Abstract
Social anxiety disorder (SAD) is highly comorbid with alcohol use disorders (AUDs) and cannabis dependence. However, the temporal sequencing of these disorders has not been extensively studied to determine whether SAD serves as a specific risk factor for problematic substance use. The present study examined these relationships after controlling for theoretically-relevant variables (e.g., gender, other Axis I pathology) in a longitudinal cohort over approximately 14 years. The sample was drawn from participants in the Oregon Adolescent Depression Project. After excluding those with substance use disorders at baseline, SAD at study entry was associated with 6.5 greater odds of cannabis dependence (but not abuse) and 4.5 greater odds of alcohol dependence (but not abuse) at follow-up after controlling for relevant variables (e.g., gender, depression, conduct disorder). The relationship between SAD and alcohol and cannabis dependence remained even after controlling for other anxiety disorders. Other anxiety disorders and mood disorders were not associated with subsequent cannabis or alcohol use disorder after controlling for relevant variables. Among the internalizing disorders, SAD appears to serve as a unique risk factor for the subsequent onset of cannabis and alcohol dependence.
Keywords: Alcohol, Marijuana, Cannabis, Social Phobia, Social Anxiety, Substance Use
I. Introduction
Social anxiety disorder (SAD) is frequently comorbid with both alcohol abuse and dependence (Davidson et al., 1993; Grant et al., 2005; Kessler et al., 1997) as well as cannabis dependence (Agosti et al., 2002; Lynskey et al., 2002). For instance, 48% of individuals with a lifetime diagnosis of SAD also meet criteria for a lifetime diagnosis of an AUD (Grant et al., 2005). The 12-month prevalence of AUDs among individuals with SAD is 13.1% (Grant et al., 2005) compared to only 8.5% among the general population (Grant et al., 2004). Similarly, findings from the National Comorbidity Study (NCS) indicate that there is a 4.2% lifetime prevalence rate for cannabis dependence in the general population, whereas among individuals with SAD, the prevalence rate of cannabis dependence is elevated to 29.0% (Agosti et al., 2002). Yet, little is known about the specificity or temporal sequencing of the relationships between SAD and these substance use disorders. Elucidation of these relationships could have important implications for the prevention and treatment of these conditions among socially anxious individuals (Heimberg & Becker, 2002).
The high rates of comorbidity between SAD and AUD and cannabis dependence are cause for concern because misuse of alcohol or cannabis tends to compound the already significant problems of patients with SAD. For example, SAD patients with AUD report more severe impairment than patients with SAD without AUD (Schneier et al., 1989) and alcoholics with SAD demonstrate more severe symptoms of alcohol dependence and display more depressive symptomatology than alcoholics without SAD (Thomas et al., 1999b). Cannabis dependence among individuals with SAD is problematic because smoking cannabis has a larger effect on respiratory function than smoking tobacco (Bloom et al., 1987; Sherrill et al., 1991), including cellular changes that may serve as a risk factor for cancer (Fligiel et al., 1997; Sarafian et al., 1999). Long-term cannabis use is associated with legal problems and increased alcohol and tobacco use (Patton et al., 2002; Reilly et al., 1998) and driving under the influence of cannabis leads to increased automobile crash risk (Ramaekers et al., 2004).
Among the anxiety disorders, SAD appears to show a particularly problematic risk profile for comorbid AUD and CUD. For example, SAD is associated with higher rates of AUD relative to most other anxiety disorders (Kessler et al., 1997). SAD is also correlated with cannabis dependence at rates more than twice that of any other anxiety disorder (Agosti et al., 2002). On the matter of sequencing, evaluation of typical age of onset of SAD and AUD suggests that SAD serves as a risk factor for subsequent AUD (Kessler et al., 1997; Randall et al., 2001a; Randall et al., 2001b; Schneier et al., 1989). Additionally, in a 13-year longitudinal investigation (Crum & Pratt, 2001), individuals with subclinical symptoms of SAD showed a greater risk for AUD relative to individuals without subclinical SAD symptomatology. Unexpectedly, individuals diagnosed with SAD using DSM-III standards did not show an increased risk of subsequent AUD, but changes in diagnostic criteria for SAD from DSM-III to DSM-IV-TR complicate interpretation of these findings in relation to contemporary diagnostic definitions. In particular, DSM-III criteria included avoidance as a necessary symptom for social phobia and avoidance is no longer a necessary criterion for SAD. This change is not trivial because it may very well be those individuals with SAD who do not avoid social situations who are most vulnerable to problematic alcohol use, especially if they use alcohol in social situations in an attempt to attenuate anxiety reactions. Similarly, among German adolescents, SAD is associated with subsequent regular and hazardous alcohol use but not DSM-IV alcohol abuse or dependence at 4-year follow-up (Zimmermann et al., 2003). That study, however, did not follow participants very far into the typical period of onset of alcohol dependence, thereby limiting its interpretability. Although there are no known longitudinal investigations of the relationship between SAD and cannabis abuse and/or dependence, given that marijuana users report they use to marijuana to cope with stress and anxiety (Hathaway, 2003; Ogborne et al., 2000), it follows that a similar temporal relationship would occur between SAD and cannabis dependence.
The limited literature in this area makes it difficult to draw firm conclusions regarding the risk for alcohol and cannabis use disorders among those with SAD. Importantly, it is unknown whether the development of AUD or CUD is unique to SAD versus other forms of anxiety. The question of specificity is critical because SAD is highly comorbid with other anxiety disorders (Davidson et al., 1993; Merikangas & Angst, 1995) and other anxiety conditions are associated with increased rates of AUD (Kushner et al., 1990) and cannabis dependence (Zvolensky et al., 2006). When all anxiety disorder diagnoses were combined, anxiety disorders preceded AUD in the Oregon Adolescent Project (Rohde et al., 1996). However this study did not investigate the temporal relations among specific anxiety disorders. The few studies that have examined specific anxiety conditions and their temporal associations with AUD and CUD suggest that other anxiety disorders were more likely to be sequelae of alcohol and cannabis use, whereas SAD may serve as a risk factor for subsequent AUD and CUD. For instance, among individuals with co-occurring panic and AUD, panic onset tends to follow AUD (Kushner et al., 1990). Age of onset of panic is also later than that of CUD among individuals with both conditions (Zvolensky et al., 2006). Similarly, age of onset of substance use disorder is earlier than that of generalized anxiety disorder (GAD) among individuals with both disorders (Kessler et al., 2002).
