Abstract
Erythropoietin (EPO) produced by the kidney and the liver (in fetuses) stimulates erythropoiesis. In the central nervous system, neurons express EPO receptor (EPOR) and astrocytes produce EPO. EPO has been shown to protect primary cultured neurons from N-methyl-d-aspartate (NMDA) receptor-mediated glutamate toxicity. Here we report in vivo evidence that EPO protects neurons against ischemia-induced cell death. Infusion of EPO into the lateral ventricles of gerbils prevented ischemia-induced learning disability and rescued hippocampal CA1 neurons from lethal ischemic damage. The neuroprotective action of exogenous EPO was also confirmed by counting synapses in the hippocampal CA1 region. Infusion of soluble EPOR (an extracellular domain capable of binding with the ligand) into animals given a mild ischemic treatment that did not produce neuronal damage, caused neuronal degeneration and impaired learning ability, whereas infusion of the heat-denatured soluble EPOR was not detrimental, demonstrating that the endogenous brain EPO is crucial for neuronal survival. The presence of EPO in neuron cultures did not repress a NMDA receptor-mediated increase in intracellular Ca2+, but rescued the neurons from NO-induced death. Taken together EPO may exert its neuroprotective effect by reducing the NO-mediated formation of free radicals or antagonizing their toxicity.
Mammals respond to oxygen deficiency in many different ways (1). One strategy for survival of the individual cells under hypoxic conditions is the induction of glycolytic enzymes, facilitating ATP production by glycolysis rather than mitochondrial oxidative phosphorylation. In response to the systemic oxygen deficiency due to anemia or decreased-environmental oxygen concentration, erythropoietin (EPO) production is stimulated. EPO is a glycoprotein that stimulates differentiation and proliferation of erythroid precursor cells, and hypoxic induction of EPO production increases red blood cells, leading to better oxygen supply to tissues (2, 3). The action of EPO is mediated by binding to the specific receptor that belongs to a new family of cytokine receptors that have no tyrosine kinase domain (4). EPO regulating erythropoiesis is mainly produced by the kidney in adults and by the liver at fetal stages (2, 3).
Stimulation of red blood cell formation was thought to be the sole physiological function of EPO, but a different function in the central nervous system has been proposed (5–7). Neuronal cell lines such as PC12 and SN6 express EPO receptor (EPOR), and binding of EPO to PC12 cells increases the intracellular concentration of monoamines (8). Immunochemical staining with anti-EPOR antibody showed that EPOR is expressed in murine hippocampal and cerebral cortical areas, and also in primary cultured hippocampal and cortical neurons (6, 9). With the use of radioiodinated EPO, specific EPO binding sites were found in some defined areas of the murine brain including the hippocampus and cerebral cortex (10). Because the blood-brain barrier prevents neurons from interacting with kidney-derived serum EPO, a site for EPO production should be present in the central nervous system for the expression of EPOR in neurons to have a physiological significance. Primary cultured astrocytes have been shown to produce EPO and low oxygen tension stimulates the production of EPO through an increase in its mRNA (11, 12). EPO mRNA is expressed in the adult rat brain and the expression is hypoxia-inducible (13). Messenger RNAs of EPO and EPOR are also expressed in the primate brain (12). EPO protects primary cultured hippocampal and cerebral cortical neurons from NMDA receptor-mediated glutamate toxicity (6), which is believed to be a major cause of neuron death by ischemia (14, 15). It remains unknown, however, whether or not the endogenous brain EPO functions in vivo.
In this paper, we report in vivo evidence that EPO plays an important role in protecting neurons from ischemia-induced cell death. Experimental results on the mechanism underlying the protective effect of EPO on glutamate-induced neuron death are also reported.
MATERIALS AND METHODS
Osmotic Minipump Implantation.
Male Mongolian gerbils each weighing 70–80 g (≈12 weeks of age) were anesthetized with 1.5% halothane in a 4:3 mixture of nitrous oxide and oxygen and placed in a stereotaxic apparatus. An osmotic minipump (Alza) was implanted subcutaneously into the back of each animal, and a needle from the minipump was placed in the left lateral ventricle according to the atlas of Thiessen and Yahr (16).
Infusion of EPO and Soluble EPOR (sEPOR).
Recombinant human EPO (17) was dissolved in a vehicle consisting of 0.01 M PBS (pH 7.5) and 0.1% BSA. One unit of EPO approximately corresponds to 10 ng of EPO protein. EPO at a dose of 0.5 (n = 8), 2.5 (n = 8), 5 (n = 11), or 25 (n = 8) unit/day was infused for 7 days into the left lateral ventricle of each normothermic gerbil in which 3-min forebrain ischemia had been induced as described (18–23); control animals (one group with 3-min forebrain ischemia and one group of sham-operated animals) received vehicle infusion (n = 11 in each group). To investigate the neuroprotective effect of endogenous brain EPO on ischemic CA1 neurons, sEPOR (24) at a dose of 5, 10, 25, or 50 μg/day was infused for 7 days into the left lateral ventricles of normothermic gerbils in which 2.5-min forebrain ischemia had been induced (n = 6–8 in each group). Sham-operated (not ischemic) animals were also infused with 25 μg/day of sEPOR for 7 days. Control ischemic animals received the infusion of vehicle (n = 8) or heat-denatured sEPOR (dsEPOR; heated at 56°C for 30 min) at a dose of 5 or 25 μg/day (n = 6 for each). The infusion of EPO or sEPOR was started at 8 or 24 h before the ischemic insult in the EPO-treated groups and in the sEPOR-treated groups, respectively.
