Summary
Narcolepsy is a neurological condition with a prevalence of up to 1 per 1,000 that is characterized by irresistible bouts of sleep. Associated features include the pathological manifestations of rapid-eye-movement (REM) sleep: cataplexy, sleep paralysis, hypnagogic hallucinations, and abnormal sleep-onset REM periods and disturbed nocturnal sleep. The condition is strongly associated with the HLA-DR2 and DQw1 phenotype. The phenomenology of narcolepsy is discussed, and diagnostic procedures are reviewed. Treatment modalities involving central nervous system stimulants for somnolence and tricyclic drugs for REM-sleep abnormalities are discussed. Sleep laboratory studies on the treatment efficacy of methylphenidate, pemoline, dextroamphetamine, protriptyline, and viloxazine are presented. Data suggest that: (1) methylphenidate and dextro-amphetamine objectively improve somnolence; (2) pemoline, at doses up to 112.5 mg, is less effective in controlling somnolence but may improve certain aspects of performance; and (3) protriptyline and viloxazine are effective anticataplectic agents that produce little improvement in somnolence.
Keywords: Narcolepsy, Polysomnography, Central nervous system stimulants, Tricyclic antide-pressants
Excessive somnolence is a major health problem in the United States. Over half of the 250,000 or so patients who come to U.S. sleep disorders centers each year present with this complaint (Coleman et al., 1982; Coleman, 1983). A recent review underscored the considerable impact sleepiness can have on public safety, ranging from transportation to nuclear power (Mitler et al., 1988a). Clinicians frequently face difficult clinical and medicolegal decisions associated with the treatment and management of sleepy patients, regardless of their specific diagnosis. For example, what is the physician’s duty to the patient and to society in connection with an excessively somnolent airplane pilot or bus driver? The American Sleep Disorders Association (formerly known as The Association of Sleep Disorders Centers) formulated a policy concerning the reporting of patients whose somnolence may represent a public safety risk (Association of Sleep Disorders Centers, 1984). The policy is that excessively somnolent patients should not automatically or precipitously be reported to public health or motor vehicle authorities because (1) sleep is a reversible and normal behavioral state, (2) most forms of excessive somnolence are readily treatable, and (3) a report can be filed at the clinician’s discretion in cases of noncompliance or treatment failure. Until satisfactory symptom control is achieved, the patient should be advised not to drive or engage in other activities in which safety requires sustained attention.
Excessive daytime somnolence has been related to various social problems ranging from marital difficulty to death in traffic accidents. Narcolepsy (ICD-9-CM 347) is probably the most clearly understood diagnostic category within the population of patients who complain of excessive somnolence (Guilleminault et al., 1976; Coleman et al., 1982; Coleman, 1983). The treatments of choice for narcolepsy in current practice involve central nervous system (CNS) stimulants alone or in combination with rapid-eye-movement (REM) sleep-suppressing drugs, such as tricyclic antidepressants (Mitler et al., 1988b). There is very little research on the efficacy of pharmacotherapy for narcolepsy. Reasons for such a lack of data are numerous: (1) perceived markets for relevant drugs are too small to warrant research support by drug manufacturers; (2) narcolepsy is misperceived to be a rare and nondisabling disorder; (3) there have been no testable scientific hypotheses concerning the relationship between the pathophysiology of narcolepsy and modes of pharmacologic action; and (4) the symptom of somnolence has been too poorly characterized to warrant studies of drug-related change. Now, although it is still true that there is no incentive for drug manufacturers to support efficacy studies, there is a much better understanding of narcolepsy’s prevalence (1 in 1,000) and disabling potential (90% are at least partially disabled and 100% cannot do work that requires long-term sustained attention). Furthermore, animal models, existing sleep research, and our initial objective drug research on narcolepsy point to testable hypotheses and reliable methods for measuring changes in somnolence.
It is important to appreciate that narcolepsy is only one diagnosis associated with the symptom of excessive somnolence. According to two cooperative studies (Coleman et al., 1982; Coleman, 1983), numerically the most important categories within this patient population are: (1) sleep apnea syndrome, accounting for 40–50% of such patients; (2) narcolepsy, accounting for 20–30% of such patients; and (3) idiopathic CNS hypersomnolence, accounting for 5–10% of such patients.
Narcolepsy, as the syndrome is currently understood, was first described by the French physician Gelineau (1880). Major symptoms associated with narcolepsy include somnolence, cataplexy, hypnagogic hallucinations, and sleep paralysis. Narcolepsy is a heritable neurological disease linked to the HLA-DR2 phenotype (Honda et al., 1983; Juji et al., 1984; Honda and Juji, 1988). The disease involves disabling disregulation of wakefulness and sleep and is characterized by excessive somnolence, as well as episodic motor paralysis and perceptual distortion. Narcolepsy can be precisely diagnosed with modern polysomnographic techniques (Mitler, 1982; Mitler et al., 1988b). In humans, narcolepsy is not rare, afflicting about 1 in 1,000, or about 250,000 Americans (Dement et al., 1972, 1973). Thus, narcolepsy is about as prevalent as multiple sclerosis (Kurland et al., 1955) and can be as disabling, with profound consequences for job capability, public safety, sense of self-worth, and social image (Broughton and Ghanem, 1976; Foster-Rawlings and Dement, 1985).
Neurochemical and pharmacotherapeutic studies of naturally occurring canine narcoleptics, experimental feline models, and humans suggest that two brain abnormalities are involved (Guilleminault et al., 1976): a widespread underrelease of dopamine (Faul et al., 1986) and a brainstem-specific proliferation (up-regulation) of muscarinic acetylcholine receptors and hypersensitivity to acetylcholine (Kilduff et al., 1986). The chemotherapeutic approach of psychostimulants for somnolence and REM-sleep-suppressing drugs for ancillary symptoms is somewhat effective. However, narcoleptics in the treatment regimens we have evaluated did not perform or maintain alertness as well as normal controls. Narcoleptics must also face various psychosocial and work-related problems throughout a lifetime of treatment.
PHENOMENOLOGY
Most often, narcoleptics present with the primary complaint of falling asleep at inappropriate times. It is also possible for narcoleptics to experience insomnia or uncontrollable muscle weakness. The four classic symptoms of narcolepsy are known as the narcoleptic tetrad and consist of: (1) sleep attacks—sudden urges to sleep; (2) cataplexy—partial or generalized flaccid paralysis precipitated by anticipatory excitement, laughter, anger, or surprise; (3) hypnagogic hallucinations—frightening or menacing hallucinations that occur at sleep onset; and (4) sleep paralysis—often frightening and unpleasant generalized paralysis slightly before or at the time of falling asleep or on awakening. A fifth, frequently associated symptom that probably should be added, to make up a narcolepsy “pentad,” is disturbed nocturnal sleep. Symptoms usually appear during the late teens and twenties. Inappropriate sleep is often the first to emerge. Patients will report attacks of sleep while talking on the telephone, eating, or driving. Other symptoms may require 10 or more years to emerge.
The symptom of somnolence in narcoleptics is by far the most troublesome and is the most difficult to control. The psychological and social impact of narcolepsy is becoming increasingly appreciated (Broughton and Ghanem, 1976; Foster-Rawlings and Dement, 1985). It is also common knowledge among clinicians responsible for patient management that the narcoleptic, whose symptoms are not satisfactorily controlled, can be dangerous to himself and others by engaging in activities such as driving, caring for children, or engaging in tasks requiring a great deal of motor and intellectual precision.
PATHOPHYSIOLOGY
Much has been discovered about narcolepsy in the past 20 years. After Aserinsky and Kleitman (1953) first described REM sleep and researchers worked out the nominal sleep structure in man, others began studying abnormalities of sleep. Rechtschaffen et al. (1963) and Takahashi and Jimbo (1963) independently discovered that the most important electrographic feature of narcoleptic sleep is the tendency to go from wakefulness to REM sleep with very little or no intervening non-REM (NREM) sleep. Such a tendency is in contrast to the normal 60–90 min of intervening NREM sleep before REM sleep is first achieved. Herein lies the rationale of the Multiple Sleep Latency Test to identify the pathological somnolence (Richardson et al., 1978; Carskadon et al., 1986) and the pathological tendency to achieve REM sleep (Mitler et al., 1979).
During normal REM sleep, profound Changes occur in the motor and proprioceptive systems. The skeletal muscles are made almost completely atonic by a hyperpolarization of spinal and brainstem alpha motoneurons via a descending reticulospinal inhibitory pathway (Giaquinto et al., 1963a,b; Nakamura et al., 1978; Glenn and Dement, 1981a,b,c). Chemical stimulation studies suggest that this pathway is at least partially mediated by acetylcholine (George et al., 1964; Baxter, 1969; Mitler and Dement, 1974; Vivaldi et al., 1979; Silberman et al., 1980). This pathway also produces hyperpolarization of gamma motoneurons (Gassel and Pompeiano, 1963). Episodically, and coincident with rapid eye movements of REM sleep, there is presynaptic inhibition and further reflex suppression (Giaquinto et al., 1963a,b; Chase and Morales, 1983).
Studies in canine and human narcoleptics have shown that the reflex inhibition during cataplexy is identical to that seen during normal REM sleep. The inhibition of both alpha and gamma motoneurons may contribute to the concomitant feelings of terror patients experience by distorting accurate proprioception and perception. Cataplectic episodes are sometimes accompanied by frank hallucination similar to the narcoleptic’s perceptual experiences during a sleep-onset REM period.
Long-term polysomnographic studies (48 h or more) of narcoleptics have shown that the abnormal tendency for REM sleep manifests itself as a disruption of the diurnal sleep-wake cycle such that sleep occurs in many short and sometimes irresistible bouts (Baldy-Moulinier et al., 1976; Browman et al., 1986). Pollak (Narcolepsy: Third International Symposium, June 10, 1988, San Diego) has shown with round-the-clock studies under entrained and free-running conditions that narcoleptics do not sleep more of each 24-h cycle than do normals. Rather, they have more frequent transitions from wakefulness to sleep. Neurochemical and neuropharmacological studies of sleep in normal humans, animals, and narcoleptic patients suggest that the sleep-wake cycle is determined by some complex interaction of catecholamine-containing and serotonin-containing neurons. Anatomical sites often implicated in the normal neuronal control of sleep are situated in the pontine reticular formation in the area of the locus ceruleus and the raphe system (Jouvet, 1972). George et al. (1964), Baxter (1969), Mitler and Dement (1974), and the Harvard group (Vivaldi et al., 1979) have shown that cholinergic stimulation is important in regulating REM sleep via these midbrain and pontine areas.
