Abstract
Phospholipase Cζ (PLCζ) is a sperm-specific PLC capable of causing repetitive intracellular Ca2+ ([Ca2+]i) release ([Ca2+]i oscillations) in mammalian eggs. Accumulating evidence suggest that PLCζ is the sperm factor responsible for inducing egg activation. Nevertheless, some sperm fractions devoid of 72-kDa PLCζ showed [Ca2+]i oscillation-inducing and PLCζ-like PLC activity (Kurokawa et al., (2005) Dev. Biol. 285, 376-392). Here, we report that PLCζ remains functional after proteolytic cleavage at the X-Y linker region. We found that N-terminal (33 and 37 kDa) and C-terminal fragments (27 kDa), presumably the result of PLCζ cleavage at the X-Y linker region, were present in fresh sperm as well as in sperm extracts and remained associated as functional complexes. Protease V8 cleaved 72-kDa PLCζ into 33/37 and 27 kDa fragments, while PLC activity and [Ca2+]i oscillation-inducing activity persisted until degradation of the fragments. Immunodepletion or affinity-depletion of these fragments abolished PLC activity and [Ca2+]i oscillation-inducing activity from sperm extracts. Lastly, co-expression of cRNAs encoding residues 1-361 and 362-647 of mouse PLCζ, mimicking cleavage at the X-Y linker region, induced [Ca2+]i oscillations and embryo development in mouse eggs. Our results support the hypothesis that PLCζ is the sole mammalian sperm factor and that its linker region may have important regulatory functions during mammalian fertilization.
Keywords: calcium; egg activation; fertilization; embryo development; inositol 1,4,5-trisphosphate; phospholipase Cζ; proteolytic cleavage; sperm factor; src kinase
INTRODUCTION
In mammals, it is postulated that the fertilizing sperm delivers into the ooplasm a soluble protein, termed sperm factor, which evokes repetitive intracellular Ca2+ ([Ca2+]i) release, also known as [Ca2+]i oscillations. These [Ca2+]i oscillations induce egg activation, which comprises a series of events including the release of the cortical granules and the resumption of the cell cycle, and that ultimately leads to embryo development (Schultz and Kopf, 1995; Ducibella et al., 2002). Earlier studies have shown that the sperm factor induces [Ca2+]i oscillations by stimulating production of inositol 1,4,5-trisphosphate (IP3), an event that requires the participation of a phospholipase C (PLC) (Dupont et al., 1996; Jones et al., 1998; Rice et al., 2000). IP3 binds and gates IP3 receptors (IP3Rs), which are located in the endoplasmic reticulum, the internal Ca2+ store of eggs, thereby inducing Ca2+ release. Consistent with this proposed sequence of events, treatment of eggs with a PLC inhibitor (Dupont et al., 1996; Wu et al., 2001) or abrogation of IP3R-1 function (Miyazaki et al., 1992; Wu et al., 1997) prevents the initiation of [Ca2+]i oscillations. However, given that both sperm and eggs express several PLC isoforms, it has remained controversial whether the sperm factor is a sperm PLC or is an activator of an egg PLC (for review, see Kurokawa et al., 2004a). Toward resolving this issue, the recent discovery of the sperm-specific PLCζ in the mouse (Saunders et al., 2002), along with the findings that injection of PLCζ recombinant protein or of its cRNA into eggs initiates sperm-like [Ca2+]i oscillations (Saunders et al., 2002; Kouchi et al., 2004) and that its immunodepletion from cytosolic sperm extracts abolishes the ability of these extracts to cause oscillations (Saunders et al., 2002), has solidified the view that the sperm factor in mammals is a sperm PLC. However, whether PLCζ represents the only active component in all mammalian sperm/sperm extracts requires additional investigation.
The sperm-specific PLCζ is one of the smallest of the known PLCs consisting of only four EF-hands, the catalytic X- and Y-domains, and a C2 domain (Fig. A1), all of which are common to the other PLC isoforms (β, γ, δ, ε, η). Besides the absence of the pleckstrin homology (PH) domain, the most salient feature of PLCζ is the exceptionally high Ca2+ sensitivity of its enzymatic activity; the enzyme is ~100-fold more sensitive to Ca2+ than PLCδ whose activity is also regulated by Ca2+ (Kouchi et al., 2004). Accordingly, PLCζ should be active at the basal [Ca2+]i concentrations prevailing in cells, ~ 100 nM, which is consistent with the view that it serves as the initiator of oscillations at fertilization. Nevertheless, it is still unclear what molecular determinants of PLCζ confer such a high Ca2+ sensitivity, although mutational studies have revealed that both EF-hand and C2 domains, where important Ca2+-binding sites are located, are required for full activation of PLCζ (Kouchi et al., 2005; Nomikos et al., 2005). Likewise, it is unknown how the activity of the enzyme is regulated in vivo. Provided that PLCζ is active at resting [Ca2+]i levels, it seems important that its enzymatic activity be carefully controlled prior to interaction of the gametes so that premature acrosome reaction is averted. In this regard, Miyazaki and co-workers recently showed that in vitro PLCζ activity is diminished by interaction of the C2 domain with PI(3)P and PI(5)P, although the physiological relevance of this mechanism remains to be tested (Kouchi et al., 2005).
Fig. 1.
