Abstract
Schistosomiasis is among the most prevalent parasitic infections worldwide. However, current Global Burden of Disease (GBD) disability-adjusted life year estimates indicate that its population-level impact is negligible. Recent studies suggest that GBD methodologies may significantly underestimate the burden of parasitic diseases, including schistosomiasis. Furthermore, strain-specific disability weights have not been established for schistosomiasis, and the magnitude of human disease burden due to Schistosoma japonicum remains controversial. We used a decision model to quantify an alternative disability weight estimate of the burden of human disease due to S. japonicum. We reviewed S. japonicum morbidity data, and constructed decision trees for all infected persons and two age-specific strata, <15 years (y) and ≥15 y. We conducted stochastic and probabilistic sensitivity analyses for each model. Infection with S. japonicum was associated with an average disability weight of 0.132, with age-specific disability weights of 0.098 (<15 y) and 0.186 (≥15 y). Re-estimated disability weights were seven to 46 times greater than current GBD measures; no simulations produced disability weight estimates lower than 0.009. Nutritional morbidities had the greatest contribution to the S. japonicum disability weight in the <15 y model, whereas major organ pathologies were the most critical variables in the older age group. GBD disability weights for schistosomiasis urgently need to be revised, and species-specific disability weights should be established. Even a marginal increase in current estimates would result in a substantial rise in the estimated global burden of schistosomiasis, and have considerable implications for public health prioritization and resource allocation for schistosomiasis research, monitoring, and control.
Author Summary
Schistosomiasis is a parasitic infection caused by a flatworm that disproportionately affects the world's poorest populations. Schistosomiasis is one of the most common infections worldwide, affecting over 207 million people in 76 countries. Current international estimates indicate that schistosomiasis has a minimal impact at the population level. This has contributed to its low prioritization in global health and subsequent resource allocation for disease control. However, recent studies indicate that these measures underestimate the extent of neglected tropical diseases, including schistosomiasis. Despite World Health Organization recommendations, the burden of schistosomiasis has not been re-examined in over a decade, and there are no established estimates for different types of schistosomiasis. The impact of symptoms associated with the Asian strain, Schistosoma japonicum, remains controversial. This study was conducted to provide an alternate measure of the burden of S. japonicum. We reviewed the literature and calculated a summary estimate for S. japonicum which was seven to 46 times greater than current measures for schistosomiasis. Findings suggest that current measures severely underestimate the extent of schistosomiasis, and urgently need to be revised. Further research is needed to examine the burden of schistosomiasis and other forgotten tropical diseases affecting the world's poorest people in endemic countries.
Introduction
Schistosomiasis is one of the most prevalent parasitic infections worldwide. An estimated 779 million people are at risk for schistosomiasis, with 207 million infected in 76 countries and territories [1],[2]. Approximately 120 million people are symptomatic and 20 million have severe and debilitating disease [3]–[5]. Schistosomiasis accounts for 1.7 [6],[7] to 4.5 million disability-adjusted life years (DALYs) [8] lost each year worldwide, among the highest of all neglected tropical diseases.
Schistosomiasis japonica is caused by the trematode Schistosoma japonicum. Schistosome egg deposition in tissue and subsequent inflammatory immune response result in extensive clinical manifestations, including hepatomegaly, splenomegaly, and liver fibrosis [9]–[16], as well as “subtle” morbidities such as anemia, diarrhea, growth retardation, and cognitive deficits [10], [17]–[21]. Schistosomiasis japonica may be more pathogenic compared to other schistosomes affecting humans, due to comparatively higher egg production [22]. However, the burden of human disease due to S. japonicum infection is not well-established.
Global Burden of Disease (GBD) estimates indicate that the population-level impact of schistosomiasis is negligible. Schistosomiasis accounts for only 0.1% of the global burden of disease [23]. A major limitation of the GBD burden estimates for schistosomiasis is their restriction to the period of acute infection, excluding a number of chronic, severe, and debilitating morbidities, such as liver cirrhosis and cognitive deficits, which were included in disability estimations for other infections. As a result, age-specific GBD disability weights were estimated to be 0.005 for those <15 years (y) of age and 0.006 for those ≥15 y, on a scale from 0 (no impairment) to 1 (death) [8],[17],[19],[24]. Similar GBD weights have been assigned to relatively minor conditions such as facial vitiligo [8],[17],[19],[24]. In contrast, some studies [19],[24] have suggested that the actual burden of schistosomiasis is several-fold higher than current GBD estimates [8],[17],[19],[24]. For example, a recent systematic review and meta-analysis of all schistosome strains [19] and a community-based study of chronic schistosomiasis japonica in China [16] concluded that the GBD disability weights underestimate the extent of disability due to schistosomiasis; re-estimated disability weights for schistosomiasis were four to 30 times higher than current GBD measures [19], [23], [25]–[27].
Another limitation of the GBD study [23], [25]–[27] and subsequent schistosomiasis burden assessments [19],[24] was their estimation of disability weights for all three major schistosome species (S. japonicum, S. mansoni and S. haematobium) together, despite their distinct pathophysiology and associated morbidities. A recent community-based study in China using a standard quality of life measurement scale (EuroQol) suggested that species-specific estimation of disability weights for schistosomiasis is warranted, and that GBD values aggregated across all schistosome species may underestimate the disability associated with S. japonicum [16]. However, this study excluded nutritional and neurological morbidities, and may therefore still underestimate the burden of human disease due to S. japonicum infection.
The burden of schistosomiasis has not been re-examined in over a decade, despite three revisions to the GBD study [25]–[27] and a strong recommendation from the World Health Organization (WHO) [8]. Additionally, there is a lack of international consensus in disease burden assessment criteria, disability weights, and estimated burden for schistosomiasis. For example, in 2002, a WHO Technical Report recalculated the global burden of schistosomiasis at 4.5 million DALYs, and asserted that the previous estimate of 1.7 million DALYs lost to schistosomiasis (2001) “represents a serious underestimate and should be revised” [8]. However, the WHO continues to report the 1.7 million DALYs figure from 2001 [6],[7]. Further, there is a more than ten-fold difference between GBD and WHO estimates for schistosomiasis-related mortality, or 15,000 to 280,000 deaths per year [8],[23].
At present, no global species-specific burden assessment exists for schistosomiasis. The burden due to S. japonicum remains controversial and warrants further investigation. This study was conducted to quantify age-specific disability weight estimates for the burden of human disease due to S. japonicum infection using a decision model approach.
Methods
Literature Search Strategy
We conducted a structured literature search using MEDLINE electronic database to identify published studies from Jan 1, 1966 to May 1, 2007. A detailed summary of key words and search headings are provided in Appendix S1 . Additional sources were identified from bibliographies of published studies, hand searches of scientific meeting abstracts, expert committee reports, and unpublished manuscripts and theses at Brown University, United States. We also solicited unpublished studies from known schistosomiasis japonica researchers via e-mail to minimize publication bias. These sources were retrieved, collected, indexed, and assessed for morbidity and disability outcome data.