In 1974, a naturally occurring canine model of narcolepsy became available for study (Knecht et al., 1973; Mitler et al., 1974). Drugs useful in controlling cataplexy in humans also control cataplexy in narcoleptic dogs. Stimulant medications useful in controlling excessive somnolence in narcoleptic humans also control narcoleptic symptoms in dogs. Long-term polygraphic studies in these animals have described a disrupted sleep-wake cycle analogous to that seen in human narcoleptics. Recent neurochemical studies on narcoleptic dogs at Stanford University have disclosed evidence for catecholamine-releasing abnormalities and proliferation of acetylcholine receptors (Boehme et al., 1982; Mefford et al., 1983; Faul et al., 1986; Kilduff et al., 1986). These data are consistent with the clinical abnormalities of narcolepsy and with the therapeutic effects of drugs that either block norepinephrine uptake or cause central anticholinergic effects. Breeding studies in dogs have shown a hereditary pattern consistent with an autosomal-recessive mode of transmission in narcoleptic Doberman pinschers and narcoleptic Labrador retrievers. However, there are complicated and as yet unexplained developmental and hereditofamilial patterns in beagles and poodles with canine narcolepsy (Foutz et al., 1979; Baker et al., 1982). These genetic studies underscore the complexities of transmission of the disease in the dog and offer a perspective to the data of Kessler (1976) suggesting that humans having one narcoleptic parent are 20–60 times more likely than the general population to present with narcolepsy or some other form of excessive somnolence.
The Second International Symposium on Narcolepsy held in Palo Alto, California, in July of 1985 heralded the confirmation of the earleir studies by the Japanese group (Honda et al., 1983; Juji et al., 1984; Langdon et al., 1984) showing that there was a strong association between narcolepsy and the HLA-DR2 phenotype. Laboratories at Stanford (Holloman et al., 1987), Kings College Hospital in London (Langdon et al., 1984), the University of Montreal, and Montpelier, France (Seignalet and Billiard, 1984) have all confirmed the strong association between the diagnosis of narcolepsy and these HLA antigens (90–100% versus 20–40% for nonnarcoleptic controls). Participants in the Palo Alto Symposium generally agreed that the HLA-DR2 and HLA-Dw2 antigens seemed to show the strongest association. These findings have generated speculation that (1) narcolepsy, or some forms of narcolepsy, are inherited and (2) narcolepsy may be an autoimmune disorder, since HLA-DR2 is associated with systemic lupus erythematosus (SLE) (Reinertsen et al., 1978) and with a high incidence of drug toxicity in rheumatoid arthritis (RA) patients (Panayi et al., 1978). It may be particularly relevant that HLA-Dw2 (which is always found in HLA-DR2 subtype) is also associated with the neurologic diseases, multiple sclerosis (Opelz et al., 1977) and optic neuritis (Jersild et al., 1973). More recent work reported and/or reviewed at the Third International Symposium on Narcolepsy held in San Diego, California, in June of 1988 has affirmed the very strong (if not 100%) association between narcolepsy and the HLA-DR2 and DQw1 antigens in white and Japanese populations. However, in blacks, this strong association holds only for DQw1 (Kramer et al., 1987; Neely et al., 1987).
New data reported by Lock et al. (1988) and Holloman (personal communication) indicate that the completely sequenced clones encoding the two expressed DR2 chains and both DQw1 alpha and beta chains show no differences between narcoleptics and healthy controls. Thus, several possibilities remain to be tested: (1) narcolepsy is caused by another gene, tightly linked (i.e., in linkage disequilibrium) to the DR region of chromosome 6, that is abnormal in the DR2 haplotype; (2) narcolepsy is caused by normal DR2/DQw1 when these genes interact with another event or gene; and (3) narcolepsy is caused by unknown regulatory alterations in some DR2/DQw1 haplotypes.
CLINICAL DIAGNOSIS
A number of conditions are characterized by the complaint of excessive somnolence. It is the five auxilliary symptoms of narcolepsy that distinguish it from other conditions. However, only 20–25% of narcoleptics present the full complement of symptoms.
A history of symptoms emerging around puberty is indicative of narcolepsy. Inappropriate sleep during the day is usually first to appear. These bouts of sleep can be reduced by physical activity. Relief of narcoleptic symptoms often follows a sleep attack or a voluntary nap. This postsleep improvement distinguishes narcoleptics from patients with sleep apnea—the most common cause of excessive somnolence. It is important to gauge the severity of daytime somnolence. The sleepiness of a narcoleptic is qualitatively worse than that associated with, say, staying out late or a night of insomnia. The narcoleptic’s sleepiness cannot be brushed off and is as disabling as that stemming from several 24-h periods without sleep.
Clinicians often rely on the presence of cataplexy as the key diagnostic feature. Narcoleptics often report partial paralysis or complete postural collapse caused by emotional activities like telling a joke, swatting a bug, or catching a ball. Cataplexy is described as a draining weakness in a segment of the body, such as the face or legs. In partial attacks, a distorted facial expression or clumsy vocalization is all that may be noticed. With more generalized episodes, the patient can suddenly drop to the floor and thereby suffer physical injury.
DIFFERENTIAL DIAGNOSTIC CONSIDERATIONS
The differential diagnosis of narcolepsy, when cataplexy is involved, should always include hypotonic epileptic seizure and syncope. The key features to keep in mind are that (1) cataplectic patients often report complete wakefulness during their attacks, (2) narcoleptics do not exhibit postictal symptoms after the cataplectic episode resolves, (3) there is rapid reversibility of the cataplectic episode, and (4) there is an absence of epileptic EEG activity during the attacks and an absence of postictal EEG slowing.
Sleep apnea syndromes (ICD-9-CM codes 780.53-0 and 780.53-1) are the most common diagnostic entity of patients who present with the complaint of excessive somnolence (Coleman et al., 1982; Coleman, 1983). Most investigators now believe that the etiology of daytime somnolence stems from sleep disruption at night secondary to respiratory abnormality (Zorick et al., 1983) and/or from short-term and long-term effects of episodic hypoxemia during the night (Clark et al., 1979; Orr et al., 1979). Our earlier electrophysiological studies of somnolence and performance tests for simple addition and symbol substitution indicate that sleep apnea patients are severely impaired in performance and ability to stay awake. Many of the clinical manifestations and hemodynamic consequences of sleep apnea syndrome are improved or resolved by improvement of upper airway patency by surgical intervention or by nasal continuous positive airway pressure (Sullivan et al., 1981; Zorick et al., 1983), or by drugs, such as protriptyline, that depress REM sleep and seem to increase airway patency through elevating muscle tone (Clark et al., 1979; Orr et al., 1979).
Sleep-related breathing abnormality was described in clinical and electrographic detail by Coccagna et al. (1972). However, the relationship between somnolence and respiratory abnormality has been discussed in medical texts for over a hundred years (Hill, 1898). Sleep apnea, as defined by most sleep clinicians (Association of Sleep Disorders Centers, 1979), is characterized by obstructive apnea during sleep, loud snoring, and excessive daytime somnolence. Middle-aged, overweight males are the most common type of person affected. The disorder has been increasingly recognized in clinical practice and is now being successfully differentiated from narcolepsy. This clarity was not always present. Yoss and Daly (1957) presented narcolepsy case series data showing the onset of somnolence (narcolepsy) as a function of age. The distribution was bimodal, with a large peak at 16–20 years and another, smaller peak at 51–55 years. The most likely explanation for the second peak is the inclusion of somnolent sleep apnea patients in the narcolepsy case series. About 80% of sleep apnea patients presenting to sleep disorders centers are 45–55 years old. Another source of confusion exists between sleep apnea patients and narcolepsy patients. Our group has shown that patients with sleep apnea frequently show abnormally short latencies to REM sleep and also may complain of hypnagogic hallucinations (Mitler et al., 1982; Browman et al., 1983). These REM abnormalities disappear after sleep apnea is successfully treated.
Patients with idiopathic CNS hypersomnolence (ICD-9-CM code 780.54-7) complain of virtually constant somnolence (Association of Sleep Disorders Centers, 1979). Sleep latencies on multiple sleep latency testing objectively confirm such pathological somnolence. When recorded in sleep laboratories, these patients appear to sleep, and also sense that they sleep very deeply throughout the night. However, patients with CNS hypersomnia do not reliably show abnormally short latencies to REM sleep or other REM-sleep-related symptoms, such as cataplexy and sleep paralysis.
OBJECTIVE TESTING FOR NARCOLEPSY
The narcoleptic patient requires life-long management that often involves drugs with considerable addictive and abuse potential. Therefore, a clinical diagnosis is not sufficient; objective confirmation of narcolepsy is necessary.
As indicated above, there are other more likely causes of the complaint of excessive somnolence. Furthermore, the presence of the key clinical features is often not conclusive because only 20–25% of narcoleptics have the complete narcoleptic tetrad. For example, one symptom in the tetrad, sleep paralysis, occurs without other symptoms in 5% of aduts. Laboratory testing can resolve such issues: Unlike narcoleptics, patients with sleep paralysis rarely have an increased tendency to achieve REM sleep, although polysomnography will confirm skeletal muscle atonia during sleep paralysis. As another example, depression is characterized by abbreviated latencies to REM sleep in nocturnal sleep (Kupfer and Foster, 1972), and REM latencies are sometimes brief enough to qualify as a true sleep onset REM sleep (usually less than 30 min). Furthermore, significant daytime sleepiness is a common finding in adolescents and is thought to result from a combination of sleep deprivation and hormonal changes (Carskadon et al., 1980, 1981).
It has been argued that a clear history of cataplexy is sufficient for the diagnosis of narcolepsy, but we disagree. Almost 50% of our narcoleptic patients do not have significant cataplexy. Furthermore, it is quite easy to get a history such as, “I get weak when I laugh.” Such data are inadequate to substantiate the life-long diagnosis of narcolepsy. Therefore, some type of objective test of somnolence should always be used by sleep specialists.
Although pupillography (Schmidt and Fortin, 1982) can differentiate the unstable, small pupil diameters characteristic of somnolent patients from the wider, stable pupils of nonsomnolent patients, almost all sleep disorders centers now use nighttime and daytime polysomnography with specific scoring criteria to evaluate for the presence of various sleep disorders. Nighttime polysomnography is essential to rule out sleep-related respiratory pathology and other disorders that could cause somnolence by restricting nocturnal sleep. Periodic leg movements during sleep is one nocturnal polysomnographic finding; although sleep-disrupting, it is consistent with the diagnosis of narcolepsy (van den Hoed et al., 1981). It is believed that the leg movements coexist with and exacerbate the symptoms of narcolepsy. Hartman and Scrima (1986) have hypothesized that periodic leg movements are etiologically linked to narcolepsy by causing chronic and repetitive sleep disruption (see also Scrima, 1981). Whereas the testing of this hypothesis is not yet complete, it is certainly true that there are many somnolent patients without narcolepsy who do have periodic leg movements.
