Abstract
Wnt proteins are cysteine-rich glycosylated proteins named after the Drosophilia Wingless (Wg) and the mouse Int-1 genes that play a role in embryonic cell patterning, proliferation, differentiation, orientation, adhesion, survival, and programmed cell death (PCD). Wnt proteins involve at least two intracellular signaling pathways. One pathway controls target gene transcription through β-catenin, generally referred to as the canonical pathway and a second pathway pertains to intracellular calcium (Ca2+) release which is termed the non-canonical or Wnt/Ca2+ pathway. The majority of Wnt proteins activate gene transcription through the canonical signaling pathway regulated by pathways that include the Frizzled transmembrane receptor and the co-receptor LRP-5/6, Dishevelled, glycogen synthase kinase-3β (GSK-3β), adenomatous polyposis coli (APC), and β-catenin. In contrast, the non-canonical Wnt signaling pathway has two intracellular signaling cascades that consist of the Wnt/Ca2+ pathway with protein kinase C (PKC) and the Wnt/PCP pathway involving Rho/Rac small GTPase and Jun N-terminal kinase (JNK). Through a series of signaling pathways, Wnt proteins modulate cell development, proliferation, and cell fate. In regards to cell survival and fate through PCD, Wnt may be critical for the prevention of tissue pathology that involves cytokine and growth factor control during disorders such as neuropsychiatric disease, retinal disease, and Alzheimer's disease. Elucidation of the vital elements that shape and control the Wnt-Frizzled signaling pathway may provide significant prospects for the treatment of disorders of the nervous system.
Keywords: Adenomatous polyposis coli, Akt, alzheimer's, β-catenin, dishevelled, erythropoietin, frizzled, GSK-3β, neurons, psychiatric, retinal disease, stem cells, vascular endothelial growth factor
FUNCTIONAL CLASSES OF THE WNT FAMILY
Named after the Drosophilia Wingless (Wg) and the mouse Int-1 genes, Wnt proteins are secreted cysteine-rich glycosylated proteins that play a role in embryonic cell patterning, proliferation, differentiation, orientation, adhesion, survival, and apoptosis (Chong, ZZ and Maiese, K, 2004, Melkonyan, HS et al., 1997, Nelson, WJ and Nusse, R, 2004, Nusse, R and Varmus, HE, 1982, Patapoutian, A and Reichardt, LF, 2000, Smalley, MJ and Dale, TC, 1999, Wodarz, A and Nusse, R, 1998). Wnt proteins are divided into two functional classes based on their ability to induce a secondary body axis in Xenopus embryos and to activate certain signaling cascades that consist of the Wnt1 class and the Wnt5a class. The members of the Wnt1 class lead to a secondary body axis in Xenopus and include Wnt1, Wnt2, Wnt3, Wnt3a, Wnt8 and Wnt8a. Wnt proteins of this class facilitate activation of the Frizzled transmembrane receptor and the co-receptor lipoprotein related protein 5 and 6 (LRP-5/6). This leads to the activation of the typical canonical Wnt/β-catenin pathway. The Wnt5a class cannot induce secondary axis formation in Xenopus and includes the Wnt proteins of Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a and Wnt11. These Wnt proteins bind the Frizzled transmembrane receptor to activate heterotrimeric G proteins and increase intracellular calcium levels. In addition, the Wnt proteins can induce Rho-dependent changes in the actin cytoskeleton.
The receptors of the Wnt proteins consist of at least 10 family members termed the Frizzled proteins after the first member, Drosophila tissue polarity gene Frizzled (Adler, PN et al., 1990, Vinson, CR et al., 1989). Members of the Frizzled protein family have several characteristics. These include a N-terminal signal peptide, an extracellular domain that contains a 120-amino acids, a cysteine-rich domain followed by a hydrophilic linker region that shows little sequence similarity among family members, a highly conserved seven-transmembrane domain separated by short extracellular and cytoplasmic loops, and a cytoplasmic domain of variable size and little sequence homology among family members (Adler, PN et al., 1990, Hsieh, JC, 2004, Vinson, CR et al., 1989, Wang, Y et al., 1996, Wodarz, A et al., 1998).
The Wnt proteins bind to the activity sites of Frizzled receptor proteins that are relevant to either the canonical and non- canonical Wnt-Frizzled signaling pathways leading to specific biological functions. In addition to the Frizzled protein receptors, other obligate co-receptors also are necessary for canonical Wnt-Frizzled signaling pathway. An additional single-pass transmembrane protein named as LRP-5/6 from the low-density-lipoprotein receptor family is required for this process (Pinson, KI et al., 2000, Tamai, K et al., 2000, Wehrli, M et al., 2000). In the canonical Wnt-Frizzled signaling pathway, Wnt binds to both the Frizzled transmembrane receptor and the co-receptor LRP-5/6 (Wehrli, M et al., 2000) resulting in the inhibition of the downstream component glycogen synthase kinase-3β (GSK-3β) (Ikeda, S et al., 1998, Papkoff, J and Aikawa, M, 1998). Wnt signaling also can be transmitted through the binding of extracellular domain of LRP-5/6 to Axin, a key component in the GSK-3β complex, indicating that the LRP-5/6 receptor is an important part of the Wnt-Frizzled signaling pathway (Mao, J et al., 2001, Tolwinski, NS et al., 2003).
Another co-receptor for the canonical and non- canonical Wnt-Frizzled signaling pathway is Ryk that belongs to one of divergent members of the receptor tyrosine kinase family. Ryk not only can form a complex with Frizzled proteins such as the co-receptor LRP-5/6 resulting in activation of the canonical Wnt-Frizzled signaling pathway, but also can regulate the non-canonical Wnt-Frizzled signaling pathway through Frizzled-independent pathways (Bejsovec, A, 2005, Cheyette, BN, 2004). The molecular structure of all Ryk genes is characterized by an extracellular domain with homology to Wnt inhibitory factor-1 (WIF1), a single transmembrane-spanning sequence to which Wnt proteins bind (Patthy, L, 2000, Schneider, S et al., 1999). In addition, a conserved intracellular PDZ-binding motif exists which links Ryk to downstream molecules of Wnt-Frizzled signaling pathway, such as Dishevelled (Bejsovec, A, 2005, Cheyette, BN, 2004, Lu, W et al., 2004). Wnt proteins can bind to the extracellular domain of the Ryk receptor through the intracellular PDZ-binding domain in the Ryk receptor to result in regulating cell proliferation, differentiation, migration, polarity, survival, and death through either the canonical or the non- canonical Wnt-Frizzled signaling pathway.
THE CANONICAL AND NON-CANONICAL PATHWAYS
Wnt involves at least two intracellular signaling pathways. One pathway controls target gene transcription through β-catenin, generally referred to as the canonical pathway that involves Wnt1, Wnt3a, and Wnt8 and functions through β-catenin-dependent pathways. Another pathway pertains to intracellular calcium (Ca2+) release which is termed the non-canonical or Wnt/Ca2+ pathway consisting primarily of Wnt-4, Wnt-5a, and Wnt-11 that functions through non-β-catenin-dependent pathways, such as the planar cell polarity (PCP) pathway (Nusse, R, 1999, Patapoutian, A et al., 2000, Salinas, PC, 1999, Tada, M and Smith, JC, 2000) and the Wnt-Ca2+-dependent pathways (Katoh, M, 2002, Kuhl, M et al., 2000, Nusse, R, 1999, Patapoutian, A et al., 2000, Salinas, PC, 1999, Slusarski, DC et al., 1997).