It is also unclear whether SAD and/or other anxiety conditions demonstrate specific relationships to AUD or cannabis dependence after accounting for other types of psychopathology related to these substance use disorders. Considering that SAD is highly comorbid with mood disorders (Stein & Kean, 2000) and that depression is related to both alcohol and cannabis use problems (Buckner et al., in press), often preceding the onset of alcohol use (King et al., 2004) and cannabis use (Paton et al., 1977), it may be that the high rates of alcohol and cannabis dependence among individuals with SAD are due to co-occurring mood pathology. Likewise, externalizing disorders, particularly conduct disorder, are highly comorbid with anxiety disorders (Russo & Beidel, 1994; Zoccolillo, 1992) and predict later AUDs and CUDs (Myers et al., 1995), so they must be controlled in analyses of connections between anxiety and substance use disorders. And, of course, alcohol and cannabis use are themselves highly comorbid (Agosti et al., 2002), making it is necessary to examine the effects of one substance after controlling for effects of the other.
Further, the majority of studies in this area tend to combine alcohol abuse and alcohol dependence diagnoses (Crum & Pratt, 2001; Schneier et al., 1989), making it difficult to demarcate whether individuals with SAD are at increased risk for alcohol abuse, dependence or both. This distinction is important because alcohol dependence is a more debilitating disorder (American Psychiatric Association, 1980) and it appears that individuals with SAD are particularly vulnerable to this more severe condition. For example, epidemiological studies using DSM-IV criteria suggest SAD is more likely to be associated with increased risk of alcohol dependence than alcohol abuse (Grant et al., 2005; Kessler et al., 1997). Further, among individuals seeking treatment for alcohol-related problems, 23% to 39% meet diagnostic criteria for SAD (Kushner et al., 1990; Schneier et al., 1989; Smail et al., 1984; Thomas et al., 1999a). Individuals with higher levels of alcohol-related problems also experience significantly higher levels of social anxiety (Buckner et al., 2006c; Lewis & O’Neill, 2000). In regards to cannabis, greater SAD symptoms are associated with greater number of CUD symptoms (Buckner et al., 2006a; Buckner et al., 2006b) and the NCS data indicate that SAD is associated with increased rates of cannabis dependence but not abuse (Agosti et al., 2002).
Given the ambiguities described above, the present investigation contributes to the elucidation of the relations of anxiety disorders with AUDs and CUDs in several ways. First, the comorbidity of specific anxiety disorders and AUDs and CUDs was evaluated. Second, longitudinal analyses examined whether particular anxiety disorders serve as risk factors for subsequent AUDs or CUDs. Third, relevant variables (e.g., depression, conduct disorder) served as covariates to ensure that observed effects were not better accounted for by these conditions. Fourth, the relationships between SAD and specific AUDs and CUDs were examined after also controlling for other anxiety disorders to ensure observed relationships were not due to comorbidity of anxiety pathology. Last, analyses clarified whether observed relations applied to substance abuse, dependence, or both. Given the data suggesting that SAD is particularly associated with alcohol and cannabis dependence, we hypothesized that, after controlling for theoretically relevant variables, SAD would be significantly associated with alcohol and cannabis dependence, but not abuse. Additionally, given other theoretical and empirical work suggesting that other anxiety disorders appear to be a sequelae of substance use (Kessler et al., 2002; Zvolensky et al., 2006), it was hypothesized that SAD, but not other anxiety disorders, would be associated with the onset of subsequent alcohol and cannabis dependence, thereby demonstrating explanatory specificity.
2. Method
2.1. Participants and Procedures
The sample was drawn from the Oregon Adolescent Depression Project (Goodwin et al., 2005; Goodwin et al., 2004; Lewinsohn et al., 1993). Participants were randomly selected from nine senior high schools representative of urban and rural districts in western Oregon. A total of 1,709 adolescents completed the initial (T1) assessments between 1987 and 1989, with an overall participation rate of 61%. Approximately one half of the T1 sample was female (53.7%), with a mean age of 16.6 years (SD = 1.2). As participants reached their 24th birthday, a third wave of questionnaire and diagnostic interview assessments (T3) was conducted with a selected subset of time 2 (T2) participants. On the basis of T1-T2 diagnostic information, three groups were selected for the T3 diagnostic interview: (a) 360 participants with a T2 lifetime history of major depressive disorder, (b) 284 participants with a T2 lifetime history of nonaffective Axis I disorder, and (c) 457 participants with no history of mental disorder at T2. The no-disorder comparison group was representative of the entire group of participants with no mental disorder at T2 (n = 863) in terms of age and gender within age; all participants with non-white ethnicity were invited to participate in the T3 assessment. This sampling strategy was intentional due to the expense of running this longitudinal investigation.
At age 30 (M = 30.6 years, SD = 0.6), the participants who completed the T3 interview were invited to participate in a fourth wave (T4) of data collection. Of the 941 eligible participants, 816 (86.7%) completed the T4 diagnostic interview. The T4 participants (59% women) were primarily Caucasian (59%) and married (53%). Forty-one percent had a bachelor’s degree or higher. For the T4 assessment, the retention rates for the three groups selected for the T3 interview were as follows: 86.5% for individuals in the major depressive disorder group, 82.5% for individuals in the nonaffective Axis I disorder group, and 89.5% for individuals in the no mental disorder group. Significantly higher attrition rates were noted for men than women (16% vs. 11%), as well as for participants with a lifetime history of alcohol use disorders (17% vs. 12% for those with no lifetime alcohol use disorder), and cannabis use disorders (18% vs. 12% for those with no lifetime history of cannabis use disorder).
Given that the age of onset for alcohol abuse and dependence peaks by the early 30s, (American Psychiatric Association, 2000; Grant et al., 2004) this report concerns data collected concerning lifetime diagnostic histories at T1 and T4. By age 30, the adults had been assessed on four occasions. Written informed consent was obtained from participants (and guardians, if applicable) to conduct all assessments.
2.2. Materials
Participants were interviewed at T1 with a version of the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) that combined features of the epidemiologic version (Orvaschel et al., 1982) and the present episode version (K-SADS-P) and included additional items to derive DSM-III-R diagnoses (American Psychiatric Association, 1980). Both versions of the K-SADS have been found to demonstrate adequate psychometric properties (Ambrosini, 2000). In the present report, lifetime histories of the following disorders at T1 were examined: SAD, PD (with and without agoraphobia), GAD, obsessive compulsive disorder (OCD), specific phobia (SP), overanxious disorder (OD), and separation anxiety disorder. Given that diagnoses at the T1 assessment periods were derived using DSM-III-R criteria, in cases where DSM-III and DSM-IV criteria differed, additional data were used to ascertain DSM-IV criteria for the diagnoses of interest (American Psychiatric Association, 2000). Lifetime history of adult T4 diagnoses were derived from a joint administration of the Longitudinal Interval Follow-up Evaluation (LIFE; Keller et al., 1987) and the Structured Clinical Interview for DSM-IV, non-patient version (SCID-I/NP; First et al., 1994) to probe for new or continuing psychiatric episodes.