The most commonly used diagnostic procedure for evaluating the patient with the complaint of excessive somnolence is the Multiple Sleep Latency Test (MSLT). This test grew out of several research studies of alternative sleep-wake schedules. These studies preserved the usual human sleep-wake ratio of 1-part-sleep-to-2-parts-wake but altered the duration of the sleep-wake cycle, e.g., 1 h of sleep opportunity every 3 h (Weitzman et al., 1974) and 30 min of sleep every 90 min (Carskadon and Dement, 1975). After extensive analysis of these studies, the Stanford group concluded that such protocols could not only be viewed as studies of alternative sleep-wake cycle durations but also as repeated tests of behavioral sleep tendency throughout the 24 hours. When such repeated tests of sleep tendency were offered to narcoleptics and normals, the narcoleptics reliably distinguished themselves from normals by falling asleep much more quickly (Richardson et al., 1978) and having many more transitions to REM sleep after brief intervals of NREM sleep (Mitler et al., 1979).
The MSLT is routine throughout the world today and consists of four or five 20-min-long opportunities to sleep. The MSLT begins at least 90 min after a nighttime polysomnogram, and the opportunities are presented every 2 h. In our hands, the MSLT has proved extremely useful. Table 1 presents MSLT results from the Sleep Disorders Center at the State University of New York, Stony Brook, for 57 consecutive cases of narcolepsy and 17 normal subjects. For each test, as well as for the average test, group differences were significant beyond the 0.001 level, and there was no overlap between the groups with respect to the number of times that REM sleep was achieved.
TABLE 1.
Multiple sleep latency results
| 1000 h test | 1200 h test | 1400 h test | 1600 h test | 1800 h test | Average test | Average number REM periods | |
|---|---|---|---|---|---|---|---|
| Narcolepticsa | |||||||
| Sleep latency | 3.0 ± 2.4 | 2.9 ± 3.6 | 2.4 ± 3.0 | 2.4 ± 2.5 | 4.0 ± 5.1 | 3.0 ± 2.7 | 3.5 ± 0.9 |
| Range | 0–12 | 0–20 | 0–18 | 0–15 | 0–20 | 1–14 | 2–5 |
| Proportion who slept | 1.00 | 0.98 | 1.00 | 1.00 | 0.97 | 0.99 | — |
| Controlsb | |||||||
| Sleep latency | 14.3 ± 6.0 | 13.7 ± 6.1 | 12.2 ± 5.7 | 12.6 ± 5.2 | 14.2 ± 6.1 | 13.4 ± 4.0 | 0 |
| Range | 1–20 | 0–20 | 2–20 | 2–20 | 3–20 | 5–20 | 0 |
| Proportion who slept | 0.59 | 0.59 | 0.71 | 0.76 | 0.53 | 0.64 | — |
N = 57 (33 males); age, 43.3 + 12.3 years.
N = 17 (11 males); age, 33.4 + 9.9 years.
It is also becoming common to get HLA typing on possible narcoleptic patients. Our group (Rubin et al., 1988) confirmed the strong association between narcolepsy and HLA-DR2 and showed that the association does not extend to patients with sleep apnea-numerically the most important group with excessive somnolence. Even though it is true that there are isolated non-DR2 and non-DQw1 narcoleptics (Guilleminault et al., 1988; Rubin et al., 1988), nevertheless, DR2 and DQw1 negativity is often helpful in ambiguous cases and those involving legal proceedings.
Family Studies
There are several ongoing studies throughout the country on the hereditofamilial pattern of narcolepsy transmission. We are engaged in such a project with the collaboration of Drs. June Fry (Medical College of Pennsylvania), Christian Guilleminault, and Carl Grumet (both of Stanford University School of Medicine). The family described here contains four proven narcoleptic patients distributed over two of five generations and is illustrative of the kind of data now being evaluated. The family was chosen because it met the following criteria: (1) at least one narcoleptic with the complaint of somnolence who showed two or more REM sleep episodes on an MSLT, (2) no sleep apnea, and (3) the HLA-DR2 antigen. The family had to have 4–8 first-degree blood relatives and 12–20 second-degree relatives who were accessible to diagnostic sleep recordings and HLA phenotyping.
RC, Jr., a 49-year-old male, was selected as the index case. A family tree (Fig. 1) was constructed to delineate the index case of grandparents, parents, aunts, uncles, cousins, siblings, offspring, nephews, nieces, and grandchildren.
FIG. 1.
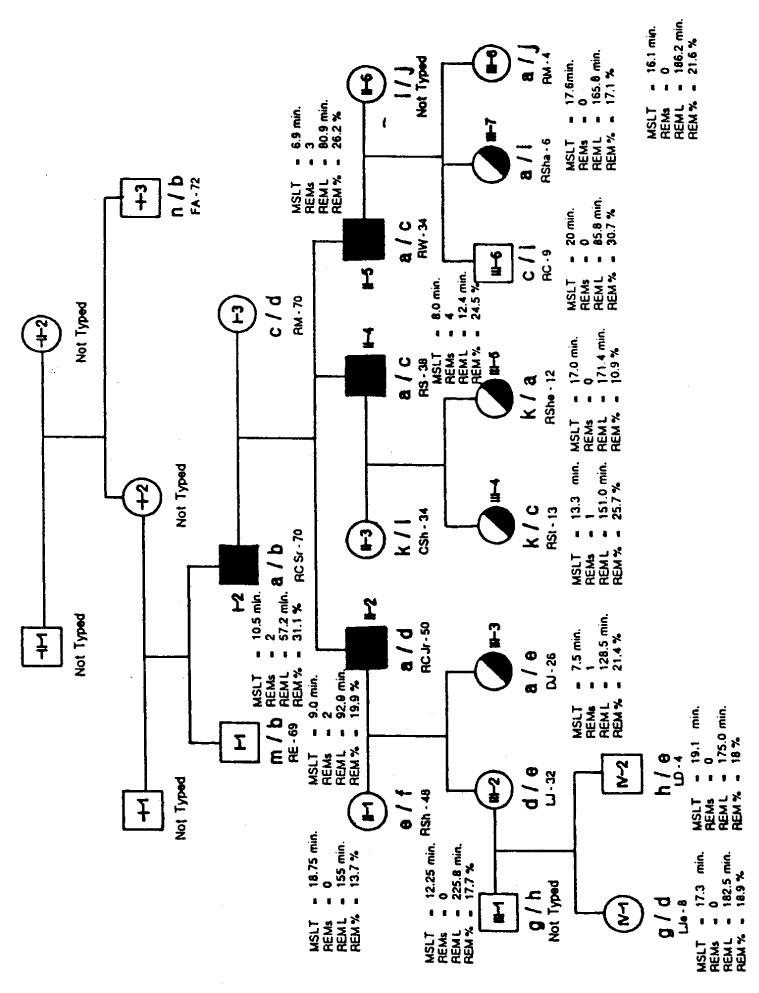
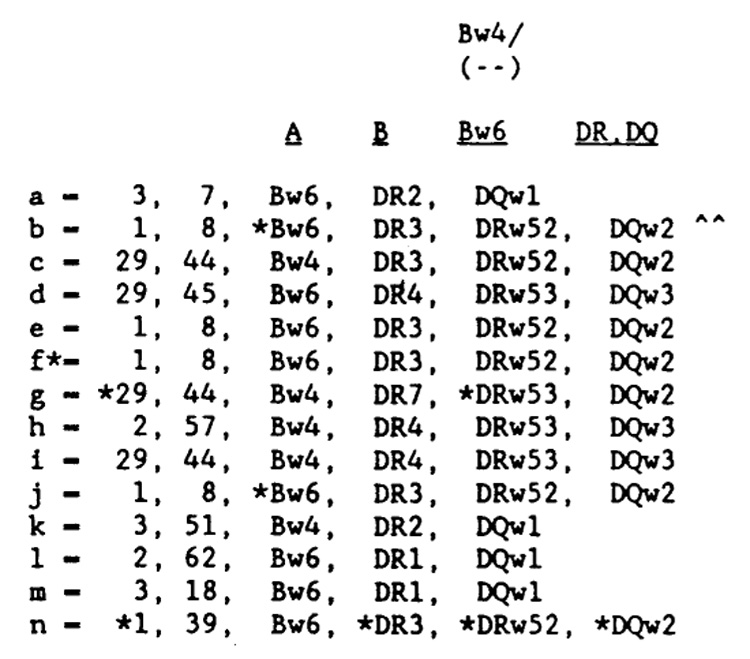
Opposite page: Family tree. The black symbols indicate clinical cases of narcolepsy, the half-black, half-white symbols indicate excessive daytime sleepiness, the white symbols indicate healthy subjects, and “a” indicates HLA-DR2-positive cases. The association between clinical narcolepsy and HLA-DR2 is seen in the cases of RC, Sr., RC, Jr., RS, and RW. The results of the nocturnal polysomnographic recording and multiple sleep latency testing are also shown. MSLT, mean sleep latency, in minutes: REMs, number of REM sleep episodes on MSLT; REML, latency from first sleep to REM sleep, in minutes, on nocturnal polysomnographic recording (NPSG): and REM %, percent of REM sleep during NPSG. Above: Explanations for the HLA codings. An asterisk indicates the most probable antigens or haplotype, an X indicates a true blank, and ^^ indicates retyping using new cells-reg isolation.
All available family members completed the study questionnaire, and all first-degree and many second-degree members (a total of 20 subjects) were examined by a physician (author R.H.). The index case and 17 members of his family were studied clinically and polysomnographically using the standard MSLT protocol published by the Association of Professional Sleep Societies. Patients previously treated with medications were recorded following a 2-week withdrawal period from all stimulants and psychoactive medications.
The index case (RC, Jr.), his father (RC, Sr.), and two younger brothers (RS and RW), were diagnosed as having classic narcolepsy with major symptomatology of excessive daytime sleepiness and sleep paralysis. Polygraphic findings showed MSLT mean latency of 9.0 min, 10.5 min, 8.0 min, and 6.9 min, repectively. The numbers of REM sleep periods during MSLT were two, two, four, and three, respectively.