The initial inclusion criteria for this review were the availability of an abstract and a focus on human infection with schistosomiasis. The abstracts of all remaining studies were reviewed and the following inclusion criteria were applied: (i) focus on S. japonicum; (ii) human studies; (iii) experimental or observational (treatment, morbidity) study designs; and (iv) description of S. japonicum morbidity measures and their respective prevalences and/or disability weights ( Figure 1 ). Additional information on publication date, author, location, population, study type, and relevant outcomes were recorded. Where available, we collected morbidity estimates based upon standardized clinical physiological data and WHO diagnostic criteria [28] such as grades I through III clinical classification for hepatic fibrosis and cirrhosis [29] or Hackett score ≥2 to define splenomegaly [30]. Although major organ pathologies such as hepatic and spleen morbidities have not been extensively studied in schistosomiasis, these morbidities were included as disability-related outcomes in the GBD disease burden assessment for other conditions. We included these morbidities in this assessment of disability-related outcomes in S. japonicum, based on our a priori hypothesis that they may impose a heavy burden on infected individuals.
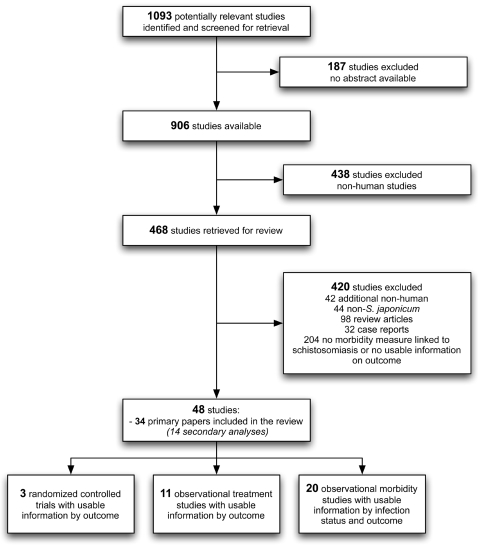
Figure 1. Literature search strategy.
Shown is a diagrammatic representation of the retrieval strategy used for identifying and selecting studies for inclusion in the final analysis.
Model Structure
We identified a broad spectrum of morbidities associated with S. japonicum, namely: diarrhea, gastrointestinal bleeding, abdominal pain, hepatomegaly (mild/moderate, severe), hepatic fibrosis and cirrhosis (mild, moderate, severe), splenomegaly, cognitive deficits, stunting, wasting, anemia (mild, moderate, severe), central nervous system disease (i.e., including non-epilepsy neurological manifestations), and epilepsy.
Decision trees were used to systematically combine a large number of prevalence and disability data for S. japonicum morbidities [31]. The disability weight for schistosomiasis was estimated in three models to represent all ages, those aged <15 y and those aged ≥15 y, in order to facilitate comparison with the GBD study. For each model, the disability weight was estimated as:
where PmorbidityA represents the probability of the Ath morbidity, DmorbidityA represents the disability weight associated with the Ath morbidity, and N is the total number of morbidities considered.
In general, we structured the decision trees to allow for all plausible combinations of morbidities, with the presence or absence of any single morbidity considered independently from other morbidities, with the exception of liver morbidity. Our model was therefore a series of successive binary branches, each depicting the presence or absence of a single morbidity. This means that although the probability of developing each morbidity was less than 1 by definition, the sum of all morbidity probabilities could exceed 1.
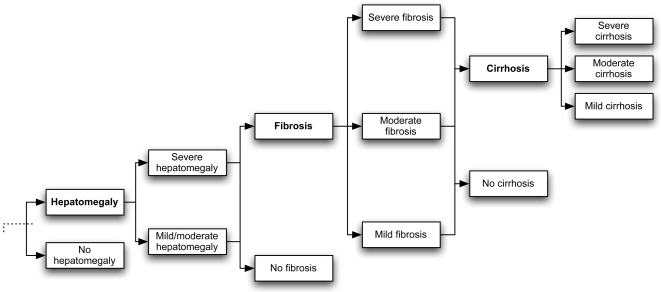
Based on available hepatic pathophysiology data, we conservatively structured the model to restrict liver pathologies according to usual liver disease progression (i.e. hepatomegaly to fibrosis to cirrhosis), and allow for the co-occurrence of only hepatomegaly and fibrosis. This is because, as the disease progresses to more severe pathology, the associated disability weight also increases. Therefore, if a combination of several liver co-morbidities had been allowed, the disability weight for liver disease could be overestimated. Figure 2 illustrates the branch of the decision tree for the liver pathologies.
Figure 2. Schematic representation of the model.
Shown is the branch of the model depicting liver pathology, which may or may not be present. If present, there may be hepatomegaly of varying degrees. Regardless of the degree of hepatomegaly, fibrosis may exist. Cirrhosis could only occur when fibrosis was present. Other comorbidities did not depend on the presence of other conditions.
Model Inputs
Probabilities
Prevalences of S. japonicum morbidities were used as the measure of occurrence, instead of incidence rates, due to the unavailability of incidence estimates and to allow comparability with prior studies [19], [23], [25]–[27]. Since prevalence is proportional to the incidence multiplied by the duration of the morbidity, measures reflected in these model inputs represent a combination of the time spent in a morbidity state and its actual occurrence.
For each morbidity, the lowest prevalence estimate identified from the literature was used as the average value of a beta (β) distribution. The lowest estimates were systematically selected for the lowest values, in order to undertake a highly conservative approach and foster comparability of findings to the GBD study. The range of each morbidity prevalence estimate reported in the literature was used to represent the variance of the β distribution. The β distribution was selected because it satisfied the a priori requirements to restrict the probability estimate range between 0 and 1, and enabled calculation and summarization of available prevalence data and the level of uncertainty associated with these estimates [32].
Disability Weights
We examined the original and revised GBD assessments to identify disability weights for morbidities associated with S. japonicum [23], [25]–[27]. For example, although schistosome infection is the only sequela considered in the GBD assessment for schistosomiasis, morbidities such as diarrhea, anemia, cognitive deficits, and cirrhosis are available in burden evaluations of other parasitic diseases (e.g., ascariasis). We compiled these disability weight estimates, supplemented findings with data derived from literature sources, and consulted experts working on schistosomiasis japonica in countries where this disease is endemic (e.g., the Philippines and China) and from the United States, to confirm conservative proxy disability weight estimates. For each morbidity, an approach similar to the one described for the prevalence of each morbidity was used to represent the value and uncertainty of the disability weights.
Base-Case Analyses
We calculated a baseline disability weight for each model using the most conservative mean estimates for disability weight inputs for all morbidities. We re-evaluated each model after systematically excluding each input to evaluate the independent contribution of each variable to the overall disability weight estimate. Model inputs were considered critical variables if they contributed more than 10% to the overall disability weight.
Sensitivity Analyses
One-way
We performed deterministic one-way sensitivity analyses for each variable, for both probability and disability weight inputs, in order to assess the degree to which variation in each model input altered findings. We re-evaluated each model at the lower and upper bounds of the 95% confidence interval (CI) for each input, and observed subsequent modifications to the model disability weight. Model inputs were considered critical variables if alteration resulted in at least 10% change in the overall disability weight.
