Abstract
Regeneration of the retina in amphibians is initiated by the transdifferentiation of the retinal pigmented epithelium (RPE) into neural progenitors. A similar process occurs in the early embryonic chick, but the RPE soon loses this ability. The factors that limit the competence of RPE cells to regenerate neural retina are not understood; however, factors normally involved in the development of the eye (ie. FGF and Pax6) have also been implicated in transdifferentiation. Therefore, we tested whether activin, a TGF-β family signaling protein shown to be important in RPE development, contributes to the loss in competence of the RPE to regenerate retina. We have found that addition of activin blocks regeneration from the RPE, even during stages when the cells are competent. Conversely, a small molecule inhibitor of the activin/TGF-β/nodal receptors can delay, and even reverse, the developmental restriction in FGF-stimulated neural retinal regeneration.
INTRODUCTION
The retinal pigmented epithelium (RPE) serves as a source of retinal regeneration in newts, salamanders and anuran (frog) tadpoles. If the retina is experimentally removed in these animals, the RPE loses pigmentation, proliferates and generates two new epithelial layers: a pigmented layer and a non-pigmented layer. The non-pigmented layer begins to express genes typical of retinal progenitor cells and undergoes extensive cell division to produce a new retina (Reh and Nagy, 1987; Reh and Nagy, 1989; Stone, 1950; Stone and Steinitz, 1957). In vitro experiments, and transplantation experiments have demonstrated that the RPE is the source of neural retinal tissue (Reh et al., 1987), and this phenomenon was one of the first well-recognized examples of “transdifferentiation” (Okada, 1981). The embryonic chick is also capable of a similar form of retinal regeneration from the RPE (Coulombre and Coulombre, 1965; Park and Hollenberg, 1989; Pittack et al., 1991). Removal of the retina from a chick embryo from stages 21 to 25 (E3.5–E4.5) causes the RPE to generate neural retinal progenitors, in a manner very similar to that observed in the amphibian. A key difference between the regeneration that is observed in amphibians and that of higher vertebrates is that while this process can occur throughout life in amphibians, it is restricted to embryonic stages in birds and mammals.
The factors that regulate regeneration from the RPE are not well characterized in either amphibians or in birds. One of the first factors demonstrated to stimulate the process of regeneration from the RPE was basic fibroblast growth factor (FGF2), which has been shown to be effective in both amphibians and chick embryos (Sakaguchi et al., 1997)(Sakaguchi et al, 1997; Pittack et al, 1991; Park and Hollenberg, 1991; 1989). More recently, Sonic hedgehog (Shh) has been shown to negatively regulate regeneration from the RPE, and antagonists to Shh can stimulate this process in chick embryos (Spence et al., 2004). Both FGF and Shh are also involved in the regulation of the initial patterning decisions in the optic vesicle that lead to the RPE and neural retinal fates (Pittack et al, 1997; Perron et al, 2003), and it is interesting that they continue to play a role in the change in cell fate that underlies neural retinal regeneration.
Another factor shown to be an important developmental signal important for the patterning of the optic vesicle domains is activin. Fuhrmann et al (2000) found that activin can replace a critical signal released from the extraocular mesenchyme that is normally important for RPE development. In addition, they reported that inhibition of activin signaling prevented normal RPE differentiation. These results led us to investigate whether there is a role for activin signaling in regeneration of neural retina from the RPE in chick embryos. We have found that activin plays a key role in restricting regeneration from the RPE, likely by stabilizing the RPE phenotype. The addition of activin blocks regeneration from the RPE, even in the presence of FGF. We went on to test the effects of a small molecule inhibitor for activin-like kinases, SB431542, on the regeneration of retina from the RPE. We found that inhibiting activin/TGFβ/nodal signaling with this compound can prolong, and even reverse, the developmental period over which FGF can stimulate neural retinal regeneration.
MATERIALS AND METHODS
Animals
The use of animals in this study was in accordance with the guidelines established by the University of Washington, IACUC, and the National Institute of Health. Mice were housed in the Department of Comparative Medicine. All procedures were carried out according to approved protocols.
RPE explant culture
The embryos were staged according to Hamburger and Hamilton (Hamburger and Hamilton, 1992). The RPE, with a small amount of associated mesenchyme, was dissected from the other ocular tissues (neural retina, extraocular mesenchyme, ciliary epithelium and lens) in HBSS- (without calcium chloride, magnesium sulfate and sodium bicarbonate) containing 5mM HEPES and additional 0.6% D-glucose. The RPE sheets were washed three times in HBSS- solution and one time in culture medium DMEM/F12 (Gibco) containing 0.9% D-glucose, 0.1125% NaHCO3, 20 mM HEPES, 5% FBS, 100 U/ml of penicillin, and 100 μg/ml of streptomycin. The RPE sheets were then placed in 24-well culture plates containing 1 ml of culture medium and mounted on a shaking device (Nutator) at 25 rpm and 37° C for up to eight days. At St 23 (St 23), the eye diameter is approximately 1 mm. At St 29, the eye diameter is approximately 3 mm. In experiments 1 and 2 (see Results), one RPE from one eye was placed in 1 ml of culture media. In experiment 3, one RPE from one eye was cut to about 1 mm2 and placed into 1 ml of culture media.
In experiment 1 (Figure 1), the RPE from St 23/24 embryos was placed in 1 ml of culture medium containing 100 ng of FGF2 (R & D Systems). On the second day, half of the culture medium was changed and an additional 100 ng FGF2 was added. In experiment 2 (Figure 2), the RPE from St 23/St24 was placed in 1 ml of culture medium with one of the following: DMSO (vehicle control); FGF2 (100 ng); FGF2 + Activin A (100 ng/ml); FGF2 + Activin A (100 ng/ml) + SB431542 (14 uM; Sigma). In experiment 3 (Figure 3), the RPE from St 23/St24 embryos was placed in 1 ml of culture medium containing several different concentrations of SB431542 (Sigma) or in DMSO as the control. The same concentration of SB431542 was added on the second day. FGF2 (100 ng) was added approximately 48 hours from the start of the explant culture. The day after the addition of FGF2, half of the culture medium was changed and another 100 ng of FGF2 was added. The RPE was fixed four days after the first addition of FGF2. In experiment 4 (Figure 4), the RPE sheet from St28–St31 embryos was placed in 1 ml of culture medium containing several different concentrations of SB431542 in 0.76 ul DMSO and 50ng FGF2. SB431542 was added every day. Half of the culture media was changed and 50ng FGF2 was added every other day.
Figure 1.