The younger daughter (DJ) and three nieces (RSt, RShe, RSha) of the index case complained of excessive daytime sleepiness. Their results on MSLT showed mean sleep latency of 7.5 min, 13.38 min, 17.0 min, and 17.6 min, respectively. The numbers of REM sleep periods during the MSLT were one, one, zero, and zero, respectively.
All subjects who were polysomnographically recorded and four other relatives (FA, RE, RM, and CSh) of the index case had complete HLA phenotyping by standard lymphocyte microcytotoxicity at the Blood Bank at Stanford University. The results of this phenotyping are also shown on the family tree (Fig. 1).
These family data underscore the significant association of DR2 with clinically manifested narcolepsy and also with the symptom of excessive daytime sleepiness. The association of excessive daytime sleepiness (EDS) only and HLA-DR2 is seen in the cases of DJ, RSt, RShe, and RSha. In addition, RShe shows to be a homozygote with DR2, DQW1 inherited from her father and her mother. The probability of this kind of combination might be as rare as 1 in 30,000. RSt, RShe’s sister, shows DR3 inherited from her father and DR2 inherited from her mother, who does not have symptoms of narcolepsy and probably represents one case of the 25–30% of the normal Caucasian population with HLA-DR2. These two interesting and unusual cases promise to give insight into the pattern of inheritance of narcolepsy. This family is and will be clinically followed, with special emphasis on these two cases.
TREATMENT
There are few demonstrably effective treatment options available to the clinician responsible for the management of narcoleptic patients on a long-term basis. The symptoms of excessive somnolence are usually treated with CNS stimulants, such as dextroamphetamine (5–60 mg distributed throughout the day), methylphenidate (5–60 mg distributed throughout the day), or pemoline (18.75–150 mg distributed throughout the day) (Mitler et al., 1988b). The symptoms related to REM sleep—cataplexy (a rapid loss of muscle tone and mobility), hypnagogic hallucination, and sleep paralysis—are controlled by tricyclic medications, such as imipramine (75–150 mg/day), and the stimulating antidepressants protriptyline (10–40 mg/day) (Schmidt et al., 1977), and fluoxetine (20–60 mg/day).
We are aware of several less common therapeutic alternatives for patients unresponsive or unsuited to the primary medication choices. For example, gamma-hydroxybutyrate (5.25–6.75 g in divided doses throughout the nocturnal sleep period) has been reported to consolidate sleep, reduce cataplexy, and reduce the need for adjutant stimulant medication (Broughton and Mamalak, 1976). However, this medication presently is in liquid form and requires one or more administrations during the hours of intended sleep. Furthermore, this drug is only available in the United States through federally approved research protocols. Kales et al. (1979) reported some success with high levels of propranolol (280–480 mg). However, in our hands, propranolol was not useful in clinical trials. In any event, the vast majority of physicians initially use stimulants alone or, when cataplexy is present, in combination with tricyclic antidepressants. Fry et al. (1986) have reported some success with codeine (150 mg/day in divided doses). Recently, Mouret et al. (1988) reported that l-tyrosine (64–120 mg/kg/day) given over a period of 6 months eventually eliminated the symptoms of daytime sleep attacks and cataplexy in eight French narcoleptics. It is, however, especially difficult to evaluate the initial l-tyrosine data from France, since the CNS stimulant medications, routinely used for narcolepsy in the United States and other countries, are not available in France. Hence, French narcoleptics have few alternative treatments to compare with l-tyrosine.
Table 2 presents the most common currently available antinarcoleptic drugs and their dosages, as well as several of the more actively investigated experimental pharmacotherapies (after Guilleminault, 1989).
TABLE 2.
Narcolepsy drugs currently available (after Guilleminault, 1989)
| Drug | Maximal dosage (all drugs administered p.o.) |
|---|---|
| Treatment of excessive daytime somnolence (EDS) | |
| Stimulants | |
| Amphetamine | <40 mg/day |
| Methylphenidate | <60 mg/day |
| Mazindol | <5 mg/day |
| Pemoline | <150 mg/day |
| Adjunct effect drugs (i.e., improve EDS if associated with stimulant) | |
| Protriptyline | <10 mg/day |
| Viloxazine | <200 mg/day |
| Treatment of cataplexy, sleep paralysis, and hypnagogic hallucinations | |
| Tricyclic antidepressants (with atropinic side-effects) | |
| Protriptyline | <20 mg/day |
| Imipramine | <200 mg/day |
| Clomipramine | <200 mg/day |
| Desipramine | <200 mg/day |
| Antidepressants (without atropinic side-effects) | |
| Viloxazine | <200 mg/day |
| Fluoxetine | <60 mg/day |
| Experimental drugs | |
| Codeine (given as stimulant) | |
| Cataplexy antagonist and mild stimulant: gamma-hydroxybutyrate |
It is believed that the stimulants function by facilitating catecholaminergic systems important for wakefulness (Jouvet, 1972; Mitler, 1976). Research studies with experimentally induced REM sleep in cats and naturally occurring narcolepsy in dogs suggest that the mode of action on REM-sleep-like symptoms of the tricyclic medications may have much to do with their anticholinergic properties (Lassen et al., 1975). However, it is possible that their capacity to block reuptake of norepinephrine and/or serotonin (Zung, 1969) contributes to their suppression of REM sleep and the dissociated components of REM sleep that trouble narcoleptic patients. This latter possibility is also supported by work from our group on viloxazine, a noradrenalin reuptake blocker.
In spite of the availability of potent stimulant medications, a sizable portion of today’s narcoleptics regard themselves as poorly controlled, according to survey statistics by the American Narcolepsy Association (personal communication; Foster-Rawlings and Dement, 1985).
Education
If narcoleptic patients and their families come to understand the neurological nature of narcolepsy, the clinician can minimize problems for the patient stemming from common misconceptions concerning low intelligence and poor motivation. The clinician should also explain that symptoms can spontaneously worsen or improve. Many patients report that symptoms worsen with aging, sleep disruption, and other changes in the work-rest schedule. On the other hand, women sometimes report improvement in symptoms after menopause. This kind of report, coupled with the fact that narcolepsy emerges during or after puberty, suggest that neuroendocrine mechanisms are somehow involved. Another important point for patient education is that changes in the severity of symptoms and medication use must be reported to the managing clinician. Such reports will enable the clinician to improve treatment and assist the patient in safety and work-related planning.
The American Narcolepsy Association (335 Quarry Road, Belmont, CA 94002) offers helpful educational materials. Furthermore, there are self-help groups throughout the country that meet local needs such as (1) identifying pharmacies with good service and adequate supplies of antinarcoleptic medication, (2) acting as an advocate in local disability and discrimination issues, and (3) providing understanding social contacts. These services are extremely important for the individual narcoleptic patient. The main problem with such self-help groups is that they frequently have no medical guidance, and some members may not actually have narcolepsy. Thus, misinformation about nonmedical treatments (e.g., special diets, exercises, etc.) is frequently exchanged in these groups—often by the testimony of a non-narcoleptic and to the detriment of bona fide narcoleptics.
Viloxazine
Viloxazine is derived from propranolol (Inderal), a cardiovascular drug with significant beta-adrenoceptor blocking activity. Viloxazine hydrochloride has a significant, and probably specific, noradrenergic reuptake blocking property. On the other hand, viloxazine does not appear to have anticholinergic properties. Viloxazine hydrochloride is rapidly and almost completely absorbed after oral ingestion. The drug’s plasma half-life is 2–4 h, with a mean of 3.5 h. In Europe it has been widely and satisfactorily used as an antidepressant drug but has not been tested systematically in narcoleptic subjects. Robert Clark (personal communication, 1983), from Columbus, Ohio, in an open, nonsystematic trial, found that some narcoleptic patients reported subjective symptomatic improvements with this drug. A systematic multicenter study was then planned, and the preliminary results were published (Guilleminault et al., 1986).
This multicenter study involved our group’s collaboration with the sleep research facilities at Stanford University (C. Guilleminault) and Hôpital du Sacre-Coeur in Montreal (J. Montplaisir). We assessed the anticataplectic effects of viloxazine (100 mg/day). We did not anticipate that viloxazine would have major alerting properties. Therefore, the protocol permitted physicians, at their discretion, to prescribe a stimulant drug (e.g., methylphenidate or dextroamphetamine) in addition to the study capsules. This adjutant medication feature was insisted upon by two institutional review boards so that subjects with disabling sleepiness could participate in the project and still drive, work, etc. If a subject was prescribed such an adjutant, he/she took the medication every day at the prescribed time throughout the project.
Admission criteria required freedom from significant medical illness other than narcolepsy and freedom from psychiatric disorders. Patients also had to have the following: (1) a history of excessive somnolence; (2) at least one of the REM sleep-related symptoms of sleep paralysis, hypnagogic hallucinations, or cataplexy; (3) nocturnal polysomnography ruling out sleep apnea syndrome; and (4) two or more transitions to REM sleep on the MSLT.
A total of 56 narcoleptic subjects were polygraphically monitored during placebo followed by viloxazine treatment and another placebo condition. Symptoms and side-effects were evaluated subjectively and objectively, using a battery of tests including the MSLT, Maintenance of Wakefulness Test (MWT), and the Wilkinson Addition Test (WAT). Data analysis was by means of nonparametric rank statistics for all parameters. Although all data analyses have not yet been done, selected results for 36 patients who underwent both MSLT and MWT appear in Table 3.
TABLE 3.
Selected results for viloxazine (100 mg) in 36 narcoleptics
| Observed means condition |
p values |
|||||
|---|---|---|---|---|---|---|
| Parameters | Placebo | Treatment | Withdrawal | Placebo versus treatment | Treatment versus withdrawal | Placebo versus withdrawal |
| MSLT sleep latency | 2.88 | 3.33 | 2.74 | 0.0796 | 0.0086 | NS |
| MWT sleep latency | 7.09 | 8.39 | 7.26 | 0.0326 | 0.0317 | NS |
| Number of MWT REM sleep periods | 2.28 | 1.58 | 2.44 | 0.0007 | 0.001 | NS |
| Percent of WAT problems solved | 89.12 | 91.84 | 92.05 | 0.0239 | NS_ | 0.0097 |
| Sleep attacks | 4.00 | 3.41 | 4.49 | 0.0435 | 0.0003 | 0.0807 |
| Cataplectic attacks | 2.94 | 1.77 | 2.62 | 0.0001 | 0.0183 | 0.0782 |
| Sleep paralysis | 2.39 | 1.47 | 2.00 | NS | NS | NS |
| Hallucinations | 2.57 | 1.37 | 2.47 | NS | NS | NS |
Scale for subjective symptom severity is as follows. For sleep and cataplectic attacks: 0, never: 1, < 1/wk; 2, 1–3/wk; 3,4–6/wk; 4, 1/day; 5, 2–3/day; 6, 4–6/day; 7, 7–10/day; 8, 11–15/day; and 9, > 15/day. For sleep paralysis and hallucinations: 0, never: 1, <1/yr; 2, 1/yr; 3, 2–11/yr; 4, 1/mo; 5, 2–3/mo; 6, 1/wk; 7, 2–6/wk; 8, 1/day; and 9, >1/day.