Occlusion of the Common Carotid Arteries.
Occlusion of the common carotid arteries was performed as described (20, 21, 25). Sham-operated animals were treated in the same manner except that the common carotid arteries were not clamped.
During forebrain ischemia, the fall in brain temperature has been shown to differ with animal, thereby affecting the number of viable CA1 neurons after ischemia (18). To avoid the effect of unstable brain temperature on ischemic neuronal loss, we kept brain and rectal temperatures at 37.0 ± 0.2°C while clamping the common carotid arteries (18–23, 25). This enabled us to induce an invariable neuronal damage in the hippocampal CA1 field even after 3-min ischemic insult (19–23, 25) and to evaluate accurately the in vivo effects of neuroprotective agents on delayed neuronal death.
Estimation of Learning Ability by Passive Avoidance Task.
Seven days after forebrain ischemia, the gerbils were trained in a conventional step-down passive avoidance apparatus that was divided into a safe platform and a chamber with a stainless-steel grid floor (19–23, 25, 26). Each animal was placed initially on the safe platform. When the gerbil stepped down onto the grid floor, it received a foot shock. Although the gerbil moved repeatedly up and down between the platform and the grid, it eventually remained on the platform. This training session lasted 5 min. Twenty-four hours later, the gerbil was again placed on the safe platform while the shock generator was turned off, and the response latency, i.e., the time until it stepped down onto the grid floor, was measured. This test session also lasted 5 min. The long response latency indicates better learning ability. The results of passive avoidance task has been shown to correlate well with the number of hippocampal CA1 neurons (19–23, 25–27).
Histopathological Study of Hippocampal CA1 Region.
One hour after the passive avoidance experiments, each animal was anesthetized with pentobarbital, and the osmotic minipump was disconnected from the needle that had been placed in the lateral ventricle. Bromophenol blue was injected through the needle to ascertain the infusion of EPO, sEPOR, dsEPOR, or vehicle into the cerebral ventricles. The animals infused with EPO or vehicle were anesthetized with pentobarbital and perfused transcardially with 4% paraformaldehyde-2.5% glutaraldehyde in 0.1 M PBS (pH 7.4) for light and electron microscopy (25). A brain region including the dorsal hippocampus was removed and kept in the same fixative overnight at 4°C. Four serial coronal sections 50 μm thick at the level 1.0–1.2 mm posterior to bregma were cut with a microslicer (Dosaka EM, Kyoto). The remaining dorsal hippocampus was embedded in paraffin, and 5-μm serial frontal sections were stained with 0.1% cresyl violet. All neurons with intact morphological appearance along a 1-mm linear length of the CA1 field in six serial paraffin sections (1.20–1.23 mm posterior to bregma) were counted. For electron microscopy, the 50 μm sections were postfixed with 1% osmium tetroxide, dehydrated, and embedded in epoxy resin. Ultrathin sections with 70-nm thick were made with a Reichert ultramicrotome (Optische Werke, Vienna, Austria) and mounted on single-slot (2 × 0.5 mm) grids, which were coated with Formbar film. Electron micrographs of the central areas (15 μm × 18.75 μm = 280 μm2) of the strata moleculare, radiatum, and oriens in the CA1 region were taken, and intact synapses in the areas were counted. All cell- and synapse-counting was done blindly with respect to experimental group.
The animals infused with sEPOR, dsEPOR, or vehicle were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) under pentobarbital anesthesia. The neurons in the hippocampal CA1 region in each animal was counted as described above, and two additional paraffin sections from each animal were processed for in situ detection of DNA fragmentation [terminal dinucleotidyltransferase-mediated UTP end labeling (TUNEL) staining] to estimate the number of CA1 neurons that degenerated after the termination of sEPOR infusion. Briefly, the sections were treated as follows: deparaffinized in xylene and a graded series of ethanol, incubated with proteinase K (20 μg/ml, Sigma) for 15 min; incubated with a mixture of terminal deoxynucleotidyl transferase and reaction buffer containing digoxigenin-dUTP (Oncor) in a humidified chamber for 1 h at 37°C, washed in wash buffer (Oncor) for 10 min and in three changes of 0.05 M PBS for 5 min each; and incubated with anti-digoxigenin-peroxidase (Oncor) for 1 h at room temperature, and exposed to 0.05% diaminobenzidine and 0.02% hydrogen peroxide.
