Abstract
Zinc dependent metalloproteinases comprise a large family of structurally homologous enzymes with a wide variety of biological roles. Originally described as proteinases involved in extracellular matrix (ECM) catabolism, these enzymes were later found to serve major roles as initiators of signaling pathways in many aspects of biology, ranging from cell proliferation, differentiation and communication, to pathological states associated with tumor metastasis, inflammation, tissue degeneration and cell death. From these enzymes, the tumor necrosis factor-α converting enzyme (TACE) stands out as a central shedding activity mediating the regulated release of a host of cytokines, receptors and other cell surface molecules. Selective drugs targeted at blocking TACE for treatment of rheumatoid arthritis and other disease indications are highly sought. Yet, the structural and chemical knowledge underlying its enzymatic activity is very limited. This is in part due to the fact that the catalytic zinc atom of metalloproteinases is usually spectroscopically silent and hence difficult to study using conventional spectroscopic and analytical tools. Most structural and biochemical studies, as well as medicinal chemistry efforts carried out so far were limited to non-dynamic structure/function characterization. Thus, to date, our mechanistic knowledge comes from theoretical calculations derived from static crystal structures from family members that are highly similar in their amino acid sequence and three-dimensional structure.
This review introduces the importance of real-time quantification of biophysical properties and structural kinetic behavior applied to the study of TACE and other zinc metalloproteinases to dissect their molecular mechanisms. The molecular details that link the catalytic chemistry to key kinetic, electronic and structural events have remained elusive because of the difficulties associated with probing time-dependent structure-function aspects of enzymatic reactions. Here we discuss the use of conventional and real-time structural-spectroscopic tools to study the reactive metal site during catalysis, and initial lessons on the enzymatic mechanism that we are learning. Approaches such as the ones presented here may be useful in the design of specific inhibitors as drug candidates.
Introduction
Reports on the discovery in 1994 of a proteolytic activity that can release pro-TNFα from macrophages and monocytes (1–3) started a race that resulted only three years later in the publication of the isolation and identification of the TNFα converting enzyme or TACE, and cloning of its cDNA (4, 5). This work opened the door to an intense decade of research into the biochemical properties and regulation of enzymes that shed signaling molecules and their receptors from the surface of cells. TNFα and TACE are known to be involved in septic and toxic shock, transplant rejection, rheumatoid arthritis and osteoarthritis, Crohn’s disease, multiple sclerosis, and autoimmune type diabetes, among many other conditions (6–11). Because of clinically proven role of TNFα in such a wide ranging host of inflammatory diseases that lack safe and efficacious drugs, many pharmaceutical companies began drug discovery efforts to look for compounds to specifically target TACE. Unfortunately, there is little to show from this TACE “gold rush”. Today, we still do not understand fundamental aspects of the activation, substrate specificity and regulation of this enzyme. The many papers published on these subjects since 1997 have provided only partial answers, often conflicting. Therefore, it is not entirely surprising that every effort to develop a TACE-specific inhibitory drug has been met so far by failure.
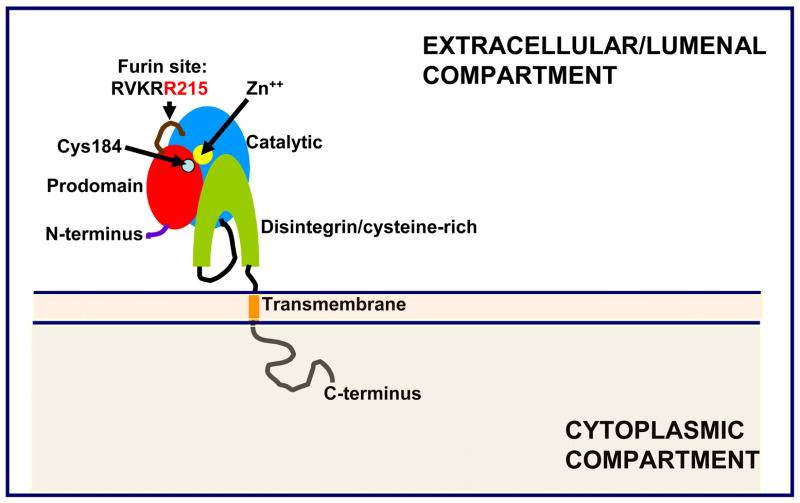
TACE is the central enzyme for the conversion of proTNFα to its secreted form and its physiological role has been demonstrated in vivo (12). TACE is also critical for the release from the plasma membrane of a large number of cell surface proteins including multiple cytokines and receptors (for recent reviews, see 13–17). Many of those ectodomains have critical signaling roles and alterations in their regulation lead to a host of inflammatory conditions, neoplasias and metabolic diseases. Yet, we have only an incipient understanding of what the regulatory events that affect TACE activity are, both at the molecular and cellular levels. The primary structure of TACE is depicted in schematic form in Figure 1. It belongs to a family of enzymes discovered by White and coworkers in the early 1990s, dubbed the ADAMs, because of the presence of a disintegrin and metalloproteinase domain in their molecular architecture (18–22). The ADAMs in turn belong to the metzincin superfamily, which includes the matrix metalloproteinases (MMPs) and is broadly characterized by the presence of a zinc metalloproteinase domain. TACE, as well as most ADAMs, contains a prodomain that is excised as part of enzyme activation (Figure 1). The transmembrane domain is followed by a cytoplasmic domain whose function remains yet to be discovered. Many reviews have been published regarding TACE, the ADAMs family and their similarities to other metzincin families (23–26), their biogenesis (27, 28) and the many substrates that they shed from cellular membranes (13–17). In this review, we will focus specifically on the role of conformational flexibility in the control of this enzyme’s activity and catalytic cycle.
Figure 1.
Domain organization of TACE as deduced from homology analysis of its primary sequence (4, 5) and structure-function studies (31, 32). The immature, full length pre-pro-protein is 824 amino acids in length; the depicted pro-protein starts at residue Ala17, the signal peptidase cleavage site; mature TACE starts at residue Arg215, the cleavage site of the pro-protein proteinase furin.
TACE zymogen inhibition and activation: initial observations
How does TACE become active and how does it turns over its substrate? We have addressed this question by focusing on the conformational properties of this enzyme and their relationship to catalysis, employing both equilibrium and kinetic spectroscopy methods, including real time experiments addressing conformation dynamics. Those studies suggested that the prodomain of TACE and as well as small, synthetic molecules can affect its conformation and function in profound ways (29–31).
