SUMMARY
The aim of our study was to determine which microsomal cytochrome P450 isozyme(s) were responsible for the microsomal oxidation of indole to indoxyl, an important intermediate in the formation of the uremic toxin indoxyl sulfate. Indole was incubated together with an NADPH-generating system and rat liver microsomes. Formation of indigo, an auto-oxidation product of indoxyl, was used to determine the indole-3-hydroxylation activity. Apparent Km and Vmax values of 0.85 mM and 1152 pmol min−1 mg−1 were calculated for the formation of indoxyl from indole using rat liver microsomes. The effects of various potential inducers and inhibitors on the metabolism of indole to indoxyl by rat liver microsomes were studied to elucidate the enzymes responsible for metabolism. Studies with general and isozyme-specific P450 inhibitors demonstrated that P450 enzymes and not FMO are responsible for the formation of indoxyl. In the induction studies, rate of indoxyl formation in the microsomes from untreated vs induced rats correlated nearly exactly with the CYP2E1 activity (4-nitrophenol 2-hydroxylation). These results suggest that CYP2E1 is the major isoform responsible for the rat microsomal oxidation of indole to indoxyl.
Keywords: Indole, indoxyl, cytochrome P450, oxidation, metabolism
INTRODUCTION
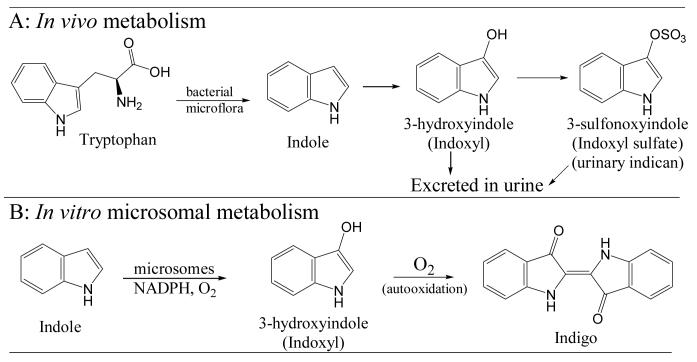
Indole, a metabolic intermediate from dietary tryptophan, is indicated as a potent co-carcinogen for the urinary bladder in animal models (1). Co-administration of indole and 2-acetylaminofluorene to rats and hamsters demonstrated that indole is a co-carcinogen for the bladder (2,3). Indole is formed from dietary tryptophan by the intestinal microflora and is excreted in the urine as the hydroxylated (3-hydroxyindole, also called indoxyl) and conjugated metabolite (indoxyl sulfate, also called indican) as summarized in Fig. 1A (4,5). Indoxyl sulfate is a uremic toxin that stimulates the progression of glomerular necrosis and renal failure in uremic patients (6-8). It is speculated that the initial tryptophan metabolite indole is absorbed, hydroxylated by cytochrome P-450 (P450, CYP) enzymes, conjugated by sulfotransferases (SULT), and excreted by the kidneys (4,9,10). However, it is not clear which isoform(s) of CYP are involved in the metabolism.
Fig. 1.
Metabolic pathways showing role of indole 3-hydroxylation in vivo (A) and in vitro (B).
Cytochrome P450 enzymes play a major role in the metabolism of a wide range of xenobiotics, drugs, and endogenous molecules (11,12). While this enzyme family plays a key role in the detoxication of xenobiotics and is responsible for the inactivation of most therapeutic drugs, P450s also transform certain xenobiotics to reactive intermediates that can cause toxicity. For example, CYP isoforms have been implicated in the metabolic activation of heterocyclic amines (CYP1A1, CYP1A2), polycyclic aromatic hydrocarbons (CYP3A4, CYP1A1), and N-nitrosamines (CYP2E1) in rodents and humans (13,14). Two reports have provided preliminary evidence that P450 enzymes are involved in the conversion of indole to indoxyl. Gillam et al. (15) observed the production of blue pigment in bacterial cultures after preparation of co-expression systems for human P450 isoforms and the redox partner, NADPH-cytochrome P450 reductase (NPR). These investigators found that expression systems containing NPR and CYP2E1 or CYP2A6 produced indigo and other metabolites in the presence of indole and NADPH (16). However, no quantitative studies were reported. Separately, Li et al. (17) described a P450 BM-3 mutant which was capable of hydroxylating indole, resulting in indigo formation. The wild-type P450 BM-3 did not catalyze the oxidation of indole.