Multi-way
We performed multi-way deterministic sensitivity analyses to explore the most extreme values for disability weight estimates in overall and age-specific models. In the conservative and pessimistic scenarios, we simultaneously considered the respective lower and upper bounds of the 95% CIs for all probability and disability weight inputs, respectively.
Probabilistic
Deterministic analysis is typically limited in its consideration of a small number of model inputs with fixed values. Conversely, probabilistic analyses allow all inputs to vary simultaneously according to their distributions. We performed probabilistic sensitivity analyses with 5,000 Monte Carlo simulations for each of the three models. In each simulation, the value for each model input was selected at random from its specified distribution. In each case, we obtained a probability density distribution for schistosomiasis japonica disability weight. We summarized the results across all simulations by graphing the mean, standard deviation, and ranges of disability weights over 5,000 Monte Carlo analyses in each model [33].
Results
Model Inputs
The retrieval search strategy for study selection and details regarding study inclusion and exclusion in this analysis are diagrammatically represented in Figure 1 . We identified 1093 potentially relevant studies from 1966 to May 1, 2007. We excluded 187 studies because no abstract was available. From the remaining 906 publications, 438 non-human studies were excluded, and 468 studies were retrieved for a detailed review. After careful examination of the publications, a total of 420 studies were excluded, including: 42 additional non-human studies, 44 non-S. japonicum, 32 case reports, 98 review articles, and 204 with no morbidity measure linked to schistosomiasis and/or no useable information on morbidity outcomes. A total of 48 studies met the inclusion criteria for this assessment, including 34 primary publications and 14 secondary papers from the same studies ( Figure 1 ).
Model Disability Weight
Base-Case Analyses
The average probability and disability weight values used in the base-case analyses are provided in Table 1 . There were more publications investigating the occurrence of schistosomiasis-associated diarrhea, anemia, hepatomegaly, liver fibrosis, and splenomegaly, compared to publications which examined schistosomiasis-associated nutritional morbidities (e.g., wasting), cognitive function, cirrhosis, and neurological manifestations.
Table 1. Probability and disability weight estimates used in the base model to estimate the disability weight of S. japonicum .
| Probabilities 1 | Disability weights 1 | References | |||||
| Overall | <15 years | ≥15 years | Overall | <15 years | ≥15 years | ||
| Diarrhea | 0.120 | 0.146 | 0.059 | 0.097 | 0.107 | 0.087 | [13], [15], [19], [23], [38], [39], [43]–[45] |
| Gastrointestinal bleeding | 0.070 | 0.070 | 0.070 | 0.010 | 0.010 | 0.010 | [46]–[48],[49] |
| Abdominal pain | 0.162 | 0.137 | 0.187 | 0.060 | 0.060 | 0.060 | [13], [15], [45], [46], [48], [50]–[54] |
| Mild/moderate hepatomegaly | 0.430 | 0.449 | 0.735 | 0.060 | 0.060 | 0.060 | [12], [13], [15], [43], [46], [48], [50], [52], [53], [55]–[72] |
| Severe hepatomegaly | 0.240 | 0.125 | 0.232 | 0.070 | 0.070 | 0.070 | |
| Mild fibrosis | 0.320 | 0.427 | 0.492 | 0.060 | 0.060 | 0.060 | [15], [18], [43], [46], [57], [58], [61], [62], [70], [72]–[74] |
| Moderate fibrosis | 0.130 | 0.121 | 0.604 | 0.060 | 0.060 | 0.060 | |
| Severe fibrosis | 0.060 | 0.023 | 0.265 | 0.070 | 0.070 | 0.070 | |
| Mild cirrhosis | 0.040 | - | 0.184 | 0.330 | 0.330 | 0.330 | [23],[45],[46],[75] |
| Moderate cirrhosis | 0.020 | - | 0.041 | 0.330 | 0.330 | 0.330 | |
| Severe cirrhosis | 0.020 | - | 0.020 | 0.330 | 0.330 | 0.330 | |
| Splenomegaly | 0.220 | 0.236 | 0.248 | 0.070 | 0.070 | 0.070 | [13], [15], [43], [44], [46], [50], [55]–[58], [60], [61], [63]–[65],[67],[70],[72],[76] |
| Cognitive deficits | 0.070 | 0.070 | 0.035 | 0.024 | 0.024 | 0.024 | [23],[46],[77] |
| Stunting | 0.100 | 0.290 | 0.100 | 0.017 | 0.024 | - | [10], [18], [20], [21], [23], [46], [49], [50], [77]–[79], [80]–[82] |
| Wasting | 0.095 | 0.235 | 0.070 | 0.030 | 0.053 | - | |
| Mild anemia | 0.140 | 0.491 | 0.165 | 0.000 | 0.000 | 0.000 | [18],[20],[23],[46],[49],[54],[80],[83] |
| Moderate anemia | 0.100 | 0.306 | 0.105 | 0.011 | 0.011 | 0.120 | |
| Severe anemia | 0.050 | 0.100 | 0.050 | 0.110 | 0.111 | 0.111 | |
| CNS disease | 0.026 | - | 0.026 | 0.070 | 0.070 | 0.070 | [46],[84] |
| Epilepsy | 0.021 | - | 0.021 | 0.125 | 0.099 | 0.150 | [23],[46],[84] |
S. japonicum was associated with an average disability weight of 0.130 in the overall model, with age-specific disability weights of 0.098 in the <15 y model and 0.198 for the ≥15 y model. In the all-ages model, major organ pathologies contributed to the largest proportion of the total disability weight, including probability and disability weight estimates for mild/moderate and severe hepatomegaly, mild fibrosis, mild cirrhosis, and splenomegaly ( Table 2 ). The contributions of the different morbidities also varied by age. In the <15 y model, diarrhea, wasting and severe anemia also contributed to a large proportion of the S. japonicum disability weight, in addition to mild/moderate hepatomegaly and splenomegaly. In contrast, major organ pathologies were the most important variables in the older cohort model, including mild/moderate hepatomegaly, mild cirrhosis, and splenomegaly ( Table 2 ).
