Abstract
BACKGROUND
A potential concern about biological pacemakers is their possible malfunction to create ventricular tachycardias (VT).
OBJECTIVE
We hypothesized that should VT complicate implantation of HCN-channel-based biological pacemakers, they would be suppressed by inhibitors of the pacemaker current, If.
METHODS
We created a chimeric channel (HCN212) containing the N- and C-termini of mouse HCN2 and the transmembrane region of mouse HCN1 and implanted it in HEK293 cells. 48h later, in whole-cell patch clamp recordings, mean steady-state block induced by 3 μM ivabradine (IVB) showed HCN1=HCN212>HCN2 currents. The HCN212 adenoviral construct was then implanted into the canine left bundle branch in 11 dogs. Complete AV block was created via radiofrequency ablation and a ventricular demand electronic pacemaker was implanted (VVI 45 bpm). ECG, 24h Holter monitoring and pacemaker log record check were performed x 11d.
RESULTS
All dogs developed rapid VT (>120 bpm, maximum rate=285±37 bpm) at 0.9±0.3 d after implantation that persisted through 5±1d. IVB, 1 mg/kg over 5 min was administered during rapid VT, and 3 dogs received a second dose 24h later. While VT terminated with IBV in all instances within 3.4±0.6 min, no effect of IVB on sinus rate was noted.
CONCLUSION
We conclude that: (1) If-associated tachyarrhythmias - if they occur with HCN-based biological pacemakers - can be controlled with If inhibiting drugs such as IVB; (2) In vitro, IVB appears to have a greater steady-state inhibiting effect on HCN1 and HCN212 isoforms than HCN4; and (3) VT originating from the HCN212 injection site is suppressed more readily than sinus rhythm. This suggests a selectivity of IVB at the concentration attained for ectopic over HCN4-based pacemaker function. This might confer a therapeutic benefit.
Keywords: Biological pacemaking, cardiac ion channels, electronic pacemakers, HCN channel chimeras, Ivabradine, ventricular tachycardia
Introduction
In recent years the possibility of developing biological pacemakers as adjuncts to or replacements for electronic pacemakers has been investigated.1–10 A potential concern about biological pacemakers is the possibility that they might malfunction to create ventricular or atrial tachyarrhythmias originating at the implant site. We have focused on the HCN family of pacemaker channel genes to create biological pacemakers and have seen no arrhythmic activity using viral vectors or adult human mesenchymal stem cells (hMSC) as delivery platforms.6–10 However, in preliminary experiments we found that canine left bundle branch implantation of an adenoviral construct of HCN212, a chimera we constructed to increase basal rates of HCN-based biological pacemakers, induces rapid ventricular tachycardia (VT). We hypothesized that if the VT were the functional result of the pacemaker current If, then ivabradine (IVB), a selective inhibitor of If11 that substantially reduces the rate of spontaneous action potential firing in sinoatrial node12 would suppress it. Moreover, previous data had suggested that IVB may show isoform-selectivity in suppressing HCN currents.13 Specifically, it expresses a greater effect to inhibit HCN1 than HCN2 or HCN4 isoforms. This suggested to us that there might be a selective effect of IVB on an HCN212-based chimera over sinus rhythm, which is largely HCN4-driven.
Methods
Experiments were performed using protocols approved by the Columbia University Institutional Animal Care and Use Committee and conform to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). IVB was kindly provided by Servier Laboratories (Courbevoie, France).
Adult rat ventricular myocytes isolation and infection
Male Wistar adult rats weighing 300–350 g (10–12 weeks old) were anesthetized before cardiectomy and freshly isolated ventricular myocytes were prepared using an enzymatic and mechanical procedure previously described by us.14 Single myocytes were then resuspended in serum-free medium (ACCTI15) and plated onto laminin-coated 9×22 mm glass coverslips. Two to three hours later they were infected with adenoviral constructs of mouse HCN2 or HCN212. In some experiments the channel was coinfected with a green fluorescent protein adenoviral construct (GFP). The culture medium was removed from the coverslips and 200 μl/coverslip of pre-warmed culture medium mixed with adenovirus was added. The coverslips were kept at 37°C in a CO2 incubator during the 2-hour adsorption period, then the inoculum was discarded and the coverslips washed and the dishes refilled with the appropriate culture medium. The multiplicity of infection (MOI, ratio of viral units to cells) was 60 for both HCN2 and HCN212 and 40 for GFP. Whole-cell patch clamp techniques were employed to record HCN2 and HCN212 currents from adult rat ventricular myocytes overexpressing the two channels 24 h after infection.
The preparation of the mouse HCN2 adenovirus has been previously described, using a shuttle vector from Microbix Systems16 and a similar approach was employed to produce an HCN212 adenovirus. The HCN1 and the chimeric HCN212 clones were provided by Dr. S. Siegelbaum. HCN212 consists of the N- and C-termini of mouse HCN2 and the transmembrane region of mouse HCN1, such that amino acids D182–L442 of HCN2 were substituted by D129–L389 of HCN1.17
HEK cells and transfection
HEK 293 cells were cultured in Dulbecco’s MEM supplemented with 10% fetal calf serum (Gibco, Invitrogen Corp.) and antibiotics (1x Penicillin-Streptomycin-Glutamine; Gibco, Invitrogen Corp.) at 37°C in 5% CO2. Murine HCN1, HCN2 or HCN212 cDNA and GFP cDNA were co-transfected in HEK cells using Gene Jammer Transfection Reagent (Stratagene). The transfection procedure was performed according to manufacturer’s instructions. Two μg of channel cDNA and 1 μg of GFP were used for each 35 mm Petri dish. Cells were used 2–3 days after transfection to permit a good level of protein expression. Cells were dispersed using the PBS-based Cell Dissociation Buffer (Gibco, Invitrogen Corp.) and re-plated at a low density onto 9×22 mm glass coverslips on the morning of each experiment. Whole-cell patch clamp was employed to record HCN2, HCN1 and HCN212 currents from HEK cells overexpressing these channels.