These preliminary observations on the metabolism of indole are of interest since indole and tryptophan have been implicated as etiological factors in experimental bladder carcinogenesis, and the ultimate metabolic product of indole, indoxyl sulfate, causes glomerular necrosis and renal failure in uremic patients. In this study we used human and induced and uninduced rat liver microsomes and P450 inhibitors to examine the catalytic activity of specific P450 isoforms in the biotransformation of indole to indoxyl. Since several P450 isoforms show genetic polymorphism (18) and are highly inducible by chemicals (11), determination of the P450 isoform(s) responsible for the formation of indoxyl is of value to understand the risk of humans exposed to this metabolite of dietary tryptophan. We focussed specifically on the 3-hydroxylation of indole because it serves as the critical intermediate in the formation of the toxin indoxyl sulfate.
MATERIALS AND METHODS
Reagents and chemicals
Nicotinamide adenine dinucleotide phosphate (NADP+), glucose-6-phosphate, glucose-6-phosphate dehydrogenase suspension, Sigma Total Protein reagent, p-nitrophenol and methimazole were obtained from Sigma Chemical Company (St. Louis, MO, USA). Indole, indigo and n-octylamine were from Aldrich Chemical Company (Milwaukee, WI, USA). Diethyldithiocarbamic acid sodium salt, aniline and N-benzylimidazole were from Acros Organics (New Jersey, USA). Haloperidol, nifedipine and tolbutamide were obtained from ICN Biomedicals Inc. (Aurora, OH, USA). SKF-525A (2-diethyl-aminoethyl-2,2-diphenylvalerate) was from Biomol Research Labs. Inc. (Plymouth Meeting, PA, USA). All other assay reagents and buffer components were of the highest grade available from commercial sources.
Hepatic microsomes
Rats (male Sprague-Dawley, CD IGS) were obtained from Charles River (Wilmington, MA, USA) and were handled with IACUC approval. Human liver samples were obtained from the John L. McClellan Memorial Veterans' Hospital Medical Center (Little Rock, AR) under IRB approval. Microsomal fractions from human liver and rat liver (uninduced) were prepared in our laboratory as described previously (19). Protein concentrations were determined by Biuret assay (Sigma Total Protein). Microsomal fractions were stored in 1 ml aliquots at −80°C and, after thawing, were kept at 4°C prior to enzyme incubations. Commercial isoniazid-induced, Arochlor 1254-induced and control (uninduced) microsomes were purchased from In Vitro Technologies (Baltimore, MD, USA). The commercial microsomes were used only in the experiments studying the effect of P450 inducers on indole 3-hydroxylation; all other experiments utilized the microsomes prepared in our laboratory.
NADPH-generating system
The NADPH-generating system consisted of 1 mM NADP+, 5 mM glucose-6-phosphate and glucose-6-phosphate dehydrogenase (1 unit/ml). Stock solutions of these components were pre-mixed and pre-incubated for 7 min to generate NADPH prior to initiating microsomal reactions. NADPH generation was measured at 340 nm, and calculated from the extinction coefficient (ε = 6.2 mM−1 cm−1). The amount of NADPH production was greater than 0.2 mM in microsomal incubations.