Upon binding to either the Frizzled receptor or a receptor complex consisting of Frizzled and LRP5/6, Wnt protein can activate one of three different signaling cascades. These cascades include the canonical Wnt signaling pathway (Nusse, R, 1999, Rattner, A et al., 1997), the Wnt/PCP pathway (Nusse, R, 1999, Rattner, A et al., 1997, Veeman, MT et al., 2003), or the Wnt/Ca2+ pathway (Katoh, M, 2002, Kuhl, M et al., 2000, Nusse, R, 1999, Patapoutian, A et al., 2000, Salinas, PC, 1999, Slusarski, DC et al., 1997). Each of pathways, although distinct, appears to be transduced initially through Dishevelled, a cytoplasmic multi-functional phosphoprotein (Axelrod, JD et al., 1998, Boutros, M and Mlodzik, M, 1999, Boutros, M et al., 1998). In mammals, the Dishevelled protein family members contains Dishevelled-1, Dishevelled-2, Dishevelled-3 in all organs. These family members have three highly conserved domains that include an N-terminal DIX domain named for Dishevelled and Axin, a central PDZ domain termed for Postsynaptic density-95, Discs-large and Zonula occludens-1, and a C-terminal DEP that is named for Dishevelled, Egl-10 and Pleckstrin (Habas, R and Dawid, IB, 2005, Wharton, KA, Jr., 2003). At the level of Dishevelled, the Wnt signaling pathway can be separated along one of three different cascades that are dependent upon the three highly conserved domains of Dishevelled. As a result, Dishevelled is a key transducer of the Wnt signal that acts at the plasma membrane or in the cytoplasm in all three Wnt-Frizzled signaling pathways. However, new work has suggested that Dishevelled also acts within the nucleus and nuclear location of Dishevelled is essential for its function in the Wnt-Frizzled signaling pathway (Itoh, K et al., 2005, Weitzman, JB, 2005).
The majority of Wnt proteins activate gene transcription through the canonical signaling pathway controlled by β-catenin. In general, all Wnt signaling pathways are initiated by interaction of Wnt proteins with Frizzled receptors, but in this pathway, the Wnt signaling pathway will only be activated if the binding of the Wnt protein to the Frizzled transmembrane receptor takes place in the presence of the co-receptor LRP-5/6 (Mao, J et al., 2001, Pinson, KI et al., 2000, Wehrli, M et al., 2000) resulting in the formation of a Wnt-Frizzled-LRP5/6 trimolecular complex. Once Wnt protein binds to the Frizzled transmembrane receptor and the co-receptor LRP-5/6, this is followed by recruitment of Dishevelled. Dishevelled is phosphorylated by casein kinase Iε to form a complex with Frat1 and inhibit GSK-3β activity (Ikeda, S et al., 1998, Kishida, M et al., 2001, Lee, E et al., 2001, Lee, JS et al., 1999, Papkoff, J et al., 1998). In addition, the formation of the Wnt-Frizzled-LRP5/6 complex also promotes the LRP5/6-mediated degradation of Axin (Mao, J et al., 2001).
The combined inhibition of GSK-3β activity with the degradation of Axin blocks the formation of the protein complex consisting of GSK-3β, Axin, and adenomatous polyposis coli (APC) tumor suppressor protein. Yet, during the absence of Wnt signaling, β-catenin is associated with the protein complex of GSK-3β, Axin and APC tumor suppressor protein. β-catenin is phosphorylated by activation of GSK-3β leading to its ubiquitination and subsequent degradation by proteosomes (Aberle, H et al., 1997, Hart, M et al., 1999, Latres, E et al., 1999, Patapoutian, A et al., 2000, Winston, JT et al., 1999). As a result, β-catenin cannot translocate into the nucleus and physically bind to DNA in order to activate the transcription of its target genes. In the absence of β-catenin in the nucleus, the T cell factor (Tcf) and lymphocyte enhancer factor (Lef) (Tcf/Lef) family members are associated with transcriptional inhibitors, such as Groucho (Cavallo, RA et al., 1998, Roose, J et al., 1998).
Yet, without the formation of the protein complex of GSK-3β, Axin and APC tumor suppressor protein, phosphorylation of β-catenin with its subsequent degradation does not occur and the accumulation of free β-catenin results for translocation to the nucleus (Akiyama, T, 2000, Cavallo, RA et al., 1998, Ikeda, S et al., 1998, Roose, J et al., 1998). Once positioned in the nucleus, the free β-catenin acts as a transcription factor and activates Tcf and Lef by forming nuclear complexes with members of the Tcf/Lef transcription factor family (Ishitani, T et al., 2003). This leads to the transcription and expression of a variety of Wnt-responsive target genes such as c-Myc (He, TC et al., 1998), cyclin D1 (Nusse, R, 1999, Shtutman, M et al., 1999, Tetsu, O and McCormick, F, 1999), and Axin 2 (Jho, EH et al., 2002, Lustig, B et al., 2002).
The non-canonical Wnt signaling pathway, also termed the atypical Wnt-Frizzled signaling pathway, has two intracellular signaling cascades that consist of the Wnt/Ca2+ pathway and the Wnt/PCP pathway. In the Wnt/Ca2+ pathway, Wnt protein binds to Frizzled transmembrane receptors on the cell surface resulting in several cellular processes that involve stimulation of heterotrimeric G proteins, increased intracellular Ca2+ release, decreased cyclic guanosine mono-phosphate (cGMP) levels, and activation of the two kinases Ca2+-calmodulin-dependent protein kinase II (CamKII) or calcineurin (CaCN) and protein kinase C (PKC). These processes can stimulate nuclear factor (NF)-AT and other transcription factors (Kuhl, M, 2004, Veeman, MT et al., 2003, Wang, HY and Malbon, CC, 2003). In the Wnt/PCP pathway, Wnt proteins bind to Frizzled transmembrane receptors on the cell surface followed by activating Rho/Rac small GTPase (Habas, R et al., 2003) and Jun N-terminal kinase (JNK) (Moriguchi, T et al., 1999) to assist in the subsequent regulation of cytoskeletal organization and gene expression (Moulin, N and Widmann, C, 2004).
WNT AND NEURONAL DEVELOPMENT
The Wnt-Frizzled signaling pathway leads to the development of the brain, spinal cord, and the extension of numerous sub-populations of sensory and motor neurons. The canonical Wnt-Frizzled signaling pathway is required for anterior neural patterning in studies with Xenopus embryos (Kiecker, C and Niehrs, C, 2001). XIdax, an inhibitor of the canonical Wnt-Frizzled signaling pathway, can reduce the expression of anterior neural markers, indicating that the canonical Wnt-Frizzled signaling pathway is crucial for the anterior neural development in Xenopus (Michiue, T et al., 2004). The co-expression of Wnt1 and Wnt3a may be necessary for the development of the dorsal neural tube since loss of these two Wnt proteins results in fewer dorsal lateral neural precursors, suggesting that the Wnt-Frizzled signaling pathway plays a vital role in regulating dorsal neural patterning (Chizhikov, VV and Millen, KJ, 2005, Ikeya, M et al., 1997, Muroyama, Y et al., 2002). Several cellular proteins in the Wnt-Frizzled signaling pathway that have been shown to be involved in the dorsal-ventral patterning of the neural tube also directly regulate patterning of the telencephalon (Grove, EA and Tole, S, 1999) and also can contribute to forebrain patterning in the developing brain (Abu-Khalil, A et al., 2004, Braun, MM et al., 2003). For example, Wnt 3a, Wnt 5a, and Wnt 2b contribute to the development of the cortical hem which forms the boundary between the hippocampus and choroids plexus in the embryonic cerebral cortex (Grove, EA et al., 1998, Lee, SM et al., 2000). Wnt genes, genes encoding Frizzled Wnt receptors, or secreted Frizzled-related proteins and Tcf/Lef-1 transcription factors, also are expressed in postnatal mouse cerebral cortex lasting into young adulthood, further indicating that the Wnt/β-catenin signaling pathway represents a major cortical input during embryonic brain development (Shimogori, T et al., 2004).
