Abstract
Most cold-sensitive subnucleus caudalis (Vc) neurons are also excited by the TRPM8 agonist menthol and the TRPA1 agonist cinnamaldehyde (CA). We investigated how interactions among menthol, CA and noxious cooling and heating of the tongue affected responses of superficial Vc units recorded in thiopental-anesthetized rats. Units responded to 1% CA which enhanced cold- and heat-evoked responses 5 min later. They responded more strongly to 10% CA which initially depressed cold responses, followed by enhancement at 5 min without affecting responses to heat. Following 10% CA, the mean response to 1% menthol was significantly lower than when menthol was tested first. After menthol, the subsequent response to CA was significantly weaker compared to the mean CA-evoked response when it was tested first. These results demonstrate mutual cross-desensitization between CA and menthol. The response to CA was enhanced following prior application of 10% ethanol (menthol vehicle). Prior application of menthol did not prevent the biphasic effect of 10% CA on cold-evoked responses, nor did prior application of CA prevent menthol enhancement of cold-evoked responses. Responses to noxious heat were unaffected by 10% CA and menthol regardless of the order of chemical presentation. These data indicate that superficial Vc neurons receive convergent input from primary afferents expressing TRPM8 and TRPA1. The mutual cross-desensitization between CA and menthol, and differential modulation of cold- vs. heat-evoked responses, suggests a direct inhibition of TRPM8 and TRPA1 expressed in peripheral nerve endings by CA and menthol, respectively, rather than a central site of interaction.
Keywords: menthol, cinnamaldehyde, TRPM8, TRPA1, cold, trigeminal
Introduction
Cinnamon and mint are food spices often used in oral hygiene products. Cinnamaldehyde, the pungent chemical in cinnamon oil, acts at the transient receptor potential (TRP) ion channel TRPA1 [2–4, 13, 24] whereas menthol extracted from mint leaves acts at TRPM8 [18, 21]. TRPM8 is a transducer of cool to cold temperatures [18, 21] and knockout mice lacking TRPM8 exhibit reduced sensitivity to cool environmental temperatures [5, 9, 12]. Menthol also elicits irritation at higher concentrations [8, 10] suggesting an additional role for TRPM8 in chemesthesis. TRPA1 was originally reported to respond to cold temperatures at a higher threshold (i.e., colder temperatures) compared to TRPM8 [24], and knockout mice lacking TRPA1 have been reported by one group [16] to exhibit a reduced behavioral sensitivity to intense cooling although this has not been confirmed [3]. We recently reported [26] that oral application of menthol excites cold-sensitive neurons in the trigeminal subnucleus caudalis (Vc), and that most cold- and menthol-responsive neurons were also excited by cinnamaldehyde. In the present study, we have further investigated the interactions between menthol and cinnamaldehyde as well as their effects on responses of Vc neurons to thermal stimuli.
Materials and Methods
Under a protocol approved by the University of California, Davis Institutional Animal Care and Use Committee, 14 adult male Sprague-Dawley rats (454–570 g), were anesthetized with sodium thiopental (induction: 85 mg/kg ip; maintenance: 10 mg · kg-1 · h-1 iv) and prepared for single-unit recording as described previously [26]. We isolated single units in superficial laminae of dorsomedial Vc that responded to cold and noxious heat stimuli delivered to the anterior tongue by a feedback-controlled Peltier thermode (Physitemp NTE-2A, 13 mm diameter). The thermode was programmed by computer to cool the tongue over a 45 sec period, thereby reaching a thermode-tongue interface temperature of ~0°C, and to heat the tongue to 53°C over a period of 13 sec. Chemical stimuli were delivered to the anterior tongue by continuous superfusion for 1 min at a rate of ~0.5 ml/min. Cinnamaldehyde (CA; Sigma-Aldrich Chemical Co., St. Louis MO) was mixed with water and 5% Tween-80 (Sigma) to a final concentration of 1% (76 mM) or 10% (760 mM). A 1% (64 mM) concentration of menthol (Givaudan, Cincinnati OH) was made by dissolving it in 10% ethanol and 1% Tween-80. A 10% (2.17 M) concentration of ethanol (Sigma) was made by dissolving it in distilled water and 1% Tween-80.