Table 2. Proportion of model disability weight attributable to each morbidity.
| Overall | <15 | ≥15 | ||||
| Contribution to model disability weight | % of total disability weight (0.130) | Contribution to model disability weight | % of total disability weight (0.098) | Contribution to model disability weight | % of total disability weight (0.186) | |
| Diarrhea | 0.010 | 8 | 0.014 | 14 | 0.004 | 2 |
| Gastrointestinal bleeding | 0.010 | 8 | 0.001 | 1 | 0.001 | 1 |
| Abdominal pain | 0.009 | 7 | 0.007 | 7 | 0.009 | 5 |
| Mild/moderate hepatomegaly | 0.014 | 11 | 0.018 | 18 | 0.023 | 12 |
| Severe hepatomegaly | 0.015 | 12 | 0.007 | 7 | 0.011 | 6 |
| Mild fibrosis | 0.014 | 11 | 0.008 | 8 | 0.005 | 3 |
| Moderate fibrosis | 0.006 | 5 | 0.003 | 3 | 0.015 | 8 |
| Severe fibrosis | 0.003 | 2 | 0.001 | 1 | 0.010 | 5 |
| Mild cirrhosis | 0.013 | 10 | 0.000 | 0 | 0.054 | 27 |
| Moderate cirrhosis | 0.006 | 5 | 0.000 | 0 | 0.013 | 7 |
| Severe cirrhosis | 0.006 | 5 | 0.000 | 0 | 0.006 | 3 |
| Splenomegaly | 0.014 | 11 | 0.015 | 15 | 0.014 | 7 |
| Cognitive deficits | 0.001 | 1 | 0.002 | 2 | 0.001 | 1 |
| Stunting | 0.001 | 1 | 0.006 | 6 | 0.000 | 0 |
| Wasting | 0.002 | 2 | 0.011 | 11 | 0.000 | 0 |
| Mild anemia | 0.000 | 0 | 0.000 | 0 | 0.000 | 0 |
| Moderate anemia | 0.001 | 1 | 0.002 | 2 | 0.001 | 1 |
| Severe anemia | 0.005 | 4 | 0.010 | 10 | 0.005 | 3 |
| CNS disease | 0.002 | 2 | 0.000 | 0 | 0.001 | 1 |
| Epilepsy | 0.002 | 2 | 0.000 | 0 | 0.003 | 2 |
Sensitivity Analyses
One-way
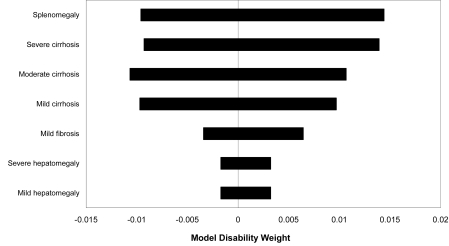
The overall model was sensitive to probability estimates for mild/moderate and severe hepatomegaly, moderate and severe cirrhosis, and splenomegaly; and disability weight estimates for mild/moderate and severe hepatomegaly and mild fibrosis ( Figure 3 ). As expected, the one-way sensitivity analyses varied by age. The <15 y model was most sensitive to probability and disability estimates for mild/moderate hepatomegaly and splenomegaly and to additional disability weight estimates for wasting and severe anemia. The ≥15 y model was also sensitive to probability and disability estimates for mild/moderate hepatomegaly, mild cirrhosis, and splenomegaly, and to the additional disability weight for moderate fibrosis.
Figure 3. One-way sensitivity analysis.
The horizontal bars depict the effect of re-evaluating the disability weight (shown on the X-axis) after changing the value of the specified parameter from the low to the high end of its range. The number of parameters that resulted in the greatest variation are shown.
Multi-way
Considering the most conservative estimate for each variable simultaneously resulted in a disability weight of 0.047 in the overall model, with age-specific estimates of 0.033 in the <15 y model, and 0.091 in the ≥15 y model. In the pessimistic scenario, we observed a maximum overall disability weight of 0.228, which ranged from 0.194 (<15 y) to 0.305 (≥15 y) in age-specific cohort analyses.
Probabilistic
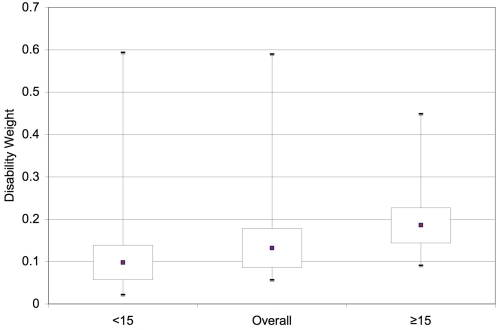
Findings from probabilistic sensitivity analyses confirmed the magnitude and robustness of estimates from deterministic analyses. In the overall model, the average disability weight was 0.132 over 5,000 Monte Carlo simulations, with age-specific estimates of 0.098 (<15 y) and 0.186 (≥15 y). No simulations produced disability weight estimates lower than 0.009, and only 2.5% of simulations in the <15 y model were less than 0.044. Median values were 0.124 (2.5–97.5%: 0.080–0.232) for all ages, with age-specific estimates ranging from 0.092 (<15 y; 2.5–97.5%: 0.044–0.174) to 0.180 (≥15 y; 2.5–97.5%: 0.123–0.287) ( Figure 4 ).
Figure 4. Probabilistic sensitivity analyses.
Boxplots depicting the results of 5,000 simulation Monte Carlo analysis for each age-group model. Boxes represent the median, 25th and 75th percentiles, and error bars extend to the 2.5th and 97.5th percentile. Means are depicted by the circles.
Discussion
Our findings indicate that age-specific disability weights of 0.098 to 0.186 would be a more appropriate estimate for the burden of human disease due to S. japonicum infection. It is noteworthy that even the most conservative estimates were seven times greater than current GBD disability weights for schistosomiasis ( Table 3 ) [23], [25]–[27].
Table 3. Disability weight estimates from review studies of all schistosome strains and schistosomiasis japonica.
| <15 years | ≥15 years | Overall | Strains | Ratio vs. GBD | |
| Global Burden of Disease [23] | 0.005 | 0.006 | - | All | - |
| King et al. , 2005 [19] | - | - | 0.020–0.150 | All | 4–30 : 1 |
| Jia et al. , 2007 [16] | 0.0951 | 0.159–0.2462 | 0.191 | S. japonicum | 19–27 : 1 |
| Current analysis | 0.098 | 0.186 | 0.130 | S. japonicum | 20–33 : 13 |
This study excluded individuals <5 years (5–14 y).
This refers to the estimated disability weights for three age groups, namely: 15–44 y (0.159), 45–59 y (0.207), and ≥60 y (0.246).
Considering the lower and upper bounds of the confidence intervals for these estimates, the ratio vs. GBD estimates ranges from 7 to 46.
Findings in this study are consistent with King et al.'s [19] meta-analysis of disability-related outcomes in all strains of schistosomiasis. Minimum re-estimated disability weights in our assessment of 0.044 (<15 y) and 0.123 (≥15 y) were similar in magnitude to King et al's estimates of 0.02 to 0.15, although our median values and upper estimates were considerably higher. This is concordant with the assertion that S. japonicum is more pathogenic than other schistosomes [22]. Our disability weight estimates are also consistent with findings from a community-based study in China that focused on chronic schistosomiasis japonica, reporting an overall disability weight of 0.191 and age-specific estimates of 0.095 (5–14 y) and 0.159 (15–44 y) [16]. Current findings and burden re-assessments by King and colleagues and Jia and colleagues are in contrast to two earlier reviews that suggested a minimal public health impact of schistosomiasis [34],[35].