Overall, our data confirmed the significant anticataleptic and REM-sleep-suppressing effects of viloxazine. There also appeared to be a mild alerting effect that was detected by both the MSLT and the MWT, according to an analysis of variance of the rank orders of sleep latencies. The MWT appeared to be slightly more sensitive than the MSLT. The placebo-viloxazine significance levels were p <0.08 for the MSLT and p < 0.03 for the MWT. Both tests detected a significant viloxazine-withdrawal difference well beyond p < 0.05.
Other Stimulants
In separate interrelated studies performed by our group, five groups of narcoleptic patients were studied: 13 narcoleptic patients on methylphenidate (5 women and 8 men; mean age, 50.7 ± 13.8 years); 14 narcoleptic patients on pemoline (10 women and 4 men; mean age, 39.8 ± 9.0 years); 10 narcoleptic patients on protriptyline (5 women and 5 men; mean age, 48.1 ± 15.1 years); 5 narcoleptic patients on dextro-amphetamine (mean age, 39.4 ± 14.4 years); and 9 control subjects with no sleep disorder (5 women and 4 men, mean age, 39.2 ± 8.4 years). Narcoleptic patients were admitted to one and only one treatment. Admission criteria were the same for the multicenter study.
Low, intermediate, and high dose levels of the study drugs were as follows: methylphenidate (10, 30, 60 mg), pemoline (18.75, 56.25, 112.5 mg), protriptyline (10, 30, 60 mg), and dextroamphetamine (10, 30,60 mg). Subjects took their medications three times per day at 0700–0800, 1100–1200, and 1500–1600 h by swallowing identically appearing opaque capsules especially prepared by our pharmacy so that each capsule contained one-third of the assigned daily dose of experimental drug. Testing occurred throughout the seventh day on each dose level. The order of dose levels was under constrained randomization from subject to subject. The control subjects were given nothing on baseline and then, on subsequent testing separated by 7 days, three placebo capsules at the appointed times. Control subjects were led to believe that the placebos contained either a low, an intermediate, or a high dose of a stimulant drug.
Testings
Subjects were studied with four day-long evaluations involving at least four separate testing sessions separated by ~2 h that comprised MWT (Browman et al., 1982; Mitler et al., 1982; Browman et al., 1983), the WAT (Wilkinson, 1968), the Digit-Symbol Substitution Test (DSST), and a clinical status questionnaire. Testing was done first when the subjects were drug-free and three more times at weekly intervals, when they were on a low, an intermediate, or a high dose of experimental drug.
Data were analyzed by means of one-way, repeated measures, analyses of variance for each group for dose level and for order of testing (i.e., first testing day versus second, first versus third, etc.). We found many significant treatment dose-level effects as well as order-of-testing effects for narcoleptic subjects. There were also order-of-testing effects for the control group. The order effects were most prominent in the performance tests and were considered to be related to practice and increased familiarity with the testing environment. It was, therefore, necessary to remove the order-of-testing effect from the data for narcoleptic subjects. This was accomplished by standardizing, with respect to testing order, the data for each narcoleptic. The standardizing process consisted of expressing each narcoleptic’s test or questionnaire scores as percentages of those control group means that corresponded to whether it was the narcoleptic’s first, second, third, or fourth testing day. For example, the mean MWT score for a narcoleptic on his second testing day, regardless of the drug or dose level assigned, was divided by the second testing day MWT mean for the control group. We then reanalyzed the standardized data for effect of dose level by one-way, repeated measures analyses of variance. Contrasts between each of the conditions (baseline, dose level 1, dose level 2, and dose level 3) were done with Dunnett’s t tests.
An alternative method of standardizing for order-of-testing effects is to create difference scores, rather than ratio scores as we have done. We have undertaken this approach and have found results to be qualitatively similar to those presented below.
Methylphenidate
The control group’s data are displayed chronologically (baseline, placebo, placebo, and placebo); the narcoleptics’ data have been standardized for order-of-testing effects and are presented as averaged percents of those control means that were appropriate for each narcoleptic’s particular sequence of testing. These average percents are displayed according to ascending dose levels (Table 4).
TABLE 4.
Methylphenidate in narcolepsy
| Baseline | Placebo or 10 mg | Placebo or 30 mg | Placebo or 60 mg | |
|---|---|---|---|---|
| Wilkinson Addition Test (problems attempted) | ||||
| Controls | 100.0 ± 24 | 118.4 ± 32 | 123.4 ± 38 | 123.4 ± 38 |
| Methylphenidate | 75.3% | 68.9% | 69.9% | 75.1% |
| Digit-Symbol Substitution Test (problems attempted) | ||||
| Controls | 731.3 ± 114 | 811.9 ± 159 | 843.2 ± 16 | 849.7 ± 154 |
| Methylphenidate | 65.1% | 70.0% | 75.4% | 78.5% |
| Maintenance of Wakefulness Test (sleep latency) | ||||
| Controls | 18.9 ± 2 | 19.2 ± 2 | 18.0 ± 4 | 17.6 ± 4 |
| Methylphenidate | 55.2% | 70.7% | 68.8% | 79.9% |
| Subjective sleepiness (rating) | ||||
| Controls | 24.0 ± 6 | 22.0 ± 5 | 20.9 ± 6 | 19.9 ± 6 |
| Methylphenidate | 191.0% | 193.3% | 194.7% | 173.1% |
| Subjective cataplexy (rating) | ||||
| Controls | 12.0 ± 0 | 12.0 ± 0 | 12.0 ± 0 | 12.0 ± 0 |
| Methylphenidate | 188.1% | 131.4% | 155.8% | 168.6% |
| Subjective symptoms overall (rating) | ||||
| Controls | 74.3 ± 8 | 71.0 ± 6 | 70.0 ± 8 | 69.6 ± 8 |
| Methylphenidate | 186.3% | 154.6% | 160.7% | 154.4% |
With respect to performance on the WAT and the DSST, narcoleptics were clearly inferior to controls in both tests on all testing sessions (p < 0.05 for all). The control group showed a practice effect on the WAT and DSST (p < 0.001), with the second, third, and fourth testing sessions all differing from baseline. However, only on the DSST did the methylphenidate group show significant treatment-related increases in the numbers of problems attempted (p < 0.05), with the 30- and 60-mg dose levels differing significantly from baseline. With respect to quality of performance (percent correct), however, methylphenidate produced no overall treatment effect. On the MWT, controls showed no adaptation effect. Narcoleptics treated with methylphenidate did show a treatment effect with the 10-mg and 60-mg conditions differing significantly from baseline (p < 0.05 and p < 0.01, respectively). With respect to subjective symptoms, the control group showed no treatment effect. The methylphenidate group showed a mild treatment-related improvement in subjective sleepiness with 60 mg differing from baseline (p < 0.05), no improvement in cataplexy, and a significant improvement in symptoms overall with every dose level differing from baseline (p < 0.05 for all).
In conclusion, in narcolepsy the psychostimulant methylphenidate improves the profound discrepancy between narcoleptics and controls on performance, ability to stay awake, and sleep-related symptoms. Specifically, methyiphenidate improves ability to remain awake and brings about a subjective improvement in narcolepsy symptoms, but it has only marginal effects on quantity of performance and does not improve quality of performance.
Pemoline
The control group’s data are displayed chronologically (baseline, placebo, placebo, and placebo). As with the methylphenidate data, pemoline data have been standardized for order-of-test effects, are presented as averaged percents of control means, and are displayed in order of ascending dose level (Table 5).
TABLE 5.
Pemoline in narcolepsy
| Baseline | Placebo or 18.75 mg | Placebo or 56.25 mg | Placebo or 112.5 mg | |
|---|---|---|---|---|
| Wilkinson Addition Test (problems attempted) | ||||
| Controls | 100.0 ± 24 | 118.4 ± 32 | 123.4 ± 38 | 123.4 ± 38 |
| Pemoline | 68.6% | 68.3% | 68.8% | 68.6% |
| Digit-Symbol Substitution Test (problems attempted) | ||||
| Controls | 731.3 ± 114 | 811.9 ± 159 | 843.2 ± 160 | 849.7 ± 154 |
| Pemoline | 72.6% | 70.7% | 74.2% | 78.3% |
| Maintenance of Wakefulness Test (sleep latency) | ||||
| Controls | 18.9 ± 2 | 19.2 ± 2 | 18.0 ± 4 | 17.6 ± 4 |
| Pemoline | 40.2% | 39.6% | 48.0% | 53.4% |
| Subjective sleepiness (rating) | ||||
| Controls | 24.0 ± 6 | 22.0 ± 5 | 20.9 ± 6 | 19.9 ± 6 |
| Pemoline | 198.5% | 238.3% | 178.8% | 172.9% |
| Subjective cataplexy (rating) | ||||
| Controls | 12.0 ± 0 | 12.0 ± 0 | 12.0 ± 0 | 12.0 ± 0 |
| Pemoline | 148.2% | 156.5% | 151.2% | 149.8% |
| Subjective symptoms overall (rating) | ||||
| Controls | 74.3 ± 8 | 71.0 ± 6 | 70.0 ± 8 | 69.6 ± 8 |
| Pemoline | 182.9% | 206.1% | 187.7% | 186.7% |
With respect to performance on the WAT and the DSST, narcoleptics were clearly inferior to controls in both tests on all testing sessions (p < 0.05 for all). Only on the DSST did the pemoline group show significant treatment-related increases in the number of problems attempted (p < 0.05), with the highest dose differing significantly from baseline. There also appeared to be a trend on the WAT for the pemoline group to perform more accurately in a dose-dependent fashion (91.5, 92.7, 93.2, 94.0% for baseline through the 112.5-mg condition, respectively). Narcoleptics treated with pemoline did show a significant treatment effect in MWT sleep latencies with the baseline and 18.75-mg conditions differing from the 112.5-mg condition (p < 0.05). The pemoline group showed a significant treatment-related improvement in subjective sleepiness, with the higher two dose levels differing from the initial dose level. There was a nonsignificant increase in symptom severity between baseline and the 18.75-mg dose level. There was no improvement in cataplexy and no significant improvement in symptoms overall.