Diagnostic interviewers were carefully selected, trained, and supervised. Interviewers had advanced degrees in a mental health discipline and completed a 70-hour course in diagnostic interviewing. Interrater reliability was evaluated by the kappa statistic (Cohen, 1960). Kappas for the T1 anxiety disorders have been reported elsewhere (Lewinsohn et al., 1997) and were acceptable. Prior to conducting interviews, all interviewers were required to demonstrate a minimum kappa of .80 across all symptoms for at least two consecutive training interviews and on one videotaped interview of a participant with evidence of psychopathology. T4 interviews were audiotaped and 15% (n = 124) were randomly selected for reliability purposes. Interrater reliability, as evaluated by the kappa statistic, was moderate to excellent for AUD (κ = .79) and CUD (κ = .90).
2.3. Statistical Procedures
First, odds ratios and confidence intervals were computed to examine the relationships between T1 predictor variables and T4 criterion variables (alcohol abuse, alcohol dependence, cannabis abuse, cannabis dependence). Next, hierarchical logistic regression analyses were performed with each of the T4 criterion variables. T1 anxiety and mood disorders found to be associated with T4 criterion variables served as predictor variables. For each model, a dichotomous variable was created representing the absence (“0”) or presence (“1”) of the covariate Axis I disorders of interest. Given that gender is associated with anxiety disorders (Lewinsohn et al., 1998) and with substance use (Siqueira et al., 2001), gender was also included as a covariate in level 1 of all regression models. For the cannabis models, participants with a T1 lifetime history of CUD were excluded from the analyses. Both cannabis models were divided into three levels. At level 1, gender was entered and at level 2, T1 “other Axis I disorder” variable was entered. At level 3, the anxiety condition of interest was entered. For the alcohol models, participants with a T1 lifetime history of AUD were excluded from the analyses and T1 lifetime history of CUD was entered at level 2 (rather than T1 AUD). These models ensured that observed effects for T1 anxiety disorder at level 3 cannot be attributed to shared variance with the variable at levels 1 (Cohen & Cohen, 1983).
Third, hierarchical logistic regression analyses were performed to examine whether the relationship between SAD and T4 criterion variables occurred above and beyond the association among SAD and other anxiety disorders. For the cannabis models, participants with a T1 lifetime history of CUD were excluded from the analyses. Gender was entered at level 1 in the model and “other Axis I disorder” was entered at level 2. This procedure ensured any observed effects were not due to these variables. For the alcohol models, participants with T1 lifetime history of AUD were excluded from the analyses. At level 2, T1 lifetime history of CUD was entered. At level 3 in the model, SAD was entered. These models ensured that observed effects for T1 SAD at level 3 cannot be attributed to shared variance with the variable at levels 1 and 2 (Cohen & Cohen, 1983).
Univariate and multivariate survival analysis methods developed to deal with censored time-to -event data were used to test time to onset of alcohol dependence and cannabis dependence. Univariate Kaplan-Meier models were used to estimate onset rates and generate survival curves for participants with and without a lifetime history of SAD at T1. Cox proportional hazards models were specified for multivariate analysis of time to onset. Survival analysis is a powerful and informative method for identifying clinically important effects that may be obscured by analytic techniques that examine the proportion of individuals who develop a disorder at a single point in time. In contrast to end-point analytic techniques that restrict the sample to individuals with known event occurrences, computation of the hazard function in time-to-event analyses includes censored participants who are lost to follow-up or who do not experience the target event during the data collection period and thereby maximizes statistical power (Willett & Singer, 1993).
The Kaplan-Meier method estimates risk of onset at a particular moment as the ratio of the number who onset at that time to the number of individuals currently at risk of onset. The Cox proportional hazard model, a regression-based method for time-to -event analysis, is a technique that allows for the simultaneous estimation of hazard ratios for multiple explanatory variables (Bull & Spiegelhalter, 1997). The hazard ratio with 95% confidence interval can be used as a measure of effect size with a positive ratio of 1.44, 2.48, and 4.28 representing small, medium, and large effects, respectively (Lipsey & Wilson, 2001). Proportional hazard models assume that the log-hazard profiles represented by all possible values of the predictors share a common shape and are mutually parallel (Singer & Willett, 1991). The proportional hazards assumption was tested by including a time x predictor interaction term in the Cox model. As recommended (Willett & Singer, 1993), if the time x predictor interaction term was significant, the term was retained in the model to ensure appropriate estimation of the effects of interest. If the proportional hazards assumption was not violated, the interaction term was removed from the model.
In accordance with the logistic regression models described above, participants with a lifetime history of alcohol dependence or cannabis dependence were excluded from each respective model. T1 anxiety, conduct, and mood disorders as well as gender were included in the Cox models as covariates.
3. Results
3.1. Descriptive Information
Table 1 summarizes demographic characteristics of the sample. Diagnostic frequencies, odds ratios, and confidence intervals were computed to examine the relations between T1 predictor variables and T4 criterion variables (alcohol abuse, alcohol dependence, cannabis abuse, cannabis dependence) (Table 2). Additionally, 84 (4.9%) reported a T1 lifetime history of AUD and 92 (5.4%) participants reported a T1 lifetime history of CUD.
Table 1.
Demographic Information for Entire Sample and by Social Anxiety Diagnosis
| Entire Sample | No Social Anxiety Diagnosis | Social Anxiety Diagnosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | % | M | SD | n | % | M | SD | n | % | M | SD |
| Age (years) | 16.56 | 1.19 | 16.56 | 1.19 | 28.76 | 10.91 | ||||||
| Gender (female) | 891 | 52.1 | 870 | 51.7 | 21 | 84.0 | ||||||
| Race/ethnicity (Caucasian) | 1557 | 91.1 | 1535 | 91.2 | 22 | 88.0 | ||||||
| Annual income | ||||||||||||
| $0-14,999 | 373 | 21.9 | 365 | 21.6 | 8 | 32.0 | ||||||
| $15,000-29,999 | 312 | 18.3 | 305 | 18.1 | 7 | 28.0 | ||||||
| $30,000+ | 248 | 14.5 | 246 | 14.6 | 2 | 8 | ||||||
Table 2.