To investigate responses to different concentrations of CA, and effects of CA on thermal responses, the stimulus sequence shown in Fig. 1 was followed. After first recording spontaneous activity for 1 min, the cold stimulus was delivered followed 2 min later by heat. CA (1%) was then superfused for one min, followed by rinse with 0.9% saline. The cooling and heating sequence was then applied 2 times starting 1 min post-rinse. At least 22 min following the end of the 1% CA infusion, 10% CA was applied in the same manner, followed by the thermal stimulus sequence as before.
Fig. 1.
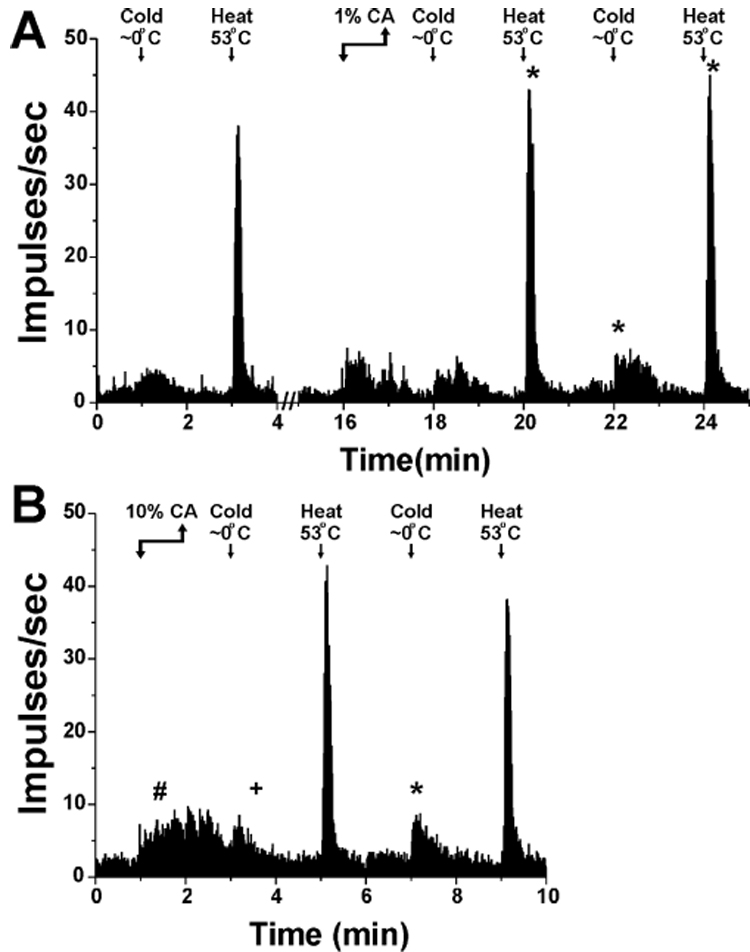
Averaged responses of 6 Vc units to thermal stimulation and 1% (A) or 10% (B) CA. The response to 10% CA was significantly larger than to 1% CA (#, p<0.001). The cold responses at 5 min post-1% and 10% CA and the heat responses at 3 and 7 min post-10% CA were significantly larger than pre-CA (*, p<0.05), however the cold response at 1 min post-10% CA was significantly smaller that the pre-CA response (+, p<0.001).
To investigate interactions between CA and menthol, the stimulus sequences illustrated in Fig. 2 were followed. CA was delivered first (not shown), and this response was used for comparison with the response to CA when it was applied after ethanol (Fig. 2A) or menthol (Fig. 2B). A second stimulus sequence was delivered in which ethanol (menthol control) was applied, followed five min later by CA. A third stimulus sequence was delivered in which menthol preceded CA to test the effects of menthol on CA-evoked responses. In the final sequence, the order of CA and menthol application was reversed to determine the effect of CA application on menthol-evoked responses. The effect of ethanol on menthol-evoked responses was not investigated presently since we previously showed that 10% ethanol did not affect responses of Vc neurons to menthol [23]. In each sequence, cold and heat stimuli were delivered before and after chemical stimuli to assess chemical modulation of thermally evoked responses. The animals were allowed to recover for a minimum of 20 minutes between chemical stimulus sequences. The responses to the first and final 10% CA applications were compared to test for CA self-desensitization. In addition, the responses to all cold and heat stimuli delivered immediately prior to each round of chemical stimulation were compared over the course of the recording to determine whether thermal responses were stable over time.
Fig. 2.