Early in our work, we found that the TACE prodomain plays a critical role during this enzyme’s biosynthesis: it is essential for the secretion (export from the endoplasmic reticulum) of the catalytic domain. We demonstrated this through reconstitution experiments employing forms of TACE lacking the prodomain: the catalytic domain secretion occurred only upon addition of the pro domain in trans from a separate expression vector (31). The prodomain also prevents premature activation via inhibition of the catalytic domain (29, 32). Other groups focusing on other family members have reported similar observations regarding the functional roles of the prodomains in the biogenesis of these enzymes and maintenance of the zymogen state: ADAM 9 (33), ADAM 10 (34), ADAM 12, including a proposed low-resolution model for the pro-catalytic domain complex (35–37), ADAM 19 (38) and ADAM 28 (39).
The activation of ADAM metalloproteinases requires proteolytic removal of the prodomain by furin or other proprotein converting enzymes of the late secretory pathway. In some cases, this process is autocatalytic (39). For activation to occur, TACE is topographically shifted from the ER to the late golgi/TGN in a phorbol ester-inducible step. How exactly such translocation occurs as well as the pathway involved in its regulation remain presently unknown, but genetic evidence appears to support the concept of topographical displacement as the key regulatory event (40). TACE prodomain displays a cysteine switch consensus motif (PKVCGY186). The cysteine switch was previously observed by many groups in prodomains of matrix metalloproteinases and other metzincins, where it was found to have a role in catalytic site inhibition by coordination of the Zn+2 ion through the thiol of the conserved cysteine reidue, preventing the ligation of the water molecule used in the nucleophilic attack of the peptide bond (41–45). Surprisingly, this motif is not essential for zymogen secretion or inhibition and it may only play a role in preventing zymogen degradation during secretion by an unidentified protease (31). In fact, the secretory and inhibitory functions of the TACE prodomain seem to invoke a common mechanism resembling molecular chaperones: the TACE prodomain seems to affect the native conformation of the catalytic domain. Fluorescence emission experiments indicate substantial exposure of tryptophan residues in the pro-catalytic domain complex relative to the free prodomain or catalytic domain (31). Therefore, the arrest in the folding of the catalytic domain mediated by the prodomain, an intramolecular chaperone, is at the heart of the inhibitory mechanism.
This inhibitory role is quite different from the previously proposed role of proteases’ prodomains in the catalysis of folding. Bryan and Agard have shown for subtilysin BPN’ and for the α-lytic protease, respectively, that the prodomains accelerate dramatically the folding of these enzymes. Once folding is completed, the prodomain is proteolyzed by the enzyme that became active and “trapped” in its native, functional state (46–51). The TACE prodomain is a very potent inhibitor of the TACE catalytic domain, with an IC50 of 75 nM. Surprisingly, the inhibitory potency drops dramatically against the whole enzyme (5 μM, 29), indicating that the disintegrin domain must somehow mediate release of the prodomain from the catalytic domain after furin cleavage.
Conformational inhibition: beyond the TACE prodomain
Unexpectedly, we then found that drug-sized synthetic molecules could also induce conformational changes in TACE preventing its activity. The small competitive inhibitor of matrix metalloproteinases, SB-3CT, was non-competitive against TACE and induced profound changes in its conformation as probed by circular dichroism spectroscopy (30). SB-3CT also induced changes in the spatial and electronic configuration of the ligands surrounding the catalytic zinc ion within the TACE active site, as determined by X-ray absorption spectroscopy (XAS), a powerful method used to probe the structure within the coordination spheres of active site metals (52).
Bioinformatic analysis indicated that the active site of TACE is substantially more electronegative than the one of several MMPs for which structural information is available. This may explain why NaCl inhibits TACE. NaCl and other small ions inhibit this enzyme competitively in the low millimolar range (IC50 for Na+= 8 mM, 32). NaCl does not affect TACE’s conformation or thermodynamic stability, as determined by circular dichroism and fluorescence spectroscopy (M. Milla, unpublished work). How can TACE be fully inhibited at physiological ionic strengths? Certainly, this is a perplexing problem that may relate to TACE regulation in vivo, since salts inhibit TACE activity less in subcellular membrane fractions enriched in this enzyme’s activity. Should TACE be part of a multi-component shedding enzyme, as our initial studies indicate, it may be possible that the substrate-binding cleft is not directly accessible from the bulk solvent.
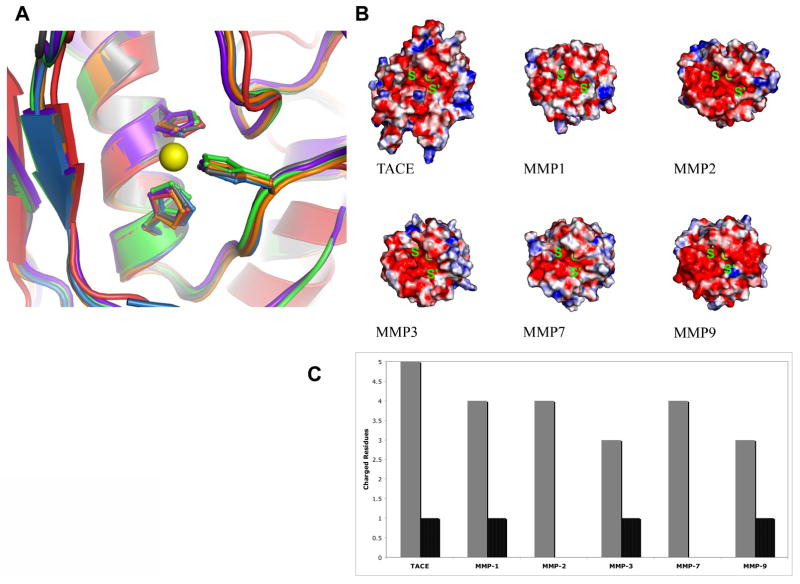
It is believed that the high structural similarity within the active sites of TACE, ADAMs and MMPs is an obstacle for the development of selective inhibitors (Figure 2A). Indeed, cross-inhibition observed with a number of known inhibitors may be at the root of failures of MMP inhibitors in clinical trials (53, 54). However, close examination of the second shell of residues surrounding the zinc ion of TACE and MMPs revealed a key difference between TACE and MMPs. It appears that while the peptide backbone structure is highly conserved among these different enzymes, the electrochemical potential induced by the active site residues is quite different. Figure 2A shows an overlap of active site residues surrounding the catalytic Zn++ of TACE, MMP-1, MMP-2, MMP-3, MMP-7 and MMP-9. Figure 2B depicts electrostatic surface potential models for those same enzymes. In TACE, the second shell residues surrounding the residues that coordinate the zinc ion are Thr404, Glu406, Gly408, Asn410, Asp416, Glu414 versus Ala402, Glu404, Gly406, Ala408, Glu412, and Ser414 for MMP-2 (30). Therefore, we propose that the microenvironment surrounding the catalytic zinc ion within TACE’s active site is substantially more polar than the one of MMP-2. The catalytic zinc site of TACE is also more polar than the catalytic sites of MMP-1, MMP-3, MMP-7, and MMP-9. This provides a molecular basis for understanding the remarkable sensitivity of TACE to inhibition by small cations presumably by affecting the ionic strength of the reaction mixture.