Associations between T1 Anxiety Disorders and T4 Criterion Variables
| Predictor Variable | T4 alcohol abuse | T4 alcohol dependence | T4 cannabis abuse | T4 cannabis dependence | Frequency (% total sample) |
|---|---|---|---|---|---|
| T1 SAD | .48 (.11-2.11) | 3.98 (1.51-10.47)** | 1.01 (.23-4.50) | 4.89 (1.82-13.15)** | 25 (1.5%) |
| T1 PD | .52 (.06-4.23) | 5.82 (1.38-24.57)* | 1.09 (.13-8.92) | 4.06 (.96-17.25) | 12 (0.7%) |
| T1 OCD | 2.44 (.41-14.72) | 5.18 (.86-31.26) | 1.91 (.21-17.24) | 4.48 (.74-27.14) | 8 (0.5%) |
| T1 OD | .28 (.04-2.12) | 1.37 (.43-4.43) | .58 (.08-4.48) | .51 (.07-3.90) | 22 (1.3%) |
| T1 SP | .78 (.22-2.73) | 1.89 (.69-5.18) | 1.65 (.46-5.84) | .41 (.05-3.11) | 34 (2.0%) |
| T1 separation anxiety disorder | .56 (.23-1.35) | .87 (.41-1.85) | .35 (.08-1.46) | 1.51 (.68-3.34) | 72 (4.2%) |
| Frequency (% total sample) | 176 (10.3%) | 185 (10.8%) | 95 (5.6%) | 107 (6.3%) | |
Note. Values are expressed as odds ratios (95% confidence interval). T1 and T4 diagnoses reflect lifetime history. Abbreviations: social anxiety disorder (SAD), panic disorder (PD), obsessive compulsive disorder (OCD), overanxious disorder (OD), specific phobia (SP).
p < .05.
p < .01.
First, relations between predictor variables were examined. Consistent with expectation, T1 SAD was significantly correlated with female gender, T1 mood disorder and T1 anxiety disorders, but not T1 conduct disorder. Contrary to expectation, T1 SAD was not associated with any T1 alcohol/cannabis variable. Second, relations among the predictor variables and the T4 alcohol abuse and alcohol dependence dependent variables were examined (Table 2). As predicted, T4 alcohol dependence was significantly associated with T1 SAD, T1 PD, T1 mood disorder, T1 conduct disorder, T1 alcohol abuse, T1 cannabis abuse, T1 cannabis dependence, and male gender. T4 alcohol abuse was not significantly associated with increased odds for T1 SAD and was only significantly associated with male gender and T1 cannabis abuse. Third, patterns of associations among the predictor variables and the T4 cannabis abuse and cannabis dependence dependent variables were examined (Table 2). Consistent with prediction, T4 cannabis dependence was significantly associated with T1 SAD, T1 mood disorder, T1 conduct disorder, T1 alcohol abuse, T1 alcohol dependence, T1 cannabis abuse, and male gender. T4 cannabis abuse was not associated T1 SAD and was only significantly associated T1 alcohol abuse and male gender.
3.2. Prediction of Lifetime History of Cannabis and Alcohol Use Disorders using Anxiety Disorder Diagnoses in Adolescence
Hierarchical logistic regression analyses were performed with each criterion variable, controlling for theoretically relevant variables. Only those T1 affective disorders found to be associated with T4 criterion variables (i.e., SAD, PD, mood disorders) served as predictor variables. Consistent with expectation, T1 SAD was the only anxiety condition to significantly predict T4 AUD or CUD after controlling for theoretically relevant variables. Specifically, T1 SAD was associated with increased odds of T4 alcohol dependence (OR = 4.47, 95% CI= 1.48-13.45, p < .01) but not T4 alcohol abuse (OR = 0.39, 95% CI= .05-3.07, p > .05). Further, T1 SAD was associated with increased odds of T4 cannabis dependence (OR = 6.58, 95% CI= 1.94-22.34, p < .01) but not T4 cannabis abuse (OR = 0.99, 95% CI= .13-7.79, p > .05).
Given that T1 PD was associated with significantly increased odds of T4 alcohol dependence, hierarchical logistic regression analyses were performed to determine whether T1 PD was significantly associated with increased odds alcohol dependence above and beyond the covariates. After controlling for T1 AUD, T1 mood disorder, T1 conduct disorder, and gender, T1 PD was not significantly associated with T4 alcohol dependence (OR = 2.36, 95% CI= .25-21.96, p > .05).
Similarly, because T1 mood disorder was also associated with significantly increased odds of T4 alcohol and cannabis dependence, hierarchical logistic regression analyses were performed with each relevant criterion variable for T1 mood disorder. After controlling for T1 AUD, T1 conduct disorder, and gender, T1 mood disorder was not significantly associated with T4 alcohol dependence (OR = 1.49, 95% CI= 1.0-2.28, p > .05) or T4 cannabis dependence (OR = .86, 95% CI= .45-1.64, p > .05).
3.3. Evaluation of the Unique Contribution of Social Anxiety Disorder Relative to Other Axis I Anxiety Disorders in Predicting the Development of Alcohol and Cannabis Use Disorders
Hierarchical logistic regression analyses were performed to examine whether the relation between T1 SAD and T4 criterion variables occurred above and beyond the associations between SAD and other anxiety disorders. After controlling for all theoretically relevant variables (including other anxiety disorder diagnoses and relevant T1 SUDs), T1 lifetime history of SAD continued to be significantly related to T4 lifetime history of alcohol dependence (OR = 3.72, 95% CI= 1.23-11.29, p < .05) but not T4 alcohol abuse (OR = 0.36, 95% CI= .05-2.78, p > .05). Similarly, T1 lifetime history of SAD continued to be significantly related to T4 lifetime history of cannabis dependence (OR = 4.88, 95% CI= 1.43-16.64, p < .05) but not T4 cannabis abuse (OR = 1.07, 95% CI= .14-8.56, p > .05).