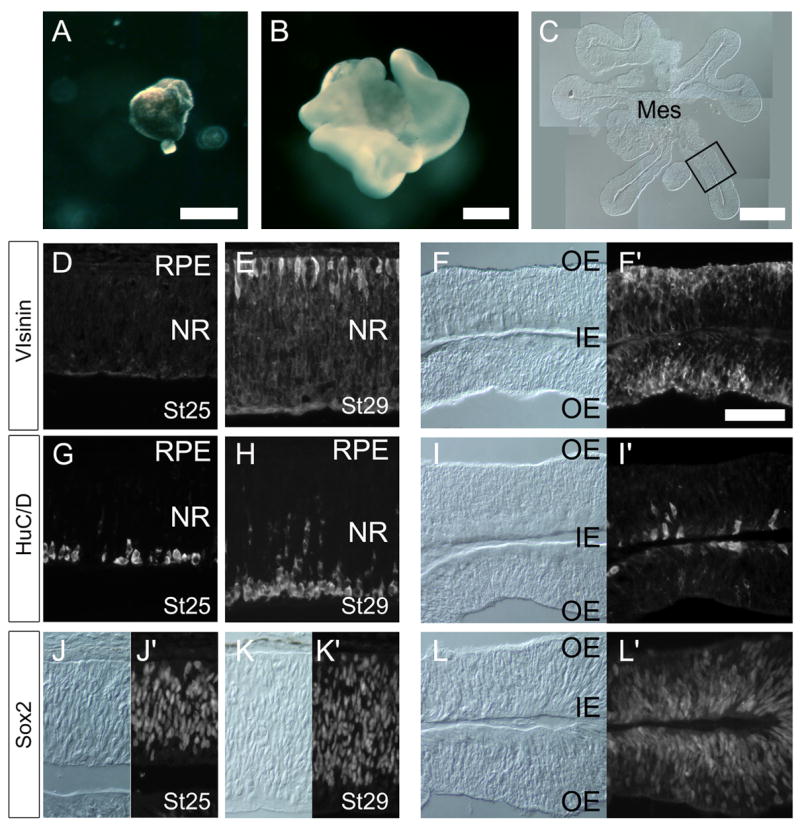
RPE is induced to generate neural retina in vitro by FGF treatment. (A) St 23/24 (E4) RPE cultured without FGF2 for 4 days. No neural retina is generated. (B) St 23/24 (E4) RPE cultures treated with 100ng/ml FGF2 for 4 days lose pigmentation and generate large neural retina. Sections (C, F, I and L) of retina derived from RPE transdifferentiation and sections (D,G,J and E,H,K) from normal chick retinas (Stage 25 and 29, respectively) for comparison. (A). Immunofluorescence for Visinin (D, E, F), Hu C/D (G,H,I), Sox2 (J,K,L). For the RPE derived retina, both bright field images (left panel) and fluorescent images (adjacent right panel) are shown for the same field (F, I and L). Scale bar 300μm in A and B, 200 μm in C; Scale bar in F′ is 50μm, and applies to all other panels as well. Outer edge (OE). Inner edge (IN), Mes = extraocular mesenchyme; NR = neural retina.
Figure 2.
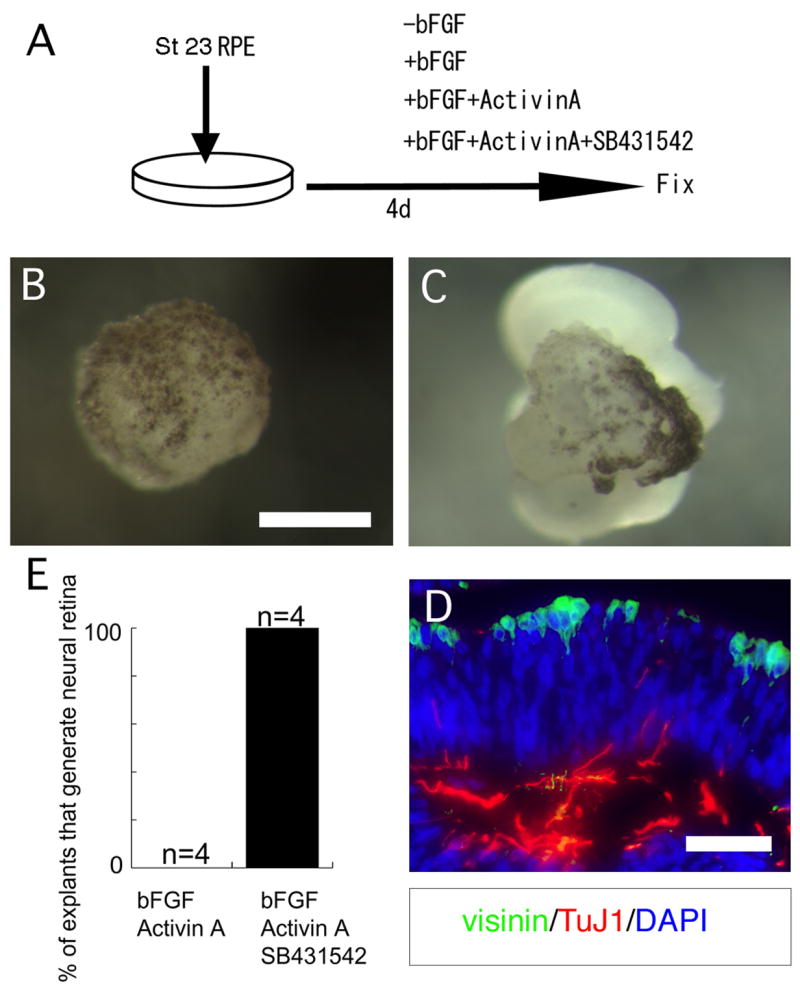
Activin signaling promotes the loss of competence of the RPE to generate neural retina when exposed to FGF2. (A) Experimental design. RPE was dissected from chick embryos at Stages 23/24 and cultured under one of four conditions: control, +FGF, +FGF and activin A, or +FGF, Activin A and SB431542. (B,E) When cultured with Activin A and FGF2, transdifferentiation was completely blocked; however, adding the activin receptor inhibitor, SB431542 reversed this inhibition and all explants showed neural retina generation from the RPE (C,D,E), as indicated by labeling for visinin and TuJ1 (D). (E) Quantitative data from explants that were sectioned and labeled with neural retinal antibodies TuJ1 and visinin. Non-parametric randomization test used to determine level of significance p=0.014. Scale bar = 300μm in B and C, and 50 μm in D.
Figure 3.
