Abstract
The present study investigated the effects of repeated days of laboratory-based smoking cue exposure on subjective and physiologic cue reactivity. Twenty non-treatment seeking moderate/heavy smokers completed three laboratory sessions approximately 7 days apart, each following a 10-hour nicotine deprivation period. Cue reactivity procedures consisted of a relaxation trial followed by two trials of in vivo cue exposure. Dependent measures included urge to smoke, a withdrawal questionnaire, mean arterial pressure (MAP), and heart rate (HR). A Condition (relaxation vs. cue exposure) by Day (1, 2, or 3) analysis of variance revealed a significant main effect of Condition (greater urge to smoke after cue exposure) but no significant main or interaction effect for Day. Similarly, MAP and HR change scores following cue exposure did not differ across test days. Cue-elicited changes in withdrawal symptoms were only observed on Day 1, but not when the interday interval was covaried. Results suggest that laboratory-based cue-elicited changes in urge to smoke, MAP, and HR are stable over three separate days.
Keywords: cue-exposure, smoking, laboratory, repeated measure, urge, physiology
Exposure to cigarette cues, such as the visual and olfactory stimuli associated with smoking, has been shown to reliably elicit strong subjective urges to smoke and modest physiological responses among smokers (Carter & Tiffany, 1999; Niaura et al., 1988; Sayette et al., 2001). The cigarette cue reactivity paradigm, designed to examine the subjective and physiological responses of smokers in a controlled laboratory setting, has demonstrated utility in eliciting urge to smoke and in predicting relapse (e.g., Waters et al., 2004). Furthermore, laboratory-based smoking cue reactivity is considered a useful endophenotype for testing mechanisms of medications’ effects on urge, exploring and isolating the neurobiology of cigarette craving, and identifying genetic factors relevant to nicotine dependence (Hutchison et al., 2002, 2006).
An important limitation of the cue reactivity paradigm is that investigators have only examined cue-elicited craving during a single exposure session and relied almost exclusively on between-subjects experimental designs for assessing effects of other variables (e.g., medication) on cue reactivity. Determining whether or not cue exposure repeated across multiple separate testing sessions results in sensitization or habituation effects on smokers’ patterns of responding has important implications for experimental designs which use repeated cue exposure under different conditions. The present study used a within-subjects experimental design to investigate the effects of laboratory-based smoking cue exposure repeated over non-consecutive days on measures of cue reactivity. This design was chosen because studies using within-subjects designs to investigate dose-dependent medication effects on cue reactivity would need separate days of testing at separate doses, with washout periods between testing days. It was hypothesized that individuals’ subjective and physiologic responses to cues would remain stable across testing sessions based theoretically on the short length of exposure and empirically on previous work that found no evidence of within-session habituation across up to 12 exposure trials (e.g., Conklin & Tiffany, 2001).
1. Method
1.1 Experimental design
Following a 10-hour overnight nicotine deprivation period, non-treatment seeking smokers underwent a laboratory-based cigarette smoking cue reactivity paradigm on three separate test sessions (the target number of days between sessions = 7; minimum = 3; maximum = 11). The spacing of the sessions was chosen to match the need to allow a washout period in a pharmacotherapy trial with repeated one-day dosing. Some flexibility in the number of days between laboratory sessions was necessary to reasonably accommodate participants’ schedules so as to minimize attrition. The number of days between sessions 1 and 2 ranged from 3 to 11 (mean = 6.2, SD = 1.8); the number of days between sessions 2 and 3 ranged from 3 to 7 (mean = 5.5, SD = 1.5). Carbon monoxide (CO) levels were assessed prior to each session to confirm abstinence (CO < 10 ppm) using the Smokerlyzer® (Bedfont Technical Instruments Ltd.).
1.2 Participants
Twenty non-treatment seeking moderate to heavy smokers, ages 20 to 67 years (M = 50.4; SD = 12.9), were recruited from the community. The sample was 85% male, 80% Caucasian, 10% African-American, and 10% Hispanic. Participants reported smoking a mean of 24 cigarettes per day (SD = 7.9; Range = 15 to 40) for an average of 23 years (SD = 15.3; Range = 2 to 50), and had a mean of 5.2 (SD = 1.5) on the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991).