3.4. Survival Curve Analyses of Social Anxiety Disorder in Predicting the Development of Alcohol Dependence
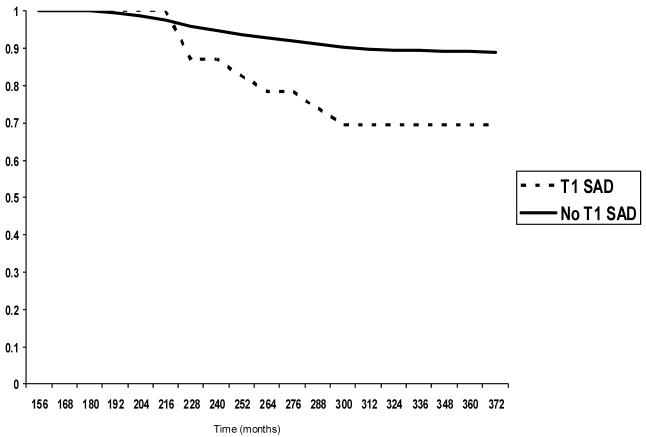
Based on cumulative sample survival probabilities from the Kaplan-Meier models, 26% of T1 participants with a SAD diagnosis developed alcohol dependence by age 24 with the steepest onset slope occurring between 18 to 19 years old. In comparison, only 8.5% of T1 participants without a SAD diagnosis developed alcohol dependence by age 24 with total cumulative onset through the T4 assessment of only 11% (see Figure 1). After covarying out the effects of participant sex and T1 anxiety, conduct, and mood disorders, T1 SAD significantly predicted time to onset of alcohol dependence in the Cox proportional hazard model (hazard ratio = 1.56; 95% CI = 1.06 - 2.31; p = .02) indicating that participants with a T1 SAD diagnosis were 1.56 times more likely to develop an alcohol dependence diagnosis over the period of observation than those without a T1 SAD diagnosis.
Figure 1.
Cumulative survival curve for alcohol dependence onset, excluding participants with T1 AUD.
3.5. Survival Curve Analyses of Social Anxiety Disorder in Predicting the Development of Cannabis Dependence
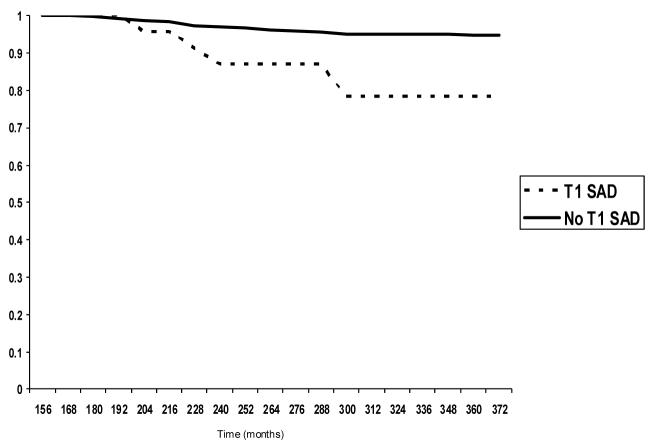
Of the participants with a T1 SAD diagnosis, 13% developed cannabis dependence by age 24 compared to only 4% of T1 participants without a SAD diagnosis. Total cumulative onset through the T4 assessment (roughly age 30) was 22% for T1 SAD participants and 5% for those without T1 SAD (see Figure 2). Findings for the Cox proportional hazard model predicting onset of cannabis dependence were similar to those predicting onset of alcohol dependence. Again, participants with a T1 SAD diagnosis were significantly more likely to develop a cannabis dependence disorder over the observation period (hazard ratio = 1.94; 95% CI = 1.21-3.13; p = .01) than those without a T1 SAD diagnosis, after controlling for relevant T1 covariates.
Figure 2.
Cumulative survival curve for cannabis dependence onset, excluding participants with T1 CUD.
3.6. Evaluation of the Specificity of Social Anxiety Symptoms in Predicting the Development of Alcohol and Cannabis Dependence relative to Other Axis I Anxiety Disorders
To determine whether SAD uniquely predicts alcohol and cannabis dependence (but not other Axis I conditions), hierarchical logistic regression analyses were performed with each criterion variable (with gender serving as level 1 covariate). For each regression, participants with a T1 lifetime history of the T4 criterion variable were excluded from the analyses. T1 lifetime history of SAD only demonstrated significantly increased odds of T4 lifetime history of separation disorder (OR = 3.96, 95% CI= 1.06-14.79, p < .05) but not mood disorder (OR = 7.36, 95% CI= .85-63.58, p > .05), CD (OR = .00, p > .05), PD (OR = 1.70, 95% CI= .37-7.86, p > .05), OCD (OR = .00, p > .05), GAD (OR = 3.17, 95% CI= .39-25.93, p > .05), or SP (OR = 4.94, 95% CI= 1.03-23.76, p = .05).
4. Discussion
The present study serves as the first known prospective investigation of the temporal relationship between current definitions of SAD and specific alcohol use and cannabis use disorders after controlling for baseline AUDs and CUDs, as well as other relevant variables. Results indicated that SAD may serve as a unique and significant risk factor for subsequent alcohol and cannabis dependence, but not abuse. These effects were above and beyond the variance accounted for by a variety of theoretically-relevant covariates, including prior CUD and AUD, mood disorders, conduct disorder, gender, and other anxiety disorders. The identification of SAD as a specific risk factor for the development of these SUDs is not trivial, as it may be that treatment of adolescent SAD could reduce the incidence of adult SUD (Kendall & Kessler, 2002).
Consistent with one of our key hypotheses, the current findings indicate that among the anxiety disorders, SAD appears to be unique in its role as a risk factor for subsequent cannabis and alcohol dependence. This finding is consistent with previous research suggesting that problematic substance use typically precedes the development of other anxiety conditions (Kessler et al., 2002; Zvolensky et al., 2006). Given the specific nature of the various anxiety disorders, it is not surprising that individuals with other anxiety disorders are less likely than individuals with SAD to use substances to self-medicate anxiety reactions. For example, patients with PD are characterized by intense fears of physiological arousal sensations, which can be brought on by illicit substances. Consequently, some patients with PD have been found to avoid cannabis, particularly when the use of cannabis has led to increased anxiety (Szuster et al., 1988). In fact, current models of the panic-cannabis nexus posit that cannabis dependence serves as a risk factor for the development of panic (Zvolensky et al., 2006). Individuals with GAD possess exaggerated worries that often focus on issues such as health and illness (Craske et al., 1989; Wells & Carter, 1999). These worries could readily focus on the consequences of substance dependence (e.g., health, legal, social stigma). Thus, fear of physiological arousal as well as heightened worry may serve as protective factors, decreasing the risk for subsequent alcohol and cannabis dependence.