Intact canine studies
Anesthesia was initiated with propofol 6 mg/kg IV and maintained with inhalational isoflurane (1.5%–2.5%) in 6 adult mongrel dogs weighing 22–25 kg. Using a steerable catheter the HCN212 viral construct was injected into the left bundle branch (LBB) as described by us previously.7 Complete atrioventricular (AV) block was induced via radiofrequency ablation of the AV junction and each site of injection was paced via the catheter electrode to permit pace-mapping of the origin of the idioventricular rhythm during follow up. Data from an additional 10 dogs (5 administered HCN2 and 5 administered saline, using the same protocol) were included as comparison groups. Although these data have been in part reported earlier8 the experiments overlapped temporally with some of the HCN212 experiments and the animals were viewed as appropriate controls.
An electronic pacemaker (Guidant, Discovery II, Flextend lead) was implanted and programmed in demand mode VVI 45 bpm. ECG(PC-EKG, Dr. Vetter GmbH, Baden-Baden, Germany), 24-h Holter monitoring (Digital Holter Analysis System, Rozinn Electronics, Inc, Glendale, NY), and pacemaker log record checks were performed daily for the 14 day duration of each experiment. If VT was observed during resting ECG recordings, then during continuous ECG recording the electronic pacemaker was turned off and IVB, 1 mg/kg was administered intravenously over 5 min. The ECG was monitored continuously for 30 min to evaluate the effect of treatment and additional ECGs were recorded 1h after IVB administration. When this acute phase of the experiment ended electronic pacing was reinitiated. The maximal rate of VT, time to reoccurrence of VT, and time to the last episode of VT after implantation was evaluated using 24-h Holter monitoring.
Statistical analysis
In cell culture studies, experimental data were compared using a Student’s t test or Chi-square test with Yates’ correction, as appropriate. When making comparisons, matched cells from the same cultures were used, and data from at least 3 separate cultures were pooled for each comparison. Data from intact animals were analyzed using two-way repeated measures ANOVA. Subsequent analysis was done using either Bonferroni’s test where equal variances were assumed, or the Games-Howell test where variances were unequal. One-way repeated measures ANOVA or independent samples t-test were used to analyze influence of one factor on a dependent variable.
Data are presented as means ± SEM. Data were analyzed using SPSS for Windows software (SPSS, Inc, Chicago, IL.). P<0.05 was considered to be significant.
Results
Comparison of HCN2 and HCN212 effects in adult rat ventricular myocytes
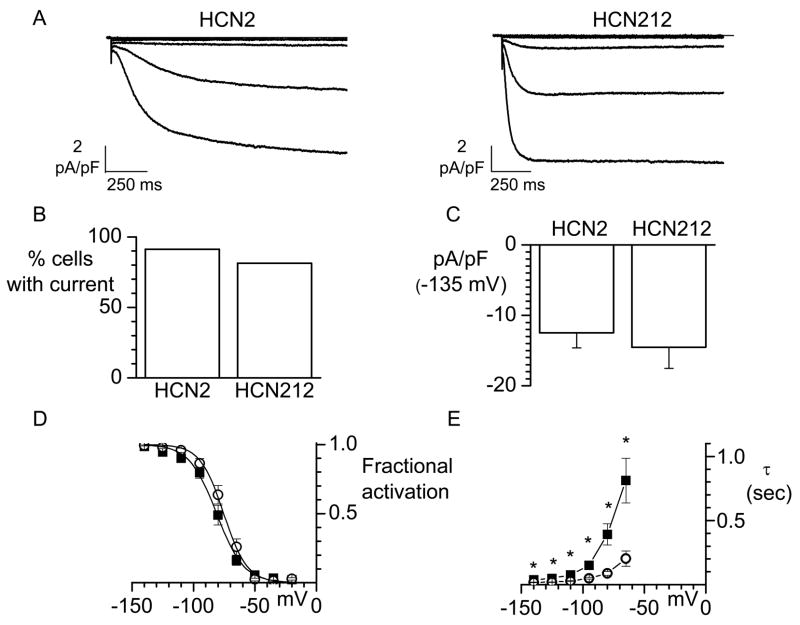
Representative normalized current traces obtained at test potentials ranging from −35 to −95 mV, from a holding potential of −35 mV, are shown in Figure 1A, which demonstrates that HCN212 current shows faster activation kinetics than HCN2. We analyzed the percentage of myocytes expressing the HCN2 and HCN212 currents (Figure 1B) and the current density at −135 mV (Figure 1C). HCN2 and HCN212 channels gave rise to currents with similar levels of expression (91.3%, n=23 vs 81.5%, n=27; p>0.05) and current density (−12.5±2.2, n=5 vs −14.5±3.0, n=9; P>0.05). That HCN212 and HCN2 channels manifest similar voltage dependence of activation is evident from the mean current-voltage relationships shown in Figure 1D. For statistical analysis individual activation curves were fit to the Boltzmann equation and the calculated midpoint (V1/2) and slope factor (s) from all cells averaged and compared. Mean parameters for HCN2- and HCN212-expressing cells (n= 5 and 9 respectively) were: V1/2 = −81.2±2.6 mV and −75.7±2.6 mV (P>0.05) and s= 10.3±1.1 mV and 8.3±1.0 mV (P>0.05). Analysis of activation kinetics revealed that the mean activation time constants of HCN212 current are faster than those of HCN2 current at each voltage analyzed (Figure 1E). Faster kinetics would be expected to result in more current being passed earlier in diastolic depolarization with HCN1 than with HCN2 so that a faster basal rate would be expected in HCN212-based biological pacemakers.