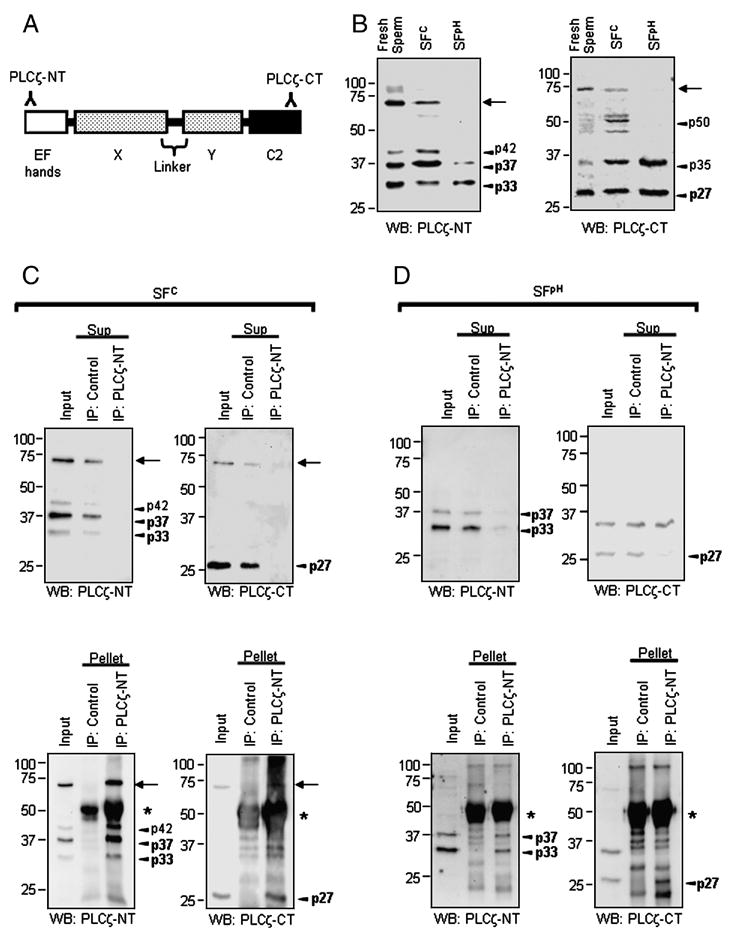
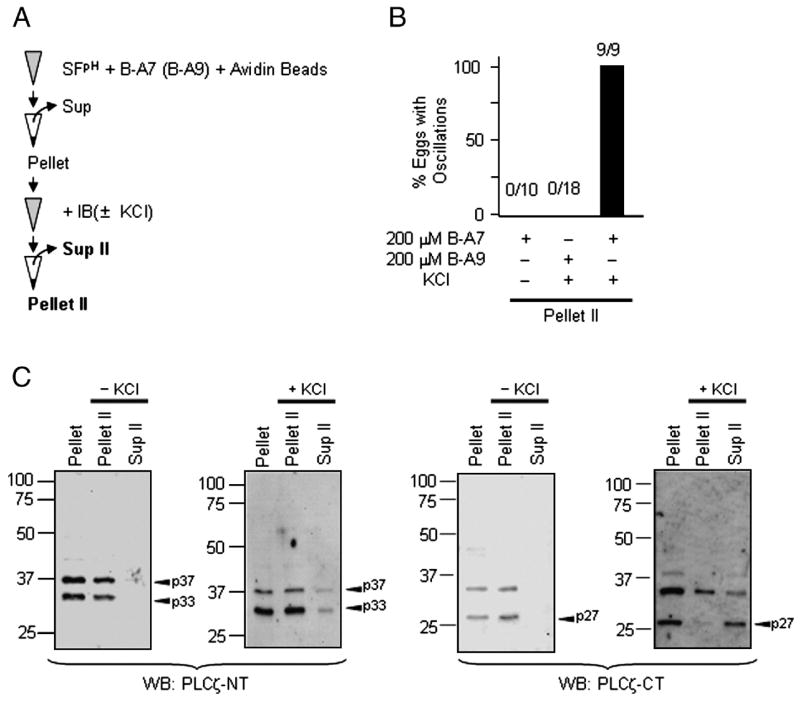
N- and C-terminal PLCζ fragments form stable complexes. A, schematic representation of PLCζ main domains: EF-hands, X- and Y-catalytic, X-Y linker and C2 domain. Two antibodies, anti-PLCζ-NT and anti-PLCζ-CT, were raised against the N- and C-terminal end of PLCζ, respectively. B, Western blotting using anti-PLCζ-NT (left panel) and anti-PLCζ-CT (right panel) shows that 72-kDa PLCζ is present in fresh live sperm as well as in SFC, but is absent in SFpH (arrows). PLCζ fragments are present in all samples (arrowheads: p27, p33, p35, p37, p42 p50). C and D, SFC (C) and SFpH (D) were precipitated (IP) with a control antibody (control) or with anti-PLCζ-NT (PLCζ-NT). The resulting supernatants (Sup in upper panels) and pellet (lower panels) were used for immunoblotting (anti-PLCζ-NT, left panel; anti-PLCζ-CT, right panel). Arrows and arrowheads indicate 72-kDa PLCζ and its fragments; the fragments most commonly and consistently seen (see text) are shown in bold. Asterisks indicate IgG heavy chains.
We have previously demonstrated that two sperm fractions (SF), the cytosolic fraction (SFC) and the high-pH soluble fraction (SFpH) can be obtained by conventional sonication/freeze-thawing and biochemical processing of porcine (p) sperm (Kurokawa et al., 2005); both SFs were equally capable of triggering [Ca2+]i oscillations and activation when microinjected into mouse eggs. To our surprise, although both SFC and SFpH showed PLCζ-like in vitro PLC activity, immunobloting studies revealed that only trace amounts of full-length PLCζ (72-kDa PLCζ) were present in SFpH compared to SFC (Kurokawa et al., 2005). Moreover, additional chromatographic processing of these fractions showed that other active fractions also lacked 72-kDa PLCζ (Kurokawa et al., 2005). Hence, we envisioned two possibilities: 1) there is an unidentified sperm-specific PLC isoform that also contributes to the sperm-induced [Ca2+]i oscillations; or 2) PLCζ undergoes some kind of post-translational modification, such as proteolytic cleavage, which disables its immunological detection but not its phosphoinositide-hydrolyzing activity. In the present study, we show evidence that PLCζ remains functional even after proteolytic cleavage at the linker region that connects the X- and Y-domains. Both SFC and SFpH contain N-terminal and C-terminal PLCζ fragments that form functional complexes, as their depletion abrogates [Ca2+]i oscillation-triggering activity as well as PLC activity from SFpH fractions. Moreover, SFC treated with Staphylococcus aureus protease V8 retains the ability to induce [Ca2+]i oscillations even after 72-kDa PLCζ, but not the fragments, is no longer detectable by immunobloting. We also found that co-expression of cRNAs encoding residues 1-361 and 362-647 of mouse PLCζ (mPLCζ), which approximates cleavage of the linker region, induces [Ca2+]i oscillations and embryo development in mouse eggs. Collectively, our results confirm that PLCζ is the only molecule in our sperm extracts capable of inducing [Ca2+]i oscillations and further show that its proteolytic processing may play a role during mammalian fertilization.
MATERIALS AND METHODS
Gamete collection
Eggs at the second stage of meiosis (MII) were obtained from CD1 female mice (8–12 weeks old) superovulated by injection of 5 IU of pregnant mare serum gonadotropin (PMSG; Sigma, St. Louis, MO) followed 48 hours later by 5 IU of human chorionic gonadotropin (hCG; Sigma). Eggs were harvested 14 hours post-hCG into a Hepes-buffered tyrode-lactate solution (TL-Hepes) supplemented with 5% heat-treated calf serum (Parrish et al., 1988). Cumulus cells were removed by brief treatment with 0.1% bovine testes hyaluronidase (Sigma). Porcine sperm were collected from freshly ejaculated semen and were immediately washed two times either with injection buffer (IB: 75 mM KCl and 20 mM HEPES, pH 7.0) for sperm extract preparation or with TL-Hepes for immunoblotting.