There are several differences in King et al.'s meta-analysis of all schistosomes [19] and the China study focusing on chronic infections due to S. japonicum [16] compared to our analysis, particularly regarding the selection of schistosomiasis-related health conditions. In contrast to King et al.'s [19] assessment, we accounted for hepato-splenic pathologies due to S. japonicum infection, and identified them as critical disability-related outcomes, as did the Chinese study [16]. The exclusions of organ pathologies in King et al.'s assessment [19] and nutritional morbidities in the China study [16] may contribute to an underestimation of disability weights, relative to our findings. We also excluded work and school performance outcomes in our assessment, due to the limited availability of such studies in S. japonicum and a lack of objective standardized measures for these outcomes. Inclusion of these functional outcomes in future studies is expected to further increase estimated disability weights for S. japonicum.
There are several study limitations. Few incidence studies were available, and results are based on review of only 34 primary papers (48 total) with usable outcome information. Due to the nature of this analysis, which is to describe the natural history of acute and chronic S. japonicum infection, we decided not to formally score each study based on the quality of the information included. Hence, we assumed that every report represented the best available information for the specified population and outcomes at the time the study was undertaken [31]. Our analysis was limited by the research designs and topical foci of earlier studies. We did not include the entire range of possible morbidities and disabilities due to S. japonicum infection. We also excluded mortality-related outcomes and did not account for treatment status, co-morbidities with other infectious or non-infectious diseases, infection intensity, re-infection, or disease progression in order to facilitate comparability of findings to the GBD assessment [23], [25]–[27]. There is a possibility of publication bias in every review. To limit this, we solicited both published and unpublished manuscripts and consulted with experts in order to limit publication bias, and intentionally biased our model estimates to the lower ranges for each model parameter, in order to obtain conservative estimates. In addition, when more than one publication described the frequency of occurrence of a specific morbidity associated with schistosomiasis, the most conservative estimate was included in the assessment. These exclusions and limitations in available studies would likely lead to an underestimation of re-estimated model disability weights.
In schistosomiasis-endemic regions, polyparasitism, malnutrition, and other infectious diseases are ubiquitous. Therefore, it is important to interpret findings in the context of concurrent infections. For example, some disability-related outcomes, including anemia and diarrhea, may be due to the presence of other parasitic infections. Although polyparasitism may have contributed to an over-estimation of disability weights attributable to S. japonicum, this is unlikely since we used highly conservative estimates in this analysis. Recent studies have challenged the ability of experts to quantify patient-centered disability, particularly in chronic conditions characterized by low mortality [17],[19],[36],[37] and extensive co-morbidities [19],[38],[39]. However, the extent of the disparity between expert opinion and patient's experience is unknown. Expert panel methodologies may also underestimate the disability-related impact of nutritional morbidities, in favor of more standardized and observable organ pathologies. Therefore, the fundamental reliance on GBD disability weights in this assessment also represents an important study limitation.
Decision model estimation is a useful analytic method for conducting disease burden assessments; however, deterministic and probabilistic sensitivity analyses are exploratory, rather than explanatory. As a result, decision model estimation does not facilitate evaluation of the statistical significance or robustness of findings, which represents a study limitation. The β-distribution was selected for this analysis based on its satisfaction of a priori requirements to restrict the estimate ranges from 0 and 1, calculation and summarization of data, and evaluation of the level of estimate uncertainty [32]. Similar to other distributions, the β-distribution is influenced by the quality, availability, and level of skewness in model inputs. Therefore, availability and quality of epidemiological and disease burden data remain study limitations.
Further research is needed to examine the broad range of morbidities associated with schistosomiasis, particularly on methods to parse attributable causation of specific infections in the context of polyparasitism and other comorbid conditions. Additionally, the separation between specific causes of disease from associated morbidities, and the exclusion of selected conditions partially attributable to other infections in the GBD assessment may contribute to an underestimation of disability weights for schistosomiasis.
Lastly, as a zoonotic disease, schistosomiasis japonica is also a veterinary and agricultural public health issue. Future interdisciplinary research should consider the direct impact on human health and the indirect impact on animal health and economic productivity. Methods described by Budke et al. [40] and Carabin et al. [41] could also be utilized to broaden S. japonicum burden assessment to incorporate cost-effectiveness measures [40]–[42].
In conclusion, a minimum disability weight of 0.033 to 0.091 would be a more accurate estimate of disability due to S. japonicum. GBD methodologies underestimate the burden of disease attributable to S. japonicum, and hence they should be revised. Even a minimal increase in current estimates would result in a substantial rise in the estimated global burden of schistosomiasis, and have considerable implications for public health prioritization, health policy, and resource allocation for research, monitoring, and control.
Supporting Information
Translation of the abstract into Chinese by Ruilan Wei.
(0.14 MB PDF)
Translation of the abstract into French by Hélène Carabin.
(0.02 MB DOC)
Translation of the abstract into Spanish by Susie Welty and Elena Gibbons.
(0.03 MB DOC)
Key words for search strategy used to review the literature on S. japonicum for Medline (1966–2007).
(0.06 MB DOC)
Footnotes