Figure 2.
Key features of the catalytic zinc-protein complex of TACE relative to selected MMPs. A) Overlay of the crystal structures of TACE (red; PDB file 1BKC), MMP1 (gray; PDB file 966C), MMP2 (orange; PDB file 1QIB), MMP3 (green; PDB file 1HY7), MMP7 (purple; PDB file 1MM) and MMP9 (blue; PDB file 1GKD). B) Surface electrostatic potential maps, showing the surface polarity of the S and S’ sites adjacent to the catalytic machinery for TACE versus MMPs. C) Total charged surface residues within 12 Å from the zinc for TACE versus MMPs. Gray: negatively charged residues; black: positively charged residues.
A lesson to be drawn at this point is that despite high structural similarity, variation in the polarity of the active site residues of the zinc-binding site in MMPs and ADAMs dictates their different electronic states. This stems from subtle differences in distances, effective charge of the catalytic metal ion and orientation of the active site’s His imidazole rings towards the zinc ion. Detailed understanding of the electronic state of the active site of TACE, polarity, chemistry, and fine structure will be of great utility in understanding the chemical basis of TACE inhibition by salts, small synthetic molecules, its own prodomain and the endogenous protein tissue inhibitor of metalloproteinases-3 (TIMP-3, 55). Differences in surface electrostatic potential at the S and S' sites in TACE and MMPs are also evident (Figure 2B and C).
Our findings argue that the seemingly high structural similarity may not be the full story in predicting the catalytic behavior of these enzymes or their predisposition toward inhibitors. The non-competitive inhibition of TACE by SB-3CT was unanticipated from structural considerations (30, 56). Closer examination of the binding modes of this compound to TACE’s active site, based on dynamic considerations, revealed a different mode of binding. This was supported by the evaluation of the electronic environment from the XAS analysis and molecular modeling analysis. The overriding conclusion is that the structural similarities among a set of related enzymes may not necessarily predict a similar behavior toward the same inhibitor. The quest for achieving selectivity in the inhibition of each of these enzymes should prove feasible by adopting a multi-pronged strategy considering factors other than mere surface complementarily of the designed inhibitor to the enzyme’s catalytic cleft.
These initial observations point to new avenues for inhibitor design, exploiting allosteric effects and TACE unique active site electrostatics. The comparative biophysical analysis of TACE versus MMPs raised the notion that highly structurally homologous enzymes may exhibit different protein conformational transitions in the presence of their substrates and during their enzymatic activities. Such variation in protein dynamics and structural kinetics may enhance enzyme selectivity and control. This hypothesis presents the field of protein dynamics with great technical challenges and the need to quantify the structure and chemical states of effective protein conformational transitions that evolve during enzymatic reactions in real-time and under physiological conditions.
Probing the structural and chemical properties of effective active site conformations: dynamic experimental tools
Protein function is strongly correlated with protein structure and dynamics. Protein motion and dynamics manifest in backbone and side-chain mobility seem to play a crucial role in protein stability, function, and reactivity. Dynamic processes occur in proteins on many different time scales. Surprisingly, very limited information is available on how to correlate protein motions occurring on short time scales (pico to nanoseconds) and longer time scales (micro to milliseconds) with protein function and enzyme catalysis. Being proteins, enzymes are flexible moieties whose structures exhibit dynamic fluctuations and structural kinetics on a wide range of time scales. The inherent mobility of a protein fold results in protein conformational transitions and has been shown to be manifested in the various steps constituting the catalytic cycle. The nature of this linkage between protein structure movement and function is undoubtedly complex and might involve the formation of a coupled network of interactions that bring the substrate closer, orient it properly, and provide a favorable electrostatic environment in which the chemical reaction can occur (57). However, the molecular basis to link protein dynamics and catalytic chemistry to key kinetic, electronic, and structural events have remained elusive because of the difficulties associated with probing time-dependent structure-function aspects of enzymatic reactions.
Following on this argument, the majority of protein structures presently solved by X-ray crystallography actually correspond to a static state (or a state average), that represents only poorly the ensemble of conformations adopted by a protein in action. Although protein structures provide information about protein folds and local structural arrangements, a single static structure is generally insufficient to enable the elucidation of enzymatic catalysis reaction mechanisms at an atomic level of detail. Typically, the catalytic cycle involves a series of intermediates and transition states that cannot be trapped by static or steady state structural methods. Furthermore, determining the energies of the various stationary points in the cycle is not trivial from a theoretical or experimental point of view.
Substantial efforts have been aimed at understanding the molecular basis by which the catalytic zinc machineries of metalloproteinases execute their enzymatic reactions (58, 59). Protein crystallography, proteomic, and theoretical studies have been instrumental in proposing reaction mechanisms (58, 60–62). Structural snapshots of protein complexes with substrate analogues and inhibitors have suggested inconsistent and controversial reaction mechanisms. In addition, the molecular details that link the catalytic chemistry to key kinetic, electronic, and structural events have remained elusive because of the difficulties associated with probing time-dependent structure-function aspects of such reactions in the presence of relatively large ligands such as peptide substrates.
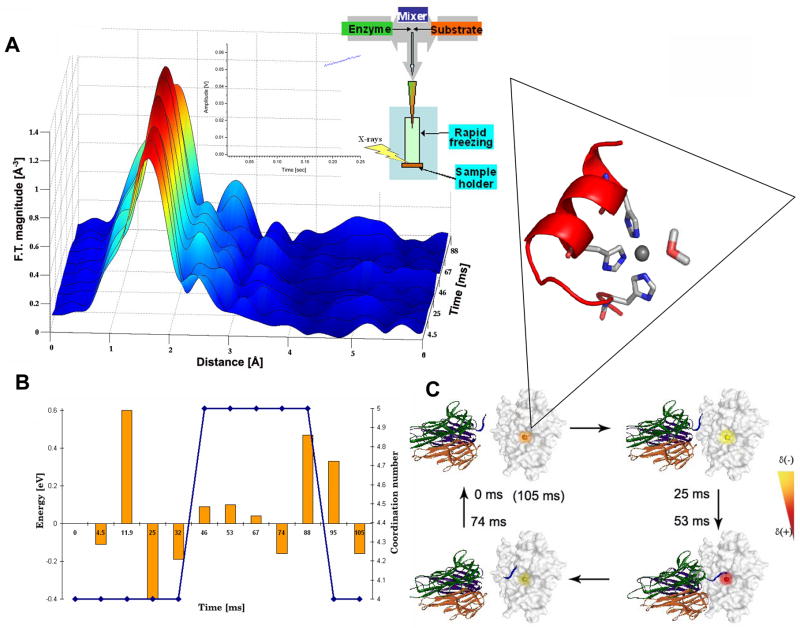
We have recently introduced a structural-dynamic experimental strategy to the field of metallaproteinases. Specifically, this strategy employs stopped-flow freeze-quench XAS in conjunction with transient kinetic studies to probe changes in the structure and reactivity of the catalytic zinc-protein complex residing in the catalytic cleft of metalloproteinases in real-time (Figure 3). XAS reports accurately on metal-protein bond distances, coordination numbers and total effective charges of designated metal centers residing in metalloproteins. XAS can be conducted on diluted protein solutions and in real-time (63).