1.3 Procedure
Each day, participants arrived at approximately 9 a.m. and were seated at a table beside a one-way mirror in a well-ventilated room. A tray with two inverted opaque covers was placed on the table in front of the participant. Under each cover was an open pack of the participant’s preferred brand of cigarettes with one cigarette out of the pack, a lighter, and an ashtray. A blood pressure cuff was attached to the nondominant arm for ongoing assessment of mean arterial pressure and heart rate and participants underwent an 8-minute period of acclimation to the cuff’s inflation/deflation cycle. Next, participants were asked to relax (“sit quietly and do nothing”) for a 4-minute baseline. At the end of the 4-minute baseline, participants completed self-report measures (see below). They were then asked to lift the first cover and look at the smoking cues. After 2 minutes, they were asked to pick up the cigarette, light it without putting it in their mouths, put down the lighter, hold it in one hand in a comfortable manner without smoking it, and look at it. After 2 minutes, the participants extinguished the cigarette, placed the cover over the cues, and completed self-report measures. The participants lifted the second cover and repeated the 4-minute cigarette exposure with a fresh cigarette, then afterwards completed the same battery of measures. Participants were monitored through a one-way mirror throughout the exposure session to ensure that the procedures were followed properly. A neutral cue exposure was not included given previous work indicating no differences in measures between relaxation and a neutral cue condition (Rohsenow et al., 2007).
1.4 Measures
The following subjective and physiologic measures were administered at baseline (i.e., resting period) and after each of the two cue-exposure trials.
1.4.1 Subjective cue reactivity measures
An 11-point Likert scale (0 = “no urge at all” to 10 = “strongest urge you’ve ever had”) assessed urge to smoke (“How much is your urge to smoke right now?”). The Minnesota Nicotine Withdrawal Scale (NWS; Hughes and Hatsukami, 1986), excluding the items insomnia (not appropriate for current state) and desire/craving (per Hughes and Hatsukami 1998 and to clearly separate the constructs of withdrawal and craving), was asked for “right now.” The mean of the six 5-point (0–4) Likert scales was used.
1.4.2 Physiological measures
Heart rate (HR) in beats per minute and mean arterial pressure (MAP) were assessed using a model of a Dinamap adult physiological monitor in STAT mode that sampled every 15 secs. During deflection, it determined systole, MAP, diastole, and HR using an oscillometric method with movement artifacts automatically eliminated. Values were averaged over each 4-minute trial and change scores from baseline were calculated, as is customary for these measures.
1.4.3 Data analytic plan
The study hypotheses for urge and NWS were tested via mixed design analyses of variance (ANOVAs) performed using the general linear model such that Condition was a two level within-subjects factor (Relaxation vs. Combined Smoking Cues), and Day was a three level within-subjects factor (Day 1, 2, or 3). The hypotheses for HR and MAP change scores were tested with one-way analyses by Day, using the general linear model. In addition, to examine the influence of time between test sessions on results, the mean number of days between each session was added as a covariate in secondary analyses. To evaluate whether individual differences in smoking level (i.e., nicotine dependence, number of years as a smoker, or number of cigarettes smoked per day) influenced reactivity to smoking cues, partial correlations were computed between each indicator of smoking level and the mean for each dependent variable across days and trials, while controlling for the corresponding mean of the same dependent measure assessed after relaxation. (For MAP and HR change scores, univariate correlations were used.) These individual differences could not be added to ANOVAs because of the small sample size. Given the study design, the current sample size (n = 20) allowed 53% power to detect a medium effect size (Delta = 0.75) and 94% power to detect a large effect size (Delta = 1.25) at alpha at .05. Analyses were conducted using SPSS 14.0, with partial eta-squared (ηp2) as an index of effect size (medium = .06 to .13, large = .14 or more).
2. Results
2.1 Correlation between smoking cue exposure trials
The mean correlations between the two cue exposure trials were as follows: urge rating, r = .91 (Range = .89 to .94); NWS, r = .99 (Range = .98 to 1.0); HR, r = .72 (Range = .65 to .80); and MAP r = .64 (Range = .40 to .83). Therefore, the two smoking cue trials were combined for all analyses.