In conclusion, narcoleptics treated with the psychostimulant pemoline show significant improvement in the ability to stay awake, the quality of performance, and subjective severity of symptoms, but only at the 112.5-mg dose level. There is also a trend toward dose-dependent improvement in quality of performance on cognitive tasks. However, with the dose levels studied, no parameter for narcoleptics even approached nonnarcoleptic control levels.
Dextroamphetamine
We have just begun to study dextroamphetamine. We report preliminary results for five subjects herein the same format as for the other drugs studied.
The control group’s data are displayed chronologically (baseline, placebo, placebo, and placebo); the dextroamphetamine data are displayed according to ascending dose levels. Because of the small number of subjects and the high intersubject variability only the DSST, MWT, and overall symptoms parameters show significant drug effects (Table 6).
TABLE 6.
Dextroamphetamine in narcolepsy
| Baseline | Placebo or 10 mg | Placebo or 30 mg | Placebo or 60 mg | |
|---|---|---|---|---|
| Wilkinson Addition Test (problems attempted) | ||||
| Controls | 100.0 ± 24 | 118.4 ± 32 | 123.4 ± 38 | 123.4 ± 38 |
| Dextroamphetamine | 83.0% | 88.9% | 77.6% | 72.9% |
| Digit-Symbol Substitution Test (problems attempted) | ||||
| Controls | 731.3 ± 114 | 811.9 ± 159 | 843.2 ± 16 | 849.7 ± 154 |
| Dextroamphetamine | 68.6% | 61.2% | 75.4% | 78.1% |
| Maintenance of Wakefulness Test (sleep latency) | ||||
| Controls | 18.9 ± 2 | 19.2 ± 2 | 18.0 ± 4 | 17.6 ± 4 |
| Dextroamphetamine | 34.8% | 32.0% | 52.2% | 70.3% |
| Subjective sleepiness (rating) | ||||
| Controls | 24.0 ± 6 | 22.0 ± 5 | 20.9 ± 6 | 19.9 ± 6 |
| Dextroamphetamine | 165.6% | 125.0% | 99.5% | 90.9% |
| Subjective cataplexy (rating) | ||||
| Controls | 12.0 ± 0 | 12.0 ± 0 | 12.0 ± 0 | 12.0 ± 0 |
| Dextroamphetamine | 151.7% | 175.0% | 198.3% | 146.7% |
| Subjective symptoms overall (rating) | ||||
| Controls | 74.3 ± 8 | 71.0 ± 6 | 70.0 ± 8 | 69.6 ± 8 |
| Dextroamphetamine | 146.6% | 167.9% | 123.4% | 108.4% |
With respect to performance on the WAT and the DSST, narcoleptics were clearly inferior to controls in both tests on all testing sessions (p < 0.05 for all). The dextroamphetamine group showed significant treatment-related increases in the number of DSST problems attempted (p < 0.05), with only the 30-mg and 60-mg sessions differing significantly from the 10-mg dose. On the MWT, controls showed no practice effect. Narcoleptics treated with dextroamphetamine did show a treatment effect with only the 30-mg and 60-mg conditions differing significantly from baseline (p < 0.05 and p < 0.01, respectively). The dextroamphetamine group showed a remarkable treatment-related improvement in subjective sleepiness and symptoms overall with the 30-mg and 60-mg dose levels differing from baseline (p < 0.05 for all).
In conclusion, the psychostimulant dextroamphetamine in narcolepsy appears to improve the profound discrepancy between narcoleptics and controls on DSST performance, ability to stay awake, and sleep-related symptoms.
Protriptyline
The control group’s data are displayed chronologically (baseline, placebo, placebo, and placebo). The protriptyline data are displayed in the same manner as for the previous drugs (Table 7).
TABLE 7.
Protriptyline in narcolepsy
| Baseline | Placebo or 10 mg | Placebo or 30 mg | Placebo or 60 mg | |
|---|---|---|---|---|
| Wilkinson Addition Test (problems attempted) | ||||
| Controls | 100.0 ± 24 | 118.4 ± 32 | 123.4 ± 38 | 123.4 ± 38 |
| Protriptyline | 71.3% | 64.0% | 67.4% | 57.0% |
| Digit-Symbol Substitution Test (problems attempted) | ||||
| Controls | 731.3 ± 114 | 811.9 ± 159 | 843.2 ± 160 | 849.7 ± 154 |
| Protriptyline | 55.9% | 57.6% | 58.7% | 52.0% |
| Maintenance of Wakefulness Test (sleep latency) | ||||
| Controls | 18.9 ± 2 | 19.2 ± 2 | 18.0 ± 4 | 17.6 ± 4 |
| Protriptyline | 49.1% | 48.2% | 49.3% | 54.5% |
| Subjective sleepiness (rating) | ||||
| Controls | 24.0 ± 6 | 22.0 ± 5 | 20.9 ± 6 | 19.9 ± 6 |
| Protriptyline | 202.1% | 183.2% | 165.7% | 176.8% |
| Subjective cataplexy (rating) | ||||
| Controls | 12.0 ± 0 | 12.0 ± 0 | 12.0 ± 0 | 12.0 ± 0 |
| Protriptyline | 212.9% | 117.5% | 104.2% | 121.7% |
| Subjective symptoms overall (rating) | ||||
| Controls | 74.3 ± 8 | 71.0 ± 6 | 70.0 ± 8 | 69.6 ± 8 |
| Protriptyline | 195.0% | 156.9% | 152.8% | 152.6% |
With respect to performance on the WAT and the DSST, narcoleptics were clearly inferior to controls in both tests on all testing sessions (p < 0.05 for all). The protriptyline group showed no significant treatment-related increases on any performance measure. Narcoleptics treated with protriptyline also showed no treatment effect in ability to stay awake. The protriptyline group showed no treatment-related improvement in subjective sleepiness but did show marked improvement over baseline in cataplexy (p < 0.01 for all) and a significant improvement in symptoms overall with every dose level differing from baseline (p < 0.05 for all).
In conclusion, the antidepressant protriptyline in narcolepsy did not improve the profound discrepancy between narcoleptics and controls on performance, ability to stay awake, and symptoms of sleepiness. However, protriptyline markedly improved the symptom of cataplexy and was also judged by patients to improve symptoms overall.
CONCLUSIONS
Our data to date indicate profound deficits in performance and ability to stay awake in narcoleptics. Clearly these findings should be replicated with more narcoleptics and a larger, matched control group.
We have demonstrated that we can measure objective and subjective therapeutic efficacy of several medications and that other medications have limited therapeutic efficacy. With some medication-parameter cells, such as with methylphenidate or dextroamphetamine and DSST or MWT scores, measurements for narcoleptics approach control levels (i.e., are normalized). Generally, our data point to the CNS stimulants dextroamphetamine and methylphenidate as treatment options that can diminish differences in some performance measures and alertness between narcoleptics and controls. At least as important, our data show that the lower doses of pemoline and doses of protriptyline up to 60 mg/day are not efficacious for the sleepiness-related symptoms of narcolepsy.
It is clear that protriptyline was the only medication that significantly improved cataplexy at all dose levels studied. In a separate study, we found that viloxazine (100 mg/day) was also quite effective in controlling cataplexy.
There are, however, several limitations in our data to date. First, we have not always used a placebo condition as a control—only a “no-drug” baseline. Second, we have not employed the most stringent inclusion criteria that are now being suggested for narcolepsy research, namely, seropositivity for HLA-DR2 and DQw1 in white and Japanese subjects and HLA-DQw1 in black subjects (Narcolepsy: Third International Symposium, June 10, 1988, San Diego). Third, we have used the MWT; although a sensitive research tool, it is not widely used throughout the field of sleep disorders research. Fourth, we have not employed a performance test that directly addresses reaction time and errors of omission—the most common types of errors reported by narcoleptic patients.
We have not tailored our medication regimes to individual patients and therefore may not have optimized the therapeutic effects our test drugs can have. In clinical practice, various nap and drug scheduling seems to greatly improve pharmacologic control of symptoms. These techniques include timing of stimulant medication to minimize nocturnal sleep disturbance, substituting an afternoon nap for an afternoon CNS stimulant, and frequent drug holidays (1–2 days without stimulant medication).
Key questions for future research include: (1) Can other drugs for narcoleptics be used alone or in combination so that patients can function at levels comparable to age, sex, and educationally matched controls? (2) Can commonly used tests such as the MSLT and widely available, easily administered computer-based attention tests be used to replicate our past work with methylphenidate, pemoline, and dextroamphetamine, and to evaluate other drugs, such as mazindol and common drug combinations? (3) How does l-tyrosine compare with the usual therapies available in the United States?
Acknowledgment
This work was supported by NINCDS grants R01 NS20459 and RR00833 and a grant from the American Narcolepsy Association. This is publication number 6106-NP from the Research Institute of Scripps Clinic.