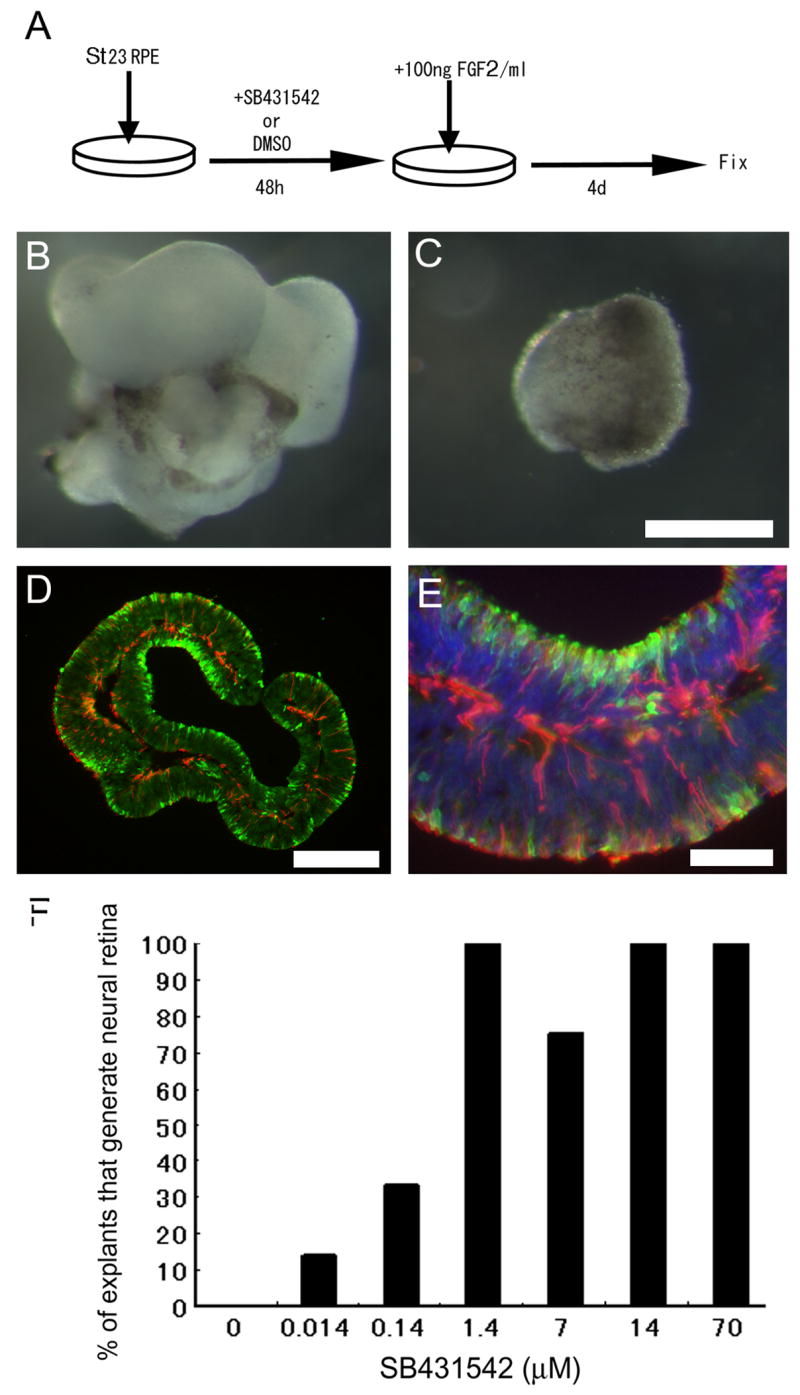
Inhibition of activin/TGFβ/nodal signaling can extend the developmental period of competence of RPE to generate neural retina. (A) Experimental design. RPE from Stages 23/24 was cultured in either SB431542 or DMSO vehicle control for 2 days prior to the addition of FGF2. (C) Cultures treated with vehicle control prior to addition of FGF2 failed to transdifferentiate into neural retina. (B,D,E) Cultures treated with the activin receptor inhibitor SB431542 prior to the addition of FGF2 showed robust transdifferentiation (B) and sections showed evidence of layered neural retina, with TuJ1 (red) and Visinin (green) labeling (D, E). (F) Dose response for SB431542 to show the percent of RPE explants that demonstrated neural retina formation is related to the concentration of SB431542. Scale bar 300 μm in B and C, 200 μm in D and 50 μm in E.
Figure 4.
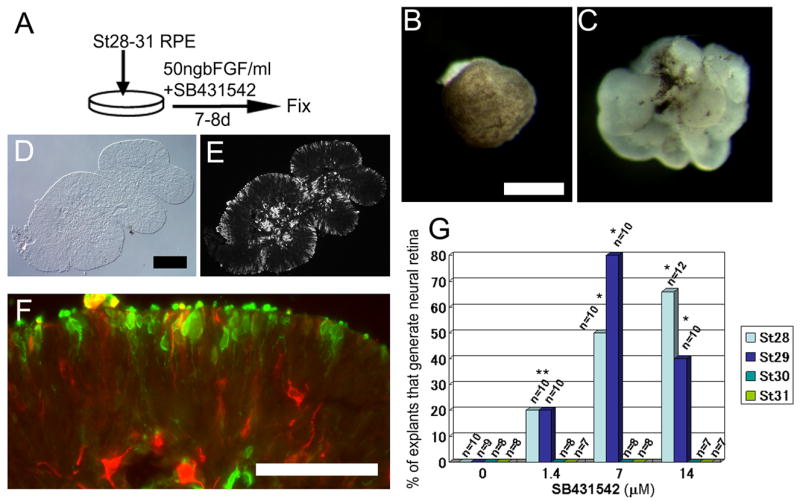
Inhibition of activin/TGFβ/nodal signaling can restore competence of RPE to generate neural retina in RPE isolated from Stages 28–31. (A) Experimental design. RPE at Stages 28–31 was cultured in a combination of SB431542 and FGF2 for 7–8 days. (B) St28 RPE explant culture treated with DMSO and 50ng FGF2 for 8 days shows no evidence for transdifferentiation. (C) St28 RPE explant culture treated with 14uM SB431542 and 50ng FGF2 for 8 days shows robust transdifferentiation. (D–F) Analysis of sections of transdifferentiated retina shows the normal layering of visinin (E and F, green) and TuJ1 (F, red) indicative of neural retina formation. (G) Graph of all experiments shows that a high percentage of RPE from either Stage 28 or 29 can be induced to generate neural retina with SB431542 and FGF2; however, we did not observe this in PRE isolated from Stages 30–31. * = p> 0.01 from non-parametric Randomization test. Scale bar = 300 μm in B,C; 200 μm in D,E; and 50 μm in F.
For the mouse RPE culture experiment (Figure 7), the RPE was dissected from E12 and E13 pigmented embryos. The neural retina and ciliary epithelium was discarded, and only clearly defined RPE sheets were used in the experiments. One RPE sheet from one eye was placed in 1ml DMEM/F12 (Gibco) containing 0.9%D-glucose, 0.1125% NaHCO3, 20mM HEPES, 5% d dialyzed FBS, 1% N2 supplement and 8mM L-glutamate, 100 U/ml of penicillin and 100 μg/ml of streptomycin. The explants were cultured for 8 days. The explants were treated with either DMSO (vehicle control), DMSO + FGF2 (50ng/ml) or FGF2 (50ng/ml) + SB431542 (14 uM).
Figure 7.