The Wnt-Frizzled signaling pathway also functions as a regulator of specific precursor cells in the developing brain (Panhuysen, M et al., 2004). Additional work also demonstrates that autoregulation of canonical Wnt/β-catenin signaling pathway can control midbrain development through the expression of transcription factor Tcf-4 isoforms that require Wnt2b, but also control Wnt2b (Kunz, M et al., 2004). Lef1/Tcf proteins also regulate the generation of dentate gyrus granule cells and the development of the hippocampus (Galceran, J et al., 2000). Other work further demonstrates roles for Dishevelled, Rac, and JNK signaling pathways during neuronal development. Wnt7b and Dishevelled can activate Rac and JNK signaling pathways to promote dendritic branching growth in cultured hippocampal neurons, since application of dominant-negative Rac, administration of dominant-negative JNK, or inhibition of JNK activity can inhibit Dishevelled-mediated dendritic growth (Rosso, SB et al., 2005).
WNT AND NEURONAL INJURY
Programmed cell death (PCD) (also known as apoptosis) is considered to be a significant component of cell death that contributes to neuronal destruction. Dysfunctions in the regulation or execution of apoptosis are implicated in a wide range of developmental abnormalities and diseases (Maiese, K and Chong, ZZ, 2004, Mattson, MP, 2004). Apoptosis also serves as a central pathway that can lead to a cell's demise in a variety of tissues (Maiese, K, 2001, Maiese, K and Chong, ZZ, 2003) and has recently been identified in organisms as diverse as plants (Hatsugai, N et al., 2004). PCD consists of membrane phosphatidylserine (PS) exposure and DNA fragmentation (Maiese, K et al., 2004) (Fig. 1). PCD can contribute significantly to a variety of disease states that especially involve the nervous system such as cerebral ischemic disease, Alzheimer's disease, and trauma (Chong, ZZ et al., 2004, Doonan, F and Cotter, TG, 2004, Ferretti, P, 2004, Koyama, R and Ikegaya, Y, 2004, Li, F et al., 2004). As an early event in the dynamics of cellular apoptosis, PS exposure may be required for embryogenesis (Bose, J et al., 2004). Yet, in mature tissues, membrane PS externalization can become a signal for the phagocytosis of cells (Hong, JR et al., 2004) (Fig. 1). In the nervous system, cells expressing externalized PS may be removed by microglia (Li, F et al., 2004, Lin, SH and Maiese, K, 2001) (Fig. 2). An additional role for membrane PS externalization in the vascular cell system is the activation of coagulation cascades (Chong, ZZ et al., 2002, Chong, ZZ et al., 2004). The externalization of membrane PS residues in endothelial cells can promote the formation of a procoagulant surface (Bombeli, T et al., 1997).
Fig. (1). The Wnt pathway controls mitochondrial cytochrome c release, caspase activity, and programmed cell death (PCD).
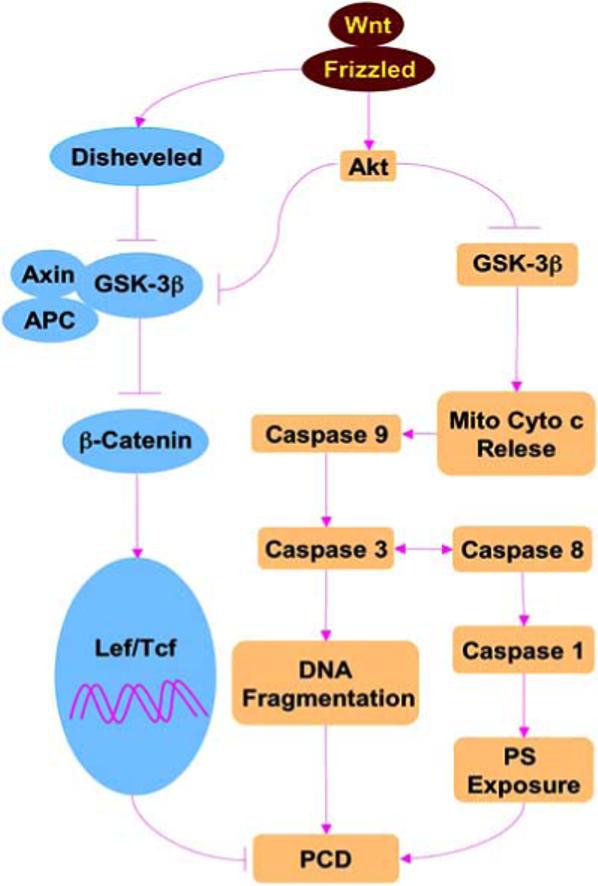
The Wnt protein binds to its Frizzled receptors activating Dishevelled followed by the inhibition of GSK-3β (glycogen synthase kinase-3β), Axin and APC (adenomatous polyposis coli) tumor suppressor protein complex. The GSK-3β, Axin and APC complex blocks phosphorylation of ß-catenin. β-catenin translocates to the cell nucleus and contributes to the formation of Lef/Tcf (lymphocyte enhancer factor/T cell factor) and β-catenin complex that can lead to cellular proliferation, differentiation, and survival. The binding of Wnt to Frizzled receptors also can activate Akt that prevents the activity of GSK-3β, prevents Mito (mitochondrial) Cyto c (cytochrome c) release, caspase activity, and PCD that involves DNA fragmentation and phosphatidylserine (PS) exposure.
Fig. (2). Wnt preserves neuronal survival and may prevent microglial activation.
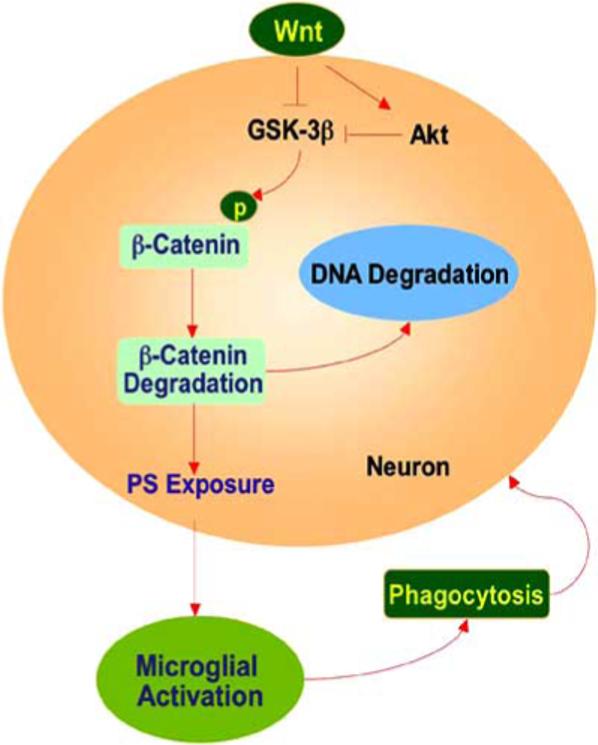
Through its receptor Frizzled, Wnt inhibits glycogen synthase kinase-3β (GSK-3β). In the absence of Wnt, GSK-3β can degrade β-catenin, promote membrane phosphatidylserine (PS) exposure, and lead to microglial activation and the phagocytosis of neurons.