All procedures involving live animal handling and euthanasia were performed according to standard animal protocols approved by the University of Massachusetts Animal Care Committee.
Preparation of porcine sperm extracts
SFC was prepared as previously described (Wu et al., 2001). Briefly, after washing, the sperm suspension was sonicated in sperm buffer (75 mM KCl, 20 mM Hepes, 1 mM EDTA, 10 mM β-glycerophosphate, 1 mM DTT, 200 μM PMSF, 10 μg/ml pepstatin, 10 μg/ml leupeptin, pH 7.0) at 4°C and the lysate ultracentrifuged. The clear supernatant was concentrated with ultrafiltration membranes (Centriprep-30; Amicon, Beverly, MA). These extracts were then mixed for 30 min at 4°C with ammonium sulfate at 50% final saturation; the precipitates were collected by centrifugation and kept at −80°C. Upon use, the pellets were resuspended with IB, washed, and re-concentrated with ultrafiltration membranes.
SFpH was obtained as we reported previously (Kurokawa et al., 2005). In brief, after sonication, the porcine sperm pellets were washed with sperm buffer twice, and then once with sperm buffer containing 1 M KCl. The resulting pellets were treated with 100 mM Na2CO3 (pH 11.5). After the treatment, the sperm suspension was neutralized, dialyzed against IB, and concentrated. Samples were aliquoted and kept at −80°C.
cDNA Constructs
Full length mPLCζ (mPLCζ WT; Accession No. AF435950), a variant lacking three N-terminal EF-hand domains (mPLCζ ΔEF; Accession No. AK006672), and the regions corresponding to residues 248-647, 362-647, and 454-647 of mPLCζ were amplified by PCR. The regions corresponding to residues 1-557 (mPLCζ ΔC2), 1-247, 1-361, and 1-453 of mPLCζ were made by introducing a stop codon to the respective sites of mPLCζ WT using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA). Likewise, D210R and S405A mutations were generated using the QuikChange kit according to the manufacturer’s instructions. All the constructs were cloned into the pCS2 or pCS2-MT plasmids (Turner and Weintraub, 1994) using EcoRI and XbaI sites; pCS2-MT has 6 repeats of myc-tag sequence at the 5′ end of MCS. cRNAs were produced as previously described (Fissore et al., 1996). In brief, the cDNAs were linearized at the NotI site, and transcribed in vitro using the mMessage/mMachine capping kit (Ambion, Austin, TX). Capped and poly(A)-tailed cRNAs were purified from the reaction mixture using the MEGAclear Kit (Ambion). The cRNAs were eluted with DEPC-treated H2O and, if necessary, further diluted with it before microinjection.
Transfection
Cos-7 cells were grown in RPMI medium with 10% FBS, Pen/strep and 2 mM L-glutamine. One day prior to transfection, cells were seeded onto 12 well plates at 30% confluence. Transfections were performed using 0.5 μg of the selected plasmid DNA per well and 1.5 μl of FuGENE6 (Hoffmann-La Roche, Nutley, NJ) as per manufacturer’s instruction. Transfected cells were grown for 48 hours, washed with PBS and extracted directly into Laemmli sample buffer (200μl/well); 10 μl were loaded per lane.
Microinjections and Ca2+ measurements
Microinjection procedures were as previously described (Wu et al., 2001). In brief, eggs from CD-1 mice were microinjected using Narishige manipulators (Medical Systems Corp., Great Neck, NY) mounted on a Nikon Diaphot microscope (Nikon Inc., Garden City, NY). Glass micropipettes were filled by suction from a microdrop containing SFs or cRNAs. Solution was expelled into the cytoplasm of eggs by pneumatic pressure (PLI-100, picoinjector, Harvard Apparatus, Cambridge, MA). The injection volume was approximately 5-10 pl and resulted in a final intracellular concentration of the injected compounds of approximately 1–2% of the concentration in the injection pipette.
Ca2+ measurements were carried out as previously described (Kurokawa et al., 2005). Eggs were loaded with 1 μM Fura-2 acetoxymethylester (Fura-2 AM; Molecular Probes, Eugene, OR) supplemented with 0.02% pluronic acid (Molecular Probes) for 20 min at room temperature. [Ca2+]i values were monitored using a Nikon Diaphot microscope fitted for fluorescence measurements. Two to fifteen eggs were simultaneously monitored using the software Image 1/FL (Universal Imaging, Downington, PA). Images were acquired using an SIT camera (Dage-MTI, Michigan City, IN) coupled to an amplifier (Video Scope International Ltd., Sterling, VA). [Ca2+]i values were not calibrated in this system and are therefore reported as the ratios of 340/380 nm fluorescence. Fluorescence ratios were obtained every 10–20 sec depending on the experiment.
Western blotting and Immunoprecipitation
Anti-PLCζ rabbit sera were raised against a 19-mer sequence at the N-terminus of pPLCζ (anti-PLCζ-NT) or a 19-mer sequence at the C-terminus of mPLCζ (anti-PLCζ-CT) (Kurokawa et al., 2005) (Fig. 1A). The antisera used were affinity-purified using the antigenic peptides. The monoclonal antibody (9E10; Santa Cruz Biotechnology, Santa Cruz, CA) was used for detection of myc-tagged proteins.
Protein samples were separated by SDS-polyacrylamide gel electrophoresis and, thereafter, transferred onto polyvinylidene difluoride membranes (Millipore Corporation, Bedford, MA). Following incubation with non-fat dry milk (6%), the membranes were probed with a primary antibody followed by a horseradish peroxidase-labeled secondary antibody (Bio-Rad Laboratories, Hercules, CA). Immunoreactivity was detected using chemiluminescence reagents (NEN Life Science Products, Boston, MA) and the Kodak Image Station 440CF (NEN Life Science Products). Western blotting procedures were repeated at least five times for each sample and the representative pictures are shown in the figures.
For immunoprecipitation, pre-immune control antibody or anti-PLCζ-NT antibody prebound to protein A sepharose beads (Sigma) was mixed with 50 μg sperm protein in lysis buffer (20 mM Tris-HCl, 150 mM NaCl, and 0.1% Triton X-100, pH 7.6) and incubated at 4°C for 6 hours. After the beads were separated from the supernatant by a short centrifugation (1000 g for 1 min), the supernatant was either subjected directly to microinjection, PLC assay, or to western blotting. The bead pellet was washed three times with lysis buffer containing 300 mM NaCl and three times with lysis buffer, and subjected to western blotting.
Peptides and affinity pull-down
Peptides A7 (SDSIQAEEWYFGKITRRE) and A9 (SDSIQAEEWYFGKIT) were prepared as previously described (Fukami et al., 1993) and biotinylated at the N-terminal end of each peptide (B-A7 and B-A9). The biotinylated peptides were dissolved in IB at 1 mM and kept at −80°C until use. Further dilutions were performed to obtain working concentrations.