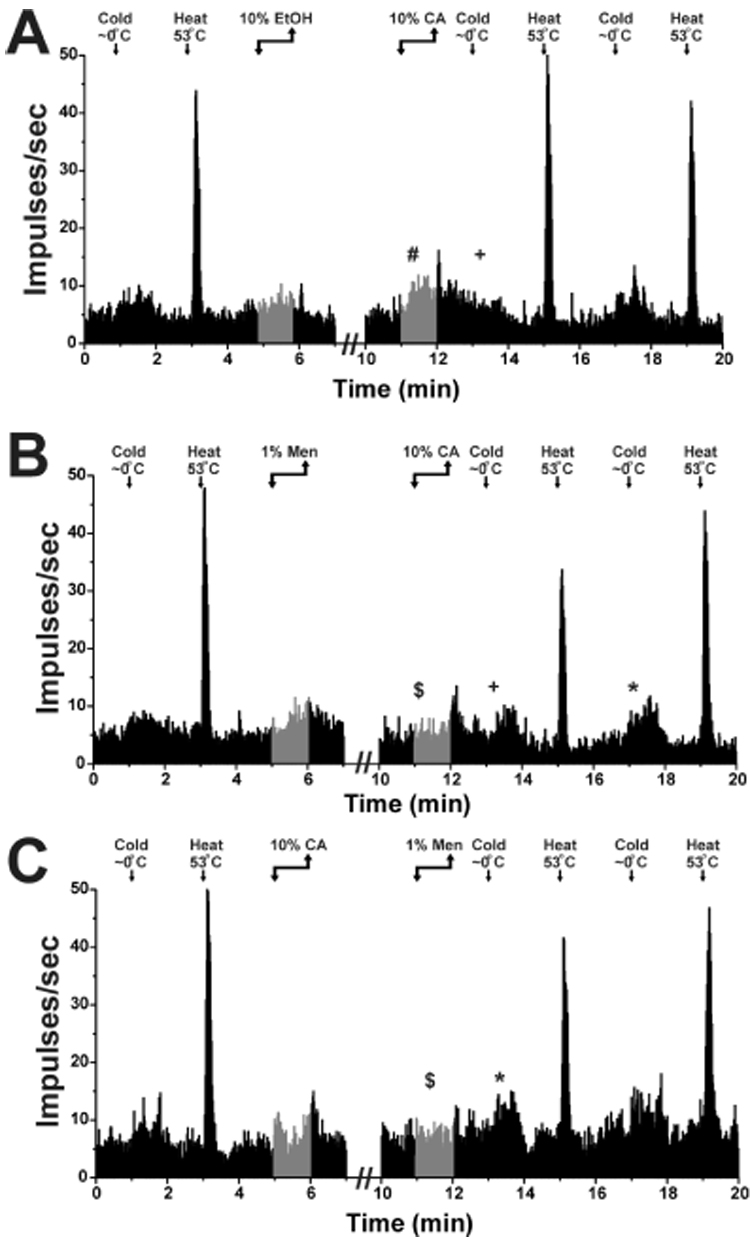
Averaged responses of 8 Vc units to thermal stimuli before and after chemical application.
A: Application of 10% EtOH followed 5 min later by 10% CA. Ethanol did not elicit a significant response (gray portion of PSTH). The CA response was significantly increased following EtOH (#, p<0.05). The cold response was significantly decreased at 1 min post-CA (+, p<0.001) as compared to pre-ethanol. There were no other significant changes in the thermal responses.
B: Application of 1% menthol followed 5 min later by 10% CA. The CA response post-menthol was significantly reduced compared to CA alone ($, p<0.001). The cold responses were similar to those following CA alone. The response at 1 min post-CA was significantly decreased (+, p<0.01) while the response at 5 min post-CA was significantly enhanced (*, p<0.05) as compared to the response pre-menthol. The heat responses were not significantly different.
C: Application of 10% CA followed 5 min later by 1% menthol. The menthol response post-CA was significantly reduced compared to menthol prior to CA ($, p<0.01; compare gray portions of PSTHs for menthol in A and B). Unlike following CA, the cold response was significantly enhanced at 1 min post-menthol (*, p<0.05), but not significantly different at 5 min post-menthol as compared to pre-CA. The heat responses did not change significantly.