Statistics.
The effects of EPO and sEPOR on response latency and CA1 neuronal density were evaluated by the two-tailed Mann-Whitney U test, which enabled us to compare the EPO- and sEPOR-treated groups with the corresponding ischemic control groups. All data were represented as mean ± SE.
Culture of Neurons.
Hippocampal and cerebral cortical neurons from brains of 19-day old fetal Wistar rats were cultured as described (6). The cells cultured for 7–10 days in the serum-free B-27/Neurobasal (GIBCO) chemically defined medium were used for experiments.
Rate of 45Ca Uptake by Cultured Neurons.
Rate of 45Ca uptake by cerebral cortical neurons were determined as described (28) with minor modification. The neurons were incubated at 37°C for 1.5, 3, and 6 min in Krebs/Hepes buffer consisting of 45Ca (370 kBq/ml), 140 mM NaCl, 4.7 mM KCl, 2.2 mM CaCl2, 1.2 mM MgCl2, 1.2 mM KH2PO4, 11 mM glucose, and 15 mM Hepes, pH 7.4. Then the neurons were washed three times with the cold Krebs/Hepes buffer containing 1 mM EGTA but no Ca2+, and recovered by trypsinization. Radioactive 45Ca incorporated into the neurons was measured by a γ-counter.
Measurement of Intracellular Calcium Concentration.
Intracellular calcium concentration was determined by the method reported (29), by using fura-2 loaded cerebral cortical neurons. The fluorescence intensity emitted by the fura-2/Ca2+ complex was measured with a CAF-100 fluorescence spectrometer (Jasco, Tokyo).
Effects of Glutamate, S-Methyl-l-Thiocitrulline (Me-TC; a NO Synthase Inhibitor), Sodium Nitroprusside (SNP; a NO-Generating Agent), and EPO on Cultured Neurons.
Hippocampal neurons were used for experiments. For incubation of the neurons with test materials, the medium was replaced with N2/Neurobasal medium (GIBCO). The neurons were incubated with 200 μM glutamate or 1 mM SNP (Dojin) for 15 min. When effect of Me-TC (Dojin, Tokyo) on glutamate toxicity was examined, the neurons were incubated with 25 μM Me-TC 30 min before glutamate challenge. When the neuroprotective effect of EPO was examined, 1 unit/ml EPO was added to cultures 24 h before incubation of neurons with test materials. After incubation in a CO2 incubator at 37°C, the medium was replaced with the fresh N2/Neurobasal medium containing no test material, and the cells were cultured for another 24 h. The cells were stained with trypan blue and fixed with 1% glutaraldehyde in PBS. Total and nonviable cells were counted under light microscopy. Nonviable cells were stained and viable cells excluded the dye.
RESULTS
Protective Effect of EPO on Ischemia-Induced Neuron Death.
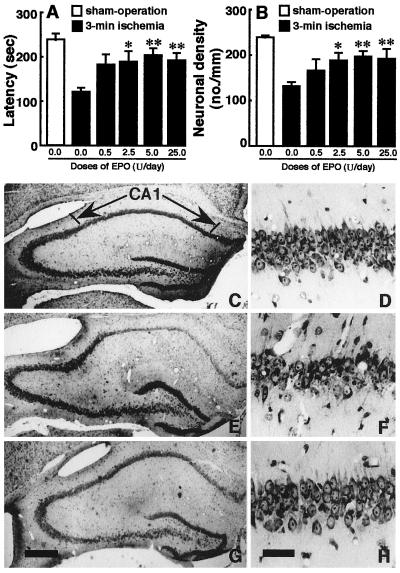
The response latency in the step-down passive avoidance task, which is an index of learning ability, and CA1 neuronal density of sham-operated animals with vehicle infusion were 240 ± 13 s and 241 ± 5 cells/mm, respectively, while those of 3-min ischemic gerbils infused with vehicle alone were 123 ± 9 s and 133 ± 11 cells/mm, respectively. There were significant differences in response latency (U = 0, P < 0.01) and CA1 neuronal density (U = 0, P < 0.01) between the two groups (Fig. 1 A, B). In histological sections, the CA1 region of 3-min ischemic gerbils exhibited a marked decline in viable neurons, when compared with the CA1 field of sham-operated animals (Fig. 1 C–F).
Figure 1.
Effects of intracerebroventricular EPO infusion on the response latency and hippocampal CA1 region of 3-min ischemic gerbils. (A) response latency; (B) CA1 neuronal density. Open columns indicate sham-operated (sham-op) animals and closed columns indicate vehicle- or EPO-infused ischemic animals. Each value represents mean ± SE (n = 8–11). *P < 0.05 and **P < 0.01, significantly different from the corresponding vehicle-infused ischemic group (statistical significance tested by the two-tailed Mann–Whitney U test). (C–H) Photomicrographs of hippocampal sections stained with cresyl violet: (C, E, and G) low magnification; (D, F and H) high magnification corresponding to C, E, and G. (C and D) A sham-operated animal; (E and F) an ischemic animal infused with vehicle; (G and H) an ischemic animal infused with 5 units/day of EPO. [Bar = 1.0 mm (C, E, and G) and 0.1 mm (D, F, and H).]