Incubation procedure for indole-3-hydroxylation
Liver microsomes (1-1.5 mg) were incubated at 37 ± 1°C for 20-30 min in 1 ml mixtures (final volume) containing phosphate buffer (100 mM, pH 7.4), NADP+ (1 mM), glucose-6-phosphate (5 mM), glucose-6-phosphate dehydrogenase (1 unit/ml), and indole (0.1 mM – 2.5 mM), at the final concentrations indicated. Reactions were started by addition of the NADPH-generating system. Enzymatic activity was terminated by transferring the incubation tubes to crushed ice and immediately extracting with chloroform (3 × 1 ml). The organic layers were pooled, the solvent evaporated under a gentle stream of nitrogen in a water bath at 40°C, and the residue was reconstituted in 1 ml DMSO. To ensure that product was not lost in the pelleted protein, the protein precipitate was washed with 1 ml DMSO. Indoxyl is quantitatively converted to indigo by auto-oxidation under these conditions (20), and formation of indoxyl was determined from the absorption of indigo at 660 nm (ε = 2.58 mM−1 cm−1).
All inhibitors were dissolved in either phosphate buffer or methanol to give final concentration of 1 mM in the incubation mixture. The final assay concentration of methanol was less than 1% (v/v). Inhibitors and concentrations were chosen to distinguish between P450 and microsomal flavin-containing monooxygenase (FMO) activities, and between specific P450 isoforms (21).
Assay for CYP2E1-mediated p-nitrophenol hydroxylation
Hydroxylation of p-nitrophenol, a specific substrate for CYP2E1, was measured by a published method (23). Liver microsomes (1.5 mg) were incubated at 37 ± 1°C in 1 ml incubation mixtures (final volume) containing potassium phosphate buffer (50 mM, pH 7.4), EDTA (1 mM), ascorbic acid (1 mM), NADP+ (1 mM), glucose-6-phosphate (5 mM), glucose-6-phosphate dehydrogenase (1 unit/ml) and p-nitrophenol (0.1 mM), at the final concentrations indicated. Reactions were started by addition of the NADPH-generating system and were stopped after 10 min by addition of 1 ml of 6% perchloric acid. Tubes were vortex-mixed and the precipitated protein removed by centrifugation (5 min at 4,000 g). Supernatant (1 ml) was transferred into a 1.5 ml polystyrene cuvette (Fisher Scientific) followed by addition of 0.2 ml of 10 N NaOH. After mixing, the absorbance was measured at 546 nm. Zero-time incubations served as blanks. Concentration of the product 4-nitrocatechol was calculated from the extinction coefficient at 546 nm (ε = 9.53 mM−1 cm−1) (23).
RESULTS
In vitro metabolism of indole to indoxyl
The metabolism of indole to indoxyl (3-hydroxyindole), precursor for indoxyl sulfate, was assessed in vitro using human and induced and uninduced rat hepatic microsomes. Formation of indoxyl was determined by an indirect UV method that measured the formation of indigo, a spontaneous auto-oxidation product of indoxyl (20), under P450 assay conditions. The rate of indoxyl formation was calculated from the initial rate of absorbance change at 660 nm (ε = 2.58 mM−1 cm−1) caused by formation of indigo. We optimized the conditions for this assay method in our laboratory. No other possible microsomal metabolites of indole absorbed at this wavelength, and indoxyl was quantitatively converted to indigo by auto-oxidation under these conditions. Under the conditions described, 3-hydroxylation of indole was linear up to 30 minutes, after which it declined. Therefore, all incubations were carried out using incubation times of 20 or 30 min. In addition, 3-hydroxylation of indole was linear with respect to microsomal protein concentration up to 1.5 mg per incubate, and therefore, all incubations were carried out with 1-1.5 mg microsomal protein per 1 ml assay.
