraoperative changes. Given the similarity between CTS affected hands, whether living or not, and the marked difference between the unaffected and CTS affected cadaver hands, we believe it is reasonable to assume similar differences between unaffected and CTS affected living hands, and that thus unaffected cadaver hands can reasonably be used as controls in the study of SSCT function.
There has recently been increased attention paid to the structure and potential function of the SSCT (Ettema et al., 2004; Ettema et al., 2006a; Guimberteau, 2001). Differences in relative motion between flexor tendons and SSCT of the magnitude noted here can, for example, be detected by ultrasound (Ettema et al., 2006b; Oh et al., 2007). However, the current resolution of ultrasound techniques is insufficient to detect smaller differences in motion. Ultrasound techniques can only average velocities within a sampling window. Small errors in window placement, out of plane movement, and other technical issues make it difficult to detect differences of the magnitude seen when comparing, for example, our normal, dissociated, and adherent groups.
We did not find a statistically significant difference in the slopes while comparing all 8 patients as a group, but this is partly because the patient slopes, while consistently different from the controls, clustered in two distinct patterns, with either greater or smaller slopes. As a result of this bimodal distribution, we needed a far larger sample size than initially anticipated. Nevertheless, we believe that this qualitative difference is worth reporting.
In five of the patients and two of the cadaver control hands, there was a distinct lag in the initiation of SSCT motion after tendon motion began, suggestive of a physical dissociation of the SSCT and tendon. We believe that this may represent the rupture of the normal tendon-SSCT connections, consistent with a possible traumatic event, such as might occur with adjacent fingers moving forcefully or at maximum amplitude in opposite directions, stretching the SSCT to failure.
Our findings suggest that patients with carpal tunnel syndrome have changes in the functioning of the SSCT which could well explain the known pathological changes seen in this disorder. If fibrosis of the SSCT does indeed result in adherence between the SSCT and the underlying tendons, then the normal gradual increase in of SSCT motion, as shown in Figures 4 and 5, will not occur. The absolute amount of SSCT motion will increase, from roughly 30% of tendon motion, as seen in the normal hands, to 60%, as seen in the adherent CTS hands, and this in turn may predispose to further shear injury between the adherent SSCT and either the subjacent tendon or the surrounding unaffected SSCT. One could then imaging a vicious cycle of further adherence and further shear, until either the tendon becomes totally fixed to the SSCT or pulls completely free. These are indeed the two motion patterns that we observed in our CTS patients.
The limitations in this study lie in the lack of normal living controls, the relatively small sample size, and large variables in cadaver patient group. However, we were reluctant to embark upon a larger study without first testing the feasibility of the measurement method. The fact that we included three cadaver CTS hands with non-idiopathic CTS, and five which had surgery, might also have skewed the results of this study. We used these specimens simply to see if the findings in cadaver hands with a history of CTS were similar to those in patients with CTS, and different from those in normal cadaver hands, since we believe strongly that the opening of carpal tunnels in normal volunteers would be unethical. The fact that the postoperative findings in the cadaver CTS hands were similar to the intraoperative findings in the patients suggests to us that the changes seen in the SSCT gliding are irreversible. The strength of this paper lies in the detailed information provided on the motion of the SSCT in patients with carpal tunnel syndrome. This abnormal motion may be a marker for carpal tunnel syndrome, and, we believe, warrants further study, as it may prove helpful in both the diagnosis and possibly even treatment of CTS.
In summary, we have analyzed the relative movement of the visceral synovium and underlying tendon in hands with and without a diagnosis of CTS, and inferred from those observations the behavior of the intervening SSCT. While the differences in motion did not reach statistical significance, qualitative differences in motion in the hands affected with CTS suggest that the SSCT fibrosis known to exist in patients with idiopathic CTS may affect tendon mechanics in the hands of individuals with CTS.