2.2 Effects of repeated cue exposure on subjective and physiologic measures
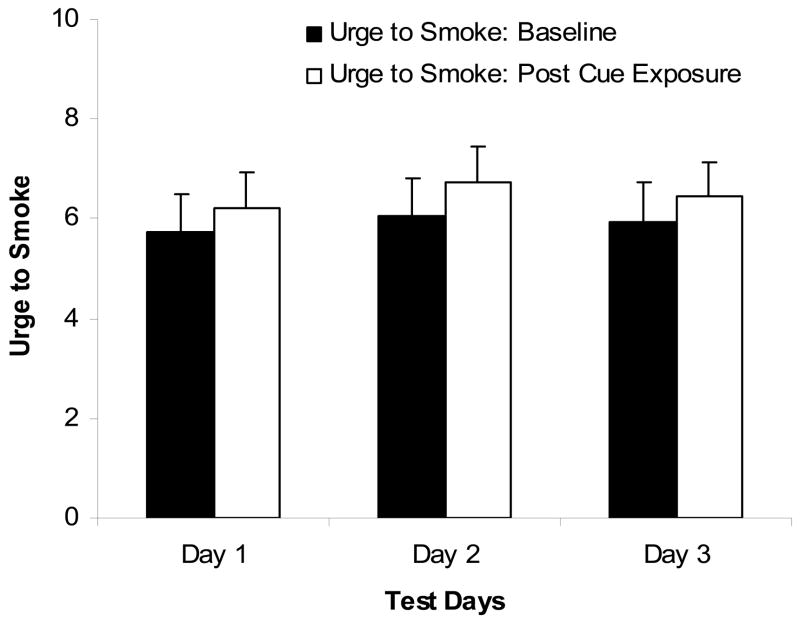
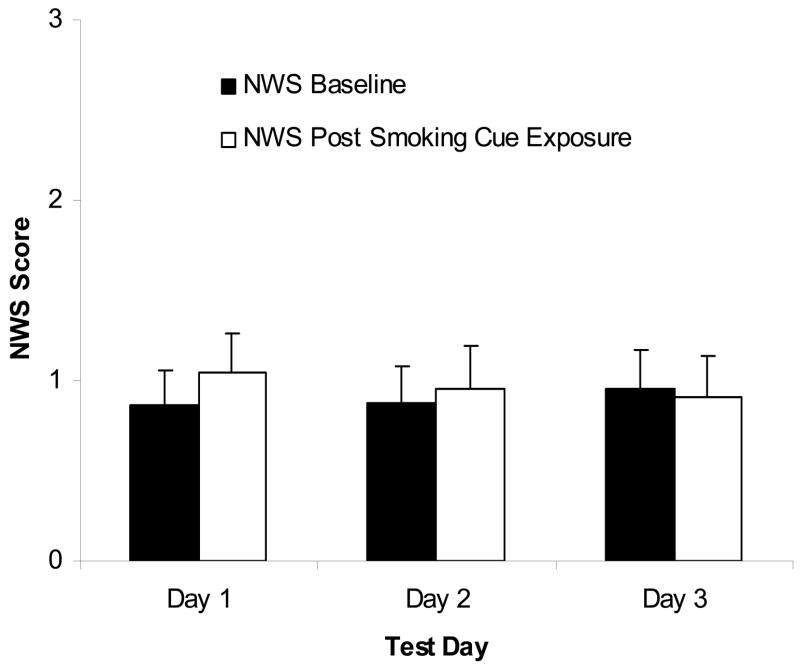
A main effect of Condition was found on urge rating: urge ratings were higher following cigarette cue exposure as compared to the relaxation period, F(1,19) = 5.21, p < .05, ηp2 = .22 (see Figure 1). There was no significant main effect of Day, F(2,18) = 1.0, ηp2 = .05, nor a Day × Condition interaction, F(2,18) = .24, ηp2 = .01, on urge rating. No significant difference in HR and MAP change scores was found across days, F(2,18) = .17, ηp2 = .08, and F(2,18) = 1.05, ηp2 = .22, respectively. Analysis of NWS showed no significant main effect of Condition, F(1,19) = 1.39, ηp2 = .07, or Day, F(2,18) = .06, ηp2 = .003, but there was a significant Condition × Day interaction, F(2,18) =5.26, p < .05, ηp2 = .22. Simple effects tests showed that NWS scores were significantly (p < .05) higher following cue exposure as compared to the relaxation period on day 1 (t = 2.15, df = 19), but not on days 2 or 3 (See Figure 2).
Figure 1.
Mean urge to smoke rating (range 0 to 11; with standard errors) for the baseline and post-cue conditions across the 3 days of repeated testing. Participants reported greater urge to smoke after cue-exposure than baseline with no significant main effect of Test Day or Day × Condition interaction.
Figure 2.
Mean smoking withdrawal rating (range 0 to 4; with standard errors) for the baseline and post-cue conditions across the 3 days of repeated testing. Participants reported greater withdrawal after cue-exposure than baseline on Day1 only. NWS = Minnesota Nicotine Withdrawal Scale
When the mean number of days between each session was added as a covariate, the Day × Condition interaction for urge to smoke was still non-significant (F [2, 17] = .11) and no significant difference in HR and MAP change scores was found across days, F(2,17) = 1.58, and F(2,17) = 1.0, respectively. Analysis of NWS continued to show no significant main effect of Condition, F(1,17) = 1.78, ηp2 = .09, or Day, F(2,17) = .64, ηp2 = .03, however, the Condition × Day interaction was no longer significant, F(2,17) = 1.29, ηp2 = .07.
Although the zero-order correlation between nicotine dependence (FTND scores) and mean urge to smoke after smoking cues was significant, the partial correlation was non-significant when controlling for urge to smoke assessed following relaxation (pr = −.07). Thus, the significant positive relationship between nicotine dependence and urge to smoke following cigarette cues was accounted for by overall higher urge levels prior to cigarette cue exposure. No other correlations or partial correlations with urge or withdrawal were significant (all r’s < .37). No correlations of change scores for HR or MAP with individual differences in smoking levels were significant except change in mean HR with number of years smoking (r = −.56, p < .05).