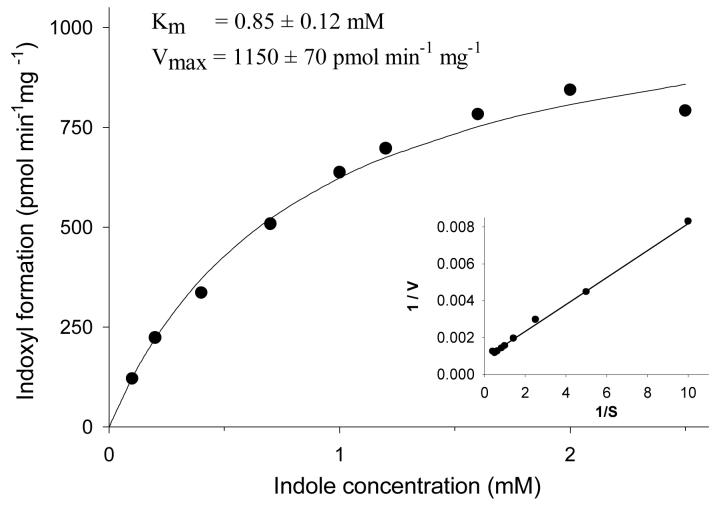
Initial velocity kinetic analysis of the 3-hydroxylation of indole by rat liver microsomes is shown in Fig. 2. Apparent Km and Vmax values were obtained by nonlinear least squares curve-fitting (24) of the data to the Michaelis-Menten equation. Based on these results, it was determined that rat liver microsomes were able to efficiently metabolize indole to indoxyl (apparent Km = 0.85 ± 0.12 mM, Vmax = 1153 ± 66 pmol min−1 mg−1).
Fig. 2.
Initial velocity for the oxidation of indole to indoxyl by the uninduced rat liver microsomes prepared in our laboratory. Symbols represent observed values while the line represents non-linear fitting of the observed data to the Michaelis-Menten equation.
Effect of various potential inhibitors in the metabolism of indole to indoxyl
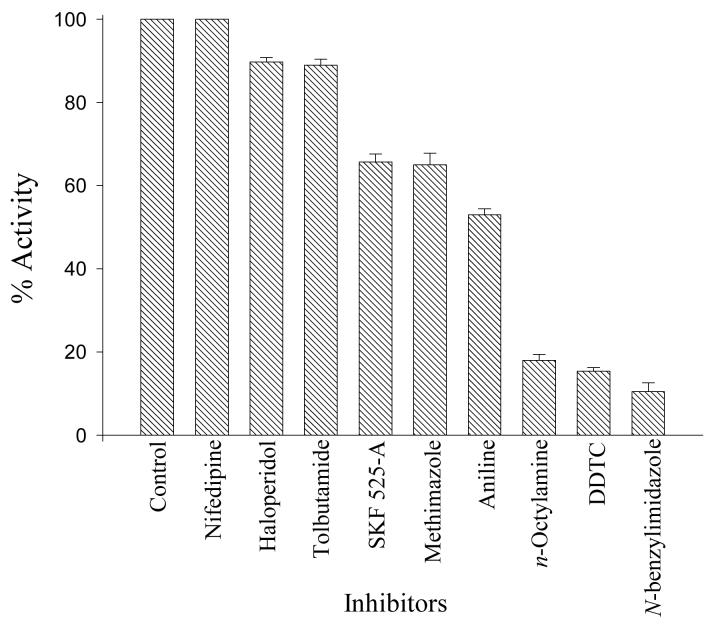
As the catalytic specificity of P450 and FMO often overlap, it is of interest to distinguish the involvement of these two enzyme families in the oxidative reactions of xenobiotics. Thus, we chose to compare the effect of several P450 and FMO inhibitors/activators on microsomal indole 3-hydroxylation. The effects of potential inhibitors on the metabolism of indole to indoxyl with rat liver microsomes are illustrated in Fig. 3. The results with n-octylamine suggest that FMO does not contribute to the microsomal 3-hydroxylation of indole; n-octylamine increases FMO activity rather than inhibits it (25). The conclusion that P450 and not FMO catalyzes the 3-hydroxylation of indole is strengthened by the results showing all of the general P450 inhibitors (N-benzylimidazole, n-octylamine, methimazole, and SKF525-A) significantly reduced 3-hydroxylation of indole (35-90%). Of the isoform-specific P450 inhibitors, DDTC was the most potent inhibitor of the formation of indoxyl from indole (85% inhibition). DDTC is a specific inhibitor of CYP2E1, and this result suggests that CYP2E1 is an important isoform involved in the microsomal oxidation of indole to indoxyl. However, the CYP2B inhibitor aniline showed 50% inhibition of the formation of indoxyl, and this result cannot rule out the involvement of CYP2B. Nifedipine, tolbutamide, and haloperidol had almost no effect (0-10% inhibition) on the formation of indoxyl, which suggests that the CYP3A, 2C, and 2D families do not significantly contribute to the microsomal 3-hydroxylation of indole.