The question arises as to why SAD is associated with dependence specifically. Patients with SAD use substances with anxiolytic properties such as alcohol and cannabis to cope with social anxiety reactions (Schneier et al., 1989; Smail et al., 1984). As a result, these individuals may come to believe that they need these substances to cope with negative affective states related to social anxiety. Consequently, they become less likely to engage in adaptive coping strategies as they are ever more reliant on substances to regulate affective states. Reliance on alcohol or cannabis to cope with social anxiety increases the likelihood that these individuals will be vulnerable to substance-related problems, thereby increasing their risk for substance dependence. The use of substances to regulate emotions appears to be particularly related to dependence. For example, using alcohol to regulate emotions is associated with the dependence symptoms of withdrawal and tolerance (Cooper, 1994; Cooper et al., 1992) and the use of alcohol and cannabis to cope with negative emotions is associated with increased risk for the development of substance-related problems regardless of quantity/frequency of alcohol use (Cooper et al., 1995).
Three additional findings are particularly noteworthy. First, the relationship between SAD and cannabis dependence emerged after controlling for the effects of AUD and the relationship between SAD and alcohol dependence remained after controlling for CUD. These data indicate that observed relationships cannot simply be accounted for by the co-occurrence of SAD and AUD (Kessler et al., 1997) or the co-occurrence of AUD and CUD (Patton et al., 2002; Reilly et al., 1998). Second, the association between SAD and alcohol/cannabis dependence emerged even after controlling for CD. Given that CD was an especially strong predictor of subsequent AUD and CUD, the current findings suggest the relationship between SAD and these substance use disorders is not better accounted for by the relationship between CD and anxiety disorders (Russo & Beidel, 1994; Zoccolillo, 1992). Third, mood disorders were not found to predict subsequent AUD or CUD after controlling for theoretically relevant variables, suggesting that SAD may not only serve as a unique risk factor for alcohol and cannabis dependence among anxiety disorders, but among internalizing disorders generally.
The present findings should be considered in light of several limitations that point to interesting areas for further work. First, there were very few subjects in some diagnostic categories, resulting in odds ratios with large confidence intervals. Although, these prevalence rates are consistent with those found in other studies of adolescent populations (McGee et al., 1990), replication with larger samples of anxiety disorders is needed. Second, although a psychometrically sound interview was employed to identify psychopathology, future work could benefit from the use of multiple measures to address the limitations inherent in the assessment of adolescent psychopathology (Loney & Lima, 2003). Third, baseline assessments were conducted using DSM-III criteria. Although sufficient information was obtained to derive DSM-IV diagnoses, future research is necessary to ascertain whether the present findings would replicate on data collected specifically using DSM-IV criteria. Fourth, given that not all students participated T1 assessments, generalizability to those students who refused to participate is limited. Fifth, attrition over the course of 14 years was high, particularly among those with substance use disorders. Although our data suggest that SAD is correlated with alcohol and cannabis dependence despite the increased attrition rates among this group, we cannot rule out the possibility that attrition may have obscured certain effects.
Despite these limitations, the findings of the present investigation suggest that individuals with SAD are at an increased risk for a lifetime history of alcohol and cannabis dependence above and beyond a rich array of relevant factors. Moreover, the relationship between SAD and alcohol and cannabis dependence appears relatively specific, as no such effect was evident for other anxiety or mood disorders. Further elucidation of the mechanisms underlying why SAD serves as a risk factor for subsequent cannabis and alcohol dependence could have important implications for the development of prevention and treatment programs for at-risk individuals.
Table 3.
Associations between T1 Covariates and T4 Criterion Variables
| Predictor Variable | T4 alcohol abuse | T4 alcohol dependence | T4 cannabis abuse | T4 cannabis dependence | Frequency (% total sample) |
|---|---|---|---|---|---|
| Gender | 1.73 (1.23-2.42)** | 1.91 (1.38-2.66)** | 1.46 (.95-2.25) | 1.68 (1.12-2.53)* | 52.0% female |
| T1 mood disorder | .72 (.49-1.06) | 1.57 (1.11-2.22)* | 1.00 (.63-1.62) | 1.78 (1.17-2.71)** | 347 (20.3%) |
| T1 conduct disorder | 3.86 (1.81-8.27)** | 6.69 (3.03-14.77)** | 3.22 (1.38-7.54)** | 16.79 (7.37-38.26)** | 56 (3.3%) |
| T1 alcohol abuse | - | 7.92 (2.71-23-08)** | 18.75 (6.36-55.28)** | 12.08 (4.29-33.98)** | 32 (1.9%) |
| T1 alcohol dependence | .91 (.36-2.25) | - | 1.55 (.58-4.14) | 10.13 (4.75-21.51)** | 53 (3.1%) |
| T1 cannabis abuse | 3.40 (1.36-8.49)** | 2.55 (1.01-6.43)* | - | 5.13 (2.01-13.06)** | 31 (1.8%) |
| T1 cannabis dependence | 1.88 (.92-3.84) | 7.69 (3.76-15.71)** | 1.90 (.81-4.46) | - | 61 (3.6%) |
| Frequency (% total sample) | 176 (10.3%) | 185 (10.8%) | 95 (5.6%) | 107 (6.3%) | |
Note. Values are expressed as odds ratios (95% confidence interval). T1 and T4 diagnoses reflect lifetime history.
p < .05.
p < .01.