Responses to each thermal or chemical stimulus were quantified by counting the number of action potentials elicited during the 1 min period of stimulus application, and subtracting spontaneous activity during the 1-min period immediately prior to stimulation. Paired t-tests were used to test whether a given response was significantly different following the conditioning stimulus, or in comparison to pre-stimulus spontaneous activity. A repeated measures ANOVA was used to analyze the stability of the thermal responses over time. Data are reported as group averages plus or minus standard error and a p-value of <0.05 was considered significant.
Results
Units were recorded at a mean depth of 107 +/− 33 (SEM) µm and were histologically localized to the superficial dorsomedial Vc as in previous studies [6, 7, 11, 26]. They all responded to noxious cooling and heating of the tongue (Fig. 1A).
Response to CA
Vc units exhibited a concentration-dependent increase in firing to lingual CA. Application of 1% CA significantly increased mean firing above the spontaneous firing rate (from 77.7 spikes/60 sec ± 29.1 SD to 223.2 spikes/60 sec ± 25.0, n= 6, Fig 1A), as did 10% CA when applied at least 22 min later (from 133.8 spikes/60 sec ±61.2 to 361.3 spikes/60 sec ±79.5; Fig. 1B). The mean response to 10% CA was significantly greater than to 1% CA (p < 0.001).
CA modulation of cold-evoked reponses
Vc units exhibited a significant increase in firing during cooling (from 106.6 spikes/60 sec ±29.6 to 171.9 spikes/60 sec ±48.4, p<0.001). The cold-evoked response 1 min after 1% CA was not significantly different from the pre-CA cold-evoked response, but was significantly larger 5 min after 1% CA (Fig. 1A; p<0.001). One min after 10% CA, the cold-evoked response was significantly smaller, and 5 min after 10% CA it was significantly larger (p < 0.001 for both), compared to the pre-CA cold response (Fig. 1B).
CA modulation of heat-evoked responses
The same units also exhibited a significant response to noxious heat (from 79.8 spikes/60 sec ±25.2 SD to 438.2 spikes/60 sec ±49.2, Fig 1A). Heat-evoked responses were significantly larger 3 min (p < 0.01) and 7 min (p < 0.05) following 1% CA compared to the pre-CA response (Fig. 1A). The mean heat-evoked response following 10% CA was numerically larger compared to pre-CA although this did not reach statistical significance (Fig. 1B).
Ethanol control
Ethanol did not significantly affect mean firing (Fig. 2A, left-hand PSTH; gray bars). Application of 10% CA post-ethanol elicited an increase in firing (Fig. 2A, right-hand PSTH, gray bars; 215.6 spikes/60 sec ±155.9 SD) that was significantly greater (p < 0.02) compared to the response of the same units to 10% CA delivered prior to 10% ethanol (data not shown; 143.1 spikes/60 sec ±46.7 SD). This indicates that 10% ethanol enhanced responses to CA. The cold-evoked response recorded 1 min after CA following ethanol was significantly reduced (p<0.001), similar to the effect observed when CA was delivered alone (Fig. 1B). Five min after CA, the cold-evoked response had recovered and was not significantly different from the cold-evoked response prior to ethanol (Fig. 2A). Mean responses to noxious heat were not significantly affected (Fig. 2A).
Cross-desensitization between CA and menthol
When menthol was applied prior to CA, it elicited a significant (p < 0.001) increase in firing (Fig. 2B, left-hand PSTH, gray bars). Subsequent application of CA elicited little or no increase in firing (Fig. 2B, right-hand PSTH, gray bars), such that the response to CA post-menthol (54.0 spikes/60 sec ±34.3 SD) was significantly smaller (p < 0.001; n= 8) compared to the response to CA applied just prior to menthol (143.1 spikes/60 sec ±46.7; Fig. 2A). The response to CA applied before menthol (final stimulus sequence) was not significantly different from the first CA response (p<0.2). This is consistent with menthol cross-desensitization of Vc responses to CA rather than tachyphylaxis from repeated CA applications.
Immediately after menthol followed by CA, the mean cold-evoked response was significantly lower compared to pre-menthol (pre-menthol: 138.2 spikes/60 sec ±41.9 SD; post-CA: 65.5 spikes/60 sec ±74.4, p<0.02). However, 5 min after CA the mean cold-evoked response was significantly larger (204.3 spikes/60 sec ±116.3, p<0.001) compared to pre-menthol. There was no significant change in the mean heat-evoked response after menthol and CA (Fig. 2B).