Affinity pull-down experiments were performed for 15 min at 4°C by mixing 25 μl of SFC or SFpH (1.0 μg/μl) with 25 μl of either B-A7 or B-A9 to obtain final peptide concentrations of 50, 100, or 200 μM. Following addition of 10 μl of 50% avidin beads (Pierce Biotechnology, Rockford, IL), the combined mixture was further incubated for 15 min at 4°C. The suspension was then centrifuged at 1000 g for 1 min and, thereby, separated into supernatant (Sup) and pellet fractions. The supernatants were either injected into mouse eggs to monitor [Ca2+]i responses or subjected to immunoblotting, whereas the pellets were subjected to immunoblot analyses. In some experiments, pellets were further washed with IB and then mixed with equal amounts of IB supplemented with 2 M KCl (pH 7.0) after which these supernatants containing high salts (Sup II) were either microinjected into mouse eggs or used for immunoblotting. The remaining pellet (Pellet II) was washed with IB and used for immunoblotting.
PLC assays
In vitro PLC assays were carried out based on the method previously described (Liu et al., 1996; Kurokawa et al., 2005). In brief, 20 μl (1 to 20 μg) of a sample was mixed with 30 μl of substrate cocktail to obtain a final concentration of 50 μM phosphatidylethanolamine (Sigma), 40 μM PIP2 (Sigma), 0.01 μCi/50 μl [3H]PIP2 (PerkinElmer Life Sciences Inc., Boston, MA), 25 mM MES (Sigma, pH 6.3), 1.0 mg/ml BSA, 2 mM EGTA and 100 nM Ca2+. The reaction proceeded for 5 min at room temperature and was stopped by addition of 2 ml chloroform/methanol (2:1; v/v). The generated IP3 was separated into the upper aqueous phase by addition of 0.5 ml of 1N HCl and radioactivity was measured with a liquid scintillation counter (Beckman, Fullerton, CA).
Protease V8 treatment
S. aureus protease V8 (Calbiochem, San Diego, CA) was dissolved in 50 mM Na3PO4 (pH 7.8). For V8 treatment of SFC, 50 μg of SFC (0.5 μg/μl) was mixed with 1 μg of V8 and incubated at 37 °C for various time. At each time point, the mixture was injected to mouse eggs or subjected to PLC assay or western blotting.
RESULTS
Immunodepletion of PLCζ fragments from SFpH abrogates the fraction’s PLC activity and the ability to trigger [Ca2+]i oscillations
We have previously shown that 72-kDa PLCζ was present in freshly collected porcine sperm and in SFC, but not in SFpH (Fig. 1B), even though both SFs showed high PLCζ-like activity and induced [Ca2+]i oscillations in mouse eggs (Kurokawa et al., 2005). Importantly, despite the absence of 72-kDa PLCζ from SFpH, lower, strongly immunoreactive products, most likely the result of partial PLCζ proteolysis, were commonly found in this fraction as well as in SFC and in preparations from fresh live sperm (Fig. 1B). Therefore, we reasoned that the PLCζ-like activity in SFpH, and perhaps also in SFC, might be ascribed to a functional complex formed by the PLCζ fragments. To test this hypothesis, we performed immunoprecipitation of PLCζ in SFC and SFpH and examined whether depletion of 72-kDa PLCζ and/or of its fragments removed the [Ca2+]i oscillation-inducing activity and PLC activity from the fractions. Immunoprecipitation was carried out with the anti-PLCζ-NT antibody and depletion was confirmed by immunoblotting of the resulting supernatants and pellets with the anti-PLCζ-CT as well as with the anti-PLCζ-NT antibody (Fig. 1A). Incubation of SFC with the anti-PLCζ-NT antibody prebound to protein A sepharose beads depleted 72-kDa PLCζ from the supernatant fractions and caused concentration of it in the pellet fractions; incubation with pre-immune control antibody failed to precipitate 72-kDa PLCζ (Fig. 1C). When SFpH was subjected to the same treatment, as expected, the 72-kDa PLCζ was not precipitated, as it was absent from these fractions (Fig. 1D), supporting our previous observation that SFpH contains only negligible amounts of 72-kDa PLCζ (Kurokawa et al., 2005). Instead, three fragments were specifically precipitated in this fraction; two of the fragments, 33 and 37 kDa, were recognized by the anti-PLCζ-NT antibody in immunoblots and thought to represent N-terminal PLCζ fragments, and a 27-kDa fragment that was detected by the anti-PLCζ-CT antibody and thought to represent a C-terminal PLCζ fragment (Fig. 1D, left and right panels, respectively). The fact that the anti-PLCζ-NT antibody precipitated both N- and C-terminal PLCζ fragments suggests that they exist as stable complexes in the sperm fractions. Importantly, these complexes retained enzymatic activity, because their depletion obliterated the ability of SFpH fractions to induce [Ca2+]i oscillations in mouse eggs (Fig. 2A), and nearly abolished their in vitro PLC activity (Fig. 2B). Similar effects were observed in SFC devoid of 72-kDa PLCζ and of its fragments (data not shown). Interestingly, fresh sperm and SFC showed an additional N-terminal fragment of approximately 42 kDa; the functional significance of this fragment remains untested (Fig. 1B and 1C). Also note that there were additional C-terminal fragments of PLCζ at 35 and 50 kDa (Fig. 1B right panel). However, we noticed that these fragment were not consistently detected in the sperm fractions (See Fig. 1C right panels). Moreover, these fragments were not co-precipitated with anti-PLCζ-NT antibody (Fig. 1D right panels). Therefore, we concluded that 35- and 50-kDa fragments did not significantly participate in PLC activity of the sperm fractions. Taken together, these results suggest that N- (33 and 37 kDa) and C-terminus (27 kDa) PLCζ fragments form stable complexes and that they retain in vitro PLC enzymatic activity.
Fig. 2.