Figure 3.
A) Schematic representation of a stopped-flow XAS experiment to monitor kinetic phases involved in the proteolytic cycle of TACE. This method allows to establish a correlation between the pre-steady state kinetic behavior of this enzyme (inset) and the structure of the evolving transient metal-protein intermediates, as well as local charge transitions of the metal ion. A) The enzyme is rapidly mixed with substrate in a stopped-flow apparatus (mixing time = 2 ms). The instrument is equipped with freeze-quench device. Enzyme/substrate time point samples are rapidly frozen (~3 ms) and mounted on an X-ray absorption sample holder. Inset: the pre-steady state kinetic trace is monitored via an in-line fluorescence detector and analyzed. X-ray absorption data are represented as a radial distribution versus time and dynamic spectral changes are then correlated with distinct kinetic phases of the reaction. No significant change in first shell peak intensity is observed during the lag phase. Remarkably, changes in first and second shell peak intensities are observed during the kinetic burst. These spectral changes are correlated with structural alternations of the catalytic zinc-protein complex. B) Schematic presentation of the curve fitting procedure employed for XAS data analysis. The change in the coordination of the zinc-protein complex is marked in blue. Changes in total effective charge, detected from the raw XAS data, are marked in orange. Remarkably, we observe a change in total effective charge of the zinc ion during the lag phase of the reaction, prior to substrate binding. This suggests the presence of long range communication pathways between the protein surface and the catalytic zinc environment. C) Schematic representation of the electronic changes detected by XAS within the active site of TACE prior to substrate binding and during proteolysis. Changes in zinc site electronics are designated via an artificial color scheme. The total effective charge of the catalytic zinc ion is modulated by a series of long-range conformational transitions induced by the interaction of TACE with membrane bound substrates such as TNF-α. Inset: the conserved catalytic zinc site of TACE. The zinc ion is bound to a water molecule and to three histidine ligands located within the active site of the enzyme.
Applying this methodology to TACE, we have quantified the structure, electronics, and lifetime of the evolving zinc-protein reaction intermediates during the peptide hydrolysis reaction using a peptide substrate (64). This allowed us to visualize changes in a catalytic center that researchers have, until now, been unable to investigate. Importantly, we correlated for the first time the distinct kinetic phases involved in the proteolytic reaction and key structural and electronic intermediates evolving at TACE active site. Using time-dependent XAS we have quantified local charge and metal-protein intermediates evolving within the catalytic metal site during distinct kinetic phases of the peptide hydrolysis reaction at the millisecond time-scale. Specifically, we have quantified changes in the catalytic zinc-protein bond distances, coordination number, and total effective charge occurring during kinetic lag and burst phases.
Unexpectedly, we found that the TACE reaction is governed by initial charge transitions of the catalytic zinc ion followed by predicted dynamic structural transformations of the tetrahedral catalytic zinc-protein complex. This is mediated by the binding of the peptide substrate to the zinc ion forming a penta-coordinated transient complex followed by product release and restoration of the tetrahedral zinc-protein complex. We demonstrated that the TACE catalytic zinc ion undergoes dynamic charge transitions prior to substrate binding to the metal ion. Thus, this work presents the importance of local charge transitions critical for proteolysis as well as long sought evidence for the proposed reaction model of peptide hydrolysis (58). Furthermore, the reported results reveal for the first time novel communication pathways taking place between distal protein sites and the enzyme catalytic core (such as substrate binding protein surfaces) and the catalytic machinery.
Using the catalytic zinc ion as a reporter, this work provides the first structural-kinetic reaction model of metalloproteinases and points out a potential new approach for drug design: selective inhibitors for TACE may be developed by targeting alternative sites different from the catalytic zinc site, in a way that blocks or critically perturbs the precursor electronic activity of the catalytic core in TACE. This approach departs from traditional drug design strategies used for metalloenzymes targeting the catalytic metal site with potent zinc chelating peptidomimetic compounds (65–74). It further suggests the use of protein or peptide inhibitors in addition to small synthetic compounds to block or constrain side chain conformational transitions occurring at catalytic exosites. This novel inhibition approach is based on the reported dynamic structural and electronic model of TACE during substrate hydrolysis but may be generalized to other related systems.
Future perspectives for the utilization of advanced technologies probing conformational dynamics
Revealing real-time structural kinetic and electronic features of TACE during substrate proteolysis extends our understanding of the physicochemical properties of TACE. The inherent protein flexibility observed in TACE may be mediated by a network of conformational transitions residing on different time scales which are utilized to direct and orient its relatively large substrates to the active site. Following on this argument, quantification of side chain conformation transitions and correlation with TACE catalytic events are of critical importance. Probing slow time scale events are of particular interest because functionally important biophysical processes, including enzyme catalysis, signal transduction, ligand binding and allosteric regulation are expected to occur within this time scale.
Application of kinetic crystallography techniques (62, 75–77) to study protein conformational transitions during enzyme catalysis adds an additional dimension to the structural information derived from static structural analysis. The detection of large protein conformational changes is somewhat limited, presumably by crystal packing. Yet, probing enzymes within a crystal matrix will greatly advance the field in providing a glimpse into protein cavity networks and ligand migration in confined protein moieties.
Structural dynamic investigation of local backbone fluctuations on the pico to nanosecond time scale have been the subject of detailed characterization using nuclear magnetic resonance (NMR) and molecular dynamic simulations. Slower motions, in the submicrosecond to second range, remained poorly understood. Relaxation dispersion has been used to only locate sites of conformational transitions between states exhibiting differences in chemical shifts (78). Recent advances in the NMR field have exploited the use of residual dipolar and hydrogen-bond scalar couplings to identify slow correlated motions in proteins (79–82). Combining this technique with molecular dynamic simulation to map native conformational landscapes in proteins brings the field towards the elucidation and quantification of slow motions in biomolecules.
In summary, the concept of detecting slow motions and large conformation transitions in proteins to understand protein function raises the notion that such information may also derived from low-resolution structural techniques such as small angle X-ray scattering (SAXS) spectroscopy. SAXS is a well-suited technique for the study of biopolymers in solution. Combining SAXS scattering profiles with computational tools provides the reconstruction of low-resolution three dimensional molecular models of small proteins as well as large clusters (83). Attempts to probe large conformational transitions of protein side chains by SAXS in real time are currently being developed by combining stopped flow techniques and computational analysis.