Figure 1.
Electrophysiological characterization of murine HCN2 and HCN212 channels overexpressed in adult cardiac myocytes. A. Sample IHCN2 (left) and IHCN212 (right) normalized current traces recorded during hyperpolarizing steps (from −35 to −95 mV (15 mV step) from a holding potential of −35 mV.
B. Percentage of cells patched expressing HCN2 or HCN212 currents. C. HCN2 and HCN212 current density measured at −135 mV. D. Mean steady-state activation curves for HCN2 (n=5, squares) and HCN212 (n=9, circles). Solid lines show fit of Boltzmann relation. E. Mean activation time constants of HCN2 (squares) and HCN212 (circles) obtained between −140 mV and −65 mV. Each point represents the mean of 3–5 values for HCN2 and 7–9 values for HCN212. *P< 0.05 vs. HCN212.
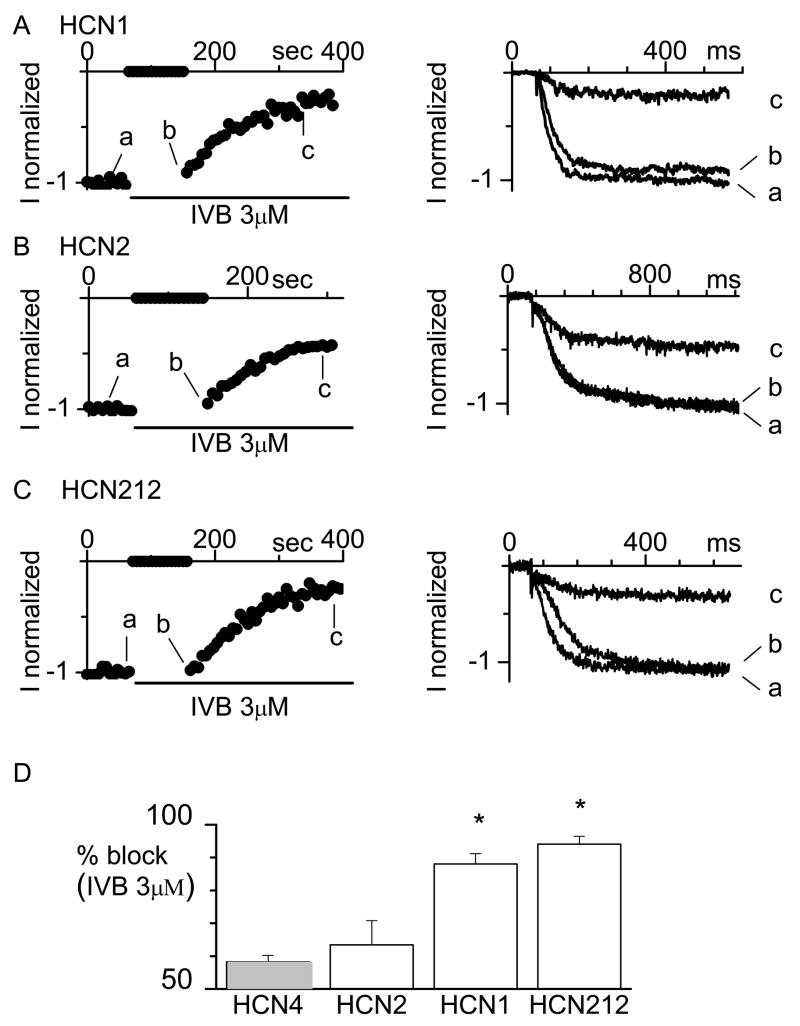
Whole-cell patch clamp was then employed to record HCN2, HCN1 and HCN212 currents from HEK cells overexpressing the channels and state-dependence of IVB block of these channels was investigated. We employed a concentration of 3×10−6M/L because this is approximately 2x the published half-maximal concentration for block of cloned HCN channels and native pacemaker current.13,18 At this concentration, IVB is selective for the pacemaker current.19 Representative time-courses of normalized HCN1, HCN2 and HCN212 current amplitude measured at −100 mV and the current traces recorded before (a) and just after resuming the pulsing protocol (b) are shown in Figure 2A, B and C. In agreement with previous results,13 IVB shows affinity for the closed conformation of HCN1 channels since the current amplitude of the trace recorded before (a) the period at −35 mV is bigger then that recorded just after (b). In n=5 cells the mean ratio b/a calculated was 0.88± 0.02 (P<0.05).
Figure 2.
Block of murine HCN1, HCN2 and HCN212 channels by IVB. Time-course of normalized IHCN1 (A, right), IHCN2 (B, right) and IHCN212 (C, right) current amplitude. We used a voltage protocol in which activating/deactivating steps (−100/+5 mV, every 6 s) were applied from a holding potential of −35 mV for tens of seconds. Then, at the time of IVB (3 μM) application membrane voltage was held at −35 mV for 90 s. At this voltage the HCN channels are closed. Then, in the continuous presence of IVB, the pulsing protocol was resumed. Sample normalized current traces recorded at the times indicated are shown on the left side of each panel. D. Mean percentage steady-state block of human HCN4, and murine HCN2, HCN1 and HCN212 currents induced by IVB 3 μM (n=4–6). Values reported for HCN4 current (gray bar) are taken from.13 *P<0.05 vs. HCN4 and HCN2.