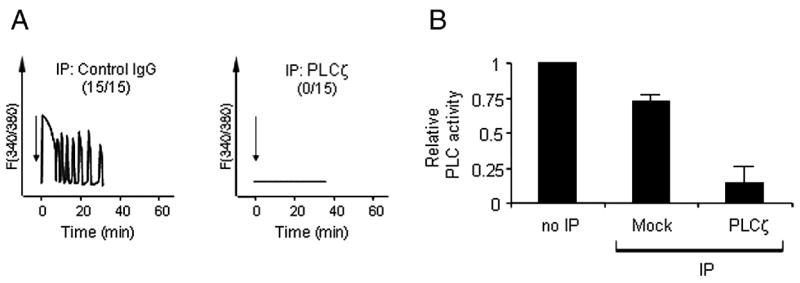
Immunodepletion of PLCζ fragments depletes the ability of SFpH to trigger [Ca2+]i oscillations and abrogates its in vitro PLC activity. A, SFpH was subjected to immunodepletion using a control antibody (left panel) or anti-PLCζ-NT (right panel), and the supernatants injected (arrows) into mouse eggs. [Ca2+]i changes were monitored. Mock-depleted SFpH (control IgG) triggered [Ca2+]i oscillations in all of the mouse eggs injected, while PLCζ fragments-depleted SFpH (PLCζ) did not induce any Ca2+ rise (numbers in parentheses indicate the number of eggs that exhibited oscillations and the number of eggs injected). B, In vitro PLC activity of SFpH after immunodepletion. PLC activity is shown relative to that of untreated SFpH (the error bars indicate standard deviation (n=3)).
V8-treated PLCζ retains in vitro PLC activity and the ability to induce [Ca2+]i oscillations
To further investigate whether or not PLCζ fragments retain PLC activity and are capable of triggering [Ca2+]i oscillations in mouse eggs, we performed limited proteolysis of 72-kDa PLCζ present in SFC followed by monitoring of the remaining activity in these fractions. Prior to selecting a protease, we estimated where the cleavage of PLCζ was taking place in our fractions. Previous reports have shown that the region connecting the X- and Y-catalytic domains, the linker region, of PLC is susceptible to proteolysis (Ellis et al., 1993; Fernald et al., 1994; Schnabel and Camps, 1998). This information, together with the size of the N- and C-terminal fragments in our fractions of ~33/37 and 27 kDa, respectively, lead us to suspect that PLCζ might be cleaved within this region. Consequently, we selected S. aureus protease V8, as it selectively cleaves peptide bonds on the carboxyl side of Asp and Glu residues, both of which are enriched in the linker region of pPLCζ (Yoneda et al., 2006). Moreover, V8 has been previously shown to preferentially cleave the X-Y linker region of other PLC isoforms (Fernald et al., 1994; Schnabel and Camps, 1998). Treatment of SFC with protease V8 led to a rapid digestion of 72-kDa PLCζ, which became almost undetectable within 30 min post-treatment (Fig. 3A). Conversely, the immunoreactivity of the 33 and 37 kDa N-terminal fragments and that of the 27 kDa C-terminal fragment remained steady or even increased during this time (Fig. 3A), suggesting that V8 was cleaving 72-kDa PLCζ at sites similar to those targeted by the endogenous proteases. Importantly, V8-treated SFC fractions were still capable of inducing [Ca2+]i oscillations in mouse eggs for more than 60 min after the treatment (Fig. 3B). Likewise, in vitro PLC activity of SFC remained unchanged for the first 30 min of V8 treatment and then gradually decreased (Fig. 3C). Importantly, after 60 min, the immunoreactivity of the V8-generated PLCζ fragments also became undetectable, which coincided with the demise of both [Ca2+]i oscillation-inducing activity and PLC activity of the SFC fractions (Fig. 3A–C).
Fig. 3.
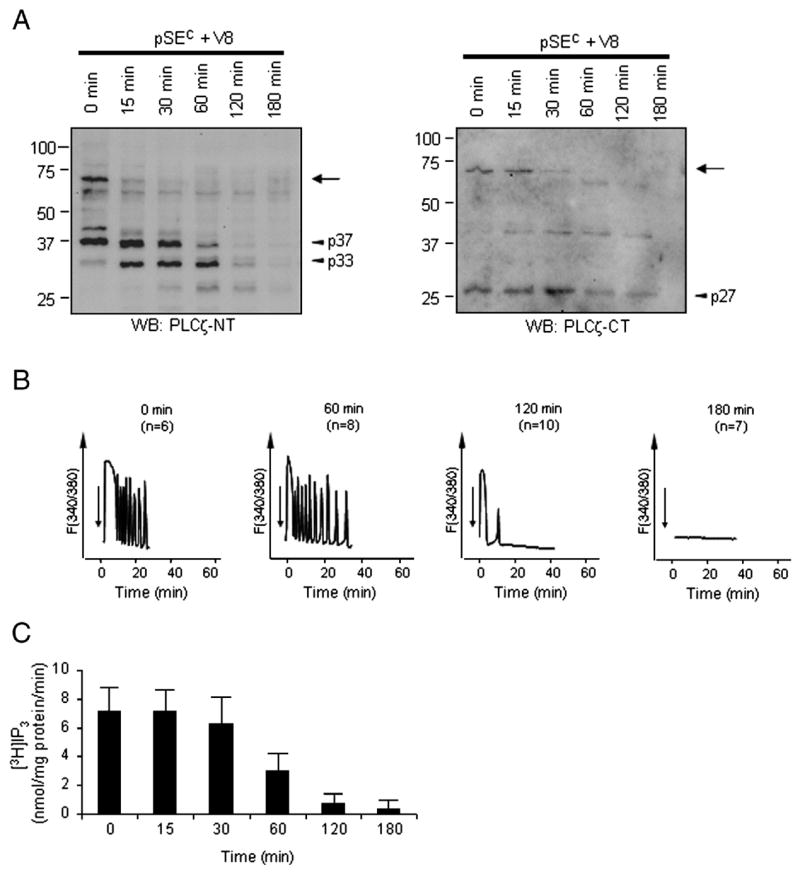
Limited proteolysis of SFC by protease V8. A, Protease V8 (10 ng/μl) was mixed with SFC (0.5 μg/μl) and samples taken at different time points. Western blotting using anti-PLCζ-NT (left panel) and anti-PLCζ-CT (right panel) shows that 72-kDa PLCζ (arrow) was cleaved to smaller, endogenous-like fragments present in SFpH (arrow heads: p27, p33, p37). B, samples as from A were injected (arrows) into mouse eggs and monitored for [Ca2+]i changes (numbers in parentheses indicate the number of eggs tested). V8 treatment of SFC for up to 60 min did not alter the pattern of [Ca2+]i oscillations (compare between Time 0 and 60 min). Nonetheless, SFC incubated with V8 for 120 min triggered only 1 to 3 Ca2+ spikes (the large first Ca2+ rise followed by 0–2 small and short rises), while SFC incubated with V8 for 180 min no longer induced any Ca2+ rise. The most representative Ca2+ patterns obtained were shown. C, samples from A were tested for in vitro PLC activity. Averages in PLC activity with standard deviation are shown (n=10).