The authors have declared that no competing interests exist.
This work was supported by the National Institutes of Health, grant TW01582, from the NIH/NSF Ecology of Infectious Disease Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.Lammie PJ, Fenwick A, Utzinger J. A blueprint for success: integration of neglected tropical disease control programmes. Trends Parasitol. 2006;22:313–321. doi: 10.1016/j.pt.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002;82:139–146. doi: 10.1016/s0001-706x(02)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Geneve: World Health Organization; 2002. The World Health Report 2002: Reducing risk, promoting healthy life. [Google Scholar]
- 6.Morel CM. Reaching maturity-25 years of the TDR. Parasitol Today. 2000;16:522–528. doi: 10.1016/s0169-4758(00)01815-9. [DOI] [PubMed] [Google Scholar]
- 7.Utzinger J, Xiao SH, N'Goran EK, Bergquist R, Tanner M. The potential of artemether for the control of schistosomiasis. Int J Parasitol. 2001;31:1549–1562. doi: 10.1016/s0020-7519(01)00297-1. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Geneva: World Health Organization; 2002. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis: Report of a WHO Expert Committee. WHO Tech Rep Ser 912. [PubMed] [Google Scholar]
- 9.Domingo EO, Tiu E, Peters PA, Warren KS, Mahmoud AA, et al. Morbidity in schistosomiasis japonica in relation to intensity of infection: study of a community in Leyte, Philippines. Am J Trop Med Hyg. 1980;29:858–867. doi: 10.4269/ajtmh.1980.29.858. [DOI] [PubMed] [Google Scholar]
- 10.McGarvey ST, Aligui G, Daniel BL, Peters P, Olveda RM, et al. Child growth and schistosomiasis japonica in northeastern Leyte, the Philippines: cross-sectional results. Am J Trop Med Hyg. 1992;46:571–581. doi: 10.4269/ajtmh.1992.46.571. [DOI] [PubMed] [Google Scholar]
- 11.Olds GR, Olveda RM, Wu G, Wiest P, McGarvey ST, et al. Immunity and morbidity in schistosomiasis japonicum infection. Am J Trop Med Hyg. 1996;55:121–126. doi: 10.4269/ajtmh.1996.55.121. [DOI] [PubMed] [Google Scholar]
- 12.Olveda RM, Daniel BL, Ramirez BD, Aligui GD, Acosta LP, et al. Schistosomiasis japonica in the Philippines: the long-term impact of population-based chemotherapy on infection, transmission, and morbidity. J Infect Dis. 1996;174:163–172. doi: 10.1093/infdis/174.1.163. [DOI] [PubMed] [Google Scholar]
- 13.Olveda RM, Tiu E, Fevidal P, Jr, de Veyra F, Jr, Icatlo FC, Jr, et al. Relationship of prevalence and intensity of infection to morbidity in schistosomiasis japonica: a study of three communities in Leyte, Philippines. Am J Trop Med Hyg. 1983;32:1312–1321. doi: 10.4269/ajtmh.1983.32.1312. [DOI] [PubMed] [Google Scholar]
- 14.Ross AGP, Bartley PB, Sleigh AC, Olds GR, Li Y, et al. Schistosomiasis. N Engl J Med. 2002;346:1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 15.Wiest PM, Wu G, Zhong S, McGarvey ST, Yuan J, et al. Impact of annual screening and chemotherapy with praziquantel on schistosomiasis japonica on Jishan Island, People's Republic of China. Am J Trop Med Hyg. 1994;51:162–169. doi: 10.4269/ajtmh.1994.51.162. [DOI] [PubMed] [Google Scholar]
- 16.Jia TW, Zhou XN, Wang XH, Utzinger J, Steinmann P, et al. Assessment of the age-specific disability weight of chronic schistosomiasis japonica. Bull World Health Organ. 2007;85:458–465. doi: 10.2471/BLT.06.033035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergquist NR, Leonardo LR, Mitchell GF. Vaccine-linked chemotherapy: can schistosomiasis control benefit from an integrated approach? Trends Parasitol. 2005;21:112–117. doi: 10.1016/j.pt.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Coutinho HM, McGarvey ST, Acosta LP, Manalo DL, Langdon GC, et al. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J Infect Dis. 2005;192:528–536. doi: 10.1086/430929. [DOI] [PubMed] [Google Scholar]
- 19.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 20.McGarvey ST, Aligui G, Graham KK, Peters P, Olds GR, et al. Schistosomiasis japonica and childhood nutritional status in northeastern Leyte, the Philippines: a randomized trial of praziquantel versus placebo. Am J Trop Med Hyg. 1996;54:498–502. doi: 10.4269/ajtmh.1996.54.498. [DOI] [PubMed] [Google Scholar]
- 21.McGarvey ST, Wu G, Zhang S, Wang Y, Peters P, et al. Child growth, nutritional status, and schistosomiasis japonica in Jiangxi, People's Republic of China. Am J Trop Med Hyg. 1993;48:547–553. doi: 10.4269/ajtmh.1993.48.547. [DOI] [PubMed] [Google Scholar]
- 22.Davis A. Schistosomiasis. In: Cook GC, Zumla AI, editors. Manson's Tropical Diseases. 21st ed: WB Saunders. 2003. pp. 1431–1469. [Google Scholar]
- 23.Murray CJL, Lopez AD, editors. Cambridge: Harvard University Press; 1996. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries and Risk Factors in 1990 and Projected to 2020. [Google Scholar]
- 24.Michaud CM, Gordon WS, Reich MR. Cambridge: Harvard Center for Population and Development Studies; 2003. The Global Burden of Disease due to Schistosomiasis. Disease Control Priorities Project Working Paper 19. [Google Scholar]
- 25.Murray CJL, Lopez AD, editors. Cambridge: Harvard University Press; 1996. Global Health Statistics: A Compendium of Incidence, Prevalence and Mortality Estimates for over 200 Conditions. [Google Scholar]
- 26.Murray CJL, Lopez AD, editors. Cambridge: Harvard University Press; 2004. Health Dimensions of Sex and Reproduction: The Global Burden of Sexually Transmitted Diseases, HIV, Maternal Conditions, Perinatal Disorders, and Congenital Anomalies. [Google Scholar]
- 27.Murray CJL, Lopez AD, Mathers CD, editors. Cambridge: Harvard University Press; 2004. The Global Epidemiology of Infectious Diseases. [Google Scholar]
- 28.WHO. Geneva: World Health Organization; 1992. International Statistical Classification of Diseases and Related Health Problems. Tenth Edition. [Google Scholar]
- 29.Doehring-Schwerdtfeger E, Mohamed-Ali G, Abdel-Rahim IM, Kardorff R, Franke D, et al. Sonomorphological abnormalities in Sudanese children with Schistosoma mansoni infection: a proposed staging-system for field diagnosis of periportal fibrosis. Am J Trop Med Hyg. 1989;41:63–69. [PubMed] [Google Scholar]
- 30.Hackett LW. Spleen measurements in malaria. J Natl Malar Soc. 1944;3:121–133. [Google Scholar]
- 31.TreeAge. Williamstown, MA: TreeAge Software; 2005. TreeAge Suite Version 0.5. [Google Scholar]
- 32.Briggs AH, Ades AE, Price MJ. Probabilistic sensitivity analysis for decision trees with multiple branches: use of the Dirichlet distribution in a Bayesian framework. Med Decis Making. 2003;23:341–350. doi: 10.1177/0272989X03255922. [DOI] [PubMed] [Google Scholar]