Conclusion
In this review, we argue that biophysical quantification of large, slow-moving protein conformational changes is of major importance for understanding molecular mechanisms and biological function. In addition, this information may be applied to the design of novel inhibitors aimed at selective conformation transitions rather than active site pocket chemistry. For enzymes like TACE, traditional structure-activity relationship approaches, although successful in the generation of initial leads showing efficacious in vivo readouts (65–74), have not delivered clinical candidates that met both safety and efficacy requirements needed for late development into marketed drugs for rheumatoid arthritis and other indications. The medical need remains unmet and fresh approaches to the problem of understanding mechanism of action and selectivity are highly desirable. Advancement in experimental procedures described here may be applied to study protein plasticity and flexibility aspects in TACE as well as in other related or non-related systems, in the search for such biochemical differentiation.
Figure 4.

Proposed mechanism for peptide hydrolysis by TACE. Panel a: kinetic lag-phase where changes in total effective charge of the zinc ion were detected. These events are supported by theoretical calculations proposing active site conformational transitions induced by the substrate upon its binding to the protease surface. Specifically, the substrate induces a conformational change at residue Glu406 that in turn enables it to perform base catalysis. Glu406 deprotonates a water molecule coordinated to the catalytic zinc ion, with generation of the hydroxyl nucleophile that attacks the Cα carbonyl group of the scissile bond. An oxyanion is formed by receiving the carbonyl electrons. Panel b: the nascent oxyanion is then coordinated to the catalytic zinc ion, thus lowering the transition state energy barrier while the extra proton on Glu406 is accepted by the amide group of the scissile bond. The last reaction step includes electron transfer from the oxyanion to the amide proton, effecting bond cleavage and substrate release. Binding of the substrate to the catalytic zinc ion forms a penta-coordinated zinc-protein complex. This complex and its life time are directly detected by time-resolved XAS during the burst phase of TACE enzyme kinetics. Curled lines: N-and C-termini of the substrate. (+) and (−):relative charge; curved arrows indicate charge flow. Dashed lines indicate hydrogen and coordination bonds.
Acknowledgments
We would like to acknowledge present and former students and postdocs of our groups for their diligent contributions to our research programs. We would also like to thank Drs. Ariel Solomon and Rotem Sertchook for help with figures. This work was partly financed by grants from the NIH (AR45949) to MEM, and the Israel Science Foundation, the Clotide and Mauricio Pontecorvo funds and by a research grant from Mr. and Mrs. Michael AMBACH to IS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.McGeehan GM, Becherer JD, Bast RC, Jr, Boyer CM, Champion B, Connolly KM, Conway JG, Furdon P, Karp S, Kidao S, McElroy AB, Nichols J, Pryzwansky KM, Schoenen F, Sekut L, Truesdale A, Verghese M, Warner J, Ways JP. Regulation of tumour necrosis factor-α processing by a metalloproteinase inhibitor. Nature. 1994;370:558–561. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- 2.Mohler KM, Sleath PR, Fitzner JN, Cerretti DP, Alderson M, Kerwar SS, Torrance DS, Otten-Evans C, Greenstreet T, Weerawarna K, Kronheim SR, Petersen M, Gerhart M, Kozlosky CJ, March CJ, Black RA. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 1994;370:218–220. doi: 10.1038/370218a0. [DOI] [PubMed] [Google Scholar]
- 3.Gearing AJH, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, Leber TM, Mangan M, Miller K, Nayee P, Owen K, Patel S, Thomas W, Wells G, Wood LM, Woolley K. Processing of tumour necrosis factor-α precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 4.Moss ML, Jin C, Milla ME, Bickett DM, Buckhart W, Carter HL, Chen WJ, Clay WC, Didsbury J, Hassler D, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Moyer M, Pahel GL, Rocque W, Seaton T, Su JL, Warner J, Willard D, Becherer D. Cloning of a disintegrin metalloproteinase that processes precursor tumor-necrosis factor-α. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 5.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour necrosis factor-α from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 6.Old LJ. Tumor necrosis factor. Science. 1985;230:630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- 7.Beutler B, Cerami A. Tumor necrosis, cachexia, shock and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 8.Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, Hariri RJ, Fahey TJ, III, Zentella A, Albert JD, Shires GT, Cerami A. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 9.Piguet PF, Grau GE, Allet B, Vasalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs-host disease. J Exp Med. 1987;166:1280–1289. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson PK, Gekker G, Chao CC, Hu S, Edelman C, Balfour HH, Jr, Verhof J. Human cytomegalovirus-stimulated peripheral blood mononuclear cells induce HIV-1 replication via a tumor necrosis factor-α-mediated mechanism. J Clin Invest. 1992;89:574–580. doi: 10.1172/JCI115623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams RO, Feldmann M, Maini R. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnaborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1279–80. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 13.Huovila APJ, Turner AJ, Pelto-Huikko M, Karkainen I, Ortiz RM. Shedding light on ADAM metalloproteinases. TIBS. 2005;30:413–22. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Blobel CP. ADAMs: key components in EGFR signaling and development. Nature Rev Mol Biol. 2005;15:598–606. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 15.Arribas J, Borroto A. Protein ectodomain shedding. Chem Rev. 2002;102:4627–4638. doi: 10.1021/cr010202t. [DOI] [PubMed] [Google Scholar]