Fig. 3.
Effect of potential cytochrome P450 inhibitors on the metabolic formation of indoxyl using rat liver microsomes. Bars represent the mean ± SD of two determinations, and 100% activity represents activity in the absence of inhibitor (325 pmol min−1 mg−1). SKF 525-A, 2-diethyl-aminoethyl-2,2-diphenylvalerate; DDTC, diethyldithiocarbamic acid.
Effect of cytochrome P450 inducers in the metabolism of indole to indoxyl
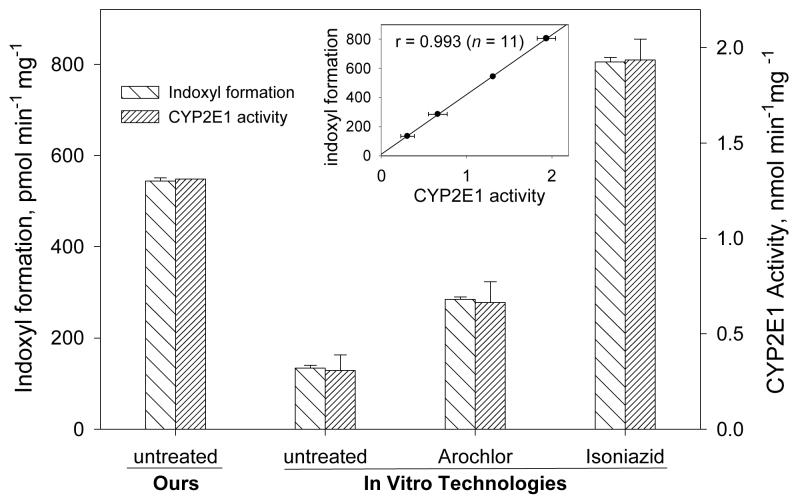
Enzyme induction causes increased rate of synthesis of an enzyme and thus an increase in total enzyme activity relative to the uninduced organism. In this study, we used microsomes from Arochlor 1254 and isoniazid treated animals in order to further establish the P450 isozymes involved in the in vitro metabolism of indole to indoxyl. Arochlor 1254 is widely used as mixed type of inducing agent that induces a wide range of P450 isoforms (26,27). Isoniazid, in contrast, has been found to be a specific inducer of the CYP2E1 isoform (28). These two inducing agents were chosen based on our inhibitor results and on the preliminary evidence that CYP2E1 expression systems formed indigo after metabolism of indole (15). The effect of these inducers in the formation of indoxyl is demonstrated in Fig. 4A where the activities are expressed as pmol of indoxyl formed min−1 mg−1. Maximum indoxyl formation was observed with the isoniazid-induced rat liver microsomes, 6-fold greater than the uninduced microsomes from In Vitro Technologies (IVT). However, the rate of indoxyl formation by the microsomes prepared in our laboratory from untreated (uninduced) animals was nearly as high as that in the IVT isoniazid-induced microsomes. Considering the inhibitor results, we used a specific substrate for CYP2E1, p-nitrophenol, to determine the level of CYP2E1 activity in our freshly prepared-microsomes and the microsomes obtained commercially. Our results demonstrated that indoxyl formation was proportional to the CYP2E1 activity in all rat liver microsomal preparations (Fig. 4A insert, r = 0.993). We also calculated the correlation of the indole 3-hydroxylation activity in rat liver microsomes with the activities provided by IVT of several other P450 isoforms (2E1, chlorzoxazone 6-hydroxylation; 2D6, dextromethorphan O-demethylation; 2C19, mephenytoin 4-hydroxylation; 1A2, phenacetin O-deethylation; 3A4, testosterone 6β-hydroxylation; 1A1, ethoxyresorufin O-deethylation). Only the activity for CYP2E1 positively correlated with microsomal indole 3-hydroxylation activity (r = 0.991); correlation constants for the others were less than 0.55. The indole 3-hydroxylation activity in the human liver microsomes from eight individuals also correlated with the CYP2E1 activity (r = x, Fig. 4B). These results suggest that CYP2E1 is the major enzyme catalyzing 3-hydroxylation of indole in rat and human liver microsomes.