Acknowledgements
This research was supported in part by NIMH awards MH40501, MH50522, and MH52588, by the John Simon Guggenheim Foundation and NIDA Grant DA12951 awarded to Dr. Peter M. Lewinsohn. This research was also supported in part by a National Research Service Award from the National Institute of Drug Abuse (F31 DA12457-01) awarded to Julia D. Buckner.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. American Journal of Drug and Alcohol Abuse. 2002;28:643–652. doi: 10.1081/ada-120015873. [DOI] [PubMed] [Google Scholar]
- Ambrosini PJ. Historical development and present status of the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:49–58. doi: 10.1097/00004583-200001000-00016. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Author; Washington, DC: 1980. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Author; Washington, DC: 2000. [Google Scholar]
- Bloom JW, Kaltenborn WT, Paoletti P, Camilli A, Lebowitz MD. Respiratory effects of non-tobacco cigarettes. British Medical Journal. 1987;295:1516–1518. doi: 10.1136/bmj.295.6612.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Keough ME, Schmidt NB. Problematic Cannabis and Alcohol Use among Young Adults: The Roles of Depression and Discomfort and Distress Intolerance. Addictive Behaviors. doi: 10.1016/j.addbeh.2006.12.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Mallott MA, Schmidt NB, Taylor J. Peer influence and gender differences in problematic cannabis use among individuals with social anxiety. Journal of Anxiety Disorders. 2006a;20:1087–1102. doi: 10.1016/j.janxdis.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB, Bobadilla L, Taylor J. Social anxiety and problematic cannabis use: Evaluating the moderating role of stress reactivity and perceived coping. Behaviour Research and Therapy. 2006b;44:1007–1015. doi: 10.1016/j.brat.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB, Eggleston AM. Social anxiety and problematic alcohol consumption: The mediating role of drinking motives and situations. Behavior Therapy. 2006c;37:381–391. doi: 10.1016/j.beth.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Bull K, Spiegelhalter DJ. Tutorial in biostatistics survival analysis in observational studies. Statistics in Medicine. 1997;16:1041–1074. doi: 10.1002/(sici)1097-0258(19970515)16:9<1041::aid-sim506>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1983. [Google Scholar]
- Cooper ML. Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychological Assessment. 1994;6:117–128. [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. Journal of Personality and Social Psychology. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Russell M, Skinner JB, Windle M. Development and validation of a three-dimensional measure of drinking motives. Psychological Assessment. 1992;4:123–132. [Google Scholar]
- Craske MG, Rapee RM, Jackel L, Barlow DH. Qualitative dimensions of worry in DSM-III-R generalized anxiety disorder subjects and nonanxious controls. Behaviour Research and Therapy. 1989;27:397–402. doi: 10.1016/0005-7967(89)90010-7. [DOI] [PubMed] [Google Scholar]
- Crum RM, Pratt LA. Risk of heavy drinking and alcohol use disorders in social phobia: A prospective analysis. American Journal of Psychiatry. 2001;158:1693–1700. doi: 10.1176/appi.ajp.158.10.1693. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Hughes DL, George LK, Blazer DG. The epidemiology of social phobia: Findings from the Duke Epidemiological Catchment Area Study. Psychological Medicine. 1993;23:709–18. doi: 10.1017/s0033291700025484. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for Axis I DSM-IV Disorders - Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1994. First, M. B., Spitzer, R. L., Gibbon, M. & Williams, J. B. (1994). Structured Clinical Interview for Axis I DSM- IV Disorders - Patient Edition (SCID-I/P, Version 2.0) [Google Scholar]
- Fligiel SE, Roth MD, Kleerup EC, Barsky SH, Simmons MS, Tashkin DP. Tracheobronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Chest. 1997;112:319–26. doi: 10.1378/chest.112.2.319. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Lewinsohn PM, Seeley JR. Cigarette smoking and panic attacks among young adults in the community: The role of parental smoking and anxiety disorders. Biological Psychiatry. 2005;58:686–693. doi: 10.1016/j.biopsych.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Lewinsohn PM, Seely JR. Respiratory Symptoms and Mental Disorders Among Youth: Results From a Prospective, Longitudinal Study. Psychosomatic Medicine. 2004;66:943–949. doi: 10.1097/01.psy.0000138123.70740.92. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug and Alcohol Dependence. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Blanco C, Stinson FS, Chou SP, Goldstein RB, Dawson DA, Smith S, Saha TD, Huang B. The epidemiology of social anxiety disorder in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2005;66:1351–1361. doi: 10.4088/jcp.v66n1102. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Ruan J, Pickering RP. Co-occurrence of 12-month alcohol and drug use disorders and personality disorders in the United States: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004;61:362–368. doi: 10.1001/archpsyc.61.4.361. [DOI] [PubMed] [Google Scholar]
- Hathaway AD. Cannabis effects and dependency concerns in long-term frequent users: A missing piece of the public health puzzle. Addiction Research and Theory. 2003;11:441–458. [Google Scholar]
- Heimberg RG, Becker RE. Cognitive-behavioral group therapy for social phobia: Basic mechanisms and clinical strategies. Guilford Press; New York: 2002. [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The longitudinal interval follow-up evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Kessler RC. The impact of childhood psychopathology interventions on subsequent substance abuse: Policy implications, comments, and recommendations. Journal of Consulting and Clinical Psychology. Special Issue: Impact of childhood psychopathology interventions on subsequent substance abuse. 2002;70:1303–1306. [PubMed] [Google Scholar]
- Kessler RC, Andrade LH, Bijl RV, Offord DR, Demler OV, Stein DJ. The effects of co-morbidity on the onset and persistence of generalized anxiety disorder in the ICPE surveys. Psychological Medicine. 2002;32:1213–1225. doi: 10.1017/s0033291702006104. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry. 1997;54:313–21. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction. 2004;99:1548–1559. doi: 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Sher KJ, Beitman BD. The Relation Between Alcohol Problems and the Anxiety Disorders. American Journal of Psychiatry. 1990;147:685–695. doi: 10.1176/ajp.147.6.685. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Gotlib IH, Lewinsohn M, Seeley JR, Allen NB. Gender differences in anxiety disorders and anxiety symptoms in adolescents. Journal of Abnormal Psychology. 1998;107:109–117. doi: 10.1037//0021-843x.107.1.109. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Hops H, Roberts RE, Seeley JR, Fischer SA. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III-R disorders in high school students. Journal of Abnormal Psychology. 1993;102:133–144. doi: 10.1037//0021-843x.102.1.133. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Zinbarg R, Seeley JR, Lewinsohn M, Sack WH. Lifetime comorbidity among anxiety disorders and between anxiety disorders and other mental disorders in adolescents. Journal of Anxiety Disorders. 1997;11:377–394. doi: 10.1016/s0887-6185(97)00017-0. [DOI] [PubMed] [Google Scholar]