- 16.Moss ML, Bartsch JW. Therapeutic benefits from targeting of ADAM family members. Biochemistry. 2004;43:7227–35. doi: 10.1021/bi049677f. [DOI] [PubMed] [Google Scholar]
- 17.Blobel CP. Functional and biochemical characterization of ADAMs and their predicted role in protein ectodomain shedding. Inflammation Res. 2002;51:83–84. doi: 10.1007/BF02684007. [DOI] [PubMed] [Google Scholar]
- 18.Wolfsberg TG, Primakoff P, Myles DG, White JM. ADAM, a novel family of membrane proteins containing a disintegrin and metalloprotease domain -multipotential functions in cell-cell and cell-matrix interactions. J Cell Biol. 1995;131:275–278. doi: 10.1083/jcb.131.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfsberg TG, Straight PD, Gerena RL, Huovila AP, Primakoff P, Myles DG, White JM. ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloproteinase domain. Devel Biol. 1995;69:378–83. doi: 10.1006/dbio.1995.1152. [DOI] [PubMed] [Google Scholar]
- 20.Venter JC, et al. The Sequence of the Human Genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 21.White JM. ADAMs: modulators of cell-cell and cell-matrix interactions. Curr Opin Cell Biol. 2003;15:598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Schlondorff J, Blobel CP. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci. 1999;112:3603–3617. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- 23.Killar L, White J, Black R, Peschon J. Adamalysins. A family of metzincins including the TNF-alpha converting enzyme (TACE) Ann NY Acad Sci. 1999;30:442–52. doi: 10.1111/j.1749-6632.1999.tb07701.x. [DOI] [PubMed] [Google Scholar]
- 24.Moss ML, Becherer JD, Milla ME, Pahel G, Lambert M, Andrews R, Frye S, Haffner C, Cowan D, Maloney P, Dixon EP, Jansen M, Mitchell JL, Leesnitzer T, Warner J, Conway J, Bickett DM, Bird M, Priest R, Reinhard J, Lin P. TNF-α convertase. In: Bradshaw D, Nixon JS, Bottomley K, editors. Metalloproteinases as Targets for Anti-inflammatory Drugs. Birkhauser Publishers; 1999. [Google Scholar]
- 25.Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: srtructures, evolution and diversification. FASEB J. 1998;12:1075–1095. [PubMed] [Google Scholar]
- 26.Becherer JD, Blobel CP. Biochemical properties and functions of membrane-anchored metalloprotease-disintegrin proteins (ADAMs) Curr Top Devel Biol. 2003;54:101–123. doi: 10.1016/s0070-2153(03)54006-6. [DOI] [PubMed] [Google Scholar]
- 27.Milla ME, Gonzales PE, Leonard JD. The TACE zymogen. Cell Biochem Biophys. 2006;44:342–8. doi: 10.1385/CBB:44:3:342. [DOI] [PubMed] [Google Scholar]
- 28.Fahrenholz F, Postina R. Alpha secretase activation. Neurodegener Dis. 2006;3:255–61. doi: 10.1159/000095264. [DOI] [PubMed] [Google Scholar]
- 29.Gonzales PE, Solomon A, Miller AB, Leesnitzer MA, Sagi I, Milla ME. Inhibition of the tumor necrosis factor-α-converting enzyme by its pro domain. J Biol Chem. 2004;279:31638–31645. doi: 10.1074/jbc.M401311200. [DOI] [PubMed] [Google Scholar]
- 30.Solomon A, Rosenblum G, Gonzales PE, Leonard JD, Mobashery S, Milla ME, Sagi I. Pronounced Chemical Diversity between the Catalytic Zinc Sites of TACE and MMPs Despite of their High Structural Homology. J Biol Chem. 2004:31646–31654. doi: 10.1074/jbc.M401310200. [DOI] [PubMed] [Google Scholar]
- 31.Leonard JD, Lin F, Milla ME. Chaperone-like properties of the prodomain of TACE and the functional role of its cysteine switch. Biochem J. 2005;387:797–805. doi: 10.1042/BJ20041727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milla ME, Leesnitzer MA, Moss ML, Clay WC, Carter HL, Miller AB, Su JL, Lambert MH, Willard DH, Sheeley DM, Kost TA, Burkhart W, Moyer M, Blackburn RK, Pahel GL, Mitchell JL, Hoffman CR, Becherer JD. Specific sequence determinants are required for the expression of functional tumor necrosis factor-α converting enzyme (TACE) J Biol Chem. 1999;274:30563–30570. doi: 10.1074/jbc.274.43.30563. [DOI] [PubMed] [Google Scholar]
- 33.Roghani M, Becherer JD, Moss ML, Atherton RE, Erdjument-Bromage H, Arribas J, Blackburn RK, Weskamp G, Tempst P, Blobel CP. Metalloprotease-disintegrin MDC9: intracellular maturation and catalytic activity. J Biol Chem. 1999;274:3531–3540. doi: 10.1074/jbc.274.6.3531. [DOI] [PubMed] [Google Scholar]
- 34.Anders A, Gilbert S, Garten W, Postina R, Fahrenholz F. Regulation of the alpha-secretase ADAM10 by its prodomain and proprotein convertases. FASEB J. 2001;15:1837–1839. doi: 10.1096/fj.01-0007fje. [DOI] [PubMed] [Google Scholar]
- 35.Loechel F, Overgaard MT, Oxvig C, Albrechtsen R, Wewer UM. Regulation of human ADAM 12 protease by the prodomain. Evidence for a functional cysteine switch. J Biol Chem. 1999;274:13427–13433. doi: 10.1074/jbc.274.19.13427. [DOI] [PubMed] [Google Scholar]
- 36.Sundberg C, Thodeti CK, Kveiborg M, Larsson C, Parker P, Albrechtsen R, Wewer UM. Regulation of ADAM12 cell-surface expression by PKC epsilon. J Biol Chem. 2004;279:51601–11. doi: 10.1074/jbc.M403753200. [DOI] [PubMed] [Google Scholar]
- 37.Wewer UM, Morgelin M, Holck P, Jacobsen J, Lydolph MC, Johnsen AH, Kveiborg M, Albrechtsen R. ADAM12 is a four-leafed clover: the excised prodomain remains bound to the mature enzyme. J Biol Chem. 2006;281:9418–22. doi: 10.1074/jbc.M513580200. [DOI] [PubMed] [Google Scholar]
- 38.Kang T, Zhao YG, Pei D, Sucic JF, Sang QXA. Intracellular activation of human adamalysin 19/disintegrin and metalloproteinase 19 by furin occurs via one of the two consecutive recognition sites. J Biol Chem. 2002;277:25583–25591. doi: 10.1074/jbc.M203532200. [DOI] [PubMed] [Google Scholar]
- 39.Howard L, Maciewicz RA, Blobel CP. Cloning and characterization of ADAM28: evidence for autocatalytic pro-domain removal and for cell surface localization of mature ADAM28. Biochemical J. 2000;348(Pt 1):21–7. [PMC free article] [PubMed] [Google Scholar]