3. Discussion
Urge to smoke ratings and physiological reactivity to smoking cues appear stable across three nonconsecutive days of brief laboratory smoking cue exposure among moderate to heavy smokers following overnight 10 hr abstinence periods. These findings support the utility of using repeated measures within-subjects designs for smoking cue exposure assessment with moderate to heavy smokers when assessing urge and physiological reactions, such as in studies of medication effects at different doses. There was some evidence of habituation with regard to nicotine withdrawal symptoms, such that participants reported significant cue-elicited increases in withdrawal symptoms only following the first cue-exposure day. However, withdrawal levels were rated as mild both prior to and after cue exposure across all test sessions and the increase in cue-elicited withdrawal was very small, even on the first day. No cue-elicited withdrawal symptoms were evident across exposure sessions after controlling for the number of days between exposure sessions. Since it is not clear how this interval could affect the first day’s cue exposure effects, the loss of the Day 1 effects could have resulted from loss of power due to adding the covariate. Taken together, while smoking cues elicited significant urge to smoke and physiological reactivity across three separate test days, these reactions did not habituate, and participants reported low levels of withdrawal prior to cue exposure with smoking cues increasing these levels only a small amount on the first day and not thereafter.
Although urge to smoke was significantly increased by smoking cues, the magnitude of this effect was modest. This finding is consistent with some other studies (e.g., Rohsenow et al., 2007; Tidey et al., 2005). A methodological concern that has been raised about smoking cue-reactivity paradigms is that smoking deprivation alone may raise craving to levels where the cue-reactivity effects are no longer noticeable due to ceiling effects, possibly explaining why weaker reactivity effects have been observed when participants are nicotine deprived as compared to non-deprived (e.g., Sayette et al., 2001; Tidey et al., 2005). However, 10 hours of smoking deprivation in the present study did not obscure cue-reactivity effects in these participants: prior to cue exposure, levels of urge were only moderately high and ratings of withdrawal were low. Furthermore, smoking cues consistently increased reports of subjective urge and physiologic responses to smoking cues. Thus, the modest effects of smoking cues on urge to smoke are not due to ceiling effects.
Limitations of the study include the relatively small sample size, which increased the possibility that null findings were due to insufficient statistical power. Second, this study compared participants’ reactivity to smoking cues with their responses following a relaxation period rather than with their responses to a neutral cue condition; although our previous work indicated that this approach should make no difference (Rohsenow et al., 2007), this methodology should be taken into consideration when generalizing these findings to other cue reactivity studies. In addition, while caffeine has known effects on physiological reactivity (but not on urge to smoke or withdrawal measures), participants were not asked to abstain from caffeine prior to the laboratory session and recent caffeine consumption was not assessed. Therefore, variance may have been added to the HR and MAP scores due to differential caffeine intake. In addition, participants were non-treatment-seeking moderate to heavy smokers and findings may not generalize to other populations, such as treatment seeking smokers, restrained smokers, or those recently abstinent. Finally, the cue-elicited effects on urge in this study were modest and these findings do not address the stability of cue elicited responses using non-deprived testing sessions. Additional research should examine whether gender and other moderators are associated with differential habituation to smoking cues.
Acknowledgments
This research was supported in part by a Research Career Scientist Award and a Senior Research Career Scientist Award, a Merit Review Grant from the Office of Research and Development’s Medical Research Service of the Department of Veterans Affairs, and by a Career Development Award from the National Institute of Alcohol Abuse and Alcoholism (1K23 AA014966). A preliminary report of these results was made at the annual meeting of the Society for Research on Nicotine and Tobacco, New Orleans, LA, February 2003.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Experimental and Clinical Psychopharmacology. 2001;9:399–408. doi: 10.1037//1064-1297.9.4.399. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, LaChance H, Niaura R, Bryan AD, Smolen A. The DRD4 VNTR influences reactivity to smoking cues. Journal of Abnormal Psychology. 2002;111:134–143. doi: 10.1037//0021-843x.111.1.134. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Ray LA, Sandman E, Rutter MC, Peters A, Swift R. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology. 2006;31:1310–1317. doi: 10.1038/sj.npp.1300917. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, MacKinnon SV, Sirota AD, Kaplan GB. High-dose transdermal nicotine and naltrexone: Effects on nicotine withdrawal, urges, smoking, and effects of smoking. Experimental and Clinical Psychopharmacology. 2007;15:81–92. doi: 10.1037/1064-1297.15.1.81. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multidimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96:1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift R. Subjective and physiological responses to smoking cues in smokers with schizophrenia. Nicotine & Tobacco Research. 2005;7:421–429. doi: 10.1080/14622200500125724. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. Journal of Consulting and Clinical Psychology. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]