In contrast, IVB did not show appreciable affinity for the closed conformation of HCN2 (panel B) and HCN212 (panel C) channels as, for both currents the traces (a and b) had similar magnitudes. The mean ratio between the current amplitudes recorded just after (a) and just before (b) was 0.97±0.01 (P>0.05, n=6) and 0.97±0.03 (P>0.05, n=6) for HCN2 and HCN212 respectively. These results argue in favor of the hypothesis that drug molecules block HCN2 and HCN212 channels only when the channel gate is open, as previously reported for HCN4 and native sinoatrial f-channels.13,18
As expected, resumption of the pulsing protocol in the presence of IVB caused the current to decrease until steady state block was reached. HCN2, HCN1 and HN212 current inhibition developed over a similar time-course with a mean time constant of 102± 27 s for HCN2 (n=6), 196 ± 36 s for HCN1 (n=4) and 184± 26 s for HCN212 (n=5) (P>.05). The mean percent steady-state current block induced by IVB 3 μM and that previously reported for HCN4 channels were compared using ANOVA as shown in Figure 2D. IVB 3 μM more completely blocked HCN1 and HCN212 than HCN2 and HCN4 (P<.05).
Intact animal studies
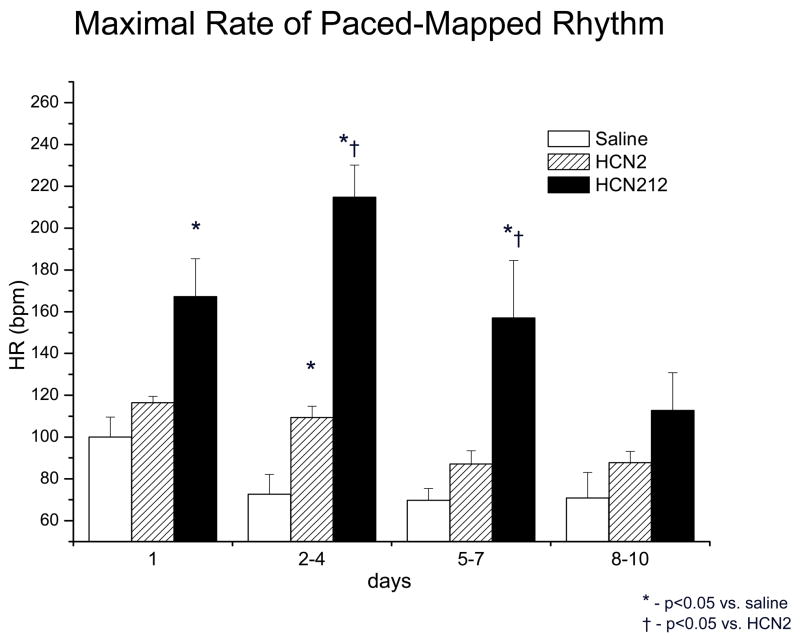
Our prior and concurrent studies using HCN2 with either viral or stem cell platform delivery demonstrated a stable, basal rhythm of 50–60 bpm.7–10 In contrast, with HCN212 we saw only VT of varying rates. The first episode of rapid, bursting VT was noted in all 6 dogs injected with HCN212 at 0.9±0.3 days after implantation and persisted for 5±1 days. The maximal VT rate was 285±37 bpm as registered during 24-h monitoring. This did not happen concurrently or subsequently in any of the animals administered HCN2 or saline and reported by us separately.8 Summary data are provided in Figure 3. The QRS configurations of the VT resembled those of impulses pace-mapped to the injection site during the implantation procedure and the VT could itself be overdrive-suppressed.
Figure 3.
Maximal rate of rhythm that pace-mapped to injection site, recorded during daily 24 h Holter monitoring. The maximal rate was significantly faster during the first week after implantation in animals injected with the murine HCN212 chimera (black bars) compared to animals receiving saline (white bars) and those injected with HNC2 gene (striped bars) on days 2–4 and 5–7.