Independent from the early externalization of membrane PS residues, the cleavage of genomic DNA into fragments is considered to be a delayed event that occurs late during apoptosis (Dombroski, D et al., 2000, Jessel, R et al., 2002, Kang, JQ et al., 2003, Maiese, K and Vincent, AM, 2000). Several enzymes responsible for DNA degradation have been differentiated based on their ionic sensitivities to zinc (Torriglia, A et al., 1997) and magnesium (Sun, XM and Cohen, GM, 1994). Calcium, a critical independent component that can determine cell survival (Weber, JT, 2004), also may determine endonuclease activity through calcium/magnesium - dependent endonucleases such as DNase I (Madaio, MP et al., 1996). Other enzymes that may degrade DNA include the acidic, cation independent endonuclease (DNase II) (Torriglia, A et al., 1995), cyclophilins (Montague, JW et al., 1997), and the 97 kDa magnesium - dependent endonuclease (Pandey, S et al., 1997). In the nervous system, three separate endonuclease activities are present that include a constitutive acidic cation-independent endonuclease, a constitutive calcium/magnesium-dependent endonuclease, and an inducible magnesium dependent endonuclease (Vincent, AM and Maiese, K, 1999). The physiologic characteristics of the magnesium dependent endonuclease, such as a pH range of 7.4−8.0, a dependence on magnesium, and a molecular weight of 95−108 kDa, are consistent with a recently described constitutive 97 kDa endonuclease in non-neuronal tissues.
Oxidative stress can lead to apoptosis in neurons through multiple cellular pathways (Chong, ZZ et al., 2005). Oxidative stress results in nuclei condensation and DNA fragmentation (Chong, ZZ et al., 2003, Goldshmit, Y et al., 2001, Pugazhenthi, S et al., 2003, Vincent, AM et al., 1999). In neurons, free radical exposure produces apoptotic death in hippocampal and dopaminergic neurons (Chong, ZZ et al., 2003, Sharma, SK and Ebadi, M, 2003, Vincent, AM and Maiese, K, 1999, Witting, A et al., 2000). Externalization of membrane PS residues also occurs in neurons during anoxia (Chong, ZZ et al., 2002), free radical exposure (Chong, ZZ et al., 2003), or during the administration of agents that induce the production of reactive oxygen species, such as 6-hydroxydopamine (Salinas, M et al., 2003).
The Wnt-Frizzled signaling pathway can regulate PCD through a variety of mechanisms that include the Wnt-bone morphogenetic protein (BMP) signaling loop (Ellies, DL et al., 2000, Golden, JA et al., 1999), secreted Frizzled-related protein-2 (SFRP2) expression (Ellies, DL et al., 2000, Jones, SE et al., 2000), Wnt-β-catenin signaling (Ahmed, Y et al., 2002, Brault, V et al., 2001, Galceran, J et al., 2000, Hari, L et al., 2002), c-Jun N-Terminal kinase signaling (Grotewold, L and Ruther, U, 2002, Lisovsky, M et al., 2002, Yeo, W and Gautier, J, 2004), GSK-3β-NF-κB signaling (Bournat, JC et al., 2000, Kozlovsky, N et al., 2002) and gene expression that involves human Dickkopf-1 (hDkk-1) (Shou, J et al., 2002), nemo (Mirkovic, I et al., 2002), sox 10 (Honore, SM et al., 2003), and tau (Jackson, GR et al., 2002).
Wnt1 signaling has been associated with the control of apoptosis during injury in some cell systems. Wnt1 prevents apoptosis through β-catenin / Tcf transcription mediated pathways (Chen, S et al., 2001, Rhee, CS et al., 2002). Overexpression of exogenous Wnt1 results in the protection of cells against c-myc induced apoptosis through induction of β-catenin, cyclooxygenase-2, and Wnt1 induced secreted protein (WISP-1) (You, Z et al., 2002). Wnt1 signaling also can inhibit apoptosis through prevention of cytochrome c release from mitochondria and the subsequent inhibition of caspase 9 activation (Chen, S et al., 2001). The APC gene also appears to represent another mechanism that regulates apoptosis. The APC gene functions to cleave β-catenin leading to the down-regulation of transactivation of Tcf/Lef (Munemitsu, S et al., 1995). Without Tcf/Lef activity, APC is then permitted to increase the activities of caspase 3, caspase 7, and caspase 9 and lead to the cleavage of poly (ADP-ribose) polymerase (PARP) to enhance the vulnerability of cells to apoptosis (Chen, T et al., 2003).
The Wnt-β-catenin signaling pathway regulates cell proliferation and differentiation, cell polarity, and specification of cell fate, such as apoptosis. Yet, different from the Wnt-BMP signaling loop, the Wnt-β-catenin signaling pathway can prevent apoptosis through the regulation of β-catenin and Tcf/Lef. In β-catenin mutant embryos, the removal of β-catenin can lead to apoptotic loss of the hindbrain, the melanocyte lineage, neural crest cells, sensory neurons and dorsal root ganglia (Brault, V et al., 2001, Hari, L et al., 2002). Over-expression of exogenous Wnt-1 results in the protection of cells against c-Myc induced apoptosis through induction of β-catenin, cyclooxygenase-2, and Wnt-1 induced secreted protein (WISP-1) (You, Z et al., 2002).
The Wnt signaling pathway also can decrease apoptosis and increase survival of neurons or neuronal cell lines by activating NF-κB (Bournat, JC et al., 2000), inhibiting GSK-3β (Kozlovsky, N et al., 2002), or blocking the release of cytochrome c (Fig. 1). In the absence of Wnt activity, GSK-3β phosphorylates β-catenin at serine or threonine residues of the N-terminal region to predispose degradation of β-catenin through ubiquination (Fig. 2). GSK-3β dependent phosphorylation of β-catenin can be promoted through phosphorylation of Axin (Yamamoto, H et al., 1999). In studies with chemotherapeutic agents, Wnt1 signaling also can inhibit apoptosis through prevention of cytochrome c release from mitochondria and the subsequent inhibition of caspase 9 activation (Chen, S et al., 2001).
The Wnt pathway also uses protein kinase B (Akt) to promote cellular differentiation and survival (Chong, ZZ et al., 2005) (Fig. 1). Since Wnt can inactivate GSK-3β and block the phosphorylation of β-catenin (Ikeda, S et al., 1998, Papkoff, J et al., 1998), this leads to the activation of β-catenin followed by transcription of its target genes for cellular protection. Akt may be necessary in pathways that involve Wnt1, since Akt inhibits the activity of GSK-3β through phosphorylation of this protein to promote cell survival (Crowder, RJ and Freeman, RS, 2000). Furthermore, neuronal cell differentiation that is dependent upon Wnt signaling appears to become stalled without Akt phosphorylation and the subsequent inactivation of GSK-3β (Fukumoto, S et al., 2001). In addition, Wnt has been demonstrated through WISP-1 to activate the anti-apoptotic signaling pathway of Akt following genomic DNA damage (Su, F et al., 2002) and to block cell injury during serum withdrawal through increased Akt phosphorylation and activity (Longo, KA et al., 2002).
Wnt and Neuropsychiatric Disorders, Retinal Disease, and Alzheimer's Disease
Wnt1 expression has been demonstrated in the brains of individuals affected by neuropsychiatric disorders (Miyaoka, T et al., 1999). In addition, retinal degeneration during retinitis pigmentosa with the progressive loss of photoreceptors has been associated with increased secretion of Frizzled-related protein-2, a Wnt inhibitory protein, suggesting that loss of Wnt signaling may contribute to retinal neurodegeneration (Jones, SE et al., 2000). Other studies demonstrate that a mutation in the membrane-type Frizzled-related protein gene may be involved in retinal photoreceptor degeneration (Kameya, S et al., 2002).
