Abstract
Models of human gait are based on adult locomotion. C. E. Bauby and A. D. Kuo (2000) proposed that adults rely on passive mechanisms at the spinal level to control motion in the anteroposterior direction and rely on direct monitoring of postural control in the lateral direction. The authors' purpose in this study was to determine if that model applies to control at the onset of walking in typically developing toddlers (n = 9) and in toddlers with Down syndrome (n = 6). Their longitudinal data suggested that toddlers control gait in a distinctly different manner than adults do. An adult pattern of control emerges with experience. In addition, the effect of experience on the emergence of that pattern is magnified by task-specific early intervention. The present data support the emergence and discovery of efficient patterns of control in this fundamental human behavior.
Keywords: Down syndrome, early intervention, gait, variability
Control mechanisms of steady-state, stable gait are complex. The goal of understanding those control mechanisms is to recognize how to change or improve performance. To gain an appreciation of the complexity of stable, mature control, one must understand how gait progresses or develops from the earliest, unstable performance toward steady, efficient behavior.
In the stable walking of adults, step-width variability is greater than step-length variability (Bauby & Kuo, 2000; Owings & Grabiner, 2004). Bauby and Kuo proposed that skilled adults rely on passive dynamics of the lower extremity for control in the sagittal plane, whereas active control is necessary in the frontal plane. They developed a two-legged mechanical model on the basis that contention and found high congruence between the step variability in the model and in humans walking over ground. Both humans and the two-legged passive model showed greater variability in mediolateral than in anteroposterior placement of the feet. The pattern of minimal variability found in the sagittal plane during highly skilled gait has led some to the claim that a neural network in the spinal cord that does not require active control directs sagittal plane motion (Duysens & Van de Crommert, 1998). Because that pattern of minimal variability is almost universal in healthy adults, many think it is an innate quality.
In a study of elicited infant stepping, Forssberg (1985) claimed that innate neural networks are responsible for the infant's leg patterns and are at work as well during adult locomotion. That is, infants and adults share the same basic control mechanisms (i.e., a pattern generator). He stated that infant stepping and adult stepping are kinematically different because the spinal network in adults is hierarchically directed by neural mechanisms in the brain and brain stem. Some researchers hold that hierarchical view today. Recently, Yang, Stephens, and Vishram (1998a, 1998b) stated that infants are ideal for studying stepping behavior because they have little cerebral influence on their motor activity.
Others offer a different explanation for infant stepping and the transition to walking. Thelen and Cooke (1987) stated that one does not need to use remodeling of the nervous system to account for changing kinematics as Forssberg (1985) suggested. Thelen and Cooke argued that the changes seen between infant stepping and independent walking emerge as the multiple subsystems involved with walking (including, but not limited to, the neurological system) develop with time and practice.
Specific gait parameters emerge with time, practice, and changing context. Whereas the detailed kinematics of stable walking takes years to develop (Sutherland, 1997), specific changes that give us insight into the control of independent walking happen quite rapidly. A mature pattern of intralimb coordination between the shank and thigh emerges by 3 months of walking experience; however, when researchers change the context by placing weights on the toddlers' ankles, toddlers with 4 weeks of walking experience display that more mature coordination pattern (Clark & Phillips, 1993). It appears that mechanisms that underlie toddler gait are not part of a rigid system but reflect an adaptive complex system that allows for the emergence of a wide range of parameters, given variations in context. That observation contrasts starkly with the concept of a rigid control system based on innate spinal neuron organization and development of the motor cortex.
The time needed for stable gait parameters to emerge is often short. The first 4−5 months of walking experience is an important time of rapid transition in gait. By 5 months of walking experience, toddlers have combined the postural requirements for remaining upright with forward progression, leading to a decrease in step width and in lateral acceleration of the center of mass (Bril & Brenière, 1992). By 2−4 months after the onset of independent walking, toddlers begin to use their legs effectively as inverted pendulums (Hallemans, Aerts, Otten, De Deyn, & De Clercq, 2004; Ivanenko et al., 2004). That observation implies that the rhythmic pendular oscillations seen in adult walking are not innate and that before that pattern can emerge, active neural control and intersegmental coordination are required (Ivanenko et al.).