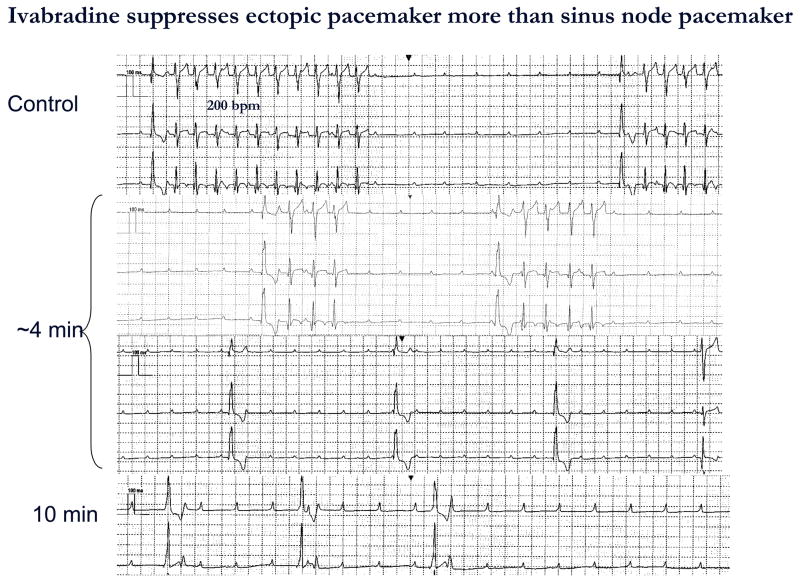
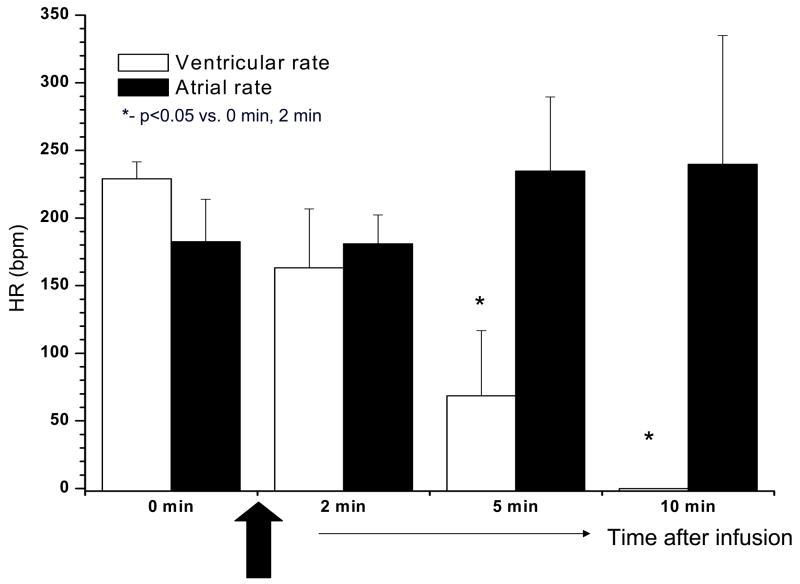
IVB was administered to all dogs and in 3 of them a second IVB administration was performed 24 h after the first treatment. For the purpose of analysis we pooled all IVB administrations (9 in 6 dogs). IVB terminated VT in all 9 episodes at 3.4±0.6 min after completing administration. VT reoccurred in all cases in 16.5±1.0 h. A representative experiment is shown in Figure 4. Of interest is that the effect of IVB on the ectopic pacemaker rate was greater than on sinus rate. Summary data are presented in Figure 5.
Figure 4.
ECG recorded before, during, and after IVB administration from a dog in complete AV block implanted with HCN212 chimera. Each panel shows continuous traces. The top panel depicts bursts of VT having a rate of 200 bpm and a QRS configuration that pace-mapped to the implantation site. Four min after completing IVB infusion (1 mg/kg IV) over 5 min (middle panel) VT slowed and bursts were shorter. Ten min after IVB infusion (bottom panel) a slow idioventricular rhythm having a wider QRS complex and apparently originating from another site was observed. Atrial rate remained stable during the entire period of observation. ECGs recorded with electronic pacemaker turned off and at paper speed = 25 mm/sec
Figure 5.
Atrial and ventricular rates recorded during and after IVB administration. Ventricular rate (white bars) significantly slowed after IVB infusion. In contrast, IVB had no significant effect on atrial rate (black bars) during the period of the experiment.
Discussion
We employed the HCN212 chimera because we were seeking a modified HCN channel that might incorporate the favorable activation kinetics of HCN1 as well as the more robust cAMP response of HCN2. We reasoned that this would create a pacemaker that had a faster basal rate than the HCN2 biological pacemaker we had previously reported6–8 and yet maintained the catecholamine sensitivity that is greater for HCN2 than HCN1. In fact, the HCN212 chimera overshot our expectations in that it manifested VT having excessively rapid rates. In other words, while the basic concept underlying the design of the chimera was sound, the specific design, itself, was flawed. The resultant VT had the following characteristics: at its maximum rate it pace-mapped to the injection site of the HCN212 construct and manifested a rate well over 200 bpm; it overdrive-suppressed both itself and the endogenous idioventricular rhythms of the dogs, resulting in long pauses; and it was highly sensitive to the If inhibitor, IVB. These characteristics all are clearly those of an automatic tachycardia and an association with If as a contributory current. While automatic tachycardias in otherwise normal ventricles are not classically associated with rates this rapid, one must remember that this is not a naturally occurring HCN isoform but rather a fabricated chimera.
Despite the undesirable outcome with HCN212 there were several novel and potentially useful findings: first, the isoform specificity of IVB for HCN1 over HCN2 and HCN4 channels in situ13 is consistent with and may contribute to the greater sensitivity of the biological than native pacemaker to the drug; second, IVB may offer a useful tool for suppressing automatic tachycardias in vivo and/or as a diagnostic identifier of automaticity in clinical arrhythmias; and third, to the extent that HCN channels might contribute to the occurrence of biological pacemaker malfunction, IVB might confer therapeutic benefit.
We shall discuss each of these points in sequence:
The isoform specificity of IVB for HCN1 over HCN2 and HCN4 channels in situ is consistent with and may contribute to the greater sensitivity of the biological than native pacemaker to the drug
An earlier study13 determined that HCN4 channels are predominantly sites of open channel block by IVB. The present study suggests strongly that this is the primary mechanism for IVB-induced channel block of HCN2 and HCN212 as well. This differs from block of HCN1, which both we and the earlier study found to be dependent on the channel being closed. Thus, the state-dependent nature of IVB block does not account for the differential sensitivity we observed between the biological and native pacemakers in vivo. Further, since HCN212 behaves like HCN2 rather than HCN1, it suggests that residues in the N- and/or C-termini are critical for open channel block by IVB. Regarding steady-state block it appears that block of HCN212 and HCN1 channels with 3μM IVB is significantly greater than that of HCN4 and HCN2 (see Figure 2D). Because we studied only a single concentration near the half-maximal response, we cannot say if this reflects an isoform difference in the dose-response relation or in the maximal inhibition. Nonetheless, one important interpretation of these results is that the pore-forming unit of the HCN212 channel is truly functional in the same way as HCN1.