In Alzheimer's disease, neurotoxicity of Aβ in hippocampal neurons has been linked to increased levels of GSK-3β and loss of β-catenin. Decreased production of Aβ can occur during the enhancement of PKC activity (Savage, MJ et al., 1998) which may be controlled by the Wnt pathway (Garrido, JL et al., 2002). The proteolytic processing of amyloid precursor protein (APP) during Alzheimer's disease also has been closely linked to the Wnt pathway through presenilin 1 (PS1) and Dishevelled. PS1 is required for the processing of APP and has been shown to down-regulate Wnt signaling and interact with β-catenin to promote its turnover (Soriano, S et al., 2001). Dishevelled also can regulate the α-secretase cleavage of APP through PKC/mitogen-activated protein kinase dependent pathways, increasing soluble production of APP (sAPP) (Mudher, A et al., 2001). Overexpression of mouse Dishevelled-1 and −2 inhibits GSK-3β mediated phosphorylation of tau protein and may thus prevent formation of neurofibrillary tangles during Alzheimer's disease (Wagner, U et al., 1997).
Potential Therapeutic Modalities Involving the Wnt Pathway
Several cytokines and trophic factors may have some dependence on the Wnt signaling pathway (Maiese, K et al., 2004, Maiese, K et al., 2005). Vascular endothelial growth factor (VEGF) can stimulate new vessel formation by promoting tyrosine phosphorylation of β-catenin (Cohen, AW et al., 1999, Roura, S et al., 1999). In addition, fibroblast growth factor 2 (FGF2) can inhibit GSK-3 activity, augment nuclear levels of β-catenin, and enhance Tcf/Lef-dependent transcription of a cyclin D1-luciferase construct, suggesting that the angiogenic properties of FGF2 are tightly regulated by β-catenin activation in the Wnt-Frizzled signaling pathway (Dono, R et al., 2002, Holnthoner, W et al., 2002). Another potential candidate is erythropoietin (EPO) (Li, F et al., 2004, Maiese, K et al., 2005). Both cell culture and animal model work have demonstrated neuronal protection with EPO (Chong, ZZ et al., 2002, Chong, ZZ et al., 2002, Genc, S et al., 2004). Systemic administration of EPO before or immediately after a retinal insult can protect retinal ganglion cells from apoptosis (Grimm, C et al., 2002) and can improve functional outcome and reduce lipid peroxidation during spinal cord injury (Kaptanoglu, E et al., 2004). EPO also can block microglial cell activation and proliferation to prevent phagocytosis of injured cells through pathways that involve cellular membrane PS exposure (Chong, ZZ et al., 2004) and the regulation of caspases (Chong, ZZ et al., 2003, Chong, ZZ et al., 2003). EPO can prevent cellular inflammation by inhibiting several pro-inflammatory cytokines, such as IL-6, tumor necrosis factor-α (TNF-α), and monocyte chemoattractant protein 1 (Chong, ZZ et al., 2002, Genc, S et al., 2004).
Components of the Wnt pathway that are utilized by EPO, such as Akt, can offer cellular protection (Maiese, K et al., 2003). EPO can phosphorylate Akt and is dependent upon the activation of PI 3-K and Janus Kinase 2 (Jak2) (Chong, ZZ et al., 2002, Witthuhn, BA et al., 1993). One of the principal pathways through which EPO prevents cellular apoptosis is through the activation of Akt (Maiese, K et al., 2004, Maiese, K et al., 2005) During anoxia or free radical exposure, expression of the active form of Akt (phospho-Akt) is increased (Kang, JQ et al., 2003, Kang, JQ et al., 2003). EPO can significantly enhance the activity of Akt during oxidative stress and prevent inflammatory activation of microglia (Chong, ZZ et al., 2003, Chong, ZZ et al., 2003, Chong, ZZ et al., 2003). This up-regulation of Akt activity during injury paradigms appears to be vital for EPO protection, since prevention of Akt phosphorylation blocks cellular protection by EPO (Chong, ZZ et al., 2003, Chong, ZZ et al., 2003, Chong, ZZ et al., 2003). Through the regulation of the PI 3-K/Akt dependent pathway, EPO can prevent cellular apoptosis following N-methyl-D-aspartate toxicity (Dzietko, M et al., 2004), hypoxia (Chong, ZZ et al., 2002), and oxidative stress (Chong, ZZ et al., 2003, Chong, ZZ et al., 2003, Chong, ZZ et al., 2003).
CONCLUSION
Wnt-Frizzled signaling consists of Wnt/β-catenin, Wnt/Ca2+, and Wnt/planar cell polarity pathways and are involved with the development of the brain, spinal cord, and the extension of sub-populations of sensory and motor neurons. The Wnt-Frizzled signaling pathway also modulates both early and late apoptotic injury paradigms in a variety of cell populations during the development of an organism as well as during acute and chronic injury. Diseases of the nervous system, such as retinal degeneration and Alzheimer's disease, may be dependent upon the Wnt pathway. In addition, cytokines and growth factors may use Wnt proteins to exert their biological effects. Identifying the vital elements that shape and control the Wnt-Frizzled signaling pathway may provide significant prospects for the treatment of disorders of the nervous system.
ACKNOWLEDGEMENTS
This research was supported by the following grants (KM): American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, Johnson and Johnson Focused Investigator Award, LEARN Foundation Award, MI Life Sciences Challenge Award, and NIH NIEHS (P30 ES06639).
REFERENCES
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16(13):3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Khalil A, Fu L, Grove EA, Zecevic N, Geschwind DH. Wnt genes define distinct boundaries in the developing human brain: implications for human forebrain patterning. J Comp Neurol. 2004;474(2):276–88. doi: 10.1002/cne.20112. [DOI] [PubMed] [Google Scholar]
- Adler PN, Vinson C, Park WJ, Conover S, Klein L. Molecular structure of frizzled, a Drosophila tissue polarity gene. Genetics. 1990;126(2):401–16. doi: 10.1093/genetics/126.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Y, Nouri A, Wieschaus E. Drosophila Apc1 and Apc2 regulate Wingless transduction throughout development. Development. 2002;129(7):1751–62. doi: 10.1242/dev.129.7.1751. [DOI] [PubMed] [Google Scholar]
- Akiyama T. Wnt/beta-catenin signaling. Cytokine Growth Factor Rev. 2000;11(4):273–82. doi: 10.1016/s1359-6101(00)00011-3. [DOI] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12(16):2610–22. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejsovec A. Wnt pathway activation: new relations and locations. Cell. 2005;120(1):11–4. doi: 10.1016/j.cell.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Bombeli T, Karsan A, Tait JF, Harlan JM. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89(7):2429–42. [PubMed] [Google Scholar]
- Bose J, Gruber AD, Helming L, Schiebe S, Wegener I, Hafner M, Beales M, Kontgen F, Lengeling A. The phosphatidylserine receptor has essential functions during embryogenesis but not in apoptotic cell removal. J Biol. 2004;3(4):15. doi: 10.1186/jbiol10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bournat JC, Brown AM, Soler AP. Wnt-1 dependent activation of the survival factor NF-kappaB in PC12 cells. J Neurosci Res. 2000;61(1):21–32. doi: 10.1002/1097-4547(20000701)61:1<21::AID-JNR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83(1−2):27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94(1):109–18. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128(8):1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Braun MM, Etheridge A, Bernard A, Robertson CP, Roelink H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development. 2003;130(23):5579–87. doi: 10.1242/dev.00685. [DOI] [PubMed] [Google Scholar]
- Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature. 1998;395(6702):604–8. doi: 10.1038/26982. [DOI] [PubMed] [Google Scholar]
- Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152(1):87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Yang I, Irby R, Shain KH, Wang HG, Quackenbush J, Coppola D, Cheng JQ, Yeatman TJ. Regulation of caspase expression and apoptosis by adenomatous polyposis coli. Cancer Res. 2003;63(15):4368–74. [PubMed] [Google Scholar]