Peptide A7 binds to PLCζ/PLCζ fragments and inhibits in vitro PLC activity in SFpH
We have previously shown that pre-incubation of SFC extracts with peptide A, a Src-family kinase (SFK) specific inhibitor, blocked the ability of these extracts to trigger [Ca2+]i oscillations in mouse eggs whereas a control peptide, peptide A9, was without effect (Kurokawa et al. 2004b). However, whether this was due to inhibition of an SFK-mediated pathway was unclear, as only high concentrations of peptide A (~200 μM) were effective and other SFK inhibitors, such as Lavendustin A and PP2, were unable to block SFC-induced [Ca2+]i oscillations (Kurokawa et al. 2004b). Given the difference in electrostatic charge between peptides A and A9 (Fig. 4A), and considering the wealth of charged residues in the linker region of pPLCζ (Yoneda et al., 2006), we reasoned that peptide A could be inhibiting SFC-mediated oscillations by interacting with PLCζ and/or its fragments. Therefore, to gain more insight into the mechanism of action of peptide A, we employed the more specific inhibitor peptide A7, which only differs from the inactive peptide A9 by 3 charged amino acids (Arginine-Arginine-Glutamic acid) in the C-terminal end (Fig. 4A). We first confirmed that peptide A7 was as effective as peptide A in inhibiting [Ca2+]i oscillations induced by injection of both SFC and SFpH into mouse eggs (data not shown). We then performed in vitro PLC assays to test that peptide A7, but not A9, caused a dose-dependent decrease in PLC activity in both SFC (data not shown) and SEpH (Fig. 4B). Lastly, we evaluated whether peptide A7 binds to the 72-kDa PLCζ and/or its fragments. Accordingly, incubation of SFC and SFpH with biotinylated versions of the peptides, B-A7 or B-A9, followed by precipitation with avidin beads resulted in precipitation of 72-kDa PLCζ as well as of its smaller fragments in a concentration-dependent manner by B-A7 but not by B-A9 (Fig. 4C; also see Fig. 5C). Correspondingly, the depletion of 72-kDa PLCζ and its fragments by 200 μM B-A7 abrogated the ability of sperm extracts to trigger [Ca2+]i oscillations (Fig. 4D).
Fig. 4.
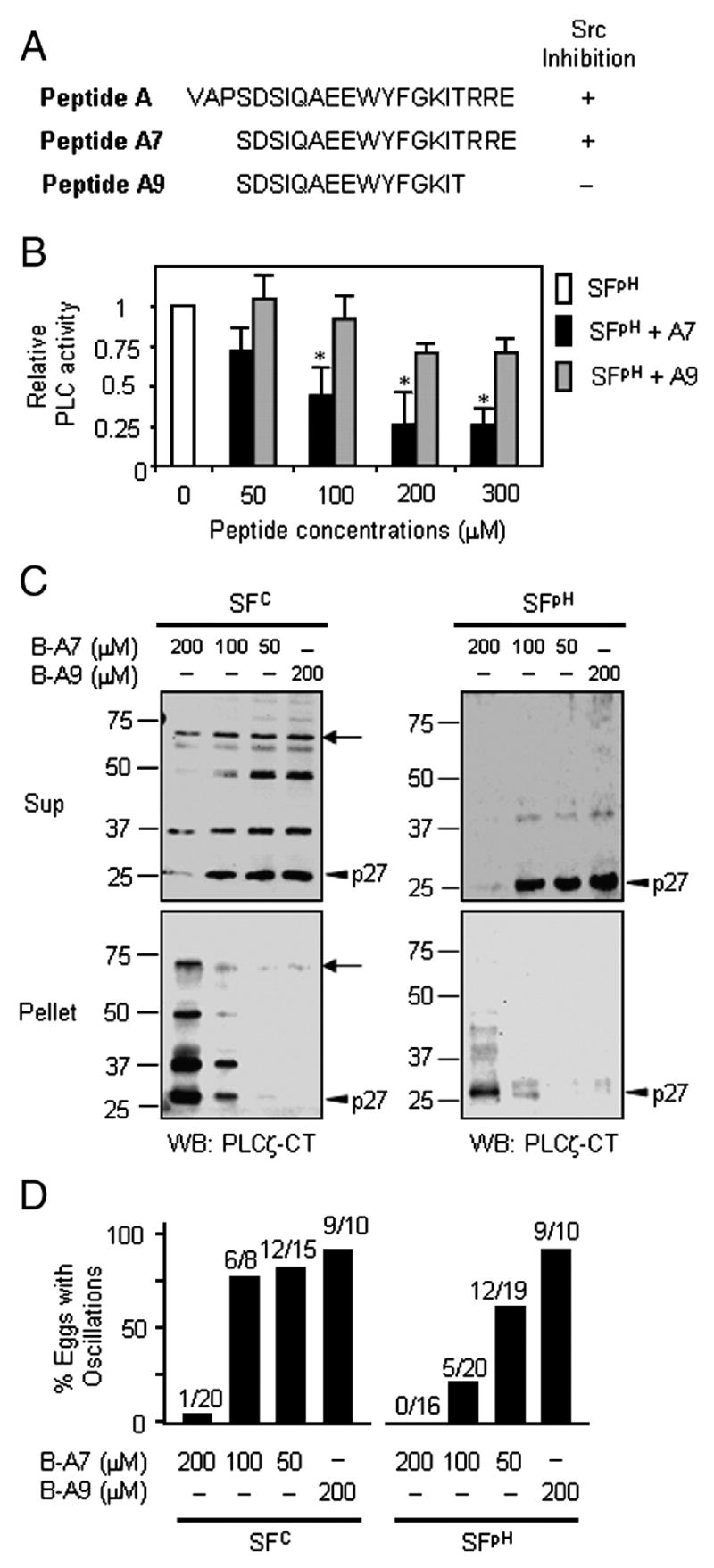
Peptide A7 binds to 72-kDa PLCζ and its fragments and inhibits in vitro PLC activity. A, Amino-acid sequences of peptides A, A7, and A9 are shown. Peptide A7 is a more specific analogue of peptide A and lacks three amino acids at the N-terminal end of peptide A, whereas peptide A9, the inactive analogue, lacks three amino acids at each end of peptide A. The ability of each peptide to inhibit Src family kinases (Fukami et al., 1993) also is indicated. B, In vitro PLC assays reveal that peptide A7 significantly inhibits SFpH PLC activity compared to its inactive control, peptide A9. Shown are means with standard deviation (n=4). The asterisks indicate P<0.05 (student’s t-test). C and D, Biotinylated peptide A7 (B-A7) binds to and precipitates PLCζ and its fragments after addition of avidin beads. SFC and SFpH were pre-incubated with B-A7 followed by avidin bead precipitation, and the resulting supernatant (Sup) and pellet were subjected to immunoblotting (C) and microinjection into mouse eggs (D). Immnoblotting with anti-PLCζ-CT antibody shows that 72-kDa PLCζ (arrows) and its fragments, especially p27 (arrowheads), were precipitated by B-A7 in a dose-dependent manner, but not by B-A9 (C). The removal of the [Ca2+]i oscillation-inducing activity from both SFC and SFpH upon B-A7/avidin precipitation also was dose dependent, whereas pre-incubation with B-A9 had no effect (D).