Interestingly, our findings also suggest that the in situ demonstration of differing isoform selectivity of IVB may have a clinical counterpart. As shown in Figure 5, atrial rate, which is primarily contributed to by HCN4 is unaffected by the dose of IVB (1mg/kg IV over 5 min) that terminates the function of the HCN212 pacemaker. There are several possible interpretations of this finding: (1) to the extent that any HCN1-based mechanisms might contribute to automatic arrhythmias in vivo, IVB would suppress them while leaving the basic sinus mechanism of the heart unaltered. This result might appear at odds with the primary recommended clinical use of IVB today, which is to slow sinus rate in the setting of angina.,20 However, the present study was not intended to explore the possibility of sinus slowing. The latter would have required a dose-response relationship using bolus plus infusion. Had a higher dose and sustained plasma level been maintained we might have seen slowing of sinus rate. (2) It is possible that catecholamine release was augmented in the setting of a rapid and unstable VT. This might have increased sinus rate, counteracting the effect of IVB to suppress the sinus pacemaker. Further, the use-dependent nature of IVB block of pacemaker channels is such that tissue firing at a faster rate would be expected to exhibit greater sensitivity than that firing at a slower rate. (3) In transfecting myocytes with HCN212 we created a population of cells whose balance between IHCN212 and resident ion channels was unique. The differing ratios of channels contributing to pacemaker function in sinus node would be anticipated to maintain sinus rhythm in a manner different from that in the transfected biological pacemaker.
IVB may offer a useful tool not only for suppressing automatic tachycardias in vivo, but as a diagnostic test of automatic versus other mechanisms for clinical arrhythmias
In making this suggestion we are accepting the premise that If is a major driver of automatic rhythms. Indeed there are significant data that support this view. The pioneering work of DiFrancesco demonstrated that pacemaker function was initiated by a hyperpolarization-activated inward current, designated If, and subsequent research catalogued both the properties of the responsible channel and the importance to its function of cAMP binding.21,22 However, it is clear that If is not the only current contributing to pacemaker function. Not only are inward Ca currents (ICa,T and ICa,L) important, but there is a modulatory action of repolarizing K currents which, as they increase or decrease may contribute respectively to decreases and increases in automatic rate (reviewed in 23). Most recently, the role of Na/Ca exchange current has been investigated and this too appears to be an important contributor.24,25 That all these currents interact to provide the pacemaker potential is part of the beauty of the system’s design: failure of one component (or its pharmacological block) may slow pacemaker rate, but the other components can still operate to ensure continuity of pacemaker function.
How does this information potentially relate to clinical cardiac arrhythmias? It is generally recognized that most clinical arrhythmias are reentrant, but it is also clear - based on characteristics of onset and offset, modification by electrophysiologic testing or by drugs, and studies of basic mechanism - that a subset are triggered by afterdepolarizations and another subset are automatic.26 Clinical diagnosis of mechanism is hampered by several obstacles: first, While pacing and transient entrainment are aids in identifying reentry (e.g.27–29) there is no “clean” pharmacologic identifier of reentrant arrhythmias. Second, the diagnosis of triggered activity has been helped by pacing and by use of various drugs, most importantly adenosine30 and flunarazine.31 However, there is no single drug or pacing response that we can say with certainty identifies an arrhythmia as being unimpeachably triggered (whether by early or delayed after depolarizations). Third, while a characteristic response to overdrive pacing is an identifier of automatic rhythms, there is no unique pharmacological identifier of these rhythms.
How does IVB fit into this setting? As the product of research on a series of compounds that have with increasing selectivity singled out If as the target current, it would be reasonable to conclude that response of an arrhythmia to IVB would suggest a role for If in that arrhythmia. This should not be overinterpreted as indicating that a positive response to IVB means If is the sole determinant of an arrhythmia. However, for the population of arrhythmias that is automatic a beneficial response to IVB (although not necessarily cessation of the arrhythmia altogether) should be expected.
To the extent that HCN channels might contribute to the occurrence of biological pacemaker malfunction, IVB might confer therapeutic benefit
In a practical sense, this is the least important of the three unique findings of this study. We state this because at present there are no clinically available biological pacemakers. Yet, we do believe that given the critical mass of investigators involved in the field1–10 and the general interest in seeing the benefits of biological pacemaking become a reality this goal will eventually be realized. And when it is realized, there is the possibility that runaway function may materialize in some cases. We state “may” because the only time we have seen this happen using HCN constructs is with a design whose clinical applicability is flawed, as with the chimera used in this study. We would expect such design flaws to be engineered out of the system before it is clinically developed. But as long as there is the possibility that tachyarrhythmia might occur, it is useful to know that there already is at least one pharmacologic agent that would be a suitable treatment.
Conclusions
We have demonstrated that rapid VT induced by a chimeric pacemaker channel HCN212 is readily suppressed by the If inhibitor, IVB. We have found as well a greater effect of the IVB concentration attained on HCN212 and HCN1 channels over HCN4 and HCN2, and that open channel block appears to be the mechanism for drug effect. To the extent that it selects for the If current over other ion channel contributors to cardiac electrical activity, IVB would appear to be a potential therapeutic agent for automatic tachycardias in general, and potentially malfunctioning biological pacemakers in particular. Although the lack of measurement of plasma levels of IVB is a limitation of this study, that the drug had a clear, reproducible and reversible effect is unequivocal.