- 40.Borroto A, Ruiz-Paz S, de la Torre TV, Borrell-Pages M, Merlos-Suarez A, Pandiella A, Blobel CP, Baselga J, Arribas J. Impaired trafficking and activation of tumor necrosis factor-alpha-converting enzyme in cell mutants defective in protein ectodomain shedding. J Biol Chem. 2003;278:25933–9. doi: 10.1074/jbc.M301673200. [DOI] [PubMed] [Google Scholar]
- 41.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;87:5578–82. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151. [PubMed] [Google Scholar]
- 43.Springman EB, Angleton EL, Birkedal-Hansen H, Van Wart HE. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc Natl Acad Sci USA. 1990;87:364–8. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen LC, Noelken ME, Nagase H. Disruption of the cysteine-75 and zinc ion coordination is not sufficient to activate the precursor of human matrix metalloproteinase 3 (stromelysin 1) Biochemistry. 1993;32:10289–95. doi: 10.1021/bi00090a003. [DOI] [PubMed] [Google Scholar]
- 45.Morgunova E, Tuuttila A, Bergmann U, Isupov M, Lindqvist Y, Schneider G, Tryggvason K. Structure of human pro-matrix metalloproteinase-2: activation mechanism revealed. Science. 1999;284:1667–1670. doi: 10.1126/science.284.5420.1667. [DOI] [PubMed] [Google Scholar]
- 46.Baker D, Sohl JL, Agard DA. A protein folding reaction under kinetic control. Nature. 1992;356:263–265. doi: 10.1038/356263a0. [DOI] [PubMed] [Google Scholar]
- 47.Cunningham EL, Jaswal SS, Sohl JL, Agard DA. Kinetic stability as a mechanism for protease longevity. Proc Natl Acad Sci USA. 1999;96:11008–11014. doi: 10.1073/pnas.96.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strausberg S, Alexander P, Wang L, Schwarz F, Bryan P. Catalysis of a protein folding reaction: thermodynamic and kinetic analysis of subtilisin BPN’ interactions with its propeptide fragment. Biochemistry. 1993;32:8112–8119. doi: 10.1021/bi00083a009. [DOI] [PubMed] [Google Scholar]
- 49.Eder J, Rheinnecker M, Fersht AR. Folding of subtilisin BPN’: characterization of a folding intermediate. Biochemistry. 1993;32:18–26. doi: 10.1021/bi00052a004. [DOI] [PubMed] [Google Scholar]
- 50.Gallagher T, Gilliland G, Wang L, Bryan P. The prosegment-subtilisin BPN’ complex: crystal structure of a specific ‘foldase’. Structure. 1995;3:907–914. doi: 10.1016/S0969-2126(01)00225-8. [DOI] [PubMed] [Google Scholar]
- 51.Bryan P, Wang L, Hoskins J, Ruvinov S, Strausberg S, Alexander P, Almog O, Gilliland G, Gallagher T. Catalysis of a protein folding reaction: mechanistic implications of the 2.0 A structure of the subtilisin-prodomain complex. Biochemistry. 1995;34:10310–10318. doi: 10.1021/bi00032a026. [DOI] [PubMed] [Google Scholar]
- 52.Strange RW, Hasnain SS. Combined use of XAFS and crystallography for studying protein-ligand interactions in metalloproteins. Methods Mol Biol. 2005;305:167–96. doi: 10.1385/1-59259-912-5:167. [DOI] [PubMed] [Google Scholar]
- 53.Hodgson J. Remodeling MMPIs. Bio/Technology. 1995;13:554–557. doi: 10.1038/nbt0695-554. [DOI] [PubMed] [Google Scholar]
- 54.Docherty AJ, Crabbe T, O’Connell JP, Groom CR. Proteases as drug targets. Biochem Soc Symp. 2003;70:147–161. doi: 10.1042/bss0700147. [DOI] [PubMed] [Google Scholar]
- 55.Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, Stephens PE, Shelley C, Hutton M, Knauper V, Docherty AJP, Murphy G. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Letters. 1998;435:39–44. doi: 10.1016/s0014-5793(98)01031-x. [DOI] [PubMed] [Google Scholar]
- 56.Maskos K, Fernandez-Catalan C, Huber R, Bourenkov GP, Bartunik H, Ellestad GA, Reddy P, Wolfson MF, Rauch CT, Castner BJ, Davis R, Clarke HRG, Petersen M, Fitzner JM, Cerretti DP, March CJ, Paxton RJ, Black RA, Bode W. Crystal structure of the catalytic domain of human tumor necrosis factor-α converting enzyme. Proc Natl Acad Sci USA. 1998;95:3408–3412. doi: 10.1073/pnas.95.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benkovic SJ, Hammes-Schiffer S. A perspective on enzyme catalysis. Science. 2003;301:1196–1202. doi: 10.1126/science.1085515. [DOI] [PubMed] [Google Scholar]
- 58.Christianson DW, Lipscomb WN. Carboxypptidase-A. Accounts of Chemical Research. 1989;22:62–69. [Google Scholar]
- 59.Kilshtain-Vardi A, Shoham G, Goldblum A. Mechanism of action of zinc proteinases: A MNDO/d/H study of alternative general-acid general-base catalytic pathways for carboxypeptidase-A. Intl J Q Chem. 2002;88:87–98. [Google Scholar]
- 60.Kotra LP, Cross JB, Shimura Y, Fridman R, Schlegel HB, Mobashery S. Insight into the complex and dynamic process of activation of matrix metalloproteinases. J Am Chem Soc. 2001;123:3108–13. doi: 10.1021/ja001896a. [DOI] [PubMed] [Google Scholar]
- 61.Pelmenschikov V, Siegbahn PE. Catalytic mechanism of matrix metalloproteinases: two-layered ONIOM study. Inorg Chem. 2002;41:5659–66. doi: 10.1021/ic0255656. [DOI] [PubMed] [Google Scholar]
- 62.Bertini I, Calderone V, Fragai M, Luchinat C, Maletta M, Yeo KJ. Snapshots of the reaction mechanism of matrix metalloproteinases. Angew Chem Int Ed Engl. 2006;45:7952–5. doi: 10.1002/anie.200603100. [DOI] [PubMed] [Google Scholar]
- 63.Kleifeld O, Frenkel A, Martin JML, Sagi I. Active site electronic and structure and dynamics during metalloenzyme catalysis. Nature Struct Biol. 2003;10:98–102. doi: 10.1038/nsb889. [DOI] [PubMed] [Google Scholar]
- 64.Solomon A, Akabayov B, Frenkel A, Milla ME, Sagi I. The TNFα converting enzyme recognizes and hydrolyzes peptides via distinct active site electronics and catalytic intermediates. Proc Natl Acad Sci USA. 2007;104 in press. [Google Scholar]
- 65.Xue CB, He X, Corbett RL, Roderick J, Wasserman ZR, Liu RQ, Jaffee BD, Covington MB, Qian M, Trzaskos JM, Newton RC, Magolda RL, Wexler RR, Decicco CP. Discovery of macrocyclic hydroxamic acids containing biphenylmethyl derivatives at P1’, a series of selective TNF-alpha converting enzyme inhibitors with potent cellular activity in the inhibition of TNF-alpha release. J Med Chem. 2001;44:3351–3354. doi: 10.1021/jm0155502. [DOI] [PubMed] [Google Scholar]