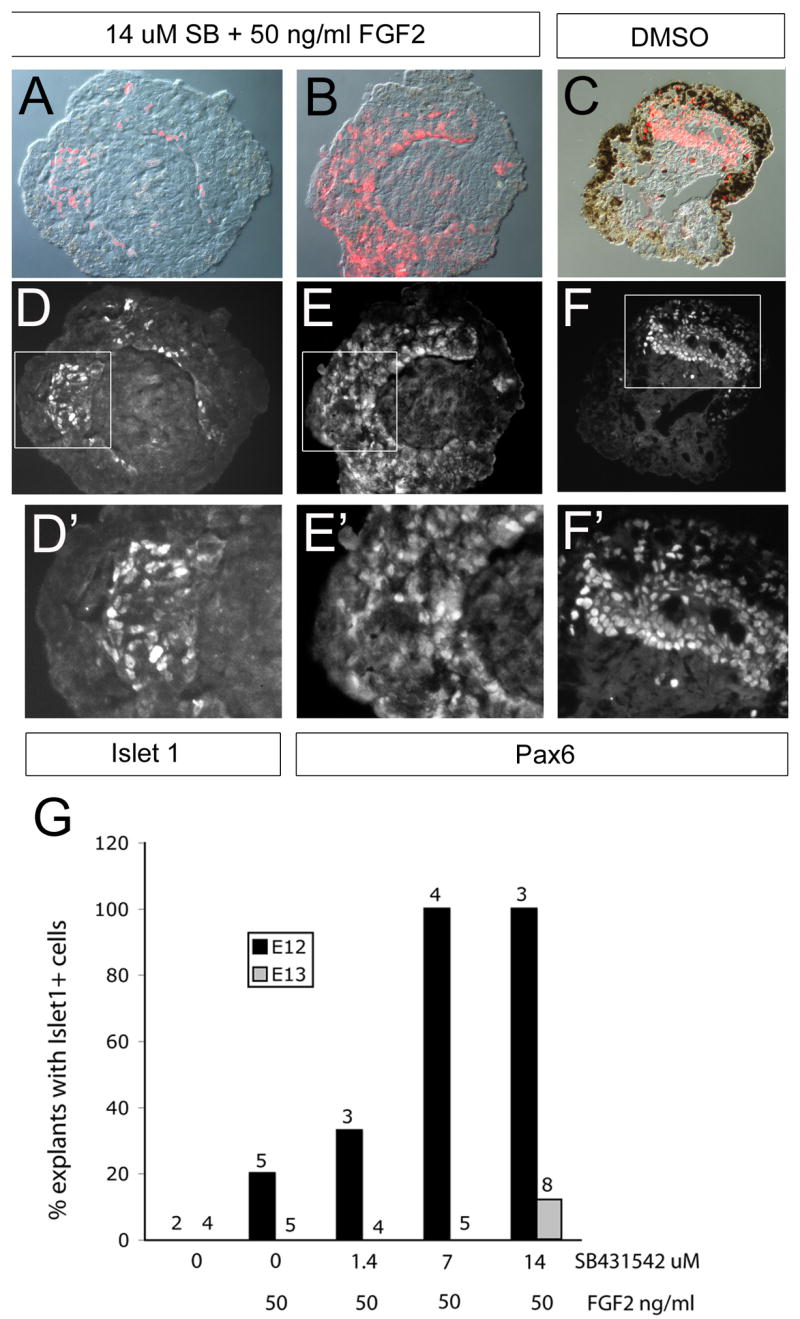
Experiment to test whether inhibition of activin signaling can promote transdifferentiation of embryonic mouse RPE. (A, D, D′) Explant of mouse E12 RPE treated with SB431542 and FGF2 for 8 days lost pigmentation and acquired Islet1+ cells (D,D′). D′ shows the boxed region in D at a higher power. (B, E, E′) Explant of mouse E12 RPE treated with FGF2 for 8 days shows Pax6+ cells (E,E′). E′ shows the boxed region of E. (C) DMSO treated explant culture of mouse RPE shows some de-pigmentation and some regions of Pax6+ cells (F,F′). F′ shows the boxed region of F at higher power. Labeled cells in D, E, and F are shown in red in A, B, and C, respectively. (G) Summary of the relationship between Islet1+ cells in explanted mouse RPE culture and SB431542/FGF2 treatment.
Tissue preparation and immunofluorescence
The explants from all experiments were analyzed by immunofluorescent labeling of frozen sections. Tissues were fixed in PBS containing 4% PFA and 5% sucrose for 5 h at 4° C and frozen for sectioning on a cryostat. For immunofluorescence, sections were blocked with PBST (0.3% Triton-X in PBS) containing 5% goat serum and 0.01% NaN3 for 20 minutes. Primary antibody incubations were carried out overnight at 20° C or 4° C (for rabbit-anti visinin and mouse anti-Tuj1) in a humidified chamber. For the Sox2 antibody incubation 40U/mL DNase1 was added for 1 h at 37° C and continued overnight at 4° C. Sections were washed three times in PBS then covered with secondary antibody solution containing 50 ug/mL Hoechst 33342 (Molecular Probes) for 40 min to 2 h at 20° C, then washed with PBS three times. Antibodies were diluted to the following concentrations: mouse anti-Pax6 (DSHB) was not diluted; mouse anti-visinin (7G4, DSHB) was not diluted; rabbit anti-visinin was diluted 1:5,000; mouse-anti Neuronal Class 3 beta-Tubulin (Tuj1) (Covance) was diluted 1:2,000; rabbit-anti calretinin (Swant) was diluted 1:2,000; rabbit anti-CRALBP (gift from Dr. J.C. Saari, University of Washington, Seattle, Washington) was diluted 1:500; goat anti-Sox2 (Santa Cruz) was diluted 1:750; mouse anti-islet (439.4D5, DSHB) was diluted 1:200; and mouse-anti HuC/D (Molecular Probes) was diluted 1:200. All secondary antibodies were purchased from Molecular Probes and were diluted in PBST at 1:500. Secondary antibodies included Alexa Fluor 488 goat anti-rabbit IgG, Alexa Fluor 568 goat anti-mouse IgG and Alexa Fluor 594 donkey anti-goat IgG. Fluorescence was detected using a fluorescence microscope (Axioplan 2, Carl Zeiss). The number of explants that displayed positive labeling for neural markers was quantified as a percentage of the total in each treatment group, and results compared with a non-parametric randomization test for significance; p values are shown for each experiment in the accompanying figure legend.
Semi-quantitative-PCR
The RPE was dissected as described above and treated with collagenase type IV (Gibco) in HBSS+ (with calcium chloride, magnesium sulfate and sodium bicarbonate) containing 5mM HEPES, additional 0.6% D-glucose and 3mM CaCl2 at room temperature under the following conditions: St 23–25 with 800 U/mL for 7–16 min; St 28 and St 29 with 8,000 U/mL for 15 min, St 31 with 8000 U/mL for 20–30min. At St 30, the RPE sheet was treated with 8,000 U/mL for 17 min or 12 min. After collagenase treatment, the RPE was placed in HBSS. A monolayer of RPE was removed from the mesenchyme using 30G needles. The purity of the RPE was checked by immunohistochemistry and stained with CRALBP. We confirmed that pure RPE was isolated from St 23–29 embryos. After St 30, the adhesion between the RPE and mesenchyme seems to change and we were unable to eliminate the mesenchymal cells. Immunohistochemistry with CRALBP showed one of three RPE samples had mesenchyme cell contamination. The contaminated mesenchyme nuclei number was 0.5% of RPE cell nuclei number. Since the RPE purity was still high, we also used St 30 and St 31 samples for PCR. Total RNA was extracted by using TriZOL (Invitrogen) according to manufacturer’s instructions and then treated with DNase (Promega). After RNA clean-up using RNeasy MiniElute Cleanup Kit (Qiagen), cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen) using oligo(dT) primers (Invitrogen). PCR was performed in 20 μl reaction mixtures containing 22.5 pM primers and 10 ul SYBR Green PCR Master Mix (Applied Biosystems). All PCR amplifications were performed with an initial 10-minute denaturation at 95° C, and forty cycles of 95° C (15 sec) and 60° C (1 min) and 64° C (45 sec). Fluorescence was detected at 64° C with an Opticon real-time PCR detection system (Bio-Rad). The mean and S.E.M of the cycle number where for three independent samples at 5 different ages is shown in Figure 6. Primer sequences are listed in table S1 and results were normalized to GAPDH levels.
Figure 6.