- 33.Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 34.Gryseels B. The relevance of schistosomiasis for public health. Trop Med Parasitol. 1989;40:134–142. [PubMed] [Google Scholar]
- 35.Tanner M. Evaluation of public health impact of schistosomiasis. Trop Med Parasitol. 1989;40:143–148. [PubMed] [Google Scholar]
- 36.AbouZahr C, Vaughan JP. Assessing the burden of sexual and reproductive ill-health: questions regarding the use of disability-adjusted life years. Bull World Health Organ. 2000;78:655–666. [PMC free article] [PubMed] [Google Scholar]
- 37.Reidpath DD, Allotey PA, Kouame A, Cummins RA. Measuring health in a vacuum: examining the disability weight of the DALY. Health Policy Plan. 2003;18:351–356. doi: 10.1093/heapol/czg043. [DOI] [PubMed] [Google Scholar]
- 38.Guerrant RL, Kosek M, Lima AA, Lorntz B, Guyatt HL. Updating the DALYs for diarrhoeal disease. Trends Parasitol. 2002;18:191–193. doi: 10.1016/s1471-4922(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 39.Guerrant RL, Kosek M, Moore S, Lorntz B, Brantley R, et al. Magnitude and impact of diarrheal diseases. Arch Med Res. 2002;33:351–355. doi: 10.1016/s0188-4409(02)00379-x. [DOI] [PubMed] [Google Scholar]
- 40.Budke CM, Jiamin Q, Zinsstag J, Qian W, Torgerson PR. Use of disability adjusted life years in the estimation of the disease burden of echinococcosis for a high endemic region of the Tibetan plateau. Am J Trop Med Hyg. 2004;71:56–64. [PubMed] [Google Scholar]
- 41.Carabin H, Budke CM, Cowan LD, Willingham AL, 3rd, Torgerson PR. Methods for assessing the burden of parasitic zoonoses: echinococcosis and cysticercosis. Trends Parasitol. 2005;21:327–333. doi: 10.1016/j.pt.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Majorowski MM, Carabin H, Kilani M, Bensalah A. Echinococcosis in Tunisia: a cost analysis. Trans R Soc Trop Med Hyg. 2005;99:268–278. doi: 10.1016/j.trstmh.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Li YS, He YK, Zeng QR, McManus DP. Epidemiological and morbidity assessment of Schistosoma japonicum infection in a migrant fisherman community, the Dongting Lake region, China. Trans R Soc Trop Med Hyg. 2003;97:177–181. doi: 10.1016/s0035-9203(03)90112-x. [DOI] [PubMed] [Google Scholar]
- 44.Li YS, Sleigh AC, Ross AGP, Williams GM, Tanner M, et al. Epidemiology of Schistosoma japonicum in China: morbidity and strategies for control in the Dongting Lake region. Int J Parasitol. 2000;30:273–281. doi: 10.1016/s0020-7519(99)00201-5. [DOI] [PubMed] [Google Scholar]
- 45.Wu Z, Shaoji Z, Pan B, Hu L, Wei R, et al. Reinfection with Schistosoma japonicum after treatment with praziquantel in Poyang lake region, China. Southeast Asian J Trop Med Public Health. 1994;25:163–169. [PubMed] [Google Scholar]
- 46.Finkelstein JL. Schistosomiasis Morbidity and Disability Assessment Questionnaire. Administered to S. japonicum Clinical Research Experts in 2005 Providence: Brown University, United States. 2005 [Google Scholar]
- 47.Genming Z, Brinkmann UK, Qingwu J, Shaoji Z, Zhide L, et al. The relationship between morbidity and intensity of Schistosoma japonicum infection of a community in Jiangxi province, China. Southeast Asian J Trop Med Public Health. 1997;28:545–550. [PubMed] [Google Scholar]
- 48.Booth M, Guyatt HL, Li Y, Tanner M. The morbidity attributable to Schistosoma japonicum infection in 3 villages in Dongting Lake region, Hunan province, PR China. Trop Med Int Health. 1996;1:646–654. doi: 10.1111/j.1365-3156.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 49.Friedman JF, Kanzaria HK, McGarvey ST. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol. 2005;21:386–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Lewert RM, Yogore MG, Jr, Blas BL. Schistosomiasis japonica in Barrio San Antonio, Basey, Samar , The Philippines. I. Epidemiology and morbidity. Am J Trop Med Hyg. 1979;28:1010–1025. doi: 10.4269/ajtmh.1979.28.1010. [DOI] [PubMed] [Google Scholar]
- 51.Ross AGP, Yuesheng L, Sleigh AS, Yi L, Williams GM, et al. Epidemiologic features of Schistosoma japonicum among fishermen and other occupational groups in the Dongting Lake region (Hunan province) of China. Am J Trop Med Hyg. 1997;57:302–308. doi: 10.4269/ajtmh.1997.57.302. [DOI] [PubMed] [Google Scholar]
- 52.Ross AGP, Li YS, Sleigh AC, McManus DP. Schistosomiasis control in the People's Republic of China. Parasitol Today. 1997;13:152–155. doi: 10.1016/s0169-4758(97)01026-0. [DOI] [PubMed] [Google Scholar]
- 53.Warren KS, Su DL, Xu ZY, Yuan HC, Peters PA, et al. Morbidity in schistosomiasis japonica in relation to intensity of infection. A study of two rural brigades in Anhui province, China. N Engl J Med. 1983;309:1533–1539. doi: 10.1056/NEJM198312223092501. [DOI] [PubMed] [Google Scholar]
- 54.Olds GR, King C, Hewlett J, Olveda RM, Wu G, et al. Double-blind placebo-controlled study of concurrent administration of albendazole and praziquantel in schoolchildren with schistosomiasis and geohelminths. J Infect Dis. 1999;179:996–1003. doi: 10.1086/314686. [DOI] [PubMed] [Google Scholar]
- 55.Li YS, Ross AGP, Yu DB, Li Y, Williams GM, et al. An evaluation of Schistosoma japonicum infections in three villages in the Dongting Lake region of China. I. Prevalence, intensity and morbidity before the implementation of adequate control strategies. Acta Trop. 1997;68:77–91. doi: 10.1016/s0001-706x(97)00077-6. [DOI] [PubMed] [Google Scholar]
- 56.Yu DB, Ross AGP, Williams GM, Li YS, McManus DP. Determinants of hepato- and spleno-megaly in Hunan, China: cross-sectional survey data from areas endemic for schistosomiasis. Ann Trop Med Parasitol. 2001;95:707–713. doi: 10.1080/00034980120103261. [DOI] [PubMed] [Google Scholar]
- 57.Wiest PM, Wu G, Zhang S, Yuan J, Peters PA, et al. Morbidity due to schistosomiasis japonica in the People's Republic of China. Trans R Soc Trop Med Hyg. 1992;86:47–50. doi: 10.1016/0035-9203(92)90437-h. [DOI] [PubMed] [Google Scholar]
- 58.Finkelstein JL. Providence: Brown University, United States; 2005. Field Data from China and Phillippines. Data Analyses Conducted as Part of Master of Public Health Thesis. [Google Scholar]
- 59.Booth M, Li Y, Tanner M. Helminth infections, morbidity indicators and schistosomiasis treatment history in three villages, Dongting Lake region, PR China. Trop Med Int Health. 1996;1:464–474. doi: 10.1046/j.1365-3156.1996.d01-93.x. [DOI] [PubMed] [Google Scholar]
- 60.Hadidjaja P, Ismid IS, Sudomo M, Syamsuddin N, Putrali J, et al. The effect of praziquantel treatment on liver and spleen size of children with schistosomiasis in Napu Valley, central Sulawesi. Southeast Asian J Trop Med Public Health. 1990;21:91–94. [PubMed] [Google Scholar]