The continuous infusion of EPO at a dose of 2.5, 5, or 25 units/day for 7 days into the lateral ventricle caused a significant prolongation in response latency time (EPO vs. vehicle in ischemic gerbils: U = 15.5, P < 0.05; U = 13.0, P < 0.01; U = 11.0, P < 0.01) (Fig. 1A). Subsequent histological examinations revealed that treatment with EPO at the dose of 2.5, 5, or 25 units/day rescued many ischemic neurons that were destined to degenerate without the treatment (EPO vs. vehicle in ischemic gerbils: U = 19.0, P < 0.05; U = 16.0, P < 0.01; U = 12.0, P < 0.01) (Fig. 1 B, G, H). The lower dose of EPO (0.5 units/day) was not significantly effective, although the mean response latency and CA1 neuronal density were higher in 0.5 units/day EPO-infused ischemic animals than in vehicle-infused ischemic animals (Fig. 1 A, B). EPO at a dose of 50 units/day or 500 units/day was ineffective in preventing the ischemia-induced reduction of response latency and neuronal loss (data not shown).
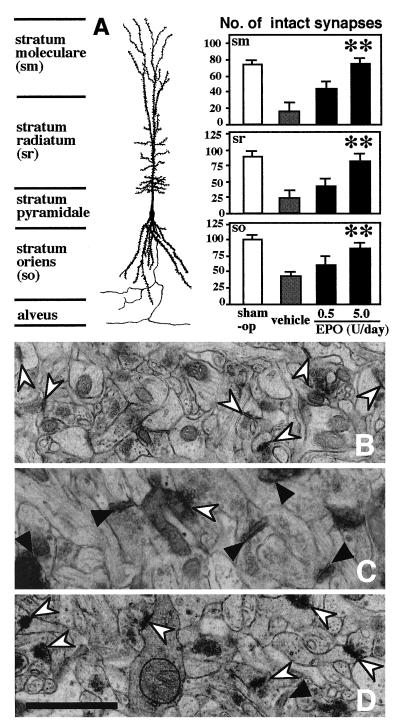
In line with the results of the passive avoidance task and light microscopic observations, electron microscopy showed that synapses within the stratum moleculare, stratum lacunosum/radiatum and stratum oriens of the hippocampal CA1 region were more numerous in EPO (5 units/day)-treated than in vehicle-treated ischemic gerbils (Fig. 2A) (5 units/day EPO vs. vehicle in the individual strata of the ischemic hippocampal CA1 field: U = 0, P < 0.01; U = 2.0, P < 0.01; U = 2.0, P < 0.01). Electron micrographs from the hippocampal CA1 field of vehicle-treated ischemic gerbils (Fig. 2C), unlike those from the hippocampus of sham-operated animals (Fig. 2B), showed many degenerating postsynaptic elements with high electron density. EPO (5 units/day) treatment apparently reduced the number of degenerating synapses in the ischemic hippocampal CA1 field (Fig. 2D).
Figure 2.
Effects of intracerebroventricular EPO infusion on the synapses in the hippocampal CA1 region of 3-min ischemic gerbils. (A) the number of synapses within the stratum moleculare (sm), stratum lacunosum/radiatum (sr) and stratum oriens (so). Open columns indicate sham-operated (sham-op) animals and stippled or solid columns indicate vehicle- or EPO-infused ischemic animals. Each value represents mean ± SE (n = 8–11). **P < 0.01, significantly different from the corresponding vehicle-infused ischemic group (statistical significance tested by the two-tailed Mann–Whitney U test). (B–D) Electron micrographs of the stratum radiatum. (B) A sham-operated animal; (C) an ischemic animal treated with vehicle; (D) an ischemic animal treated with EPO (5 units/day). Note that degenerating synapses are extremely electron dense (solid arrowheads) and more numerous in C than in B or D. Intact synapses are indicated by open arrowheads. (Bar = 1 μm.)
Stimulation of Neuron Death by sEPOR Under Mild Ischemia.