Fig. 5.
KCl treatment of SFpH/B-A7 pellets co-releases [Ca2+]i oscillation-inducing activity and PLCζ fragments p27, p33, and p37. A, [Ca2+]i oscillation-inducing activity from the B-A7/avidin SFpH pellet was recovered after washes with IB and after treatment with IB supplemented with 2 M KCl. After incubation on ice, the supernatant (Sup II) was separated from the pellet (Pellet II) and subjected to microinjection (B) or immunoblotting (C). B, Injection of Sup II following B-A7 precipitation and KCl treatment triggered [Ca2+]i oscillations in all tested mouse eggs (9 out of 9). In contrast, injection of Sup II from KCl-treated B-A9 pellets had no effects. Likewise, injection of Sup II from B-A7 pellets treated with IB without additional KCl did not cause [Ca2+]i responses. C, Immunoblotting with anti-PLCζ-NT (left) and anti-PLCζ-CT antibodies (right) shows that KCl treatment of SFpH/B-A7 pellets partially released PLCζ fragments into Sup II, although detectable amounts of the fragments still remained in Pellet II. PLCζ fragments are indicated by arrowheads (p27, p33, and p37).
Given that the association between peptide A7 and PLCζ/PLCζ fragments is likely mediated via opposite ionic charges in the molecules, increasing the ionic concentration of the solution should interfere with the interaction of the molecules and lead to the recovery of PLCζ/PLCζ fragments and [Ca2+]i oscillation-inducing activity into the supernatants (Sup II; see Fig. 5A). Consequently, injection of Sup II, which was obtained after incubation of B-A7/SFpH pellets with IB supplemented with 2 M KCl (Fig. 5A), resulted in [Ca2+]i oscillations in all tested eggs (Fig. 5B). Importantly, this treatment also resulted in the partial recovery of PLCζ fragments (p27, p33, p37) into Sup II (Fig. 5C). In contrast, supernatants obtained after treatment of B-A7 pellets with IB without additional KCl supplementation failed to trigger any [Ca2+]i responses (Fig. 5B). Likewise, KCl treatment of B-A9 pellets did not release [Ca2+]i oscillation-inducing activity (Fig. 5B) nor did this treatment release PLCζ fragments into Sup II (Fig. 5C). Together, these results suggest that peptide A7 exerts its inhibitory effect on sperm extracts by interacting with PLCζ and/or its fragments and further support our immunodepletion data showing that the 33/37-kDa and 27-kDa fragments form a functional PLC complex.
Co-expression of mPLCζ (1-361) and mPLCζ (362-647) triggers [Ca2+]i oscillations as well as egg activation and embryo development
To ascertain which PLCζ fragment(s) was responsible for triggering [Ca2+]i oscillations in eggs, we generated various mutants of mPLCζ (Fig. 6) and expressed their cRNAs in mouse eggs. Expression of wild type mPLCζ cRNA (mPLCζ WT) induced [Ca2+]i oscillations within 1 hour in all injected eggs (Fig. 7A). In contrast, microinjection of cRNAs that lacked either the region encoding the three most N-terminal EF-hand domains (mPLCζ ΔEF; Fig. 6), or a part of the C2 domain (mPLCζ ΔC2; Fig. 6) failed to induce any [Ca2+]i responses (Figs. 7B and C). As expected, none of the eggs injected with mPLCζ ΔEF or mPLCζ ΔC2 cRNA showed signs of egg activation, whereas nearly all eggs injected with mPLCζ WT cRNA were activated (Table 1). These results are consistent with previous reports (Saunders et al., 2002; Kouchi et al., 2005; Nomikos et al., 2005) indicating that both EF-hand and C2 domains are required for PLCζ to achieve maximal enzymatic activity.
Fig. 6.
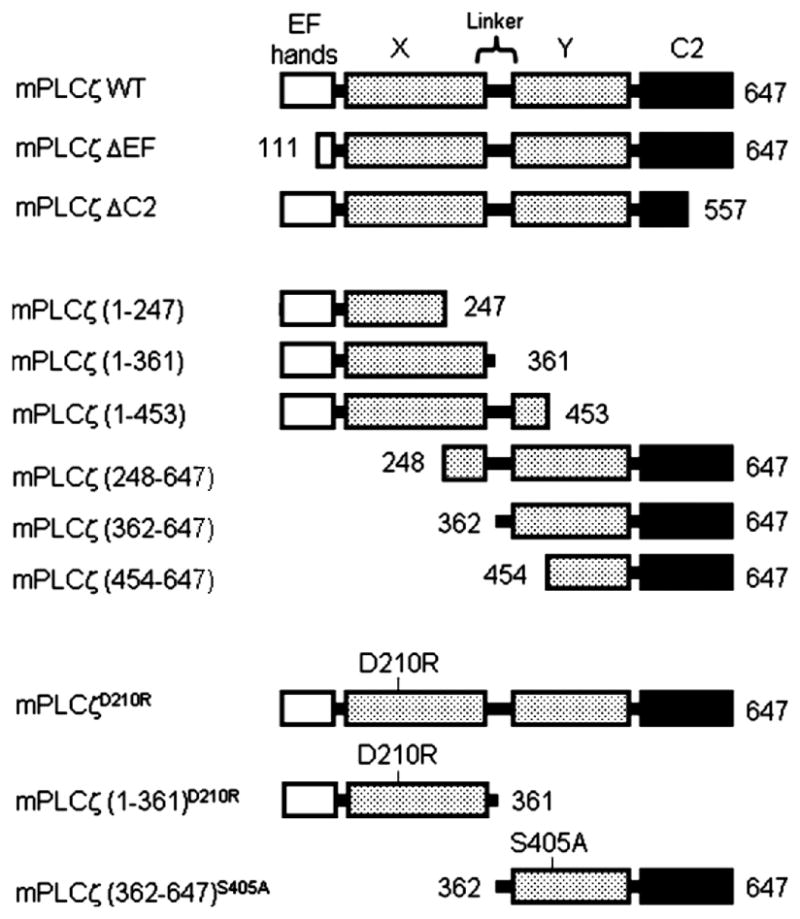
Schematic representation of wild type (WT) and mutant mPLCζ constructs. WT and mutant versions of mPLCζ were generated and cRNAs prepared (see Materials and Methods). Where present, numbers indicate the first and/or last amino acid within construct.
Fig. 7.