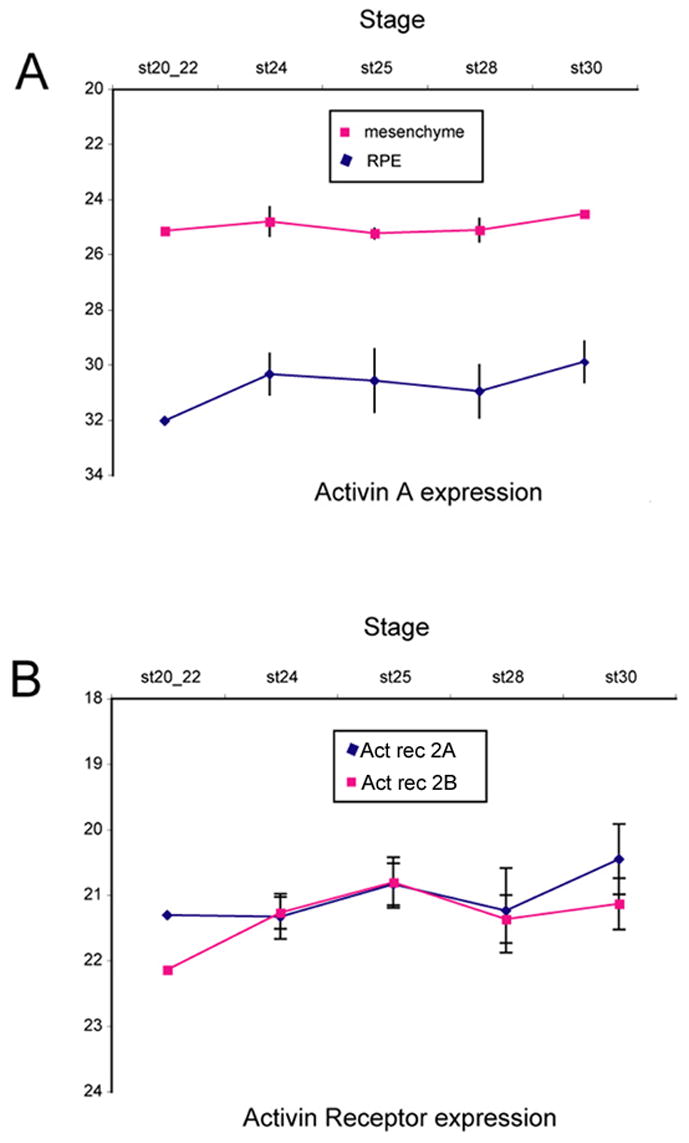
Semi-quantative real time PCR analysis of Activin βA (A) and Activin receptor (B) expression in the developing RPE and mesenchyme as a function of Stage. The level of expression is shown as relative cycle number (see Methods). (A) Activin βA is expressed more highly in the adjacent mesenchyme than in the RPE. (B) Both the A and B subunits of the activin receptor 2 show a gradual increase in expression in the RPE over the stages of development tested in this study.
Western blots
RPE and extraocular mesenchyme were dissected from St 25 chick embryos in cold HBSS(+) (HBSS containing 0.6% D-glucose and 5mM HEPES, without sodium bicarbonate). Tissue was incubated at 37°C for 25 min. in control (HBSS(+)) or activin (50ng/mL in HBSS (+)). Protein was extracted in RIPA buffer (50mM Tris HCl pH 8; 150mM NaCl; 1% NP-40; 0.5% sodium deoxycholate; 1% SDS) containing Phosphatase Inhibitor Cocktail 2 (1:100; Sigma) and Protease Inhibitor Cocktail (1:100; Sigma). Protein was quantified using BCA Protein Assay Kit (Pierce) according to manufacturers instructions and loaded in each well (5μg). Membranes were blocked in 2% bovine serum albumin (Sigma) containing Phosphatase Inhibitor Cocktail 2 and Protease Inhibitor Cocktail and incubated with primary antibodies rabbit anti-phosphoSmad2 (1:1000; Cell Signaling Technology) and mouse anti-Smad2/3 (1:1000; BD Biosciences). Secondary antibodies were goat anti-mouse alkaline phosphatase and goat ant-rabbit alkaline phosphatase from the Bio-Rad Immun-Star Chemiluminescent Kit.
RESULTS
FGF2 induces neural retina from RPE at E4
Previous studies have shown that FGF2 induces chicken RPE to generate neural retina when treated prior to St 25 (see Introduction). To confirm these earlier reports, we made explant cultures of RPE from St 23/24 chicken embryos and treated them with 100 ng FGF2. After 4 days of culture, explants maintained in control medium retained their pigmentation and simple RPE morphology (Figure 1A). By contrast, the RPE explants maintained in medium containing FGF2 gave rise to an extensively folded neuroepithelium, morphologically similar to the neural retina (Figure 1B;C). The neuroepithelium that was derived from the FGF2-treated RPE was organized like the normal embryonic retina, with labeling for the photoreceptor marker, visinin, at the outer edge (OE, Figure 1F, right) and labeling for the amacrine and ganglion cell marker, HuC/D, at the inner edge (Figure 1I, right). Labeling with the same antibodies in normal retinal sections at Stage 25 and Stage 29 is shown for comparison (Figure 1D,E; G,H; J,K). Although the retina that was derived from the RPE has expanded considerably from the original explant, the inner edge is adjacent to the original extraocular mesenchyme in some places (Figure 1C). This orientation is inverted when compared with the normal retina, consistent with the findings of Coulombre and Coulombre (1965). The neural retina that was derived from RPE also expressed the progenitor marker, Sox2 (Figure 1L), and had a similar labeling pattern to that of the normal embryonic retina (Figures 1J and 1K).
Activin signaling and RPE transdifferentiation: maintenance of competence
The ability of RPE to generate neural retina in response to FGF2 is lost in embryos after St 25 (Pittack et al, 1991). While the molecular basis for this loss in the competence of the RPE to regenerate retina in birds and mammals is not known, Araki et al (Araki et al., 2002) reported that an interaction with the adjacent extra-ocular mesenchyme is critical for limiting RPE transdifferentiation. In addition, Fuhrmann et al (2000) reported that an activin-like factor released from the extra-ocular mesenchyme during development is necessary and sufficient for the induction of the RPE fate in the proximal part of the optic vesicle. Taken together, we hypothesized that activin signaling might be important in the loss in competence of the RPE to generate neural retina after St 25. To test this possibility, we first tested whether the addition of activin A could inhibit the ability of FGF2 to induce transdifferentiation. We cultured St 23 chick embryo RPE explants with FGF2, FGF2 and Activin A or FGF2, Activin A and SB431542. We found that Activin A inhibited the FGF2 induced retinal generation (Figure 2 B); the RPE explants cultured in the presence of both FGF2 and Activin A resembled those cultured without FGF (Figure 1A). The inhibition of FGF2 induced retinal regeneration could be reversed by the addition of SB431542 (Figure 2C,D,E). Although the cultures treated with FGF2, Activin A and SB431542 did not show as robust neural retinal formation as those treated with FGF2 alone, they all showed both TuJ1 (amacrine and ganglion cells) and visinin (photoreceptors) labeling (Figure 2 D). These results confirm and extend the findings of Fuhrmann et al, 2000, showing that Activin A can antagonize FGF signaling to promote and/or maintain the RPE fate. The data further show that the ability of Activin to block FGF-induced regeneration can be reversed by SB431542, confirming that this compound can inhibit Activin signaling.
The fact that activin can inhibit FGF-induced retinal generation from the RPE supports the idea that this signal normally limits RPE competence for generation of neural retina. We next tested whether inhibition of activin signaling would maintain the competence of RPE cells to generate neural retina, using the experimental design shown in Figure 3A. To determine whether St 23 RPE explants lose their competence to generate neural retina in vitro, we maintained St 23 RPE explants in medium containing DMSO for 2 days prior to the addition of FGF2 (the equivalent of St 25), and then cultured the explants for an additional 4 days. We found that under these conditions, the RPE is no longer able generate neural retina even in the presence of FGF2 (Figure 3C). However, if St 23 RPE explant cultures are kept in media containing SB431542 for the 2 days prior to treatment with FGF2, and then cultured an additional 4 days, the RPE is still able to generate neural retina (Figure 3). In the SB431542 treated explants, the RPE-derived neural retina has the same morphology and expression of visinin+ and TuJ1+ cells as the normal retina (Figure 3D and E). This effect is dose dependent (Figure 3F), with the response saturating at 1.4 uM. These results show that SB431542 has the ability to maintain the competence of the RPE to generate neural retina for at least 2 days past the time when this competence would normally be lost.