Fig. 4.
Effect of potential cytochrome P450 inducers on the metabolic formation of indoxyl using rat liver microsomes (A) and human liver microsomes (B) and comparison with CYP2E1 activity. The left axis represents indoxyl formation, and the right axis represents CYP2E1 activity (4-nitrophenol 2-hydroxylation). Bars represent the mean ± SD of two-four determinations. Rat liver microsomes were prepared in our lab from untreated animals or obtained from In Vitro Technologies from untreated, Arochlor 1244-treated, or isoniazid-treated animals. Insert shows the correlation of CYP2E1 activity with 3-hydroxylation of indole in all samples.
DISCUSSION
Cytochrome P450 2E1 (CYP2E1) is a phase I detoxification enzyme, which is expressed in human liver and extrahepatic tissues such as the kidney, lungs and lymphocytes. CYP2E1 exhibits significant inter-individual variation in expression, and is induced by chronic alcohol consumption. Moreover, CYP2E1 mRNA levels are elevated in response to pathophysiological conditions in which lipids and ketone bodies are increased (such as diabetes, high-fat diet, and fasting) primarily due to stabilization of the CYP2E1 mRNA (29,30). CYP2E1 is responsible for the metabolic activation of numerous foreign compounds to intermediates that can be toxic to cells (11,31). CYP2E1 has been purified from several species including rodents and humans and the amino acid sequence similarity is ∼80% (32). The function and regulation of CYP2E1 are well conserved among mammalian species. We now report an additional toxicological role for rat and human liver CYP2E1, metabolizing the tryptophan metabolite indole to 3-hydroxyindole, an intermediate in the formation of indoxyl sulfate. Indoxyl sulfate is a known uremic toxin that stimulates the progression of glomerular necrosis in humans and rat models and may also be involved in the etiology of bladder carcinogenesis.
In the present study, the microsomal metabolism of indole to indoxyl (3-hydroxyindole) was investigated using rat and human microsomes and various P450 inducers and inhibitors. Our results indicate that FMO does not contribute to the microsomal biotransformation of indole to indoxyl, rather that the reaction is dependent on P450 activity. Recent studies (16) with bacterial expression systems for human P450 isoforms have indicated that indigo formation can be catalyzed in cultures containing CYP2A6, 2C19, 2E1, or 2D6. However, our results indicate that only CYP2E1 contributes significantly to the hepatic microsomal 3-hydroxylation of indole in rats and humans. Since 3-hydroxyindole is the only oxidative metabolite in the pathway for production of the uremic toxin indoxyl sulfate, and CYP2E1 activity varies widely among individuals, CYP2E1 activity may be an additional risk factor in individuals susceptible to progressive renal dysfunction.
ACKNOWLEDGEMENTS
This study was supported by Gazi University and the University of Rhode Island Research Foundation.