- Lewis BA, O’Neill HK. Alcohol expectancies and social deficits relating to problem drinking among college students. Addictive Behaviors. 2000;25:295–299. doi: 10.1016/s0306-4603(99)00063-5. [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical meta-analysis. Sage; Thousand Oaks, CA: 2001. [Google Scholar]
- Loney BR, Lima EN. Classification and assessment. In: Essau CA, editor. Conduct and oppositional defiant disorders: Epidemiology, risk factors, and treatment. Lawrence Erlbaum Associates; Mahwah, NJ: 2003. pp. 3–31. [Google Scholar]
- Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PAF, Slutske WS, Statham DJ, Martin NG. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychological Medicine. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- McGee R, Feehan M, Williams S, Partridge F, Silva PA, Kelly J. DSM-III disorders in a large sample of adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;39:611–619. doi: 10.1097/00004583-199007000-00016. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Angst J. Comorbidity and social phobia: Evidence from clinical, epidemiologic, and genetic studies. European Archives of Psychiatry and Clinical Neuroscience. 1995;244:297–303. doi: 10.1007/BF02190407. [DOI] [PubMed] [Google Scholar]
- Myers MG, Brown SA, Mott MA. Preadolescent conduct disorder behaviors predict relapse and progression of addiction for adolescent alcohol and drug abusers. Alcoholism: Clinical and Experimental Research. 1995;19:1528–1536. doi: 10.1111/j.1530-0277.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Ogborne AC, Smart RG, Weber T, Birchmore-Timney C. Who is using cannabis as a medicine and why: An exploratory study. J Psychoactive Drugs. 2000;32:435–443. doi: 10.1080/02791072.2000.10400245. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich JC,WJ, Tabrizi MA, Johnson R. Retrospective assessment of prepubertal major depression with the Kiddie-SADS-E. Journal of the American Academy of Child Psychiatry. 1982;21:392–397. doi: 10.1016/s0002-7138(09)60944-4. [DOI] [PubMed] [Google Scholar]
- Paton S, Kessler R, Kandel D. Depressive mood and adolescent illicit drug use: A longitudinal analysis. Journal of Genetic Psychology. 1977;131:267–289. doi: 10.1080/00221325.1977.10533299. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in younger people: Cohort study. British Medical Journal. 2002;325:1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Berghaus G, van Laar M, Drummer OH. Dose related risk of motor vehicle crashes after cannabis use. Drug and Alcohol Dependence. 2004;73:109–119. doi: 10.1016/j.drugalcdep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Randall CL, Johnson MR, Thevos AK, Sonne SC, Thomas SE, Willard SL, Brady KT, Davidson JR. Paroxetine for social anxiety and alcohol use in dual-diagnosed patients. Depression and Anxiety. 2001a;14:255–262. doi: 10.1002/da.1077. [DOI] [PubMed] [Google Scholar]
- Randall CL, Thomas S, Thevos AK. Concurrent alcoholism and social anxiety disorder: A first step toward developing effective treatments. Alcoholism: Clinical and Experimental Research. 2001b;25:210–220. [PubMed] [Google Scholar]
- Reilly D, Didcott P, Swift W, Hall W. Long-term cannabis use: Characteristics of users in an Australian rural area. Addiction. 1998;93:837–846. doi: 10.1046/j.1360-0443.1998.9368375.x. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Psychiatric comorbidity with problematic alcohol use in high school students. Journal of the American Academy of Child and Adolescent Psychiatry. 1996:1101–109. doi: 10.1097/00004583-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Russo MF, Beidel DC. Comorbidity of childhood anxiety and externalizing disorders: Prevalence, associated characteristics, and validation issues. Clinical Psychology Review. 1994;14:199–221. [Google Scholar]
- Sarafian TA, Magallanes JA, Shau H, Tashkin D, Roth MD. Oxidative stress produced by marijuana smoke. An adverse effect enhanced by cannabinoids. American Journal of Respiratory Cell and Molecular Biology. 1999;20:1286–1293. doi: 10.1165/ajrcmb.20.6.3424. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Martin LY, Liebowitz MR, Gorman JM, Fyer AJ. Alcohol abuse in social phobia. Journal of Anxiety Disorders. 1989;3:15–23. [Google Scholar]
- Sherrill DL, Krzyzanowski M, Bloom JW, Lebowitz MD. Respiratory effects of non-tobacco cigarettes: a longitudinal study in general population. International Journal of Epidemiology. 1991;20:132–137. doi: 10.1093/ije/20.1.132. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Modeling the days of our lives: Using survival analysis when designing and analyzing longitudinal studies of duration and the timing of events. Psychological Bulletin. 1991;110:268–290. [Google Scholar]
- Siqueira L, Diab M, Bodian C, Rolnitzky L. The relationship of stress and coping methods to adolescent marijuana use. Substance Abuse. 2001;22:157–166. doi: 10.1080/08897070109511455. [DOI] [PubMed] [Google Scholar]
- Smail P, Stockwell T, Canter S, Hodgson R. Alcohol dependence and phobic anxiety states: I. A prevalence study. British Journal of Psychiatry. 1984;144:53–57. doi: 10.1192/bjp.144.1.53. [DOI] [PubMed] [Google Scholar]
- Stein MB, Kean YM. Disability and quality of life in social phobia: Epidemiologic findings. American Journal of Psychiatry. 2000;157:1606–1613. doi: 10.1176/appi.ajp.157.10.1606. [DOI] [PubMed] [Google Scholar]
- Szuster RR, Pontius EB, Campos PE. Marijuana sensitivity and panic anxiety. Journal of Clinical Psychiatry. 1988;49:427–429. [PubMed] [Google Scholar]
- Thomas SE, Thevos AK, Randall CL. Alcoholics with and without social phobia: A comparison of substance use and psychiatric variables. Journal of Studies on Alcohol. 1999a;60:472–479. doi: 10.15288/jsa.1999.60.472. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Thevos AK, Randall CL. Alcoholics with and without social phobia: a comparison of substance use and psychiatric variables. Journal of Studies on Alcohol. 1999b;60:472–479. doi: 10.15288/jsa.1999.60.472. [DOI] [PubMed] [Google Scholar]
- Wells A, Carter K. Preliminary tests of a cognitive model of generalized anxiety disorder. Behaviour Research and Therapy. 1999;37:585–594. doi: 10.1016/s0005-7967(98)00156-9. [DOI] [PubMed] [Google Scholar]
- Willett JB, Singer JD. Investigating onset, cessation, relapse, and recovery: Why you should, and how you can, use discrete-time survival analysis to examine event occurrence. Journal of Consulting and Clinical Psychology. 1993;61:952–965. doi: 10.1037//0022-006x.61.6.952. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Wittchen H-U, Höfler M, Pfister H, Kessler RC, Lieb R. Primary anxiety disorders and the development of subsequent alcohol use disorders: A 4-year community study of adolescents and young adults. Psychological Medicine. 2003;33:1211–1222. doi: 10.1017/s0033291703008158. [DOI] [PubMed] [Google Scholar]
- Zoccolillo M. Co-occurrence of conduct disorder and its adult outcomes with depressive and anxiety disorders: A review. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:547–556. doi: 10.1097/00004583-199205000-00024. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bernstein A, Sachs-Ericsson N, Schmidt NB, Buckner JD, Bonn-Miller MO. Lifetime associations between cannabis, use, abuse, and dependence and panic attacks in a representative sample. Journal of Psychiatric Research. 2006;40:477–786. doi: 10.1016/j.jpsychires.2005.09.005. [DOI] [PubMed] [Google Scholar]