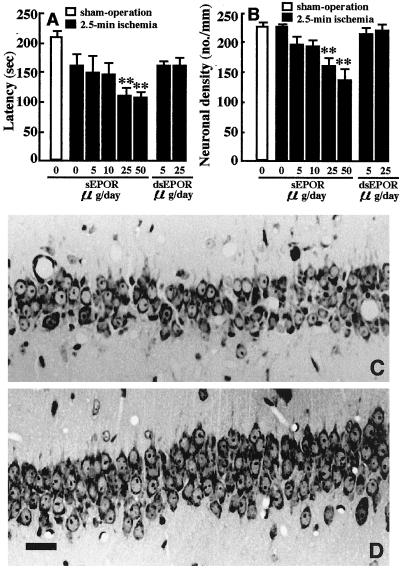
As described above, the infused recombinant EPO protects neurons from ischemic damage. This result suggests a possible function of EPO but does not lead us to conclude that brain EPO supports neuron survival in the central nervous system. We found that ischemia for 2.5 min did not reduce neuronal cell density (224 ± 8 cells/mm), while there was a significant reduction in 3-min ischemia (241 in controls to 133 cells/mm; see also Fig. 1B). This finding has made it possible to examine whether the infusion of sEPOR capable of binding with EPO provokes neuron damage in 2.5-min ischemia, which may provide conclusive evidence that brain EPO functions as a neuroprotective agent. The continuous infusion of sEPOR at a dose of 5 or 10 μg/day for 7 days into the cerebral ventricles did not show a detrimental effect but the infusion of higher doses (25 or 50 μg/day) caused a significant reduction in response latency time (U = 15.0, P < 0.01; U = 4.0, P < 0.01) (Fig. 3A) and a significant decrease in hippocampal CA1 neuron density (U = 13.0, P < 0.01; U = 4.0, P < 0.01) (Fig. 3B), when compared with those of 2.5-min ischemic animals infused with vehicle. Neither the response latency nor neuronal density was altered by the infusion of the inactive dsEPOR (5 or 25 μg/day) in 2.5-min ischemic gerbils (Fig. 3 A, B). The infusion of sEPOR (25 μg/day) in normal gerbils did not induce neuronal death in the hippocampal CA1 field (data not shown).
Figure 3.
Effects of the intracerebroventricular infusion of sEPOR and dsEPOR on the response latency and hippocampal CA1 region of 2.5-min ischemic gerbils. (A) Response latency; (B) CA1 neuronal density. Open columns indicate sham-operated animals and stippled or solid columns indicate vehicle-sEPOR- or dsEPOR-infused ischemic animals. Each value represents mean ± SE (n = 6–8). **P < 0.01, significantly different from the corresponding vehicle-infused ischemic group (statistical significance tested by the two-tailed Mann–Whitney U test). (C and D) Photomicrographs of the hippocampal CA1 field stained with cresyl violet; (C) an ischemic animal infused with sEPOR at a dose of 25 μg/day; (D) an ischemic animal infused with vehicle. Note that the infusion of sEPOR resulted in a significant decrease in hippocampal CA1 pyramidal neurons. (Bar = 0.1 mm.)
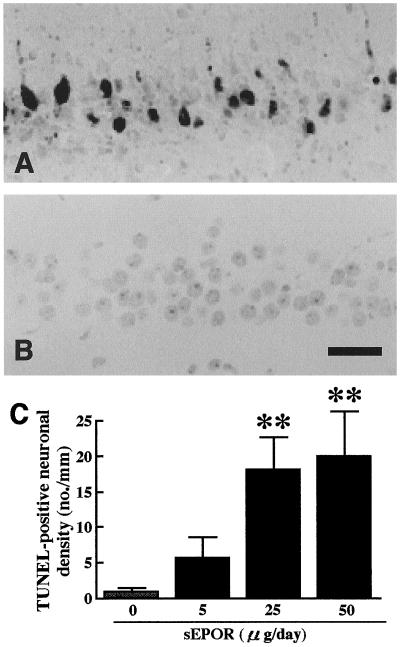
The number of degenerating CA1 neurons after the 7-day infusion of sEPOR into the cerebral ventricles of 2.5-min ischemic gerbils, was examined by TUNEL staining. sEPOR (25 or 50 μg/day) infusion in 2.5-min ischemic gerbils induced DNA fragmentation in the hippocampal CA1 field as observed 1 week after ischemia, whereas the infusion of vehicle or dsEPOR did not result in any staining (Fig. 4 A, B). Thus, TUNEL-positive neurons in the hippocampal CA1 field of sEPOR (25 or 50 μg/day)-infused animals with 2.5-min ischemia were more numerous than those in vehicle- or dsEPOR-infused ischemic gerbils (Fig. 4C) (U = 0, P < 0.01; U = 5.0, P < 0.01). Approximately 12% of CA1 neurons were in the course of degeneration even after the termination of sEPOR (25 μg/day) infusion.
Figure 4.
TUNEL staining of the hippocampal CA1 field of 2.5-min ischemic gerbils after 7 day infusion of sEPOR. (A) An ischemic animal infused with sEPOR at a dose of 25 μg/day; (B) an ischemic animal infused with vehicle. TUNEL-positive neurons were observed only in the ischemic gerbil infused with sEPOR. (C) TUNEL-positive neurons in the hippocampal CA1 field of 2.5-min ischemic animals. The stippled column indicates vehicle-infused ischemic animals and solid columns indicate sEPOR-infused animals. Each value represents mean ± SE (n = 6–8). **P < 0.01, significantly different from the corresponding vehicle-infused ischemic group (statistical significance tested by the two-tailed Mann–Whitney U test). (Bar = 0.1 mm.)