Our purpose in this study was to determine if the control pattern, as measured by step variability, observed in adult gait is innate or if it demonstrates a unique developmental trajectory over time as toddlers explore and practice that skill. If the pattern of control seen in adults were innate, as suggested by a hierarchical view, then we would expect the foot placement of newly walking infants to show more variability in the frontal than in the sagittal plane. If, however, the pattern of control were an emergent property, then we would expect to see different patterns of variability in adults and newly walking toddlers.
METHOD
The four groups of toddlers who participated in this study were part of two separate, larger studies. We combined the data from toddlers in those two studies to determine the consistency and robust nature of the findings across samples and contexts. In the new walker (NW) study, we followed 9 toddlers with typical development (TD) and 6 toddlers with Down syndrome (DS) for 6−8 months, starting when they could take three to six consecutive independent steps (onset of walking). The treadmill training (TT) study involved two groups of infants with DS who were trained on treadmills until the onset of walking and then followed for 1 more year. Ten infants were in the generalized low-intensity group (for more information, see Ulrich, Ulrich, Angulo-Kinzler, & Yun, 2001) and 10 were in an individualized high-intensity group in which training time, training speed, and ankle weight were increased over time. We recruited toddlers with TD from the local community and children with DS through fliers distributed to Down syndrome support groups as well as notices in their newsletters. We conducted all the data collection in the Motor Development Laboratory at the University of Michigan. In the NW study, children with TD participated in the study monthly, from the onset of walking through 6 months of walking experience. Children with DS took part in six testing sessions, at initial walking as well as at 1, 3, 4, 6, and 8 months of walking experience. Children in the TT study participated in four testing sessions. The first visit occurred when the children could take 8−10 independent steps (which may take 2 or 3 months after the onset of walking), and then the second, third, and fourth visits were 3, 6, and 12 months, respectively, after the first visit. The average months of walking experience across both training groups were 2.6, 5.8, 8.7, and 14.7, respectively, from Visits 1−4. No significant difference was found between the two groups of toddlers with DS in terms of the months of walking experience at each visit. The Institutional Review Board of the University of Michigan granted approval for both studies. The parents or guardians signed informed consent forms.
Before data collection, we removed all of the toddler's clothes except the diaper and attached reflective markers bilaterally to the temporomandibular joint, shoulder, elbow, hip, knee, and second metatarsal head. Toddlers in the NW study also had midshank and heel markers, whereas toddlers in the TT study had ankle markers. At each visit, toddlers walked along a computerized GAITRite mat (CIR, Havertown, PA) for 4−10 trials, and we used a six-camera Peak Motus real-time system (Peak Performance Technologies, Centennial, CO) to collect kinematic data at a sampling rate of 60 Hz.
Analysis included only trials without incidences of falling, stopping, walking off the walkway, or turning around. We defined a complete stride as two consecutive initial foot contacts of the same foot (line AB in Figure 1). We determined step length and step width by first determining the line between the heels during one stride. We then calculated step width by measuring the perpendicular distance between the heel of the opposite foot and that line (line CD in Figure 1). Step length was the distance covered by forward displacement of two consecutive initial contacts (line AD in Figure 1). In the NW study, we primarily used the Peak kinematic data to calculate step length and width, and the GAITRite data only if the Peak data did not adequately track the heel or toe marker because of an obstructed view. We used a customized MATLAB program (The Math-Works, Natick, MA) to calculate step length and width. In the TT study, we determined step length and width by using the GAITRite data because heel markers were not used in that Peak protocol. The dependent variables in this study were step-length variability and step-width variability, as determined by the coefficient of variation (standard deviation/mean).
FIGURE 1.