- Cheyette BN. Ryk: another heretical Wnt receptor defies the canon. Sci STKE. 2004;2004(263):pe54. doi: 10.1126/stke.2632004pe54. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ. Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev Biol. 2005;277(2):287–95. doi: 10.1016/j.ydbio.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. J Hematother Stem Cell Res. 2002;11(6):863–71. doi: 10.1089/152581602321080529. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106(23):2973–9. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Hematopoietic Factor Erythropoietin Fosters Neuroprotection Through Novel Signal Transduction Cascades. J Cereb Blood Flow Metab. 2002;22(5):503–514. doi: 10.1097/00004647-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c, and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003;23(3):320–30. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138(6):1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-x(L) and caspase 1, 3, and 9. Exp Cell Res. 2004;296(2):196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Essential cellular regulatory elements of oxidative stress in early and late phases of apoptosis in the central nervous system. Antioxid Redox Signal. 2004;6(2):277–87. doi: 10.1089/152308604322899341. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Activating Akt and the brain's resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20(1):299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75(3):207–46. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003;71(5):659–69. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Kang JQ, Maiese K. The tyrosine phosphatase SHP2 modulates MAP kinase p38 and caspase 1 and 3 to foster neuronal survival. Cell Mol Neurobiol. 2003;23(4−5):561–78. doi: 10.1023/A:1025158314016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Targeting WNT, protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system. Histol Histopathol. 2004;19(2):495–504. doi: 10.14670/hh-19.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AW, Carbajal JM, Schaeffer RC., Jr. VEGF stimulates tyrosine phosphorylation of beta-catenin and small-pore endothelial barrier dysfunction. Am J Physiol. 1999;277(5 Pt 2):H2038–49. doi: 10.1152/ajpheart.1999.277.5.H2038. [DOI] [PubMed] [Google Scholar]
- Crowder RJ, Freeman RS. Glycogen synthase kinase-3 beta activity is critical for neuronal death caused by inhibiting phosphatidylinositol 3-kinase or Akt but not for death caused by nerve growth factor withdrawal. J Biol Chem. 2000;275(44):34266–71. doi: 10.1074/jbc.M006160200. [DOI] [PubMed] [Google Scholar]
- Dombroski D, Balasubramanian K, Schroit AJ. Phosphatidylserine expression on cell surfaces promotes antibody- dependent aggregation and thrombosis in beta2-glycoprotein I-immune mice. J Autoimmun. 2000;14(3):221–9. doi: 10.1006/jaut.2000.0365. [DOI] [PubMed] [Google Scholar]
- Dono R, Faulhaber J, Galli A, Zuniga A, Volk T, Texido G, Zeller R, Ehmke H. FGF2 signaling is required for the development of neuronal circuits regulating blood pressure. Circ Res. 2002;90(1):E5–E10. [PubMed] [Google Scholar]
- Doonan F, Cotter TG. Apoptosis: A potential therapeutic target for retinal degenerations. Curr Neurovasc Res. 2004;1(1):41–53. doi: 10.2174/1567202043480215. [DOI] [PubMed] [Google Scholar]
- Dzietko M, Felderhoff-Mueser U, Sifringer M, Krutz B, Bittigau P, Thor F, Heumann R, Buhrer C, Ikonomidou C, Hansen HH. Erythropoietin protects the developing brain against N-methyl-D-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15(2):177–87. doi: 10.1016/j.nbd.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Ellies DL, Church V, Francis-West P, Lumsden A. The WNT antagonist cSFRP2 modulates programmed cell death in the developing hindbrain. Development. 2000;127(24):5285–95. doi: 10.1242/dev.127.24.5285. [DOI] [PubMed] [Google Scholar]
- Ferretti P. Neural stem cell plasticity: Recruitment of endogenous populations for regeneration. Curr Neurovasc Res. 2004;1(3):215–229. doi: 10.2174/1567202043362397. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee ME. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276(20):17479–83. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- Galceran J, Miyashita-Lin EM, Devaney E, Rubenstein JL, Gross-chedl R. Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development. 2000;127(3):469–82. doi: 10.1242/dev.127.3.469. [DOI] [PubMed] [Google Scholar]
- Garrido JL, Godoy JA, Alvarez A, Bronfman M, Inestrosa NC. Protein kinase C inhibits amyloid beta peptide neurotoxicity by acting on members of the Wnt pathway. FASEB J. 2002;16(14):1982–4. doi: 10.1096/fj.02-0327fje. [DOI] [PubMed] [Google Scholar]
- Genc S, Koroglu TF, Genc K. Erythropoietin as a novel neuroprotectant. Res Neur Neurosci. 2004;22:105–119. [PubMed] [Google Scholar]
- Golden JA, Bracilovic A, McFadden KA, Beesley JS, Rubenstein JL, Grinspan JB. Ectopic bone morphogenetic proteins 5 and 4 in the chicken forebrain lead to cyclopia and holoprosencephaly. Proc Natl Acad Sci USA. 1999;96(5):2439–44. doi: 10.1073/pnas.96.5.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H(2)O(2). Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J Biol Chem. 2001;276(49):46379–85. doi: 10.1074/jbc.M105637200. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Reme CE. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8(7):718–24. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- Grotewold L, Ruther U. The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. EMBO J. 2002;21(5):966–75. doi: 10.1093/emboj/21.5.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove EA, Tole S. Patterning events and specification signals in the developing hippocampus. Cereb Cortex. 1999;9(6):551–61. doi: 10.1093/cercor/9.6.551. [DOI] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125(12):2315–25. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB. Dishevelled and Wnt signaling: is the nucleus the final frontier? J Biol. 2005;4(1):2. doi: 10.1186/jbiol22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17(2):295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari L, Brault V, Kleber M, Lee HY, Ille F, Leimeroth R, Paratore C, Suter U, Kemler R, Sommer L. Lineage-specific requirements of beta-catenin in neural crest development. J Cell Biol. 2002;159(5):867–80. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, Polakis P. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9(4):207–10. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, Nishimura M, Hara-Nishimura I. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science. 2004;305(5685):855–8. doi: 10.1126/science.1099859. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Holnthoner W, Pillinger M, Groger M, Wolff K, Ashton AW, Albanese C, Neumeister P, Pestell RG, Petzelbauer P. Fibroblast growth factor-2 induces Lef/Tcf-dependent transcription in human endothelial cells. J Biol Chem. 2002;277(48):45847–53. doi: 10.1074/jbc.M209354200. [DOI] [PubMed] [Google Scholar]
- Hong JR, Lin GH, Lin CJ, Wang WP, Lee CC, Lin TL, Wu JL. Phosphatidylserine receptor is required for the engulfment of dead apoptotic cells and for normal embryonic development in zebrafish. Development. 2004;131(21):5417–27. doi: 10.1242/dev.01409. [DOI] [PubMed] [Google Scholar]
- Honore SM, Aybar MJ, Mayor R. Sox10 is required for the early development of the prospective neural crest in Xenopus embryos. Dev Biol. 2003;260(1):79–96. doi: 10.1016/s0012-1606(03)00247-1. [DOI] [PubMed] [Google Scholar]
- Hsieh JC. Specificity of WNT-receptor interactions. Front Biosci. 2004;9:1333–8. doi: 10.2741/1321. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17(5):1371–84. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389(6654):966–70. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23(4):1379–89. doi: 10.1128/MCB.23.4.1379-1389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J Biol. 2005;4(1):3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson GR, Wiedau-Pazos M, Sang TK, Wagle N, Brown CA, Massachi S, Geschwind DH. Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron. 2002;34(4):509–19. doi: 10.1016/s0896-6273(02)00706-7. [DOI] [PubMed] [Google Scholar]