REFERENCES
- 1.Bryan GT. The role of urinary tryptophan metabolites in the etiology of bladder cancer. Am. J. Clin. Nutr. 1971;24:841–847. doi: 10.1093/ajcn/24.7.841. [DOI] [PubMed] [Google Scholar]
- 2.Dunning WF, Curtis MR. The role of indole in incidence of 2-acetylaminofluorene-induced bladder cancer in rats. Proc. Soc. Exp. Biol. Med. 1958;99:91–95. doi: 10.3181/00379727-99-24258. [DOI] [PubMed] [Google Scholar]
- 3.Oyasu R, Kitajima T, Hopp ML, Sumie H. Enhancement of urinary bladder tumorigenesis in hamsters by coadministration of 2-acetylaminofluorene and indole. Cancer Res. 1972;32:2027–2033. [PubMed] [Google Scholar]
- 4.Sims J, Renwick AG. The effects of saccharin on the metabolism of dietary tryptophan to indole, a known cocarcinogen for the urinary bladder of the rat. Toxicol. Appl. Pharmacol. 1983;67:132–151. doi: 10.1016/0041-008x(83)90252-1. [DOI] [PubMed] [Google Scholar]
- 5.Lawrie CA, Renwick AG, Sims J. The urinary excretion of bacterial amino-acid metabolites by rats fed saccharin in the diet. Food Chem. Toxicol. 1985;23:445–450. doi: 10.1016/0278-6915(85)90138-3. [DOI] [PubMed] [Google Scholar]
- 6.Niwa T, Nomura T, Sugiyama S, Miyazaki T, Tsukushi S, Tsutsui S. The protein metabolite hypothesis, a model for the progression of renal failure: an oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int. Suppl. 1997;62:S23–S28. [PubMed] [Google Scholar]
- 7.Niwa T, Aoyama I, Takayama F, Tsukushi S, Miyazaki T, Owada A, Shiigai T. Urinary indoxyl sulfate is a clinical factor that affects the progression of renal failure. Miner Electrolyte Metab. 1999;25:118–122. doi: 10.1159/000057433. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki T, Aoyama I, Ise M, Seo H, Niwa T. An oral sorbent reduces overload of indoxyl sulphate and gene expression of TGF-beta1 in uraemic rat kidneys. Nephrol. Dial. Transplant. 2000;15:1773–1781. doi: 10.1093/ndt/15.11.1773. [DOI] [PubMed] [Google Scholar]
- 9.Stanfel LA, Gulyassy PF, Jarrard EA. Determination of indoxyl sulfate in plasma of patients with renal failure by use of ion-pairing liquid chromatography. Clin. Chem. 1986;32:938–942. [PubMed] [Google Scholar]
- 10.Niwa T, Ise M, Miyazaki T. Progression of glomerular sclerosis in experimental uremic rats by administration of indole, a precursor of indoxyl sulfate. Am. J. Nephrol. 1994;14:207–212. doi: 10.1159/000168716. [DOI] [PubMed] [Google Scholar]
- 11.Parkinson A. Biotransformation of xenobiotics. In: Klaasen CD, editor. Casarett and Doull's Toxicology. McGraw-Hill; New York: 1996. pp. 113–186. [Google Scholar]
- 12.Wong LL. Cytochrome P450 monooxygenases. Curr. Opin. Chem. Biol. 1998;2:263–268. doi: 10.1016/s1367-5931(98)80068-9. [DOI] [PubMed] [Google Scholar]
- 13.Guengerich FP. Oxidation of toxic and carcinogenic chemicals by human cytochrome P450 enzymes. Chem. Res. Toxicol. 1991;4:391–407. doi: 10.1021/tx00022a001. [DOI] [PubMed] [Google Scholar]
- 14.Lieber CS. Cytochrome P4502E1. Its physiological and pathological role. Physiol. Rev. 1997;77:517–544. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 15.Gillam EMJ, Aguinaldo AMA, Notley LM, Kim D, Mundkowski RG, Volkov AA, Arnold FH, Soucek P, DeVoss JJ, Guengerich FP. Formation of indigo by recombinant mammalian cytochrome P450. Biochem. Biophys. Res. Commun. 1999;265:469–472. doi: 10.1006/bbrc.1999.1702. [DOI] [PubMed] [Google Scholar]
- 16.Gillam EMJ, Notley LM, Cai H, DeVoss JJ, Guengerich FP. Oxidation of indole by cytochrome P450 enzymes. Biochem. 2000;39:13817–13824. doi: 10.1021/bi001229u. [DOI] [PubMed] [Google Scholar]