ACKNOWLEDGEMENTS
This study was funded by grants from NIH (AR49823) and Mayo Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- AAEM Guidelines in electrodiagnostic medicine. American association of electrodiagnostic medicine. Muscle Nerve. 1992;15:229–253. doi: 10.1002/mus.880150218. [DOI] [PubMed] [Google Scholar]
- AAEM. AAN. AAPMR Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: Summary statement. American association of electrodiagnostic medicine, american academy of neurology, american academy of physical medicine and rehabilitation. Muscle Nerve. 1993;16:1390–1391. doi: 10.1002/mus.880161219. [DOI] [PubMed] [Google Scholar]
- Abbas MA, Afifi AA, Zhang ZW, Kraus JF. Meta-analysis of published studies of work-related carpal tunnel syndrome. Int J Occup Environ Health. 1998;4:160–167. doi: 10.1179/oeh.1998.4.3.160. [DOI] [PubMed] [Google Scholar]
- Armstrong TJ, Castelli WA, Evans FG, Diaz-Perez R. Some histological changes in carpal tunnel contents and their biomechanical implications. J Occup Med. 1984;26:197–201. [PubMed] [Google Scholar]
- Diao E, Shao F, Liebenberg E, Rempel D, Lotz JC. Carpal tunnel pressure alters median nerve function in a dose-dependent manner: A rabbit model for carpal tunnel syndrome. J Orthop Res. 2005;23:218–223. doi: 10.1016/j.orthres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Erel E, Dilley A, Greening J, Morris V, Cohen B, Lynn B. Longitudinal sliding of the median nerve in patients with carpal tunnel syndrome. J Hand Surg [Br] 2003;28:439–443. doi: 10.1016/s0266-7681(03)00107-4. [DOI] [PubMed] [Google Scholar]
- Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg [Am] 2004;86:1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- Ettema AM, Amadio PC, Zhao C, Wold LE, O'Byrne MM, Moran SL, An KN. Changes in the functional structure of the tenosynovium in idiopathic carpal tunnel syndrome: A scanning electron microscope study. Plast Reconstr Surg. 2006a;118:1413–1422. doi: 10.1097/01.prs.0000239593.55293.c7. [DOI] [PubMed] [Google Scholar]
- Ettema AM, Belohlavek M, Zhao C, Oh SH, Amadio PC, An KN. High-resolution ultrasound analysis of subsynovial connective tissue in human cadaver carpal tunnel. Journal of Orthopaedic Research. 2006b;24:2011–2020. doi: 10.1002/jor.20252. [DOI] [PubMed] [Google Scholar]
- Ettema AM, Zhao C, Amadio PC, O'Byrne MM, An KN. Gliding characteristics of flexor tendon and tenosynovium in carpal tunnel syndrome: A pilot study. Clin Anat. 2007;20:292–299. doi: 10.1002/ca.20379. [DOI] [PubMed] [Google Scholar]
- Freeland AE, Tucci MA, Barbieri RA, Angel MF, Nick TG. Biochemical evaluation of serum and flexor tenosynovium in carpal tunnel syndrome. Microsurgery. 2002;22:378–385. doi: 10.1002/micr.10065. [DOI] [PubMed] [Google Scholar]
- Gelberman RH, Hergenroeder PT, Hargens AR, Lundborg GN, Akeson WH. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg [Am] 1981;63:380–383. [PubMed] [Google Scholar]
- Guimberteau JC. The sliding system. Vascularized flexor tendon transfers. France: 2001. New ideas in hand flexor tendon surgery. [Google Scholar]
- Kerr CD, Sybert DR, Albarracin NS. An analysis of the flexor synovium in idiopathic carpal tunnel syndrome: Report of 625 cases. J Hand Surg [Am] 1992;17:1028–1030. doi: 10.1016/s0363-5023(09)91053-x. [DOI] [PubMed] [Google Scholar]
- Lluch AL. Thickening of the synovium of the digital flexor tendons: Cause or consequence of the carpal tunnel syndrome? J Hand Surg [Br] 1992;17:209–212. doi: 10.1016/0266-7681(92)90091-f. [DOI] [PubMed] [Google Scholar]
- Nakama LH, King KB, Abrahamsson S, Rempel DM. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J Orthop Res. 2005;23:1199–1205. doi: 10.1016/j.orthres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Nakamichi K, Tachibana S. Histology of the transverse carpal ligament and flexor tenosynovium in idiopathic carpal tunnel syndrome. J Hand Surg [Am] 1998;23:1015–1024. doi: 10.1016/s0363-5023(98)80009-9. [DOI] [PubMed] [Google Scholar]