- Jessel R, Haertel S, Socaciu C, Tykhonova S, Diehl HA. Kinetics of apoptotic markers in exogeneously induced apoptosis of EL4 cells. J Cell Mol Med. 2002;6(1):82–92. doi: 10.1111/j.1582-4934.2002.tb00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Jomary C, Grist J, Stewart HJ, Neal MJ. Modulated expression of secreted frizzled-related proteins in human retinal degeneration. Neuroreport. 2000;11(18):3963–7. doi: 10.1097/00001756-200012180-00012. [DOI] [PubMed] [Google Scholar]
- Kameya S, Hawes NL, Chang B, Heckenlively JR, Naggert JK, Nishina PM. Mfrp, a gene encoding a frizzled related protein, is mutated in the mouse retinal degeneration 6. Hum Mol Genet. 2002;11(16):1879–86. doi: 10.1093/hmg/11.16.1879. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003;74(1):37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003;64(3):557–69. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- Kaptanoglu E, Solaroglu I, Okutan O, Surucu HS, Akbiyik F, Beskonakli E. Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: effect on lipid peroxidation and early ultra-structural findings. Neurosurg Rev. 2004;27(2):113–20. doi: 10.1007/s10143-003-0300-y. [DOI] [PubMed] [Google Scholar]
- Katoh M. Regulation of WNT signaling molecules by retinoic acid during neuronal differentiation in NT2 cells: threshold model of WNT action. Int J Mol Med. 2002;10(6):683–7. [PubMed] [Google Scholar]
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128(21):4189–201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- Kishida M, Hino S, Michiue T, Yamamoto H, Kishida S, Fukui A, Asashima M, Kikuchi A. Synergistic activation of the Wnt signaling pathway by Dvl and casein kinase Iepsilon. J Biol Chem. 2001;276(35):33147–55. doi: 10.1074/jbc.M103555200. [DOI] [PubMed] [Google Scholar]
- Koyama R, Ikegaya Y. Mossy fiber sprouting as a potential therapeutic target for epilepsy. Curr Neurovasc Res. 2004;1(1):3–10. doi: 10.2174/1567202043480242. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G. GSK-3 and the neuro-developmental hypothesis of schizophrenia. Eur Neuropsychopharmacol. 2002;12(1):13–25. doi: 10.1016/s0924-977x(01)00131-6. [DOI] [PubMed] [Google Scholar]
- Kuhl M. The WNT/calcium pathway: biochemical mediators, tools and future requirements. Front Biosci. 2004;9:967–74. doi: 10.2741/1307. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16(7):279–83. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Kunz M, Herrmann M, Wedlich D, Gradl D. Autoregulation of canonical Wnt signaling controls midbrain development. Dev Biol. 2004;273(2):390–401. doi: 10.1016/j.ydbio.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Latres E, Chiaur DS, Pagano M. The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene. 1999;18(4):849–54. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kirschner MW. Physiological regulation of [beta]-catenin stability by Tcf3 and CK1epsilon. J Cell Biol. 2001;154(5):983–93. doi: 10.1083/jcb.200102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Ishimoto A, Yanagawa S. Characterization of mouse dishevelled (Dvl) proteins in Wnt/Wingless signaling pathway. J Biol Chem. 1999;274(30):21464–70. doi: 10.1074/jbc.274.30.21464. [DOI] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127(3):457–67. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Erythropoietin on a Tightrope: Balancing Neuronal and Vascular Protection between Intrinsic and Extrinsic Pathways. Neurosignals. 2004;13(6):265–89. doi: 10.1159/000081963. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front Biosci. 2004;9:2500–2520. doi: 10.2741/1412. [DOI] [PubMed] [Google Scholar]
- Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21(3):262–75. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Lisovsky M, Itoh K, Sokol SY. Frizzled receptors activate a novel JNK-dependent pathway that may lead to apoptosis. Curr Biol. 2002;12(1):53–8. doi: 10.1016/s0960-9822(01)00628-5. [DOI] [PubMed] [Google Scholar]
- Longo KA, Kennell JA, Ochocinska MJ, Ross SE, Wright WS, MacDougald OA. Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J Biol Chem. 2002;277(41):38239–44. doi: 10.1074/jbc.M206402200. [DOI] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119(1):97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22(4):1184–93. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaio MP, Fabbi M, Tiso M, Daga A, Puccetti A. Spontaneously produced anti-DNA/DNase I autoantibodies modulate nuclear apoptosis in living cells. Eur J Immunol. 1996;26(12):3035–41. doi: 10.1002/eji.1830261232. [DOI] [PubMed] [Google Scholar]
- Maiese K. The dynamics of cellular injury: transformation into neuronal and vascular protection. Histol Histopathol. 2001;16(2):633–44. doi: 10.14670/HH-16.633. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24(5):228–32. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ. Insights into oxidative stress and potential novel therapeutic targets for Alzheimer disease. Restor Neurol Neurosci. 2004;22(2):87–104. [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Kang J. Transformation into treatment: Novel therapeutics that begin within the cell. In: Maiese K, editor. In: Neuronal and Vascular Plasticity: Elucidating Basic Cellular Mechanisms for Future Therapeutic Discovery. Kluwer Academic Publishers; Norwell, MA: 2003. pp. 1–26. [Google Scholar]
- Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25(11):577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293(1):90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J Neurosci Res. 2000;59(4):568–80. doi: 10.1002/(SICI)1097-4547(20000215)59:4<568::AID-JNR13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7(4):801–9. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430(7000):631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkonyan HS, Chang WC, Shapiro JP, Mahadevappa M, Fitzpatrick PA, Kiefer MC, Tomei LD, Umansky SR. SARPs: a family of secreted apoptosis-related proteins. Proc Natl Acad Sci USA. 1997;94(25):13636–41. doi: 10.1073/pnas.94.25.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiue T, Fukui A, Yukita A, Sakurai K, Danno H, Kikuchi A, Asashima M. XIdax, an inhibitor of the canonical Wnt pathway, is required for anterior neural structure formation in Xenopus. Dev Dyn. 2004;230(1):79–90. doi: 10.1002/dvdy.20037. [DOI] [PubMed] [Google Scholar]
- Mirkovic I, Charish K, Gorski SM, McKnight K, Verheyen EM. Drosophila nemo is an essential gene involved in the regulation of programmed cell death. Mech Dev. 2002;119(1):9–20. doi: 10.1016/s0925-4773(02)00289-7. [DOI] [PubMed] [Google Scholar]
- Miyaoka T, Seno H, Ishino H. Increased expression of Wnt-1 in schizophrenic brains. Schizophr Res. 1999;38(1):1–6. doi: 10.1016/s0920-9964(98)00179-0. [DOI] [PubMed] [Google Scholar]
- Montague JW, Hughes F,, Jr., Cidlowski JA. Native recombinant cyclophilins A, B, and C degrade DNA independently of peptidylprolyl cis-trans-isomerase activity. Potential roles of cyclophilins in apoptosis. J Biol Chem. 1997;272(10):6677–84. doi: 10.1074/jbc.272.10.6677. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Kawachi K, Kamakura S, Masuyama N, Yamanaka H, Matsumoto K, Kikuchi A, Nishida E. Distinct domains of mouse dishevelled are responsible for the c-Jun N-terminal kinase/stress-activated protein kinase activation and the axis formation in vertebrates. J Biol Chem. 1999;274(43):30957–62. doi: 10.1074/jbc.274.43.30957. [DOI] [PubMed] [Google Scholar]
- Moulin N, Widmann C. Islet-Brain (IB)/JNK-interacting proteins (JIPs): Future targets for the treatment of neurodegenerative diseases? Curr Neurovasc Res. 2004;1(2):111–127. doi: 10.2174/1567202043480161. [DOI] [PubMed] [Google Scholar]
- Mudher A, Chapman S, Richardson J, Asuni A, Gibb G, Pollard C, Killick R, Iqbal T, Raymond L, Varndell I, Sheppard P, Makoff A, Gower E, Soden PE, Lewis P, Murphy M, Golde TE, Rupniak HT, Anderton BH, Lovestone S. Dishevelled regulates the metabolism of amyloid precursor protein via protein kinase C/mitogen-activated protein kinase and c-Jun terminal kinase. J Neurosci. 2001;21(14):4987–95. doi: 10.1523/JNEUROSCI.21-14-04987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92(7):3046–50. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroyama Y, Fujihara M, Ikeya M, Kondoh H, Takada S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 2002;16(5):548–53. doi: 10.1101/gad.937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. WNT targets. Repression and activation. Trends Genet. 1999;15(1):1–3. doi: 10.1016/s0168-9525(98)01634-5. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Pandey S, Walker PR, Sikorska M. Identification of a novel 97 kDa endonuclease capable of internucleosomal DNA cleavage. Biochemistry. 1997;36(4):711–20. doi: 10.1021/bi962387h. [DOI] [PubMed] [Google Scholar]