Activin signaling and RPE transdifferentiation: restoration of competence
The above experimental design allowed us to test whether the inhibition of activin signaling would prevent the loss of competence to generate neural retina that occurs in RPE cells after St 25. We also carried out experiments to determine whether inhibition of activin signaling could reverse the loss in competence for retinal regeneration in more mature RPE. For these experiments, we dissected RPE from embryonic day 5–7 (H&H Stages 28–31) and put these into explant culture, with both SB431542 and FGF2 added to the medium for 7 to 8 days (Figure 4A). We found that treatment of the more mature RPE with both SB431542 and FGF2 restored the competence for neural retinal regeneration in RPE of embryos as late as Stage 29/E6 (Figure 4C–F). The neural retina generated by the St 29 RPE showed a similar morphology and immunolabeling for visinin and TuJ1 to that derived from earlier stages of RPE treated with FGF alone. Visinin expressing cells are located at the outer edge (Figure 4E,F; green), while the amacrine and ganglion cell marker, Hu C/D, was observed at the inner edge, next to the mesenchyme (Figure 4F; Figure 5 A,D). Quantitation of this effect is shown in Figure 4G; the optimum concentration for SB431542 was 7 uM, with 80% of the explants showing neural retinal formation from Stage 29 RPE. We do not know why a higher dose of SB431542 was required to reverse the RPE fate, compared with the lower dose required to maintain competence to generate neural retina; this is perhaps due to the fact that the older tissue may be less permeable to the compound. We also tested RPE from Stages 30 and 31 with the same treatment, but were not able to elicit neural retina from these later stages (Figure 4G). Other markers of neural retina formation were also present in the FGF2/SB431542 treated RPE explants from Stage 28 embryos. Calretinin, a marker for ganglion cells, amacrine cells and horizontal cells in the chick, was also expressed in many cells within the RPE-derived retina (Figure 5B,C,E,F). Many cells within the RPE-derived neural retina were also immunoreactive for the neural progenitor markers Pax6 (Figure 5I,J) and Sox2 (Figure 5G,H). Not all the RPE underwent transdifferentiation to neural retina, and in regions where RPE persisted, the neural retina that had been derived from the RPE was continuous with the remaining RPE layer (Figure 5A). In some regions, pigment granules remained in some cells intermixed with neural retinal cells (Figure 5C).
Figure 5.
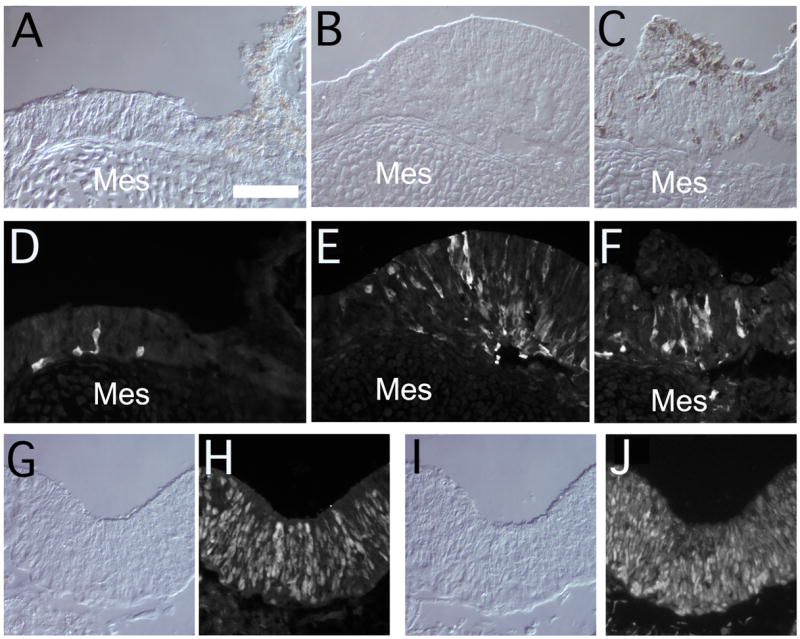
Further examples of sections through transdifferentiated Stage 28 RPE to show additional neural markers in paired bright field and fluorescent micrographs: (A,D) HuC, (B,E;C,F) calretinin, (G,H) Sox2 and (I,J) Pax6. Note that the neural retinal epithelium is frequently continuous with RPE that has retained some pigmentation. Mes = mesnchyme. Scale bar (in A) = 50 μm in all panels.
Activin and activin receptors expression in RPE and mesenchyme
These data, along with previous studies, indicates that there are at least two restriction points in the competence of RPE to generate neural retina. Until Stage 25, treatment with FGF2 is sufficient to generate neural retina. After Stage 25, inhibition of activin signaling can overcome this block and explants treated with inhibitors of activin receptors can generate neural retina to Stage 29. These results suggest that some change in activin or its receptors might normally precipitate the loss of competence for neural retinal regeneration from the RPE. Fuhrmann et al, (2000), demonstrated that both type IIA and IIB Activin receptors are expressed in the developing RPE, but the levels of activin and the activin receptors in the RPE at the later stages of development tested in this study have not been described. Therefore, we analyzed the levels of expression of Activin βA, Activin Receptor IIA and Activin Receptor IIB in the RPE and the adjacent mesenchyme using semi-quantitative real time-PCR. The results of our analysis are shown in Figure 6. All three activin signaling components that we tested had detectable levels of expression from Stage 20 to Stage 30 in both the RPE and the mesenchyme (Figure 6). However, the expression level of activin A in the mesenchyme is approximately 100 times (6–7 cycles) higher than that of RPE at all stages (Figure 6A). This is consistent with previous evidence that the activin signal that promotes RPE development emanates from the extra-ocular mesenchyme (Fuhrmann et al, 2000). By contrast, Activin Receptor IIA and IIB are expressed at approximately the same level in the RPE and the mesenchyme. We did not detect a clear change in Activin βA expression during the stages when the RPE loses its competence to generate neural retina. We found a progressive increase in expression of both receptor subunits in the RPE during these stages (Figure 6B).
We also found that one of the key downstream events in activin signaling, Smad2/3 phosphorylation, occurs in the RPE at St 23–25. Supplemental Figure 1 shows labeling with an antibody against Phospho-Smad2 in both the RPE and retina (A–D). It is interesting that the labeling is strongest in the posterior RPE, and only scattered cells are observed in the anterior RPE. This correlates with the posterior to anterior progression in RPE maturation. At this stage, the anterior RPE retains the ability to generate neural retina, and does not have active Smad signaling. Treatment of the RPE with activin further stimulates this pathway, as evidenced by an almost two-fold increase in the ratio of Phospho-Smad2:total Smad2/3 (E), thus confirming that the effects we have observed with activin on the RPE are mediated through the Activin receptor/Smad pathway.