Acknowledgments
The authors express their gratitude to Drs. Florence Mahlberg-Gaudin and Jean-Paul Vilaine of Servier Laboratories (France) for useful discussions regarding ivabradine and for providing the drug to us. We also express our gratitude to Nimee Bhat for her assistance with certain of the experiments and to Laureen Pagan and Eileen Franey for their careful attention to the preparation of the manuscript.
These studies were supported by USPHS-NHLBI grant HL-28958, Boston Scientific Corporation, Guidant Corporation and Servier Laboratories
LIST OF ABBREVIATIONS USED IN MS
- AV
atrioventricular
- GFP
green fluorescent protein
- HCN
hyperpolarization-activated, cyclic nucleotide-gated
- hMSC
adult human mesenchymal stem cell
- If
hyperpolarization-activated inward pacemaker current (“funny” current)
- IVB
ivabradine
- MOI
multiplicity of infection
- VT
ventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edelberg JM, Aird WC, Rosenberg RD. Enhancement of murine cardiac chronotropy by the molecular transfer of the human β2-adrenergic receptor cDNA. J Clin Invest. 1998;101:337–343. doi: 10.1172/JCI1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelberg JM, Huang DT, Josephson ME, Rosenberg RD. Molecular enhancement of porcine cardiac chronotropy. Heart. 2001;86:559–562. doi: 10.1136/heart.86.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miake J, Marbán E, Nuss HB. Gene therapy: biological pacemaker created by gene transfer. Nature. 2002;419:132–133. doi: 10.1038/419132b. [DOI] [PubMed] [Google Scholar]
- 4.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–2389. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 5.Tse HF, Xue T, Lau CP, Siu CW, Wang K, Zhang QY, Tomaselli GF, Akar FG, Li RA. Bioartificial sinus node constructed via in vivo gene transfer of an engineered pacemaker HCN Channel reduces the dependence on electronic pacemaker in a sick-sinus syndrome model. Circulation. 2006;114:1000–1011. doi: 10.1161/CIRCULATIONAHA.106.615385. [DOI] [PubMed] [Google Scholar]
- 6.Qu J, Plotnikov AN, Danilo P, Jr, Shlapakova I, Cohen IS, Robinson RB, Rosen MR. Expression and function of a biological pacemaker in canine heart. Circulation. 2003;107:1106–1109. doi: 10.1161/01.cir.0000059939.97249.2c. [DOI] [PubMed] [Google Scholar]
- 7.Plotnikov AN, Sosunov EA, Qu J, Shlapakova IN, Anyukhovsky EP, Liu L, Janse MJ, Brink PR, Cohen IS, Robinson RB, Danilo P, Jr, Rosen MR. Biological pacemaker implanted in canine left bundle branch provides ventricular escape rhythms that have physiologically acceptable rates. Circulation. 2004;109:506–512. doi: 10.1161/01.CIR.0000114527.10764.CC. [DOI] [PubMed] [Google Scholar]
- 8.Bucchi A, Plotnikov AN, Shlapakova I, Danilo P, Jr, Kryukova Y, Qu J, Lu Z, Liu H, Pan Z, Potapova I, KenKnight BK, Girouard S, Cohen IS, Brink PR, Robinson RB, Rosen MR. Wild-Type and mutant HCN channels in a tandem biological-electronic cardiac pacemaker. Circulation. 2006;114:992–999. doi: 10.1161/CIRCULATIONAHA.106.617613. [DOI] [PubMed] [Google Scholar]
- 9.Potapova I, Plotnikov A, Lu Z, Danilo P, Jr, Valiunas V, Qu J, Doronin S, Zuckerman J, Shlapakova IN, Gao J, Pan Z, Herron AJ, Robinson RB, Brink PR, Rosen MR, Cohen IS. Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circ Res. 2004;94:952–959. doi: 10.1161/01.RES.0000123827.60210.72. [DOI] [PubMed] [Google Scholar]
- 10.Plotnikov AP, Shlapakova I, Szabolcs MJ, Danilo P, Jr, Lorell B, Potapova IA, Lu Z, Rosen AB, Mathias RT, Brink PR, Robinson RB, Cohen IS, Rosen MR. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation. 2007;116:706–713. doi: 10.1161/CIRCULATIONAHA.107.703231. [DOI] [PubMed] [Google Scholar]
- 11.Bois P, Bescond J, Renaudon B, Lenfant J. Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol. 1996;118:1051–1057. doi: 10.1111/j.1476-5381.1996.tb15505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thollon C, Bedut S, Villeneuve N, Coge F, Piffard L, Guillaumin JP, Brunel-Jacquemin C, Chomarat P, Boutin J-A, Peglion J-L, Vilaine J-P. Use-dependent inhibition of hHCN4 by ivabradine and relationship with reduction in pacemaker activity. Br J Pharmacol. 2007;150:37–46. doi: 10.1038/sj.bjp.0706940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucchi A, Tognati A, Milanesi R, Baruscotti M, DiFrancesco D. Properties of ivabradine-induced block of HCN1 and HCN4 pacemaker channels. J Physiol. 2006;572:335–346. doi: 10.1113/jphysiol.2005.100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuznetsov V, Pak E, Robinson RB, Steinberg SF. β2-Adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circ Res. 1995;76:40–52. doi: 10.1161/01.res.76.1.40. [DOI] [PubMed] [Google Scholar]