Neuroprotective Effect of EPO on Cultured Neurons.
For better understanding of the molecular mechanism underlying the neuroprotective action of EPO, we further investigated effect of EPO on cultured neurons. A massive increase of intracellular Ca2+ concentration evoked by glutamate-induced NMDA receptor activation plays a critical role in triggering intracellular events that elicit cell destruction (30–32). EPO protects primary cultured neurons from NMDA receptor-mediated glutamate toxicity (6). EPO may exhibit its neuroprotective action by repressing glutamate-induced increase in Ca2+ concentration. Glutamate stimulated the rate of Ca2+ uptake when compared with the rate in the presence of MK801 (a potent NMDA receptor antagonist), but EPO did not reduce glutamate-mediated stimulation (data not shown). Likewise, glutamate induced an increase in intracellular Ca2+ concentration but EPO failed to repress this increase.
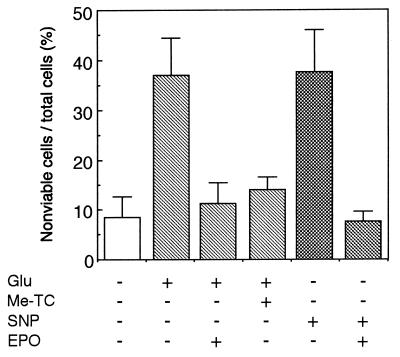
As reported (6), EPO protected neurons from glutamate-induced death (Fig. 5). Me-TC, an inhibitor of NO synthase (33), also neutralized the glutamate toxicity almost completely, suggesting that the glutamate toxicity is mediated by NO. We examined whether or not EPO could rescue neurons from NO toxicity by using SNP (34), a NO-generating agent. Incubation of neurons with SNP caused significant neuronal loss, but pretreatment of neurons with EPO resulted in almost complete survival of the neurons (Fig. 5). In contrast, EPO did not protect SNP-induced neuron death when added to the culture medium simultaneously with SNP (data not shown).
Figure 5.
EPO protects cultured hippocampal neurons from cell toxicity of SNP-derived NO. Neurons were incubated with test materials as described in the Materials and Methods. Glu, glutamate; Me-TC, a NO synthase inhibitor. Total and nonviable cell number was counted. Each value is the mean ± SD of triplicate experiments.
DISCUSSION
The present study demonstrated that the cerebroventricular infusion of EPO (2.5–25 units/day) prevents, in a dose-dependent manner, the ischemia-induced learning disability and rescues hippocampal CA1 neurons from lethal ischemic damage. The neuroprotective action of EPO was confirmed by the large number of synapses in the hippocampal CA1 region. However, EPO at a dose of 50 units/day or 500 units/day was ineffective in ameliorating ischemic neuronal damage. This suggests that hippocampal neurons with EPOR respond to EPO within a limited concentration range in vivo and that high concentrations of EPO induce a rapid down-regulation of EPOR, failing to transmit EPO-mediated signals to the neurons. Previous studies dealing with the neurotrophic actions of basic fibroblast growth factor, interleukins, tumor necrosis factor, and prosaposin also showed narrow effective concentration ranges (35–39), although the molecular basis has not been clarified.
In 2.5-min ischemic gerbils given vehicle or dsEPOR infusion, there were no detectable changes in the hippocampal CA1 region under light microscopy. In contrast, the continuous cerebroventricular infusion of sEPOR in 2.5-min ischemic gerbils caused a significant reduction in learning ability and neuronal loss in the hippocampal CA1 field. These findings indicate that the infused sEPOR forms a complex with endogenous brain EPO as expected from our previous in vitro studies (11, 24), thereby inhibiting the binding of brain EPO with neuronal EPOR, and that brain EPO functions as a trophic agent essential for neuronal survival when the brain is loaded with a sublethal ischemic insult. The neuroprotective action of brain EPO was further reinforced by the subsequent TUNEL-staining experiments that showed a significant number of TUNEL-positive neurons in the hippocampal CA1 field of sEPOR-infused but not vehicle- or dsEPOR-infused gerbils with 2.5-min ischemia. Thus, brain EPO plays a pivotal role in the maintenance and/or recovery of neuronal viability during and after nonlethal brain ischemia. Astrocytes produce EPO in a hypoxia-inducible manner (11, 12).
A glutamate-mediated increase in intracellular Ca2+ concentration activates neuronal NO synthase that requires Ca2+-calmodulin complex (34), and the increased NO mediates in vitro glutamate neurotoxicity (40). This occurs also in vivo, because the intracerebroventricular infusion of NO synthase inhibitors has been shown to rescue ischemic hippocampal CA1 neurons and mice deficient in neuronal NO synthase are tolerant to brain ischemia (41–44). The present in vitro experiments demonstrated that EPO prevents SNP (a NO-generating agent)-induced neuronal death, but it does not block NMDA receptor-mediated Ca2+ influx into neurons, indicating that suppression of NO toxicity but not intracellular calcium increase is involved in the neuroprotective action of EPO. The simultaneous application of EPO and SNP in the culture medium did not attenuate SNP-induced neuronal degeneration, in agreement with our previous finding (6) that de novo syntheses of protein and RNA are required for EPO to exert its neuroprotective action. Thus EPO, like platelet-derived growth factor (45), may increase the activities of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase and catalase in neurons. Further studies of neuronal gene expression up-regulated by EPO treatment are necessary.