- 61.Li YS, Sleigh AC, Ross AGP, Li Y, Williams GM, et al. Two-year impact of praziquantel treatment for Schistosoma japonicum infection in China: re-infection, subclinical disease and fibrosis marker measurements. Trans R Soc Trop Med Hyg. 2000;94:191–197. doi: 10.1016/s0035-9203(00)90274-8. [DOI] [PubMed] [Google Scholar]
- 62.Li YS, Sleigh AC, Li Y, Tanner M, Dessein A, et al. Five-year impact of repeated praziquantel treatment on subclinical morbidity due to Schistosoma japonicum in China. Trans R Soc Trop Med Hyg. 2002;96:438–443. doi: 10.1016/s0035-9203(02)90386-x. [DOI] [PubMed] [Google Scholar]
- 63.Ohmae H, Tanaka M, Hayashi M, Matsuzaki Y, Kurosaki Y, et al. Ultrasonographic and serologic abnormalities in Schistosoma japonicum infection in Leyte, the Philippines. Am J Trop Med Hyg. 1992;46:89–98. doi: 10.4269/ajtmh.1992.46.89. [DOI] [PubMed] [Google Scholar]
- 64.Liu J, Zhao G, Wu Z, Tao B, Jiang Q. The morbidity investigation of residents in a highly endemic village of schistosomiasis in Poyang Lake region. Chin J Parasitol Parasit Dis. 1998;16:197–200 (in Chinese). [PubMed] [Google Scholar]
- 65.Lin D, Zhang S, Murakami H, Wu Z, Totsuya T, et al. Impact mass chemotherapy with praziquantel on schistosomiasis control in Fanhu village, People's Republic of China. Southeast Asian J Trop Med Public Health. 1997;28:274–279. [PubMed] [Google Scholar]
- 66.Lin D, Murakami H, Zhang S, Wu Z, Ning A, et al. Pilot study of schistosomiasis control in Poyang Lake region. Chin J Parasitol Parasit Dis. 1999;17:167–171. [PubMed] [Google Scholar]
- 67.Wiest PM, Wu G, Zhong S, McGarvey ST, Tan E, et al. Schistosomiasis japonica on Jishan Island, Jiangxi Province, People's Republic of China: persistence of hepatic fibrosis after reduction of the prevalence of infection with age. Trans R Soc Trop Med Hyg. 1993;87:290–294. doi: 10.1016/0035-9203(93)90133-b. [DOI] [PubMed] [Google Scholar]
- 68.Takemura Y, Kikuchi S, Inaba Y. Epidemiologic study of the relationship between schistosomiasis due to Schistosoma japonicum and liver cancer/cirrhosis. Am J Trop Med Hyg. 1998;59:551–556. doi: 10.4269/ajtmh.1998.59.551. [DOI] [PubMed] [Google Scholar]
- 69.Ross AGP, Li Y, Booth M, Sleigh AC, Williams GM, et al. Five year impact of chemotherapy on morbidity attributable to Schistosoma japonicum infection in the Dongting Lake region. Trop Med Int Health. 1998;3:837–841. doi: 10.1046/j.1365-3156.1998.00305.x. [DOI] [PubMed] [Google Scholar]
- 70.Zeng QR, Hou JW, He YK, Luo XS, Zhang SK, et al. Analysis on morbidity and chemotherapy effects of Schistosoma japonicum infection in fishermen on Dongting Lake. Chin J Parasitol Parasit Dis. 2004;22:199–203 (in Chinese). [PubMed] [Google Scholar]
- 71.Wu GL, Yuan JH, He Q, Wu FD, Zhang SJ, et al. Clinico-epidemiological investigation of schistosome-induced hepatosplenomegaly: a community-based study in Jishan, Xinjian County, Jiangxi. Chin J Parasitol Parasit Dis. 1991;9:274–277 (in Chinese). [PubMed] [Google Scholar]
- 72.Kardorff R, Olveda RM, Acosta LP, Duebbelde UJ, Aligui GD, et al. Hepatosplenic morbidity in schistosomiasis japonica: evaluation with Doppler sonography. Am J Trop Med Hyg. 1999;60:954–959. doi: 10.4269/ajtmh.1999.60.954. [DOI] [PubMed] [Google Scholar]
- 73.Hirayama K, Chen H, Kikuchi M, Yin T, Gu X, et al. HLA-DR-DQ alleles and HLA-DP alleles are independently associated with susceptibility to different stages of post-schistosomal hepatic fibrosis in the Chinese population. Tissue Antigens. 1999;53:269–274. doi: 10.1034/j.1399-0039.1999.530307.x. [DOI] [PubMed] [Google Scholar]
- 74.Mott KE, Chen MG, Abdel-Wahab F, Burki A, Dixon H, et al. Liver ultrasound findings in a low prevalence area of S. japonicum in China: comparison with history, physical examination, parasitological and serological results. Acta Trop. 1992;51:65–84. doi: 10.1016/0001-706x(92)90021-o. [DOI] [PubMed] [Google Scholar]
- 75.Kurniawan AN, Hardjawidjaja L, Clark RT. A clinico-pathologic study of cases with Schistosoma japonicum infection in Indonesia. Southeast Asian J Trop Med Public Health. 1976;7:263–269. [PubMed] [Google Scholar]
- 76.Sleigh AC. Manila, the Philippines.: WHO; 1990. Schistosomiasis research and control. Mission Report to WHO/WPRO National Workshop on Schistosomiasis in Lake Regions, Nanchang, China. [Google Scholar]
- 77.Nokes C, McGarvey ST, Shiue L, Wu G, Wu H, et al. Evidence for an improvement in cognitive function following treatment of Schistosoma japonicum infection in Chinese primary schoolchildren. Am J Trop Med Hyg. 1999;60:556–565. doi: 10.4269/ajtmh.1999.60.556. [DOI] [PubMed] [Google Scholar]
- 78.Zhou H, Ross AGP, Hartel GF, Sleigh AC, Williams GM, et al. Diagnosis of schistosomiasis japonica in Chinese schoolchildren by administration of a questionnaire. Trans R Soc Trop Med Hyg. 1998;92:245–250. doi: 10.1016/s0035-9203(98)90997-x. [DOI] [PubMed] [Google Scholar]
- 79.Zhou H, He Y, Ohtsuka R. Sex differences in the malnourished status of Chinese children due to schistosomiasis infections and inadequate dietary intake. Environ Sci. 2005;12:145–153. [PubMed] [Google Scholar]
- 80.Coutinho HM, Acosta LP, McGarvey ST, Jarilla B, Jiz M, et al. Nutritional status improves after treatment of Schistosoma japonicum-infected children and adolescents. J Nutr. 2006;136:183–188. doi: 10.1093/jn/136.1.183. [DOI] [PubMed] [Google Scholar]
- 81.Coutinho HM, Leenstra T, Acosta LP, Su L, Jarilla B, et al. Pro-inflammatory cytokines and C-reactive protein are associated with undernutrition in the context of Schistosoma japonicum infection. Am J Trop Med Hyg. 2006;75:720–726. [PubMed] [Google Scholar]
- 82.Zhou H, Watanabe C, Ohtsuka R. Impacts of dietary intake and helminth infection on diversity in growth among schoolchildren in rural south China: a four-year longitudinal study. Am J Hum Biol. 2007;19:96–106. doi: 10.1002/ajhb.20588. [DOI] [PubMed] [Google Scholar]
- 83.Kanzaria HK, Acosta LP, Langdon GC, Manalo DL, Olveda RM, et al. Schistosoma japonicum and occult blood loss in endemic villages in Leyte, the Philippines. Am J Trop Med Hyg. 2005;72:115–118. [PubMed] [Google Scholar]
- 84.Chen MG, Mott KE. Progress in the assessment of morbidity due to Schistosoma japonicum infection: a review of recent literature. Trop Dis Bull. 1989;85:R1–R56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Translation of the abstract into Chinese by Ruilan Wei.
(0.14 MB PDF)
Translation of the abstract into French by Hélène Carabin.
(0.02 MB DOC)
Translation of the abstract into Spanish by Susie Welty and Elena Gibbons.
(0.03 MB DOC)
Key words for search strategy used to review the literature on S. japonicum for Medline (1966–2007).
(0.06 MB DOC)