Application of 10% CA evoked a significant increase in firing (Fig. 2C, left-hand PSTH, gray bars). When menthol was applied 5 min later, it did not elicit a significant change in firing (Fig. 2C, right-hand PSTH, gray bars). The response to menthol post-CA was significantly lower (Fig. 2C; $; p < 0.002) compared to the mean response to menthol when it was applied prior to CA (Fig. 2B; left-hand PSTH; gray bars; (112.4 spikes/60 sec ±51.9 pre-CA vs. 28.0 spikes/60sec ±34.5 5 min post-CA). This is consistent with CA cross-desensitization of Vc responses to menthol.
After CA followed by menthol, the cold-evoked response was significantly larger (Fig. 2C, *; p < 0.05) compared to the pre-CA cold-evoked response (pre-CA: 158.2 spikes/60 sec ±67.2 SD; post-CA and menthol: 221.4 spikes/60 sec ±71.1) consistent with our previous study (Zanotto et al., 2007). The subsequent response to cold, recorded 5 min post-menthol, was numerically larger, but not significantly different compared to the pre-CA cold-evoked response. There was no significant change in the heat-evoked responses following CA and menthol (Fig. 2C).
The thermal stimulus sequence was always applied immediately prior to each successive round of chemical stimulation (left-hand responses to cold and heat in Figs. 2A–C). There were no significant differences in the cold- or heat-evoked responses preceding each chemical stimulus when compared over the course of the experiment.
Discussion
The broadly tuned thermal and chemical responsiveness of the present superficial Vc units is consistent with our previous studies [6, 7, 11, 26]. We presently focused on Vc neuronal responses to CA and menthol, respective agonists of TRPA1 and TRPM8. The Vc units were excited in a concentration-dependent manner by CA, which reduced the immediately following response to noxious cold (Fig. 1B). This is consistent with human psychophysical data showing cold hypoalgesia following cutaneous application of 10% (0.8 M) CA [20]. The mechanism for this is not known but may speculatively involve CA desensitization of cold gating of TRPA1 [24] expressed in cold-sensitive nociceptors. Interestingly, the second cold stimulus following CA was enhanced suggesting that activation of TRPA1 can lead to delayed cold hyperalgesia. This is consistent with our recent observation of a weak enhancement of cold pain following application of CA (0.2%; 16 mM) to the tongue in human subjects [1].
Our data indicate lack of self-desensitization or tachyphylaxis at an interstimulus interval of greater than 20 minutes. We previously observed that repeated application of CA at a shorter (5 min) interstimulus interval did result in significant self-desensitization [7 and authors’ unpublished observations]. These data are consistent with human psychophysical data showing self-desensitization of CA-evoked oral irritation lasting at least 10 min [22].
Following 1% (but not 10%) CA, responses to noxious heat were enhanced (Fig. 1A), consistent with our recent finding that CA enhanced the perceived intensity of lingual heat pain [1]. The mechanism might speculatively involve a peripheral interaction between TRPA1 and TRPV1 co-expressed in thermal nociceptors, or central sensitization of Vc neurons. The latter is supported by the enhanced responses to both noxious cold and heat after 1% CA (Fig. 1A), but is mitigated by the lack of significant enhancement of heat responses after 10% CA (Fig. 1B).
Menthol excited cold-sensitive Vc neurons consistent with our recent report [26]. Vc responses to a second application of menthol were reduced indicating self-desensitization [26]. Moreover, as shown here, menthol cross-desensitized Vc responses to CA. Likewise, CA cross-desensitized responses to menthol. The mutual cross-desensitization is unlikely to involve a direct interaction between TRPM8 and TRPA1 since they do not appear to be co-expressed in DRG or trigeminal ganglion cells [15, 19]. It also does not appear to involve central inhibition since cold-evoked responses were enhanced, rather than depressed, following CA and menthol regardless of the order in which they were applied. One plausible explanation comes from a recent observation that CA inhibits TRPM8 [17], providing a peripheral site at which CA might reduce menthol activation of TRPM8-expressing primary afferents projecting to Vc. However, the cold-evoked response immediately post-menthol was significantly enhanced (Fig. 2C) as we recently reported [26]. Therefore, although prior CA may inhibit gating of TRPM8 by menthol, this is apparently not sufficient to prevent menthol enhancement of the response to cooling. Similarly, the recent demonstration that menthol inhibits TRPA1 [17] might explain menthol cross-desensitization of Vc responses to CA, via a peripheral depression of CA activation of TRPA1-expressing nociceptors.