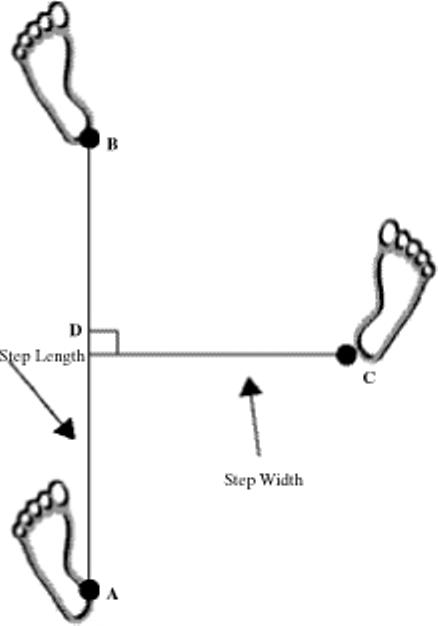
Step-length and step-width measurements made on the basis of (A) initial foot contact, (B) first ipsilateral contact, and (C) first consecutive contralateral contact.
RESULTS
New Walker (NW) Study
Every child in the NW study displayed greater variability in step length than in step width at the onset of independent walking (Table 1). On average, the coefficient of variation for step length (CVSL) was four times as high as the coefficient of variation for step width (CVSW). We ran a 2 (group) × 5 (time) × 2 (direction) analysis of variance (ANOVA) with repeated measures on time. Step variability (CVSL, CVSW) was the dependent variable. Results showed a significant direction effect, F(1, 13) = 75.03, p < .0001, and a significant Time × Direction interaction, F(1, 127) = 31.64, p < .0001. At walking onset, step-length variability for both groups was higher than step-width variability, as can be seen in Figure 2. Step length variability decreased as step width variability increased over time, converging or crossing after months of practice.
TABLE 1.
Step-Length Variability (CVSL) and Step-Width Variability (CVSW) at the First Visit for the New Walker (NW) Study at Initial Walking and the Treadmill Training (TT) Study at 1 Month of Walking Experience
| Participant | Study | Group | CVSL | CVSW |
|---|---|---|---|---|
| 1 | NW | TD | 0.59 | 0.12 |
| 2 | NW | TD | 0.67 | 0.10 |
| 3 | NW | TD | 0.19 | 0.05 |
| 4 | NW | TD | 0.47 | 0.08 |
| 5 | NW | TD | 0.18 | 0.08 |
| 6 | NW | TD | 0.32 | 0.19 |
| 7 | NW | TD | 0.32 | 0.09 |
| 8 | NW | TD | 0.21 | 0.08 |
| 9 | NW | TD | 0.47 | 0.10 |
| 10 | NW | DS | 0.73 | 0.11 |
| 11 | NW | DS | 0.37 | 0.09 |
| 12 | NW | DS | 0.22 | 0.19 |
| 13 | NW | DS | 0.94 | 0.05 |
| 14 | NW | DS | 0.33 | 0.10 |
| 15 | NW | DS | 0.18 | 0.04 |
| 1 | TT | HI | 0.28 | 0.12 |
| 2 | TT | HI | 0.18 | 0.18 |
| 3 | TT | HI | 0.30 | 0.10 |
| 4 | TT | HI | 0.32 | 0.17 |
| 5 | TT | HI | 0.38 | 0.09 |
| 6 | TT | HI | 0.28 | 0.15 |
| 7 | TT | HI | 0.21 | 0.15 |
| 8 | TT | HI | 0.29 | 0.12 |
| 9 | TT | HI | 0.28 | 0.12 |
| 10 | TT | HI | 0.43 | 0.12 |
| 11 | TT | LO | 0.26 | 0.15 |
| 12 | TT | LO | 0.21 | 0.16 |
| 13 | TT | LO | 0.25 | 0.11 |
| 14 | TT | LO | 0.23 | 0.13 |
| 15 | TT | LO | 0.34 | 0.11 |
| 16 | TT | LO | 0.22 | 0.09 |
| 17 | TT | LO | 0.30 | 0.08 |
| 18 | TT | LO | 0.42 | 0.11 |
| 19 | TT | LO | 0.45 | 0.11 |
| 20 | TT | LO | — | — |
Note. TD = Typical development; DS = Down syndrome, no training; HI = Down syndrome, high-intensity training; LO = Down syndrome, low-intensity training. Dash indicates missed
FIGURE 2.