REFERENCES
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility and concomitant phenomena during sleep. Science. 1953;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- Association of Sleep Disorders Centers. Committee on somnolent patients (Mitler MM, chairman). Resolution on reporting patients with excessive somnolence. Assoc Sleep Disord Cent Newslett. 1984;6:14–16. [Google Scholar]
- Association of Sleep Disorders Centers. Diagnostic classification of sleep and arousal disorders prepared by the sleep disorders classification committee (Roffwarg HP, chairman), 1st ed. Sleep. 1979;2:1–137. [PubMed] [Google Scholar]
- Baker T, Foutz A, McNerney V, Mitler M, Dement W. Canine model of narcolepsy: genetic and developmental determinants. Exp Neurol. 1982;75:729–742. doi: 10.1016/0014-4886(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Baldy-Moulinier M, Arguner A, Basset A. Ultradian and circadian rhythms in sleep and wakefulness. In: Guilleminault C, Dement W, Passouant P, Weitzman E, editors. Narcolepsy. Holliswood, NY: Spectrum Publications; 1976. pp. 485–488. (Advances in sleep research; vol 3.) [Google Scholar]
- Baxter B. Induction of both emotional behavior and a novel form of REM sleep by chemical stimulation applied to cat mesencephalon. Exp Neurol. 1969;23:220–229. doi: 10.1016/0014-4886(69)90059-4. [DOI] [PubMed] [Google Scholar]
- Boehme R, Baker TL, Mefford I, Ciaranello R, Dement W. Muscarinic cholinergic receptor abnormalities in canine narcolepsy. Sleep Res. 1982;11:45. [Google Scholar]
- Broughton R, Ghanem Q. The impact on compound narcolepsy in the life of the patient. In: Guilleminault C, Dement W, Passouant P, Weitzman E, editors. Narcolepsy. Holliswood, NY: Spectrum Publications; 1976. pp. 201–220. (Advances in sleep research. vol 3.) [Google Scholar]
- Broughton R, Mamalak M. Gamma-hydroxy-butyrate in the treatment of narcolepsy: a preliminary report. In: Guilleminault C, Dement W, Passouant P, Weitzman E, editors. Narcolepsy. Holliswood, NY: Spectrum Publications; 1976. pp. 659–667. (Advances in sleep research. vol. 3.) [Google Scholar]
- Browman C, Gujavarty K, Sampson M, Mitler M. REM sleep episodes during the maintenance of wakefulness test in patients with sleep apnea syndrome and patients with narcolepsy. Sleep. 1983;6:23–28. doi: 10.1093/sleep/6.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman C, Gujavarty K, Yolles S, Sampson M, Mitler M. Narcolepsy daytime performance, and ability to maintain wakefulness: evaluation of treatment and placebo effects. Sleep Res. 1982;12:229. [Google Scholar]
- Browman CP, Gujavarty KS, Yolles SF, Mitler MM. Forty-eight hour polysomnographic evaluation of narcolepsy. Sleep. 1986;9:183–188. doi: 10.1093/sleep/9.1.183. [DOI] [PubMed] [Google Scholar]
- Browman CP, Mitler MM. Hypersomnia and the perception of sleep-wake states: some preliminary findings. Percept Mot Skills. 1988;66:463–470. doi: 10.2466/pms.1988.66.2.463. [DOI] [PubMed] [Google Scholar]
- Carskadon M, Dement W. Sleep studies on a 90-minute day. Electroencephalogr Clin Neurophysiol. 1975;39:145–155. doi: 10.1016/0013-4694(75)90004-8. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC, Mitler MM, Roth R, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–524. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Harvey K, Dement WC. Sleep loss in young adolescents. Sleep. 1981;4:299–312. doi: 10.1093/sleep/4.3.299. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Harvey K, Duke P, Anders RF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2:453–460. doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- Chase MH, Morales FR. Subthreshold excitatory activity and motoneuron discharge during REM periods of active sleep. Science. 1983;221:1195–1198. doi: 10.1126/science.6310749. [DOI] [PubMed] [Google Scholar]
- Clark RW, Schmidt HS, Schaal SF, Boudoulas H, Schuller DE. Sleep apnea: treatment with protriptyline. Neurology. 1979;29:1287–1292. doi: 10.1212/wnl.29.9_part_1.1287. [DOI] [PubMed] [Google Scholar]
- Coccagna G, Mantovani M, Brignani F, Parchi C, Lugaresi E. Continuous recording of the pulmonary and systemic arterial pressure during sleep in syndromes of hypersomnia with periodic breathing. Bull Physiopathol Respir. 1972;8:1159–1172. [PubMed] [Google Scholar]
- Coleman R. Diagnosis, treatment, and follow-up of about 8,000 sleep/wake disorders patients. In: Guilleminault C, Lugaresi E, editors. Sleep/wake disorders: natural history, epidemiology, and long-term evolution. New York: Raven Press; 1983. pp. 87–97. [Google Scholar]
- Coleman R, Roffwarg H, Kennedy S, et al. Sleep-wake disorders based on a polysomnographic diagnosis: a national co-operative study. JAMA. 1982;247:997–1003. [PubMed] [Google Scholar]
- Dement W, Carskadon M, Ley R. The prevalence of the narcolepsy II. Sleep Res. 1973;2:147. [Google Scholar]
- Dement W, Zarcone V, Varner V, et al. The prevalence of narcolepsy. Sleep Res. 1972;1:148. [Google Scholar]
- Faul KF, Zeller-DeAmicis LC, Radde L, Bowersox SS, Baker TL, Kilduff TS, Dement WC. Biogenic amine concentration in the brains of normal and narcoleptic canines: current studies. Sleep. 1986;9:107–110. doi: 10.1093/sleep/9.1.107. [DOI] [PubMed] [Google Scholar]
- Foster-Rawlings S, Dement WC. An ethography of narcolepsy. Paper presented at The Second International Symposium on Narcolepsy; July 6–7; Stanford University; 1985. [Google Scholar]
- Foutz A, Mitler M, Cavalli-Sforza L, Dement W. Genetic factors in canine narcolepsy. Sleep. 1979;1:413–422. doi: 10.1093/sleep/1.4.413. [DOI] [PubMed] [Google Scholar]
- Fry JM, Pressman MR, DiPhillipo MA, Forst-Paulus M. Treatment of narcolepsy with codeine. Sleep. 1986;9:269–274. doi: 10.1093/sleep/9.1.269. [DOI] [PubMed] [Google Scholar]
- Gassel M, Pompeiano O. Fusimotor function during sleep in unrestrained cats. Arch Ital Biol. 1963;103:347–348. [PubMed] [Google Scholar]
- Gelineau J. De la narcolepsie. Gaz d Hop (Paris) 1880;53:626–628. [Google Scholar]
- George M, Haslett W, Jenden D. A cholinergic mechanism in the brain stem reticular formation: Induction of paradoxical sleep. Int J Pharmacol. 1964;3:541–552. doi: 10.1016/0028-3908(64)90076-0. [DOI] [PubMed] [Google Scholar]
- Giaquinto S, Pompeiano O, Somogyi I. Reflex activity of extensor and flexor muscles following muscular afferent excitation during sleep and wakefulness. Experientia. 1963a;19:481–482. doi: 10.1007/BF02150660. [DOI] [PubMed] [Google Scholar]
- Giaquinto S, Pompeiano O, Somogyi I. Supraspinal inhibitory control of spinal reflexes during natural sleep. Experientia. 1963b;19:652–653. doi: 10.1007/BF02151302. [DOI] [PubMed] [Google Scholar]
- Glenn L, Dement W. Membrane potential, synaptic activity and excitability of hindlimb motoneurons during wakefulness and sleep. J Neurophysiol. 1981a;46:839–854. doi: 10.1152/jn.1981.46.4.839. [DOI] [PubMed] [Google Scholar]
- Glenn L, Dement W. Membrane resistance and rheobase of hindlimb motoneurons during wakefulness and sleep. J Neurophysiol. 1981b;46:1076–1088. doi: 10.1152/jn.1981.46.5.1076. [DOI] [PubMed] [Google Scholar]
- Glenn L, Dement W. Group I excitatory and inhibitory potentials in hindlimb motoneurons during wakefulness and sleep. J Neurophysiol. 1981c;46:1089–1101. doi: 10.1152/jn.1981.46.5.1089. [DOI] [PubMed] [Google Scholar]
- Guilleminault C. Narcolepsy syndrome. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Saunders; 1989. pp. 338–346. [Google Scholar]
- Guilleminault C, Dement W, Passouant P, Weitzman E, editors. Narcolepsy. Holliswood, NY: Spectrum Publications; 1976. (Advances in sleep research. vol 3.) [Google Scholar]
- Guilleminault C, Holloman J, Grumet C, Kilduff T, McDevitt HO, Dement WC, Mitler MM. HLA-DR2 and the narcolepsy syndrome: the Stanford experience. In: Honda Y, Juji T, editors. HLA in narcolepsy. Berlin-Heidelberg: Springer-Verlag; 1988. pp. 108–113. [Google Scholar]
- Guilleminault C, Mancuso J, Salva MAQ, Hayes B, Mitler M, Poirer G, Montplaisir J. Viloxazine hydrochloride in narcolepsy: a preliminary report. Sleep. 1986;9:275–279. doi: 10.1093/sleep/9.1.275. [DOI] [PubMed] [Google Scholar]
- Hartman PG, Scrima L. Muscle activity in the legs (MAL) associated with frequent arousals in narcoleptics, nocturnal myoclonus and obstructive sleep apnea (OSA) patient. Clin Electroencephalogr. 1986;17:181–186. [PubMed] [Google Scholar]
- Hill W. On some causes of backwardness and stupidity in children. Br Med J. 1898;2:711–712. doi: 10.1136/bmj.2.1500.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloman JD, Bell JI, Kilduff TS, Dement WC, Guilleminault C, McDevitt HO. HLA-DR restriction fragment-length polymorphisms in narcolepsy. J Neurosci Res. 1987;18:239–244. doi: 10.1002/jnr.490180134. [DOI] [PubMed] [Google Scholar]
- Honda Y, Asaka A, Tanaka Y, Juji T. Discrimination of narcoleptic patients by using genetic markers and HLA. Sleep Res. 1983;12:254. [Google Scholar]
- Honda Y, Juji T, editors. HLA in narcolepsy. Tokyo: Springer-Verlag; 1988. [Google Scholar]
- Jersild C, Fog T, Hansen GS, Thomsen M, Svejgaard A, DuPont B. Histocompatibility determinants in multiple sclerosis with special reference to clinical course. Lancet. 1973;2:1221. doi: 10.1016/s0140-6736(73)90970-7. [DOI] [PubMed] [Google Scholar]
- Jouvet M. Biogenic amines in sleep. Ergeb Physiol. 1972;64:166–307. doi: 10.1007/3-540-05462-6_2. [DOI] [PubMed] [Google Scholar]
- Juji T, Satake M, Honda Y, Doi Y. HLA antigens in Japanese patients with narcolepsy. Tissue Antigens. 1984;24:316–319. doi: 10.1111/j.1399-0039.1984.tb02144.x. [DOI] [PubMed] [Google Scholar]