- Neal NC, McManners J, Stirling GA. Pathology of the flexor tendon sheath in the spontaneous carpal tunnel syndrome. J Hand Surg [Br] 1987;12:229–232. doi: 10.1016/0266-7681_87_90020-9. [DOI] [PubMed] [Google Scholar]
- Oh J, Zhao C, Amadio PC, An KN, Zobitz ME, Wold LE. Vascular pathologic changes in the flexor tenosynovium (subsynovial connective tissue) in idiopathic carpal tunnel syndrome. J Orthop Res. 2004;22:1310–1315. doi: 10.1016/j.orthres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Oh J, Zhao C, Amadio PC, An KN, Zobitz ME, Wold LE. Immunolocalization of collagen types in the subsynovial connective tissue within the carpal tunnel in humans. J Orthop Res. 2005;23:1226–1231. doi: 10.1016/j.orthres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Oh S, Belohlavek M, Zhao C, Osamura N, Zobitz ME, An KN, Amadio PC. Detection of differential gliding characteristics of the flexor digitorum superficialis tendon and subsynovial connective tissue using color doppler sonographic imaging. Journal of Ultrasound in Medicine. 2007;26:149–155. doi: 10.7863/jum.2007.26.2.149. [DOI] [PubMed] [Google Scholar]
- Phalen GS. The carpal-tunnel syndrome. Seventeen years' experience in diagnosis and treatment of six hundred fifty-four hands. J Bone Joint Surg [Am] 1966;48:211–228. [PubMed] [Google Scholar]
- Saleh SS, Fuortes L, Vaughn T, Bauer EP. Epidemiology of occupational injuries and illnesses in a university population: A focus on age and gender differences. Am J Ind Med. 2001;39:581–586. doi: 10.1002/ajim.1057. [DOI] [PubMed] [Google Scholar]
- Sanz J, Lizaur A, Sanchez Del Campo F. Postoperative changes of carpal canal pressure in carpal tunnel syndrome: A prospective study with follow-up of 1 year. J Hand Surg [Br] 2005;30:611–614. doi: 10.1016/j.jhsb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Schuind F. Canal pressures before, during, and after endoscopic release for idiopathic carpal tunnel syndrome. J Hand Surg [Am] 2002;27:1019–1025. doi: 10.1053/jhsu.2002.36541. [DOI] [PubMed] [Google Scholar]
- Stal M, Hansson GA, Moritz U. Wrist positions and movements as possible risk factors during machine milking. Appl Ergon. 1999;30:527–533. doi: 10.1016/s0003-6870(99)00015-0. [DOI] [PubMed] [Google Scholar]
- Stevens JC. Aaem minimonograph #26: The electrodiagnosis of carpal tunnel syndrome. American association of electrodiagnostic medicine. Muscle Nerve. 1997;20:1477–1486. doi: 10.1002/(sici)1097-4598(199712)20:12<1477::aid-mus1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Sud V, Freeland AE. Biochemistry of carpal tunnel syndrome. Microsurgery. 2005;25:44–46. doi: 10.1002/micr.20071. [DOI] [PubMed] [Google Scholar]
- Szabo RM. Carpal tunnel syndrome as a repetitive motion disorder. Clin Orthop. 1998;351:78–89. [PubMed] [Google Scholar]
- Szabo RM, Chidgey LK. Stress carpal tunnel pressures in patients with carpal tunnel syndrome and normal patients. J Hand Surg [Am] 1989;14:624–627. doi: 10.1016/0363-5023(89)90178-0. [DOI] [PubMed] [Google Scholar]
- Tucci MA, Barbieri RA, Freeland AE. Biochemical and histological analysis of the flexor tenosynovium in patients with carpal tunnel syndrome. Biomed Sci Instrum. 1997;33:246–251. [PubMed] [Google Scholar]
- Werner R, Armstrong TJ, Bir C, Aylard MK. Intracarpal canal pressures: The role of finger, hand, wrist and forearm position. Clin Biomech. 1997;12:44–51. doi: 10.1016/s0268-0033(96)00044-7. [DOI] [PubMed] [Google Scholar]
- Wu JZ, Dong RG, Smutz WP, Schopper AW. Modeling of time-dependent force response of fingertip to dynamic loading. J Biomech. 2003;36:383–392. doi: 10.1016/s0021-9290(02)00427-x. [DOI] [PubMed] [Google Scholar]