The menthol vehicle, 10% ethanol, did not excite Vc units consistent with our recent report [26]. Unexpectedly, Vc unit responses to 10% CA were enhanced following ethanol (Fig. 2A). Since ethanol was reported to excite TRPV1 [25], a possible explanation for our result is that the ethanol acted through TRPV1 to enhance CA activation of TRPA1 co-expressed in the same nociceptive lingual nerve endings by an as yet unknown cellular interaction.
Acknowledgments
This study was supported by grants from the National Institute of Dental Research (DE-013685) and California Tobacco-Related Disease Research Program (11RT-0053).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albin K, Iodi Carstens M M, Carstens E. Modulation of oral heat and cold pain by irritant chemicals. Chem. Sens. 2007 doi: 10.1093/chemse/bjm056. in press. [DOI] [PubMed] [Google Scholar]
- 2.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 3.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 6.Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by application of different classes of irritant chemicals to the oral and ocular mucosa. J. Neurophysiol. 1998;80:465–492. doi: 10.1152/jn.1998.80.2.465. [DOI] [PubMed] [Google Scholar]
- 7.Carstens E, Mitsuyo T. Neural correlates of oral irritation by mustard oil and other pungent chemicals: a hot topic. Chem Senses Suppl. 2005;1:i203–i204. doi: 10.1093/chemse/bjh185. [DOI] [PubMed] [Google Scholar]
- 8.Cliff MA, Green BG. Sensory irritation and coolness produced by menthol: evidence for selective desensitization of irritation. Physiol Behav. 1994;56:1021–1029. doi: 10.1016/0031-9384(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 9.Colburn RW, Lubin ML, Stone DJ, Jr., Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Dessirier J-M, O'Mahony M, Carstens E. Oral irritant properties of menthol: sensitizing and desensitizing effects of repeated application and cross-desensitization to nicotine. Physiol Behav. 2001;73:25–36. doi: 10.1016/s0031-9384(01)00431-0. [DOI] [PubMed] [Google Scholar]
- 11.Dessirier J-M, Simons CT, Sudo M, Sudo S, Carstens E. Sensitization, desensitization and stimulus-induced recovery (SIR) of responses of rat trigeminal caudalis neurons to repeated oral application of capsaicin and nicotine. J. Neurophysiol. 2000;84:1851–1862. doi: 10.1152/jn.2000.84.4.1851. [DOI] [PubMed] [Google Scholar]
- 12.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 14.Katsura H, Tsuzuki K, Noguchi K, Sakagami M. Differential expression of capsaicin-, menthol-, and mustard oil-sensitive receptors in naive rat geniculate ganglion neurons. Chem Senses. 2006;31:681–688. doi: 10.1093/chemse/bjl009. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 16.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Mizushima T, Obata K, Katsura H, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Mashimo T, Noguchi K. Noxious cold stimulation induces mitogen-activated protein kinase activation in transient receptor potential (TRP) channels TRPA1- and TRPM8-containing small sensory neurons. Neuroscience. 2006;140:1337–1348. doi: 10.1016/j.neuroscience.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 19.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 20.Namer B, Siefert F, Handwerker HO, Maihofner C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport. 2005;16:955–959. doi: 10.1097/00001756-200506210-00015. [DOI] [PubMed] [Google Scholar]
- 21.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Anderson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 22.Prescott J, Swain-Campbell N. Responses to repeated oral irritation by capsaicin, cinnamaldehyde and ethanol in PROP tasters and non-tasters. Chem Senses. 2000;25:239–246. doi: 10.1093/chemse/25.3.239. [DOI] [PubMed] [Google Scholar]
- 23.Simons CT, Iodi Carstens M, Carstens E. Oral irritation by mustard oil: self-desensitization and cross-desensitization with capsaicin. Chem Senses. 2003;28:459–465. doi: 10.1093/chemse/28.6.459. [DOI] [PubMed] [Google Scholar]
- 24.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 25.Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- 26.Zanotto KL, Merrill AW, Iodi Carstens M, Carstens E. Neurons in superficial trigeminal subnucleus caudalis responsive to oral cooling, menthol, and other irritant stimuli. J Neurophysiol. 2007;97:966–978. doi: 10.1152/jn.00996.2006. [DOI] [PubMed] [Google Scholar]