Homozygous mice carrying null mutations in the EPO or EPOR genes died around embryonic day 13 due to deficiency in erythropoiesis in the fetal liver (46, 47), providing no information on the possible functions of EPO in adults. The use of a soluble receptor, as in the present study, would be useful to find a function of the ligand, when disruption of the ligand or its receptor gene results in fetal death.
Acknowledgments
This project was supported by grants-in-aids from the Ministry of Education, Science, Sports and Culture of Japan, by Kato Memorial Bioscience Foundation to S. Masuda, and by Snow Brand Milk Products Co., Ltd.
ABBREVIATIONS
- EPO
erythropoietin
- NMDA
N-methyl-d-aspartate
- EPOR
EPO receptor
- sEPOR
soluble EPOR (an extracellular domain of EPOR)
- dsEPOR
denatured sEPOR
- Me-TC
S-methyl-l-thiocitrulline
- SNP
sodium nitroprusside
- TUNEL
terminal dinucleotidyltransferase-mediated UTP end labeling
References
- 1.Bunn H F, Poyton R O. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 2.Krantz S B. Blood. 1991;77:419–434. [PubMed] [Google Scholar]
- 3.Jelkmann W. Physiol Rev. 1992;72:449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- 4.Youssoufian H, Longmore G, Neumann D, Yoshimura A, Lodish H F. Blood. 1993;81:2223–2236. [PubMed] [Google Scholar]
- 5.Nagao M, Masuda S, Ueda M, Sasaki R. Cytotechnology. 1995;18:83–91. doi: 10.1007/BF00744323. [DOI] [PubMed] [Google Scholar]
- 6.Morishita E, Masuda M, Nagao M, Yasuda Y, Sasaki R. Neuroscience. 1997;76:105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 7.Masuda S, Chikuma M, Sasaki R. Brain Res. 1997;746:63–70. doi: 10.1016/s0006-8993(96)01186-9. [DOI] [PubMed] [Google Scholar]
- 8.Masuda S, Nagao M, Takahata K, Konishi Y, Gallyas F, Jr, Tabira T, Sasaki R. J Biol Chem. 1993;268:11208–11216. [PubMed] [Google Scholar]
- 9.Morishita E, Narita H, Nishida M, Kawashima N, Yamagishi K, Masuda S, Nagao M, Hatta H, Sasaki R. Blood. 1996;88:465–471. [PubMed] [Google Scholar]
- 10.Digicaylioglu M, Bichet S, Marti H H, Wenger R H, Rivas L A, Bauer C, Gassmann M. Proc Natl Acad Sci USA. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda S, Okano M, Yamagishi K, Nagao M, Ueda M, Sasaki R. J Biol Chem. 1994;269:19488–19493. [PubMed] [Google Scholar]
- 12.Marti H H, Wenger R H, Rivas L A, Straumann U, Digicaylioglu M, Henn V, Yonekawa Y, Bauer C, Gassmann M. Eur J Neurosci. 1996;8:666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 13.Tan C C, Eckardt K U, Firth J D, Ratcliffe P J. Am J Physiol. 1992;263:F474–F481. doi: 10.1152/ajprenal.1992.263.3.F474. [DOI] [PubMed] [Google Scholar]
- 14.Choi D W. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 15.Choi D W, Rothman S M. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 16.Thiessen D, Yahr P. A Stereo-Taxic Brain Atlas of the Gerbil. Austin, TX: University of Texas; 1977. p. 138. [Google Scholar]
- 17.Goto M, Akai K, Murakami A, Hashimoto C, Tsuda E, Ueda M, Kawanishi G, Takahashi N, Ishimoto A, Chiba H, Sasaki R. Bio/Technology. 1988;6:67–71. [Google Scholar]
- 18.Mitani A, Andou Y, Matsuda S, Kataoka K. Neurosci Lett. 1991;131:171–174. doi: 10.1016/0304-3940(91)90606-t. [DOI] [PubMed] [Google Scholar]
- 19.Sano A, Matsuda S, Wen T-C, Kotani Y, Kondoh K, Ueno S, Kakimoto Y, Yoshimura H, Sakanaka M. Biochem Biophys Res Commun. 1994;204:994–1000. doi: 10.1006/bbrc.1994.2558. [DOI] [PubMed] [Google Scholar]
- 20.Wen T-C, Matsuda S, Yoshimura H, Aburaya J, Kushihata F, Sakanaka M. Neuroscience. 1995;65:513–521. doi: 10.1016/0306-4522(94)00499-u. [DOI] [PubMed] [Google Scholar]
- 21.Wen T-C, Matsuda S, Yoshimura H, Kawabe T, Sakanaka M. Neurosci Lett. 1995;191:55–58. doi: 10.1016/0304-3940(95)11574-8. [DOI] [PubMed] [Google Scholar]