FGF2 and SB431542 treatment promotes islet-1 expression in mouse RPE
A previous study reported that FGF2 can stimulate transdifferentiation of RPE explant cultures from embryonic rat, at E12 and E13 (Zhao et al., 1995). We therefore tested whether FGF2 would stimulate embryonic mouse RPE to generate neural retina and whether this was sensitive to activin signaling. We made RPE explants from E12 and E13 mouse embryos, and treated them with FGF2 (Figure 7 B,E,E′) or the combination of FGF2 and SB431542 for 8 days (Figure 7A,D,D′). At E12, the RPE of the mouse is lightly pigmented; pigmentation progresses over the 8 day culture period (Figure 7C) in the explants cultured in DMSO alone. The RPE treated with FGF2 or the combination of FGF2 and SB431542 lost most of its pigmentation, when compared with control explants treated with DMSO. The ganglion cell marker Islet-1 was expressed in E12 RPE treated with the combination of FGF2 and SB431542 (Figure 7A,D,D′). By contrast, FGF2 treatment alone or DMSO did not induce Islet1 expression, but we did find many cells that expressed Pax6 in both the FGF2 treated and the DMSO treated explants. We quantified the percentage of explants that had Islet-1+ cells, and the data are shown in Figure 7G. The ability of SB431542 to induce Islet is dose-dependent (Figure 7G). In the E13 explants, only one of eight RPE treated with FGF2 and 14 uM SB431542 showed Islet-1 positive cells. These data support a role for activin signaling in the loss of competence of mouse RPE to generate neural cells, and corroborate our findings in chick embryos; however, even with the activin signaling pathway blocked, full transdifferentiation does not occur in the mouse RPE.
DISCUSSION
In amphibians, severe damage to the retina initiates a process of regeneration in which the RPE undergoes a remarkable process of dedifferentiation to generate embryonic-like retinal progenitor cells. A remnant of this process is retained in the embryonic chick, and some mammals. There are two key differences that distinguish the process of retinal regeneration in amphibians and that of other vertebrates. First, in amphibians the RPE is duplicated early in the process so that both a new retina and a new RPE are generated, whereas in the chick embryo, there is a direct conversion of the RPE into neural retinal progenitors and subsequently retinal neurons. Second, the dedifferentiation of the RPE occurs in the amphibian retina throughout life, whereas this only occurs in the first few days of embryogenesis in the chick.
There is little known about the molecular basis of the developmental loss in the potential of the RPE in warm-blooded vertebrates to generate neural retina. The factors that have been identified to date that regulate retinal regeneration in early embryonic chick are normally important in the control of neural retinal and/or RPE fate in the optic vesicle. Members of the FGF family can stimulate the RPE to generate neural retina (Park and Hollenberg, 1989;1991; Pittack et al, 1991) and are necessary for the development of the neural retinal domain of the optic vesicle (Pittack et al, 1997). Antagonism of Shh with cyclopamine (Spence et al, 2004) expands the amount of RPE that transdifferentiates to neural retina, when co-applied with FGF. In frogs, a member of the hedgehog family is normally important in promoting or stabilizing the RPE phenotype in ventral optic vesicle (Perron et al, 2003). We now report that antagonism of activin/TGFβ/nodal signaling with a small molecule of this pathway can extend the period of competence to generate neural retina from the RPE, which is consistent with a previous report that activin signaling promotes/stabilizes the RPE phenotype during optic vesicle formation (Fuhrmann et al, 2000). Taken together, these studies support the idea that the molecular signals that regulate the identity of the neural and RPE domains of the optic vesicle are also important for the stabilization of these distinct phenotypes, which ultimately restricts regeneration.
In addition to these signaling factors, there are also several transcription factors that induce RPE cells to develop as retina cells, including neurons and photoreceptors. Over-expression of Pax6, a key regulator of eye development, can direct RPE to generate neural retina as late as Stage 35 (E8; Azuma et al, 2005). Over-expression of another homeodomain transcription factor, Six6/Optx2, in RPE explant cultures as late as E7 (stages 31–32) is also sufficient to induce some retinal-specific genes, including visinin+ photoreceptor-like cells (Toy et al, 1998). Over-expression of NeuroD1 in chick RPE up to E6 causes many cells to directly convert from the RPE phenotype to photoreceptors (Yan and Wang, 2000). Over-expression of NSCL1 or Cath5 (atonal), along with FGF treatment can also stimulate the production of neurons from the RPE up to E6 (Ma et al, 2004). These data indicate that critical transcriptional targets for the proneural transcription factors are still accessible for several days after the RPE cells no longer demonstrate plasticity in their fate in response to critical signaling factors, like FGF and activin, and suggest that there may be several independent restriction points that limit neural retinal generation from the RPE in chicks.
Taken together, the data lead to a model of eye development and fate restriction. The early optic vesicle expresses several eye field transcription factors, including Pax6 and Six6. The distal part of the optic vesicle retains the expression of these eye field transcription factors, while the more proximal part, under the influence of hedgehog and an activin-like signal, loses expression of the EFTFs, and instead expresses a pigment cell specific transcription factor, micro-opthalmia transcription factor (MiTF). Under the continued influence of the three key signaling factors, the fates of the optic vesicle domains are progressively stabilized. Thus, at early stages, soon after the cell fates have been determined, they retain a certain level of plasticity, such that the RPE is capable of generating neural retinal cells. However, there is a progressive restriction in the potential of RPE cells in the chick. Soon after pigmentation of the RPE, coincident with the loss of Pax6 expression (Stage 22–25), there is a loss in the ability of FGF to re-direct the RPE into a neural retinal identity. This period of competence for neurogenesis can be extended by two to three days by treatment with inhibitors to the activin signaling pathway, or by over-expression of proneural transcription factors, such as Pax6, NeuroD1, NSCL1, Cath5, or Optx2. However, eventually, the RPE fate becomes stable, even when proneural transcription factors are over-expressed, perhaps through epi-genetic limits on promoter/enhancer accessibility.
What is the molecular nature of these restriction points? A key factor appears to be Pax6. Pax6 is expressed transiently in the RPE during normal embryogenesis and is required at an early stage for proper RPE development. However, as noted above, over-expression of Pax6 can drive RPE to a neural retinal fate, even very late in its development, and therefore appears to be sufficient for neurogenesis in this system. In this light, it is interesting that the normal developmental down-regulation of Pax6 expression in the RPE at E3.5–E4 correlates with the loss in the ability of FGF to stimulate neurogenesis from this tissue (Spence et al., 2007a). In addition, treatment of the RPE with FGF2 causes a rapid up-regulation of Pax6. We tested whether treatment of the RPE with SB431542 led to an increase in Pax6 expression, but we did not find that the treated explants expressed a higher level of Pax6 than controls (data not shown). Therefore, it is unlikely that inhibition of activin signaling leads to increased competence for neurogenesis in the RPE simply because of a maintenance of Pax6 expression. Another possibility is that inhibition of activin signaling with SB431542 might lead to a down-regulation of critical factors necessary for the development or maintenance of the RPE phenotype. The transcription factor, MiTF is normally critical for RPE development. Mice or quails with mutations in this gene fail to develop a normal RPE, and show spontaneous transdifferentiation to neural retina. Moreover, Fuhrmann et al (2000) found that activin signaling from the extra-ocular mesenchyme promotes RPE development, in part through stimulation in MiTF expression. Several lines of evidence indicate that Pax6 and MiTF act antagonistically, and can repress one another. Therefore, it is possible that one reason SB431542 can reverse the St 25 restriction on transdifferentiation is through a suppression of MiTF. While this is an attractive hypothesis, MiTF is spontaneously downregulated in the absence of the neural retina or in explant culture (Spence et al., 2007b) and so this is unlikely to be the explanation for the loss in neurogenic competence in cultures of RPE or during embryonic retinal regeneration in ovo.