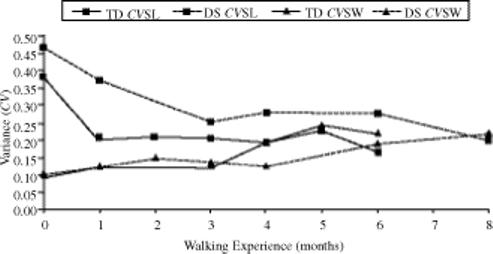
Mean step-length and step-width variability (CVSL and CVSW) over time for the children with typical development (TD) and with Down syndrome (DS) in the new walker
To explore the Time × Measure interaction, we quantified the relationship between CVSL and CVSW as the difference between those variables. We then ran a 2 (group) × 5 (time) ANOVA with repeated measures on time and with the difference score as the dependent variable. Results showed that the DS group had a significantly larger difference between the variables than the TD group did, F(1, 40.163) = 11.510, p = .002. There was also a significant time effect, F(4, 28.889) = 5.926, p = .001. Pairwise comparison showed a significant decrease between initial walking and 6 months of walking experience (p < .001). Note, however, that although the results are informative, one must interpret them with caution because the use of difference scores is less reliable than is the use of raw scores in statistical analysis.
Treadmill Training (TT) Study
The toddlers in the TT study also displayed more step-length variability than step-width variability at their first visit (Table 1). We ran a 2 (group) × 4 (time) × 2 (direction) ANOVA with repeated measures on time and with step variability (CVSL, CVSW) as the dependent variable. Results showed a significant direction effect, F(1, 14) = 21.99, p = .0003, and a significant Time × Direction interaction, F(1, 106) = 3.08, p < .0001.
Again, we ran a 2 (group) × 4 (time) ANOVA with repeated measures on time and with the difference between CVSL and CVSW as the dependent measure. Our difference measure showed a significant time effect, F(4, 27.609) = 13.963, p < .001. Pairwise comparison showed a significant decrease in the difference between initial walking and 13 months of walking experience (p < .001). The children in the low-intensity (LO) group appeared to reach no difference between CVSL and CVSW between 6 and 7 months of walking experience. The children in the high-intensity (HI) group reached that point between 5 and 6 months of walking experience (see Figure 3).
FIGURE 3.
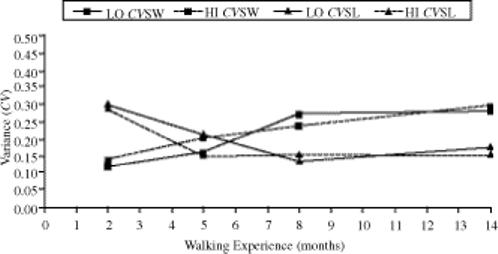
Mean step-length and step-width variability (CVSL and CVSW) over time for the high-intensity (HI) and low-intensity (LO) treadmill training groups.
Slopes and Intercepts
To examine the rate of change and starting point between all four of the groups, we compared the difference between step length and step width in those groups over a period of 1−9 months by using a random slopes and intercepts model. Overall, there was a significant time effect, F(1, 31) = 52.46, p < .0001, and a group effect, F(3, 69) = 4.29, p = .0078. We used planned contrasts to compare differences in the slopes and intercepts between groups. There were no significant differences in the rate of change of the difference measure. At 1 month of walking experience, the difference between CVSL and CVSW was significantly less in the TD group than in the DS group, F(1, 69) = 7.28, p = .0088; in the TD group compared with the LO group, F(1, 69) = 9.50, p = .003; and in the TD group compared with the HI group, F(1, 69) = 4.40, p = .0395 (see Figure 4).
FIGURE 4.
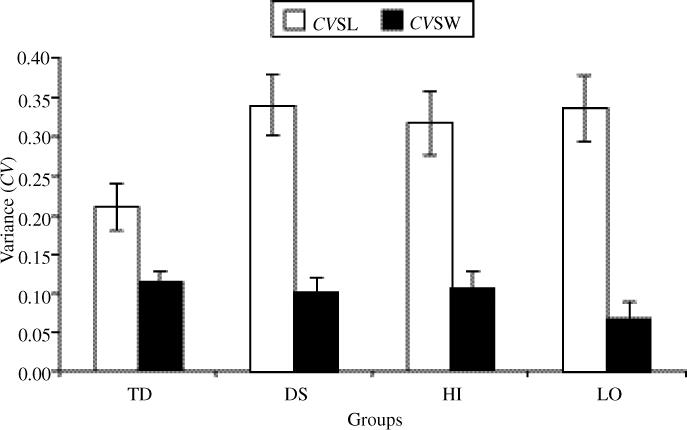
Mean step-length and step-width variability (CVSL and CVSW) in all groups at 1 month of walking experience. The Y intercept was used in calculating variance. Bars indicate standard error.