- Panhuysen M, Vogt Weisenhorn DM, Blanquet V, Brodski C, Heinzmann U, Beisker W, Wurst W. Effects of Wnt1 signaling on proliferation in the developing mid-/hindbrain region. Mol Cell Neurosci. 2004;26(1):101–11. doi: 10.1016/j.mcn.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Papkoff J, Aikawa M. WNT-1 and HGF regulate GSK3 beta activity and beta-catenin signaling in mammary epithelial cells. Biochem Biophys Res Commun. 1998;247(3):851–8. doi: 10.1006/bbrc.1998.8888. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Curr Opin Neurobiol. 2000;10(3):392–9. doi: 10.1016/s0959-4388(00)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthy L. The WIF module. Trends Biochem Sci. 2000;25(1):12–3. doi: 10.1016/s0968-0004(99)01504-2. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407(6803):535–8. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Jambal P, Audesirk G, Kern M, Cabell L, Eves E, Rosner MR, Boxer LM, Reusch JE. Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J Neurochem. 2003;84(5):982–96. doi: 10.1046/j.1471-4159.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA. 1997;94(7):2859–63. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin J, Corr M, Carson DA. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. 2002;21(43):6598–605. doi: 10.1038/sj.onc.1205920. [DOI] [PubMed] [Google Scholar]
- Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, van de Wetering M, Destree O, Clevers H. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 1998;395(6702):608–12. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8(1):34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274(51):36734–40. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278(16):13898–904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- Salinas PC. Wnt factors in axonal remodelling and synaptogenesis. Biochem Soc Symp. 1999;65:101–9. [PubMed] [Google Scholar]
- Savage MJ, Trusko SP, Howland DS, Pinsker LR, Mistretta S, Reaume AG, Greenberg BD, Siman R, Scott RW. Turnover of amyloid beta-protein in mouse brain and acute reduction of its level by phorbol ester. J Neurosci. 1998;18(5):1743–52. doi: 10.1523/JNEUROSCI.18-05-01743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Buchert M, Georgiev O, Catimel B, Halford M, Stacker SA, Baechi T, Moelling K, Hovens CM. Mutagenesis and selection of PDZ domains that bind new protein targets. Nat Biotechnol. 1999;17(2):170–5. doi: 10.1038/6172. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Ebadi M. Metallothionein attenuates 3-morpholinosydnonimine (SIN-1)-induced oxidative stress in dopaminergic neurons. Antioxid Redox Signal. 2003;5(3):251–64. doi: 10.1089/152308603322110832. [DOI] [PubMed] [Google Scholar]
- Shimogori T, VanSant J, Paik E, Grove EA. Members of the Wnt, Fz, and Frp gene families expressed in postnatal mouse cerebral cortex. J Comp Neurol. 2004;473(4):496–510. doi: 10.1002/cne.20135. [DOI] [PubMed] [Google Scholar]
- Shou J, Ali-Osman F, Multani AS, Pathak S, Fedi P, Srivenugopal KS. Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene. 2002;21(6):878–89. doi: 10.1038/sj.onc.1205138. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96(10):5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390(6658):410–3. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- Smalley MJ, Dale TC. Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev. 1999;18(2):215–30. doi: 10.1023/a:1006369223282. [DOI] [PubMed] [Google Scholar]
- Soriano S, Kang DE, Fu M, Pestell R, Chevallier N, Zheng H, Koo EH. Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing. J Cell Biol. 2001;152(4):785–94. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002;16(1):46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XM, Cohen GM. Mg(2+)-dependent cleavage of DNA into kilobase pair fragments is responsible for the initial degradation of DNA in apoptosis. J Biol Chem. 1994;269(21):14857–60. [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127(10):2227–38. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407(6803):530–5. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4(3):407–18. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Torriglia A, Chaudun E, Chany-Fournier F, Jeanny JC, Courtois Y, Counis MF. Involvement of DNase II in nuclear degeneration during lens cell differentiation. J Biol Chem. 1995;270(48):28579–85. doi: 10.1074/jbc.270.48.28579. [DOI] [PubMed] [Google Scholar]
- Torriglia A, Chaudun E, Courtois Y, Counis MF. On the use of Zn2+ to discriminate endonucleases activated during apoptosis. Biochimie. 1997;79(7):435–8. doi: 10.1016/s0300-9084(97)86153-6. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5(3):367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Maiese K. Direct temporal analysis of apoptosis induction in living adherent neurons. J Histochem Cytochem. 1999;47(5):661–72. doi: 10.1177/002215549904700508. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Maiese K. Nitric oxide induction of neuronal endonuclease activity in programmed cell death. Exp Cell Res. 1999;246(2):290–300. doi: 10.1006/excr.1998.4282. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338(6212):263–4. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- Wagner U, Brownlees J, Irving NG, Lucas FR, Salinas PC, Miller CC. Overexpression of the mouse dishevelled-1 protein inhibits GSK-3beta-mediated phosphorylation of tau in transfected mammalian cells. FEBS Lett. 1997;411(2−3):369–72. doi: 10.1016/s0014-5793(97)00733-3. [DOI] [PubMed] [Google Scholar]
- Wang HY, Malbon CC. Wnt signaling, Ca2+, and cyclic GMP: visualizing Frizzled functions. Science. 2003;300(5625):1529–30. doi: 10.1126/science.1085259. [DOI] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Abella BS, Andreasson K, Worley P, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J Biol Chem. 1996;271(8):4468–76. doi: 10.1074/jbc.271.8.4468. [DOI] [PubMed] [Google Scholar]
- Weber JT. Calcium homeostasis following traumatic neuronal injury. Curr Neurovasc Res. 2004;1(2):151–171. doi: 10.2174/1567202043480134. [DOI] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O'Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407(6803):527–30. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Weitzman JB. Dishevelled nuclear shuttling. J Biol. 2005;4(1):1. doi: 10.1186/jbiol21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton KA., Jr. Runnin' with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol. 2003;253(1):1–17. doi: 10.1006/dbio.2002.0869. [DOI] [PubMed] [Google Scholar]
- Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13(3):270–83. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn BA, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O, Ihle JN. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74(2):227–36. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Witting A, Muller P, Herrmann A, Kettenmann H, Nolte C. Phagocytic clearance of apoptotic neurons by Microglia/Brain macrophages in vitro: involvement of lectin-, integrin-, and phosphatidylserine-mediated recognition. J Neurochem. 2000;75(3):1060–70. doi: 10.1046/j.1471-4159.2000.0751060.x. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274(16):10681–4. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- Yeo W, Gautier J. Early neural cell death: dying to become neurons. Dev Biol. 2004;274(2):233–44. doi: 10.1016/j.ydbio.2004.07.026. [DOI] [PubMed] [Google Scholar]
- You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan KL, MacDougald OA, Brown AM, Evan G, Kitajewski J, Wang CY. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol. 2002;157(3):429–40. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]