As noted above, there is a profound difference among species in the ability of the RPE to undergo transdifferentiation to a neurogenic state. Might a difference in activin signaling underlie the different competencies? Mouse RPE treated with SB431542 lost pigmentation, like the embryonic chick explants, and at least one neural marker, Islet1, was induced with FGF2 in the SB431542 treated cultures. This suggests that activin signaling is also critical for the stabilization of the RPE phenotype in the mammalian eye. However, we did not find a correlation between the loss of neurogenic competency of the chick RPE and a significant increase in expression of activin in the RPE. Instead we found that there was a small, but steady increase in the expression of activin receptors by the RPE. This suggests that throughout the period of cell fate specification and maturation of the RPE, a consistent, sustained activin signal is necessary to maintain the phenotype, but that some additional mechanisms are responsible for the later restriction in RPE potential in the avian eye that are not present in amphibians. Further molecular characterization of RPE at these later stages might provide some insight into the factors that limit retinal regeneration in warm-blooded vertebrates.
Supplementary Material
Table 1.
Summary of the different experimental protocols used in this study.
Acknowledgments
We would like to thank all the members of the Reh lab for their constructive comments during this research. We would also like to thank Dr. Olivia Bermingham-McDonogh for more general criticisms throughout the period of this research. This work was supported by NIH grant EY13475 to T.A. R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki M, Takano T, Uemonsa T, Nakane Y, Tsudzuki M, Kaneko T. Epithelia-mesenchyme interaction plays an essential role in transdifferentiation of retinal pigment epithelium of silver mutant quail: localization of FGF and related molecules and aberrant migration pattern of neural crest cells during eye rudiment formation. Dev Biol. 2002;244:358–71. doi: 10.1006/dbio.2002.0591. [DOI] [PubMed] [Google Scholar]
- Azuma N, Tadokoro K, Asaka A, Yamada M, Yamaguchi Y, Handa H, Matsushima S, Watanabe T, Kida Y, Ogura T, Torii M, Shimamura K, Nakafuku M. Transdifferentiation of the retinal pigment epithelia to the neural retina by transfer of the Pax6 transcriptional factor. Hum Mol Genet. 2005;14:1059–68. doi: 10.1093/hmg/ddi098. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev Biol. 1965;12:79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Levine EM, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development. 2000;127:4599–609. doi: 10.1242/dev.127.21.4599. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–72. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Keefe JR. An analysis of urodelian retinal regeneration. I. Studies of the cellular source of retinal regeneration in Notophthalmus viridescens utilizing 3 H-thymidine and colchicine. J Exp Zool. 1973a;184:185–206. doi: 10.1002/jez.1401840206. [DOI] [PubMed] [Google Scholar]
- Keefe JR. An analysis of urodelian retinal regeneration. II. Ultrastructural features of retinal regeneration in Notophthalmus viridescens. J Exp Zool. 1973b;184:207–32. doi: 10.1002/jez.1401840207. [DOI] [PubMed] [Google Scholar]
- Ma W, Yan RT, Xie W, Wang SZ. bHLH genes cath5 and cNSCL1 promote bFGF-stimulated RPE cells to transdifferentiate toward retinal ganglion cells. Dev Biol. 2004;265:320–8. doi: 10.1016/j.ydbio.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Okada TS. Phenotypic expression of embryonic neural retinal cells in cell culture. Vision Res. 1981;21:83–6. doi: 10.1016/0042-6989(81)90140-1. [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ. Basic fibroblast growth factor induces retinal regeneration in vivo. Dev Biol. 1989;134:201–5. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ. Induction of retinal regeneration in vivo by growth factors. Dev Biol. 1991;148:322–33. doi: 10.1016/0012-1606(91)90341-y. [DOI] [PubMed] [Google Scholar]
- Perron M, Boy S, Amato MA, Viczian A, Koebernick K, Pieler T, Harris WA. A novel function for Hedgehog signalling in retinal pigment epithelium differentiation. Development. 2003;130:1565–77. doi: 10.1242/dev.00391. [DOI] [PubMed] [Google Scholar]
- Pittack C, Grunwald GB, Reh TA. Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development. 1997;124:805–16. doi: 10.1242/dev.124.4.805. [DOI] [PubMed] [Google Scholar]
- Pittack C, Jones M, Reh TA. Basic fibroblast growth factor induces retinal pigment epithelium to generate neural retina in vitro. Development. 1991;113:577–88. doi: 10.1242/dev.113.2.577. [DOI] [PubMed] [Google Scholar]
- Reh TA, Nagy T. A possible role for the vascular membrane in retinal regeneration in Rana catesbienna tadpoles. Dev Biol. 1987;122:471–82. doi: 10.1016/0012-1606(87)90311-3. [DOI] [PubMed] [Google Scholar]
- Reh TA, Nagy T. Characterization of Rana germinal neuroepithelial cells in normal and regenerating retina. Neurosci Res Suppl. 1989;10:S151–61. doi: 10.1016/0921-8696(89)90017-0. [DOI] [PubMed] [Google Scholar]
- Reh TA, Nagy T, Gretton H. Retinal pigmented epithelial cells induced to transdifferentiate to neurons by laminin. Nature. 1987;330:68–71. doi: 10.1038/330068a0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi DS, Janick LM, Reh TA. Basic fibroblast growth factor (FGF-2) induced transdifferentiation of retinal pigment epithelium: generation of retinal neurons and glia. Dev Dyn. 1997;209:387–98. doi: 10.1002/(SICI)1097-0177(199708)209:4<387::AID-AJA6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Spence JR, Aycinena JC, Del Rio-Tsonis K. Fibroblast growth factor-hedgehog interdependence during retina regeneration. Dev Dyn. 2007a;236:1161–74. doi: 10.1002/dvdy.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Madhavan M, Aycinena JC, Del Rio-Tsonis K. Retina regeneration in the chick embryo is not induced by spontaneous Mitf downregulation but requires FGF/FGFR/MEK/Erk dependent upregulation of Pax6. Mol Vis. 2007b;13:57–65. [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Madhavan M, Ewing JD, Jones DK, Lehman BM, Del Rio-Tsonis K. The hedgehog pathway is a modulator of retina regeneration. Development. 2004;131:4607–21. doi: 10.1242/dev.01298. [DOI] [PubMed] [Google Scholar]
- Stone LS. Neural retina degeneration followed by regeneration from surviving retinal pigment cells in grafted adult salamander eyes. Anat Rec. 1950;106:89–109. doi: 10.1002/ar.1091060108. [DOI] [PubMed] [Google Scholar]
- Stone LS, Steinitz H. Regeneration of neural retina and lens from retina pigment cell grafts in adult newts. J Exp Zool. 1957;135:301–17. doi: 10.1002/jez.1401350206. [DOI] [PubMed] [Google Scholar]
- Toy J, Yang JM, Leppert GS, Sundin OH. The optx2 homeobox gene is expressed in early precursors of the eye and activates retina-specific genes. Proc Natl Acad Sci U S A. 1998;95:10643–8. doi: 10.1073/pnas.95.18.10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan RT, Wang SZ. Expression of an array of photoreceptor genes in chick embryonic retinal pigment epithelium cell cultures under the induction of neuroD. Neurosci Lett. 2000;280:83–6. doi: 10.1016/s0304-3940(99)01003-4. [DOI] [PubMed] [Google Scholar]
- Zhao S, Thornquist SC, Barnstable CJ. In vitro transdifferentiation of embryonic rat retinal pigment epithelium to neural retina. Brain Res. 1995;677:300–10. doi: 10.1016/0006-8993(95)00163-k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