- Kales A, Soldatos C, Cadieux R, Bixler E, Tjiauw-Ling T, Scharf M. Propranolol in the treatment of narcolepsy. Ann Intern Med. 1979;91:742–743. doi: 10.7326/0003-4819-91-5-741. [DOI] [PubMed] [Google Scholar]
- Kessler S. Genetic factors in narcolepsy. In: Guilleminault C, Dement W, Passouant P, Weitzman E, editors. Narcolepsy. Holliswood, NY: Spectrum Publications; 1976. pp. 285–302. (Advances in sleep research, vol 3.) [Google Scholar]
- Kilduff TS, Bowersox SS, Kaitin KI, Baker TL, Ciaranello RD, Dement WC. Muscarinic cholinergic receptors and the canine model of narcolepsy. Sleep. 1986;9:102–106. doi: 10.1093/sleep/9.1.102. [DOI] [PubMed] [Google Scholar]
- Knecht G, Oliver J, Redding R, Selcer R, Johnson G. Narcolepsy in a dog and a cat. J Vet Med Assoc. 1973;62:1052–1053. [PubMed] [Google Scholar]
- Kramer RE, Dinner DS, Braun WE, Zachary AA, Teresi GA. HLA DR2 and narcolepsy. Arch Neurol. 1987;44:853–855. doi: 10.1001/archneur.1987.00520200055019. [DOI] [PubMed] [Google Scholar]
- Kupfer D, Foster F. Interval between the onset of sleep and rapid eye movement as an indicator of depression. Lancet. 1972;2:684–686. doi: 10.1016/s0140-6736(72)92090-9. [DOI] [PubMed] [Google Scholar]
- Kurland LT, Mulder DW, Westlund KB. Multiple sclerosis and amyotrophic lateral sclerosis: etiologic significance of recent epidemiologic and genetic studies. N Engl J Med. 1955;252:649–697. doi: 10.1056/NEJM195504212521601. [DOI] [PubMed] [Google Scholar]
- Langdon N, Welsh KI, Van Dam M, Vaughn RV, Parkes D. Genetic markers in narcolepsy. Lancet. 1984;2:1178–1180. doi: 10.1016/s0140-6736(84)92742-9. [DOI] [PubMed] [Google Scholar]
- Lassen L, Peterson E, Kjellberg B, Olsson S. Comparative studies of the new 5-hydroxytryptamine uptake inhibitor and some tricyclic thymoleptics. Eur J Pharmacol. 1975;3:108–115. doi: 10.1016/0014-2999(75)90329-5. [DOI] [PubMed] [Google Scholar]
- Lock CB, So AK, Welsh KI, Parkes JD, Trowsdale J. MHC class II sequences of an HLA-DR2 narcoleptic. Immunogenetics. 1988;27:449–455. doi: 10.1007/BF00364432. [DOI] [PubMed] [Google Scholar]
- Mefford I, Baker T, Boehme R, Foutz A, Ciaranello R, Barchas J, Dement W. Narcolepsy: biogenic amine deficits in an animal model. Science. 1983;220:629–632. doi: 10.1126/science.6188216. [DOI] [PubMed] [Google Scholar]
- Mitler M. Toward an animal model of narcolepsy-cataplexy. In: Guilleminault C, Dement W, Passouant P, Weitzman E, editors. Narcolepsy. Holliswood, NY: Spectrum Publications; 1976. pp. 387–409. (Advances in sleep research, vol 3.) [Google Scholar]
- Mitler M. The multiple sleep latency test as an evaluation for excessive somnolence. In: Guilleminault C, editor. Sleeping and waking disorders: indications and techniques. Menlo Park, CA: Addison-Wesley; 1982. pp. 145–153. [Google Scholar]
- Mitler M, Boysen B, Campbell L, Dement W. Narcolepsy-cataplexy in a female dog. Exp Neurol. 1974;45:332–340. doi: 10.1016/0014-4886(74)90122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitler M, Carskadon MA, Czeisler CA, Dement WC, Dinges DF, Graeber C. Sleep, catastrophes and public policy. Sleep. 1988a;11:100–109. doi: 10.1093/sleep/11.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitler M, Dement W. Cataplectic-like behavior in cats after microinjections of carbachol in pontine reticular formation. Brain Res. 1974;69:335–343. doi: 10.1016/0006-8993(74)90402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitler M, Gujavarty K, Browman C. Maintenance of wakefulness test: a polysomnographic technique for evaluating treatment in patients with excessive somnolence. Electroencephalogr Clin Neurophysiol. 1982;53:658–661. doi: 10.1016/0013-4694(82)90142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitler MM, Nelson S, Hajdukovic R. Narcolepsy: diagnosis treatment and management. In: Erman MK, editor. Psychiatric Clinics of North America: sleep disorders. Philadelphia, PA: W. B. Saunders; 1988b. pp. 593–606. [PubMed] [Google Scholar]
- Mitler M, van den Hoed J, Carskadon M, Richardson G, Park R, Guilleminault C, Dement W. REM sleep episodes during the Multiple Sleep Latency Test in narcoleptic patients. Electroencephalogr Clin Neurophysiol. 1979;46:479–481. doi: 10.1016/0013-4694(79)90149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouret J, Lemoine P, Sanchez P, Robeline N, Taillard J, Canini F. Treatment of narcolepsy with l-tyrosine. Lancet. 1988;2:1458–1459. doi: 10.1016/s0140-6736(88)90935-x. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Goldberg LJ, Chandler SH, Chase MH. Intracellular analysis of trigeminal motoneuron activity during sleep in the cat. Science. 1978;199:204–207. doi: 10.1126/science.202025. [DOI] [PubMed] [Google Scholar]
- Neely S, Rosenberg R, Spire JP, Antel J, Arnason BGW. HLA antigens in narcolepsy. Neurology. 1987;37:1858–1860. doi: 10.1212/wnl.37.12.1858. [DOI] [PubMed] [Google Scholar]
- Opelz G, Terasaki P, Myers L, Ellison G, Ebers G, Zabriskie J, Weiner H, Kempe H, Sibley W. The association of HLA antigens A3, B7, and Dw2 with 330 multiple sclerosis patients in the United States. Tissue Antigens. 1977;9:54–58. doi: 10.1111/j.1399-0039.1977.tb01080.x. [DOI] [PubMed] [Google Scholar]
- Orr W, Martin R, Imes N, Rogers R, Stahl M. Hypersomnolent and non-hypersomnolent patients with upper airway obstruction during sleep. Chest. 1979;75:418–422. doi: 10.1378/chest.75.4.418. [DOI] [PubMed] [Google Scholar]
- Panayi GS, Wooley P, Batchelor JR. Genetic basis of rheumatoid disease: HLA antigens, disease manifestations and toxic reactions to drugs. Br Med J. 1978;2:1326–1328. doi: 10.1136/bmj.2.6148.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Wolpert W, Dement W, Mitchell S, Fischer C. Nocturnal sleep of narcoleptics. Electroencephalogr Clin Neurophysiol. 1963;15:599–609. doi: 10.1016/0013-4694(63)90032-4. [DOI] [PubMed] [Google Scholar]
- Reinertsen JL, Klippel JH, Johnson AH, Steinberg AD, Decker JL, Mann DL. Blymphocyte antigens associated with SLE. N Engl J Med. 1978;299:515–518. doi: 10.1056/NEJM197809072991004. [DOI] [PubMed] [Google Scholar]
- Richardson G, Carskadon M, Flagg W, van den Hoed J, Dement W, Mitler M. Excessive daytime sleepiness in man: multiple sleep latency measurement in narcoleptic and control subjects. Electroencephalogr Clin Neurophysiol. 1978;45:621–627. doi: 10.1016/0013-4694(78)90162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RL, Hajdukovich RM, Mitler MM. HLA-DR2 association with excessive somnolence in narcolepsy does not generalize to sleep apnea and is not accompanied by systemic autoimmune abnormalities. Clin Immunol Immunopathol. 1988;49:149–158. doi: 10.1016/0090-1229(88)90104-3. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Clark R, Hyman P. Protriptyline: an effective agent in the treatment of narcolepsy-cataplexy syndrome and hypersomnia. Am J Psychiatry. 1977;134:183–185. doi: 10.1176/ajp.134.2.183. [DOI] [PubMed] [Google Scholar]
- Schmidt HS, Fortin LD. Electronic pupillography in disorders of arousal. In: Guilleminault C, editor. Sleeping and waking disorders: indications and techniques. Menlo Park, CA: Addison-Wesley; 1982. pp. 127–143. [Google Scholar]
- Scrima L. An etiology of narcolepsy-cataplexy and a proposed cataplexy neuromechanism. Int J Neurosci. 1981;15:69–86. doi: 10.3109/00207458108985846. [DOI] [PubMed] [Google Scholar]
- Seignalet J, Billiard M. Possible association between HLA-B7 and narcolepsy. Tissue Antigens. 1984;23:188–189. doi: 10.1111/j.1399-0039.1984.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Silberman EK, Vivaldi E, Garfield J, McCarley RW, Hobson JA. Carbachol triggering of desynchronized sleep phenomena: enhancement via small volume infusions. Brain Res. 1980;191:215–224. doi: 10.1016/0006-8993(80)90324-8. [DOI] [PubMed] [Google Scholar]
- Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Jimbo M. Polygraphic study of narcoleptic syndrome, with special reference to hypnagogic hallucinations and cataplexy. Folia Psychiatr Neurol Jpn. 1963;7 (suppl):343. [Google Scholar]
- van den Hoed J, Kraemer H, Guilleminault C, Zarcone VP, Miles LE, Dement WC, Mitler MM. Disorders of excessive daytime somnolence: polysomnographic and clinical data for 100 patients. Sleep. 1981;4:23–37. doi: 10.1093/sleep/4.1.23. [DOI] [PubMed] [Google Scholar]
- Vivaldi E, McCarley R, Hobson J. Evocation of desynchronized sleep signs by carbachol filled micropipettes in the pontine brain stem. Sleep Res. 1979;8:88. [Google Scholar]
- Weitzman E, Nogeire C, Perlow M, Fukushima D, Sassin J, McGregor P, Gallagher T, Hellman L. Effects of a prolonged three-hour sleep-wake cycle on sleep stages, plasma cortisol, growth hormone and body temperature in man. J Clin Endocrinol Metab. 1974;38:1018–1030. doi: 10.1210/jcem-38-6-1018. [DOI] [PubMed] [Google Scholar]
- Wilkinson R. Sleep deprivation: performance test for partial and selective sleep deprivation. In: Abt L, Reiss B, editors. Progress in clinical psychology. New York: Grune & Stratton; 1968. pp. 28–43. [Google Scholar]
- Yoss R, Daly D. Criteria for the diagnosis of the narcoleptic syndrome. Staff Meetings Mayo Clinic. 1957;32:320–328. [PubMed] [Google Scholar]
- Zorick F, Rohers T, Conway W, Fujita S, Wittig R, Roth T. Effects of uvulopalatalpharyngeoplasty on daytime sleepiness associated with sleep apnea syndrome. Clin Respir Physiol. 1983;19:600–603. [PubMed] [Google Scholar]
- Zung W. Antidepressant drugs and sleep. Exp Med Surg. 1969;27:124–137. [PubMed] [Google Scholar]