DISCUSSION
Our results suggest that the pattern of leg control demonstrated by adults, as reflected in the variability of foot placement (Bauby & Kuo, 2000; Owings & Grabiner, 2004), is not an inherent property of the system. Extant data show that adult walkers produce greater foot placement variability in the mediolateral direction (step width) than in the anteroposterior direction (step length). Our data showed that new walkers with TD, as well as those with DS, demonstrate precisely the opposite pattern seen in adults. Bauby and Kuo proposed that the adult variability pattern occurs because walkers take advantage of the passive dynamics available to them in the sagittal plane via the pendular mechanical characteristics of the system. Thus, much of the control comes “for free.” Variability is greater in the frontal plane because the performer must more actively engage in maintaining alignment in that direction. Our data extend Bauby and Kuo's data by illustrating that the variability is not an inherent property; rather, it emerges over time as the toddler discovers how best to use those passive dynamics.
At the onset of walking, toddlers show significantly higher variability in their step lengths than in their step widths. As they continue to practice managing their system during repeated attempts to locomote, they show a reduction in step-length variability but an increase in step-width variability. Over time, the developmental trajectories of those two parameters cross and begin to take on the more adult-like relation. That mixed pattern, particularly the increase in variability for step width, is not a common developmental trajectory. Like step length, most gait parameters become more stable with practice. Bril and Brenière (1992) suggested that the first 4−5 months of walking experience are a period of integration. During that time, toddlers experience a new task and discover how to control many components through practice (Bril & Brenière; Thelen & Ulrich, 1991). As a result, many parameters stabilize, including intralimb coordination, interlimb coordination, and speed (Bril & Brenière; Clark & Phillips, 1993; Hallemans et al., 2004; Ivanenko et al., 2004). So, why might step width become less stable as step length stabilizes?
Our principal argument is that control and coordination are emergent properties, ones that arise from the constraints on the system but require repeated cycles of perceiving and acting. Ultimately, walkers discover how to take advantage of the passive dynamics of their pendulum-like system as they explore and practice that task. The problem for novice walkers is how to organize all of their body segments to work together and stay upright while making forward progress. The difficulty of producing the right amount of force in the desired direction of motion, forward, is high. Yet, one has to have some mediolateral motion to shift one's weight onto one foot, allowing the other to be lifted off the ground and swung forward. We propose that the demands of controlling the forces involved in the forward component motion are so high initially that the solution is to limit variability in the mediolateral direction while exploring more resolutely the goal of controlling forward motion. Over time, the tradeoff reverses. Improvements in control emerge in the anteroposterior direction, and walkers begin to use the passive pendular dynamics. Concurrently, that change relaxes the constraints on mediolateral motion, allowing increased freedom to explore options for fine-tuning system control in that direction. As skill improves, absolute step width reduces, but the relative variability increases, reflecting an effective option for the system to make fine adjustments to its alignment.
All of the groups tested showed an increase in step-width variability and a decrease in step-length variability over time. However, the level of variability observed at the onset of walking reflects the ability of focused practice to enhance the emergence of the control pattern seen at the onset of walking. Our data suggest that intervention before walking onset can advance the level of control in the sagittal plane, that is, reduce variability. Infants and children with Down syndrome have inherently greater postural control problems and show higher variability on many motor tasks than do their peers with typical development, at all ages. Toddlers in our DS and LO groups had more sagittal plane variability than did toddlers in the TD group. However, there was no difference between toddlers in the HI and the TD groups. Sufficiently rigorous (HI) targeted training allowed those toddlers with Down syndrome to move from the higher levels of variability seen in the untrained DS group toward levels of variability similar to those of toddlers with TD. If only an innate unfolding of neural maturation modulated change in the stepping pattern, then the early intervention would not have affected variability. Our data do not support the notion that a basic pattern generator is in place at birth or at the onset of independent walking. Instead, they suggest that control is emergent. Change in the stability of a movement pattern, as seen in both the step length and step width of all toddlers we studied, is an irrefutable indicator of a fundamental shift in control strategy. That plasticity is magnified by training, which argues for the emergence of the control of foot placement within a process of perceiving and acting rather than through innate underlying control systems. Furthermore, whereas training advanced the emergence of adult-like control of foot placement, the pattern of emergence was similar in all groups tested, indicating that that phenomenon is not limited to typically developing children but seems to generalize across populations and contexts.