- 17.Li QS, Schwaneberg U, Fischer P, Schmid RD. Directed evolution of the fatty-acid hydroxlase P450 BM-3 into an indole-hydroxylating catalyst. Chemistry. 2000;6:1531–1535. doi: 10.1002/(sici)1521-3765(20000502)6:9<1531::aid-chem1531>3.3.co;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Ingelman-Sundberg M, Oscarson M, McLellan RA. Polymorphic human cytochrome P450 enzymes: an opportunity for individualized drug treatment. Trends Pharmacol. Sci. 1999;20:342–49. doi: 10.1016/s0165-6147(99)01363-2. [DOI] [PubMed] [Google Scholar]
- 19.Turesky RJ, Lang NP, Butler MA, Teitel CH, Kadlubar FF. Metabolic activation of carcinogenic heterocyclic amines by human liver and colon. Carcinogenesis. 1991;12:1839–1845. doi: 10.1093/carcin/12.10.1839. [DOI] [PubMed] [Google Scholar]
- 20.Russel GA, Kaupp G. Oxidation of carbanions. IV. Oxidation of indoxyl to indigo in basic solution. J. Am. Chem. Soc. 1969;91:3851–3859. [Google Scholar]
- 21.Gorrod JW, Patterson LH. The metabolism of 4-substituted N-ethyl-N-methylanilines III. Effect of various potential inhibitors, activators and inducers on α-C- and N-oxidation. Xenobiotica. 1983;13:521–529. doi: 10.3109/00498258309052292. [DOI] [PubMed] [Google Scholar]
- 22.Tierney DJ, Haas AL, Koop DR. Degradation of cytochrome P-450 2E1: Selective loss after labilization of the enzyme. Arch. Biochem. Biophys. 1992;293:9–16. doi: 10.1016/0003-9861(92)90358-4. [DOI] [PubMed] [Google Scholar]
- 23.Koop DR. Hydroxylation of p-nitrophenol by rabbit ethanol-inducible cytochrome P450 isozyme 3a. Mol. Pharmacol. 1992;29:399–404. [PubMed] [Google Scholar]
- 24.Perella FW. A practical curve-fitting microcomputer program for the analysis of enzyme kinetic data on IBM-PC compatible computers. Anal. Biochem. 1988;174:437–447. doi: 10.1016/0003-2697(88)90042-5. [DOI] [PubMed] [Google Scholar]
- 25.Grothusen A, Hardt J, Brautigam L, Lang D, Bocker R. A convenient method to discriminate between cytochrome P450 enzymes and flavin-containing monooxygenases in human liver microsomes. Arch. Toxicol. 1996;71:64–71. doi: 10.1007/s002040050359. [DOI] [PubMed] [Google Scholar]
- 26.Chen TS, Dubois KP. Studies on the enzyme inducing effect of polychlorinated biphenyls. Toxicol. Appl. Pharmacol. 1973;26:504–512. doi: 10.1016/0041-008x(73)90288-3. [DOI] [PubMed] [Google Scholar]
- 27.Okey AB. Enzyme induction in the cytochrome P450 system. Pharmacol. Ther. 1990;45:241–298. doi: 10.1016/0163-7258(90)90030-6. [DOI] [PubMed] [Google Scholar]
- 28.Ryan DE, Ramanathan L, Iida S, Thomas PE, Haniu M, Shively JE, Lieber CS, Levin W. Characterization of a major form of rat hepatic microsomal cytochrome P450 induced by isoniazid. J. Biol. Chem. 1985;260:6385–6393. [PubMed] [Google Scholar]
- 29.Song BJ, Veech RL, Saenger P. Cytochrome P450IIE1 is elevated in lymphocytes from poorly controlled insulin-dependent diabetics. J. Clin. Endocrinol. Metab. 1990;71:1036–1040. doi: 10.1210/jcem-71-4-1036. [DOI] [PubMed] [Google Scholar]
- 30.Wang T, Shankar K, Ronis MJ, Mehendale HM. Potentiation of thioacetamide liver injury in diabetic rats is due to induced CYP2E1. J. Pharmacol. Exp. Ther. 2000;294:473–479. [PubMed] [Google Scholar]
- 31.Yoo JSH, Yang CS. Enzyme specificity in the metabolic activation of N-nitrosodimethylamine to a mutagen for Chinese hamster V79 cells. Cancer Res. 1985;45:5569–5574. [PubMed] [Google Scholar]
- 32.Gonzales FJ. The molecular biology of P450s. Pharmacol. Rev. 1989;40:243–288. [PubMed] [Google Scholar]