- 22.Kotani Y, Matsuda S, Wen T-C, Sakanaka M, Tanaka J, Maeda N, Kondon K, Ueno S, Sano A. J Neurochem. 1996;66:2197–2200. doi: 10.1046/j.1471-4159.1996.66052197.x. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda S, Wen T-C, Morita F, Otsuka H, Igase K, Yoshimura H, Sakanaka M. Neurosci Lett. 1996;204:109–112. doi: 10.1016/0304-3940(96)12340-5. [DOI] [PubMed] [Google Scholar]
- 24.Nagao M, Masuda S, Abe S, Ueda M, Sasaki R. Biochem Biophys Res Commun. 1992;188:888–897. doi: 10.1016/0006-291x(92)91139-h. [DOI] [PubMed] [Google Scholar]
- 25.Wen T-C, Yoshimura H, Matsuda S, Lim J-H, Sakanaka M. Acta Neuropathol. 1996;91:15–22. doi: 10.1007/s004010050387. [DOI] [PubMed] [Google Scholar]
- 26.Araki H, Nojiri M, Kawashima K, Kimura M, Aihara H. Physiol Behav. 1986;38:89–94. doi: 10.1016/0031-9384(86)90136-8. [DOI] [PubMed] [Google Scholar]
- 27.Lim J-H, Wen T-C, Matsuda S, Tanaka J, Maeda N, Peng H, Aburaya J, Ishihara K, Sakanaka M. Neurosci Res. 1997;28:191–200. doi: 10.1016/s0168-0102(97)00041-2. [DOI] [PubMed] [Google Scholar]
- 28.Artalejo C R, Garcia A G, Aunis D. J Biol Chem. 1987;262:915–926. [PubMed] [Google Scholar]
- 29.Katoh H, Watabe A, Sugimoto Y, Ichikawa A, Negishi M. Biochim Biophys Acta. 1995;1244:41–48. doi: 10.1016/0304-4165(94)00182-w. [DOI] [PubMed] [Google Scholar]
- 30.Choi D W. Neurosci Lett. 1985;58:293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- 31.Choi D W. J Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siesjo B K. Ann NY Acad Sci. 1988;522:638–661. doi: 10.1111/j.1749-6632.1988.tb33410.x. [DOI] [PubMed] [Google Scholar]
- 33.Furfine E S, Harmon M F, Paith J E, Knowles R G, Salter M, Kiff R J, Duffy C, Hazelwood R, Oplinger J A, Garvey E P. J Biol Chem. 1994;269:26677–26683. [PubMed] [Google Scholar]
- 34.Dawson V L, Dawson T M, London E D, Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison R S, Sharma A, deVellis J, Bradshaw R A. Proc Natl Acad Sci USA. 1986;83:7537–7541. doi: 10.1073/pnas.83.19.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rousselet A, Fetler L, Chamak B, Prochiantz A. Dev Biol. 1988;129:495–504. doi: 10.1016/0012-1606(88)90395-8. [DOI] [PubMed] [Google Scholar]
- 37.Araujo D M, Cotman C W. Soc Neurosci Abstr. 1991;17:1199. [Google Scholar]
- 38.Araujo D M, Cotman C W. Brain Res. 1993;600:49–55. doi: 10.1016/0006-8993(93)90400-h. [DOI] [PubMed] [Google Scholar]
- 39.Kotani Y, Matsuda S, Sakanaka M, Kondoh K, Ueno S, Sano A. J Neurochem. 1996;66:2019–2025. doi: 10.1046/j.1471-4159.1996.66052019.x. [DOI] [PubMed] [Google Scholar]
- 40.Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buisson A, Margaill I, Callebert J, Plotkine M, Boulu R G. J Neurochem. 1993;61:690–696. doi: 10.1111/j.1471-4159.1993.tb02174.x. [DOI] [PubMed] [Google Scholar]
- 42.Caldwell M, O’Neill M, Earley B, Leonard B. Eur J Pharmacol. 1994;260:191–200. doi: 10.1016/0014-2999(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 43.Huang Z, Huang P L, Panahian N, Dalkara T, Fishman M C, Moskowitz M A. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 44.Kohno K, Ohta S, Kohno K, Kumon Y, Mitani A, Sakaki S, Kataoka K. Brain Res. 1996;738:275–280. doi: 10.1016/s0006-8993(96)00794-9. [DOI] [PubMed] [Google Scholar]
- 45.Cheng B, Mattson M P. J Neurosci. 1995;15:7095–7104. doi: 10.1523/JNEUROSCI.15-11-07095.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H, Liu X, Jaenisch R, Lodish H F. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 47.Lin C-S, Lim S-K, D’Agati V, Costantini F. Genes Dev. 1996;10:154–164. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]