NOTE
Grants from the National Institutes of Health (HD042728-03) to B. Ulrich, the U.S. Office of Special Education and Rehabilitative Services (H424C010067) to D. Ulrich and R. Angulo Barroso, and to D. Ulrich from the March of Dimes Birth Defects Foundation supported this research. We thank the participants and their families as well as the local Down syndrome support groups for their help.
REFERENCES
- Bauby CE, Kuo AD. Active control of lateral balance in human walking. Journal of Biomechanics. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Bril B, Brenière Y. Postural requirements and progression velocity in young walkers. Journal of Motor Behavior. 1992;24:105–116. doi: 10.1080/00222895.1992.9941606. [DOI] [PubMed] [Google Scholar]
- Clark JE, Phillips SJ. A longitudinal study of intralimb coordination in the first year of independent walking: A dynamical systems analysis. Child Development. 1993;64:1143–1157. [PubMed] [Google Scholar]
- Duysens J, Van de Crommert HWAA. Neural control of locomotion: Pt. 1. The central pattern generator from cats to humans. Gait & Posture. 1998;7:131–141. doi: 10.1016/s0966-6362(97)00042-8. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Ontogeny of human locomotor control I. Infant stepping, supported locomotion and transition to independent locomotion. Experimental Brain Research. 1985;57:480–493. doi: 10.1007/BF00237835. [DOI] [PubMed] [Google Scholar]
- Hallemans A, Aerts P, Otten B, De Deyn PP, De Clercq D. Mechanical energy in toddler gait. A trade-off between economy and stability? The Journal of Experimental Biology. 2004;207(Pt 14):2417–2431. doi: 10.1242/jeb.01040. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Dominici N, Cappellini G, Dan B, Cheron G, Lacquaniti F. Development of pendulum mechanism and kinematic coordination from the first unsupported steps in toddlers. Journal of Experimental Biology. 2004;207(Pt 21):3797–3810. doi: 10.1242/jeb.01214. [DOI] [PubMed] [Google Scholar]
- Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. Journal of Biomechanics. 2004;37:935–938. doi: 10.1016/j.jbiomech.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Sutherland D. The development of mature gait. Gait & Posture. 1997;6:163–170. [Google Scholar]
- Thelen E, Cooke DW. Relationship between newborn stepping and later walking: A new interpretation. Developmental Medicine and Child Neurology. 1987;29:380–393. doi: 10.1111/j.1469-8749.1987.tb02492.x. [DOI] [PubMed] [Google Scholar]
- Thelen E, Ulrich BD. Hidden skills: A dynamic systems analysis of treadmill stepping during the first year. Monographs of the Society for Research in Child Development. 1991;56:1–98. [PubMed] [Google Scholar]
- Ulrich DA, Ulrich BD, Angulo-Kinzler RM, Yun J. Treadmill training of infants with Down syndrome: Evidence-based developmental outcomes. Pediatrics. 2001;108:E84. doi: 10.1542/peds.108.5.e84. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stephens MJ, Vishram R. Infant stepping: A method to study the sensory control of human walking. The Journal of Physiology. 1998a;507(Pt 3):927–937. doi: 10.1111/j.1469-7793.1998.927bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Stephens MJ, Vishram R. Transient disturbances to one limb produce coordinated, bilateral responses during infant stepping. Journal of Neurophysiology. 1998b;79:2329–2337. doi: 10.1152/jn.1998.79.5.2329. [DOI] [PubMed] [Google Scholar]