- 66.Rabinowitz MH, Andrews RC, Becherer JD, Bickett DM, Bubacz DG, Conway JG, Cowan DJ, Gaul M, Glennon K, Lambert MH, Leesnitzer MA, McDougald DL, Moss ML, Musso DL, Rizzolio MC. Design of selective and soluble inhibitors of tumor necrosis factor-alpha converting enzyme (TACE) J Med Chem. 2001;44:4252–4267. doi: 10.1021/jm0102654. [DOI] [PubMed] [Google Scholar]
- 67.Duan JJ, Chen L, Wasserman ZR, Lu Z, Liu RQ, Covington MB, Qian M, Hardman KD, Magolda RL, Newton RC, Christ DD, Wexler RR, Decicco CP. Discovery of gamma-lactam hydroxamic acids as selective inhibitors of tumor necrosis factor alpha converting enzyme: design, synthesis, and structure-activity relationships. J Med Chem. 2002;45:4954–4957. doi: 10.1021/jm0255670. [DOI] [PubMed] [Google Scholar]
- 68.Xue CB, Voss ME, Nelson DJ, Duan JJ, Cherney RJ, Jacobson IC, He X, Roderick J, Chen L, Corbett RL, Wang L, Meyer DT, Kennedy K, DeGrado WF, Hardman KD, Teleha CA, Jaffee BD, Liu RQ, Copeland RA, Covington MB, Christ DD, Trzaskos JM, Newton RC, Magolda RL, Wexler RR, Decicco CP. Design, synthesis, and structure-activity relationships of macrocyclic hydroxamic acids that inhibit tumor necrosis factor alpha release in vitro and in vivo. J Med Chem. 2001;44:2636–2660. doi: 10.1021/jm010127e. [DOI] [PubMed] [Google Scholar]
- 69.Levin JI, Chen JM, Du MT, Nelson FC, Killar LM, Skala S, Sung A, Jin G, Cowling R, Barone D, March CJ, Mohler KM, Black RA, Skotnicki JS. Anthranilate sulfonamide hydroxamate TACE inhibitors. Part 2: SAR of the acetylenic P1’ group. Bioorg Med Chem Lett. 2002;12:1199–1202. doi: 10.1016/s0960-894x(02)00136-1. [DOI] [PubMed] [Google Scholar]
- 70.Nelson FC, de los Santos E, Levin JI, Chen JM, Skotnicki JS, DiJoseph JF, Sharr MA, Sung A, Killar LM, Cowling R, Jin G, Roth CE, Albright JD. Benzodiazepine inhibitors of the MMPs and TACE. Bioorg Med Chem Lett. 2002;12:2867–2870. doi: 10.1016/s0960-894x(02)00633-9. [DOI] [PubMed] [Google Scholar]
- 71.Musso DL, Andersen MW, Andrews RC, Austin R, Beaudet EJ, Becherer JD, Bubacz DG, Bickett DM, Chan JH, Conway JG, Cowan DJ, Gaul MD, Glennon KC, Hedeen KM, Lambert MH, Leesnitzer MA, McDougald DL, Mitchell JL, Moss ML, Rabinowitz MH, Rizzolio MC, Schaller LT, Stanford JB, Tippin T, Warner JR, Whitesell LG, Wiethe RW. N-hydroxyformamide peptidomimetics as TACE/matrix metalloprotease inhibitors: oral activity via P1’ isobutyl substitution. Bioorg Med Chem Lett. 2001;11:2147–2151. doi: 10.1016/s0960-894x(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 72.Conway JG, Andrews RC, Beaudet B, Bickett DM, Boncek V, Brodie TA, Clark RL, Crumrine RC, Leesnitzer MA, McDougald DM, Han B, Hedeen K, Lin P, Milla M, Moss M, Pink H, Rabinowitz MH, Tippin T, Scates PW, Selph J, Stimpson SA, Warner J, Becherer JD. Inhibition of tumor necrosis factor-alpha (TNF-alpha) production and arthritis in the rat by GW3333, a dual inhibitor of TNF-alpha-converting enzyme and matrix metalloproteinases. J Pharmacol Exp Ther. 2001;298:900–908. [PubMed] [Google Scholar]
- 73.Thabet MM, Huzinga TW. Drug evaluation: Apratastat, a novel TACE/MMP inhibitor for rheumatoid arthritis. Curr Opin Investig Drugs. 2006;7:1014–9. [PubMed] [Google Scholar]
- 74.Duan JJ, Chen L, Lu Z, Jiang B, Asakawa N, Sheppeck JE, Liu RQ, Covington MB, Pitts W, Kim SH, Decicco CP. Discovery of low nanomolar non-hydroxamate inhibitors of tumor necrosis factor-alpha converting enzyme (TACE) Bioorg Med Chem Lett. 2007;17:266–71. doi: 10.1016/j.bmcl.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 75.Bourgeois D, Royant A. Advances in kinetic protein crystallography. Curr Opin Struct Biol. 2005;15:538–47. doi: 10.1016/j.sbi.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Meilleur F, Myles DA, Blakeley MP. Neutron Laue Macromolecular Crystallography. Eur Biophys J. 2006;35:611–20. doi: 10.1007/s00249-006-0074-6. [DOI] [PubMed] [Google Scholar]
- 77.Bourgeois D, Vallone B, Arcovito A, Sciara G, Schotte F, Anfinrud PA, Brunori M. Extended subnanosecond structural dynamics of myoglobin revealed by Laue crystallography. Proc Natl Acad Sci USA. 2006;103:4924–9. doi: 10.1073/pnas.0508880103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bernado P, Blackledge M. Local dynamic amplitudes on the protein backbone from dipolar couplings: toward the elucidation of slower motions in biomolecules. J Am Chem Soc. 2004;126:7760–1. doi: 10.1021/ja048785m. [DOI] [PubMed] [Google Scholar]
- 79.Tolman JR, Ruan K. NMR residual dipolar couplings as probes of biomolecular dynamics. Chem Rev 2006. 2006;106:1720–36. doi: 10.1021/cr040429z. [DOI] [PubMed] [Google Scholar]
- 80.Meiler J, Prompers JJ, Griesinger C, Bruschweiler R. Model-free approach to the dynamic interpretation of residual dipolar couplings in globular proteins. J Am Chem Soc. 2001;123:6098–107. doi: 10.1021/ja010002z. [DOI] [PubMed] [Google Scholar]
- 81.Bouvignies G, Bernado P, Meier S, Cho K, Grzesiek S, Bruschweiler R, Blackledge M. Identification of slow correlated motions in proteins using residual dipolar and hydrogen-bond scalar couplings. Proc Natl Acad Sci USA. 2005;102:13885–90. doi: 10.1073/pnas.0505129102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bernado P, Blanchard L, Timmins P, Marion D, Ruigrok RW, Blackledge M. A structural model for unfolded proteins from residual dipolar couplings and small-angle x-ray scattering. Proc Natl Acad Sci USA. 2005;102:17002–7. doi: 10.1073/pnas.0506202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koch MH, Vachette P, Svergun DI. Small-angle scattering: a view on the properties, structures and structural changes of biological macromolecules in solution. Q Rev Biophys. 2003;36:147–227. doi: 10.1017/s0033583503003871. [DOI] [PubMed] [Google Scholar]