- 15.Ellingsen O, Davidoff AJ, Prasad SK, Berger H-J, Springhorn JP, Marsh JD, Kelly RA, Smith TW. Adult rat ventricular myocytes cultured in defined medium: phenotype and electromechanical function. Am J Physiol. 1993;265:H747–H754. doi: 10.1152/ajpheart.1993.265.2.H747. [DOI] [PubMed] [Google Scholar]
- 16.Qu J, Barbuti A, Protas L, Santoro B, Cohen IS, Robinson RB. HCN2 over-expression in newborn and adult ventricular myocytes: distinct effects on gating and excitability. Circ Res. 2001;89:e8–e14. doi: 10.1161/hh1301.094395. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Chen S, Siegelbaum SA. Regulation of hyperpolarization-activated HCN channel gating and cAMP modulation due to interactions of COOH terminus and core transmembrane regions. J Gen Physiol. 2001;118:237–250. doi: 10.1085/jgp.118.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucchi A, Baruscotti M, DiFrancesco D. Current-dependent block of rabbit sino-atrial node If channels by ivabradine. J Gen Physiol. 2002;120:1–13. doi: 10.1085/jgp.20028593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiFrancesco D, Camm JA. Heart rate lowering by specific and selective I(f) current inhibition with ivabradine: a new therapeutic perspective in cardiovascular disease. Drugs. 2004;64:1757–1765. doi: 10.2165/00003495-200464160-00003. [DOI] [PubMed] [Google Scholar]
- 20.Borer JS, Fox K, Jaillon P, Lerebours G for the ivabradine Investigators Group. Antianginal and antiischemic effects of ivabradine, an If inhibitor, in stable angina. A randomized, double-blind, multicentered, placebo-controlled trial. Circulation. 2003;107:817–823. doi: 10.1161/01.cir.0000048143.25023.87. [DOI] [PubMed] [Google Scholar]
- 21.DiFrancesco D. Block and activation of the pace-maker channel in calf Purkinje fibres: effects of potassium, caesium and rubidium. J Physiol. 1982;329:485–507. doi: 10.1113/jphysiol.1982.sp014315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- 23.Cohen IS, Robinson RB. Pacemaker current and automatic rhythms: toward a molecular understanding. Handb Exp Pharmacol. 2006;171:41–71. doi: 10.1007/3-540-29715-4_2. [DOI] [PubMed] [Google Scholar]
- 24.Bogdanov KY, Maltsev VA, Vinogradova TM, Lyashkov AE, Spurgeon HA, Stern MD, Lakatta EG. Membrane potential fluctuations resulting from submembrane Ca2+ releases in rabbit sinoatrial nodal cells impart an exponential phase to the late diastolic depolarization that controls their chronotropic state. Circ Res. 2006;99:979–987. doi: 10.1161/01.RES.0000247933.66532.0b. [DOI] [PubMed] [Google Scholar]
- 25.Lyashkov AE, Juhaszova M, Dobrzynski H, Vinogradova TM, Maltsev VA, Juhasz O, Spurgeon HA, Sollott SJ, Lakatta EG. Calcium cycling protein density and functional importance to automaticity of isolated sinoatrial nodal cells are independent of cell size. Circ Res. 2007;100:1723–1731. doi: 10.1161/CIRCRESAHA.107.153676. [DOI] [PubMed] [Google Scholar]
- 26.Cabo C, Wit AL. Cellular electrophysiologic mechanisms of cardiac arrhythmias. Cardiol Clin. 1997;15:517–538. doi: 10.1016/s0733-8651(05)70360-x. [DOI] [PubMed] [Google Scholar]
- 27.Olshansky B, Okumura K, Hess PG, Waldo AL. Demonstration of an area of slow conduction in human atrial flutter. J Am Coll Cardiol. 1990;16:1639–1648. doi: 10.1016/0735-1097(90)90314-f. [DOI] [PubMed] [Google Scholar]
- 28.Waldo AL, Plumb VJ, Arciniegas JG, MacLean WA, Cooper TB, Priest MF, James TN. Transient entrainment and interruption of the atrioventricular bypass pathway type of paroxysmal atrial tachycardia. A model for understanding and identifying reentrant arrhythmias. Circulation. 1983;67:73–83. doi: 10.1161/01.cir.67.1.73. [DOI] [PubMed] [Google Scholar]
- 29.Kowey PR, Waxman HL, Greenspon A, Greenberg R, Poll D, Kutalek S, Gessman L, Muenz L. Value of electrophysiologic testing in patients with previous myocardial infarction and nonsustained ventricular tachycardia. Philadelphia Arrhythmia Group. Am J Cardiol. 1990;65:594–598. doi: 10.1016/0002-9149(90)91036-6. [DOI] [PubMed] [Google Scholar]
- 30.Iwai S, Markowitz SM, Stein KM, Mittal S, Slotwiner DJ, Das MK, Cohen JD, Hao SC, Lerman BB. Response to adenosine differentiates focal from macroreentrant atrial tachycardia: validation using three-dimensional electroanatomic mapping. Circulation. 2002;106:2793–2799. doi: 10.1161/01.cir.0000040587.73251.48. [DOI] [PubMed] [Google Scholar]
- 31.Vos MA, Gorgels AP, Leunissen JD, van der Nagel T, Halbertsma FJ, Wellens HJ. Further observations to confirm the arrhythmia mechanism-specific effects of flunarizine. J Cardiovasc Pharmacol. 1992;19:682–690. [PubMed] [Google Scholar]