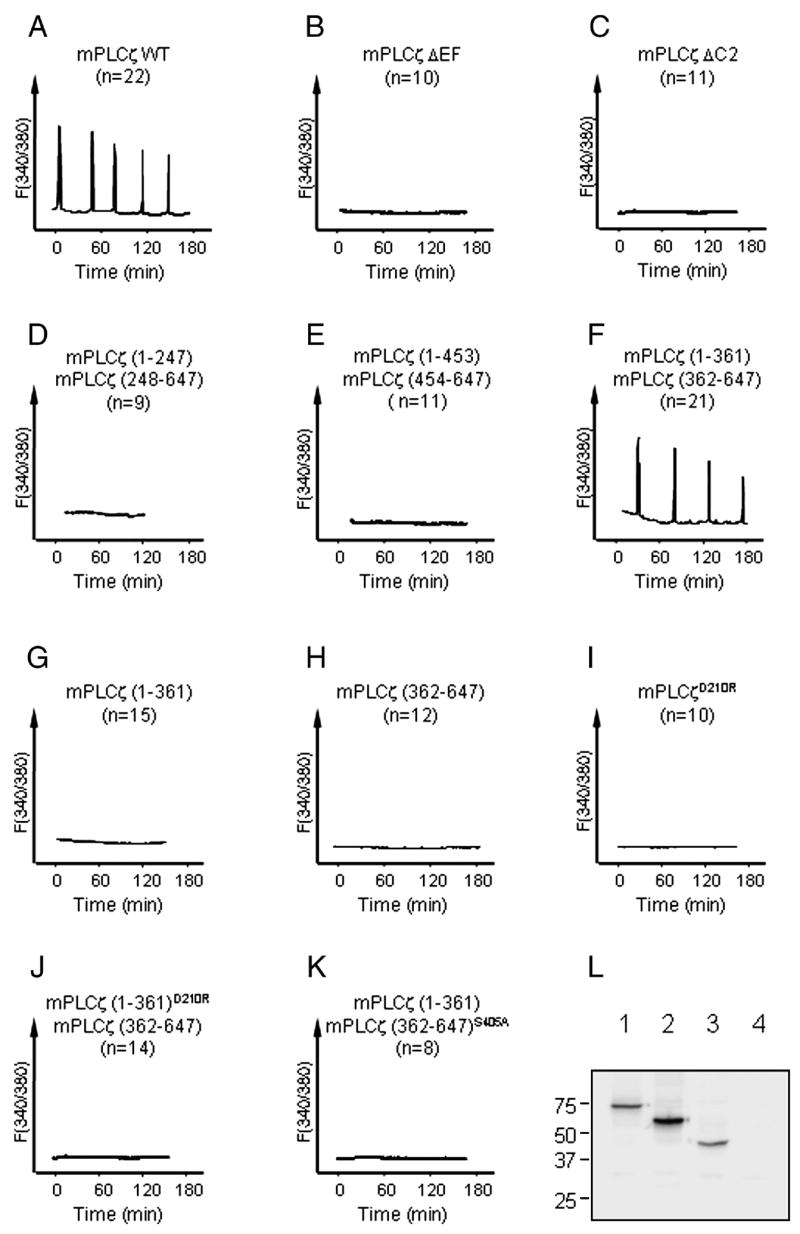
Co-expression of mPLCζ (1-361) and mPLCζ (362-647) cRNAs triggers [Ca2+]i oscillations in mouse eggs. A-K, cRNAs encoding WT and mutant PLCζs prepared as above were produced, and injected into mouse eggs. cRNAs were injected at 0.3 μg/μl (concentration in the pipette). L, mPLCζ ΔC2 (lane 1), mPLCζ (1-361)D210R (lane 2), mPLCζ (362-647)S405A (lane 3) with myc-tag were transfected to Cos-7 cells. Forty-eight hours later, cells were harvested and subjected to SDS-PAGE followed by western blotting with anti-myc antibody. As a control, mPLCζ (362-647) without myc-tag was similarly treated and loaded in lane 4.
Table 1.
Mouse egg activation following injection of mPLCζ cRNA constructs.
| cRNA constructs | No. eggs injected | No. Activation (%) |
|---|---|---|
| mPLCζ WT | 65 | 59 (91) |
| mPLCζ ΔEF | 30 | 0 (0) |
| mPLCζ ΔC2 | 54 | 0 (0) |
| mPLCζ (1-247) + mPLCζ (248-647) | 21 | 0 (0) |
| mPLCζ (1-361) + mPLCζ (362-647) | 69 | 69 (97) |
| mPLCζ (1-453) + mPLCζ (454-647) | 21 | 0 (0) |
| mPLCζD210R | 58 | 0 (0) |
| mPLCζ (1-361) | 26 | 0 (0) |
| mPLCζ (362-647) | 24 | 0 (0) |
| mPLCζ (1-361)D210R + mPLCζ (362-647) | 32 | 0 (0) |
| mPLCζ (1-361) + mPLCζ (362-647)S405A | 36 | 0 (0) |
Egg activation was assessed by PN formation at 6 hours post-injection. All cRNAs were injected at 0.3 μg/μl (concentration in the pipette); when two different cRNAs were injected, the total cRNA concentration in the pipette was of 0.6 μg/μl.
Next, we tested whether co-expression of two cRNAs encoding different but complementary portions of mPLCζ fragments, i.e. one cRNA encoded for an N-terminal fragment and the other for a complementary C-terminal portion that restored the full length of the molecule, was able to trigger [Ca2+]i oscillations and egg activation. Expression of cRNAs pairs encoding mPLCζ residues 1-247 and 248-647, or residues 1-453 and 454-647 (Fig. 6) failed to induce [Ca2+]i responses or egg activation (Fig. 7D and E and Table 1). Remarkably, co-injection of cRNAs encoding mPLCζ residues 1-361 and 362-647 (Fig. 6) induced persistent [Ca2+]i oscillations (Fig. 7F) and egg activation at similar rates to those injected with mPLCζ WT cRNA (Table 1 and Fig. 8, top panel, 6 hours), whereas injection of either cRNA alone did not induce any Ca2+ release (Fig. 7G and H) or egg activation (Table 1). Furthermore, substitution of Asp210 with Arg (Fig. 6), which is known to abrogate PLC activity in mPLCζ (mPLCζD210R in Fig. 7I and Table 1), abolished the ability of the cRNA combination (mPLCζ (1-361)D210R + (362-647)) to trigger [Ca2+]i oscillations and egg activation (Fig. 7J and Table 1). Likewise, substitution of Ser405 by Ala (Fig. 6), which also significantly decreases PLC activity (Ellis et al., 1998), abolished the ability of mPLCζ (1-361) + (362-647)S405A cRNA combination to initiate [Ca2+]i oscillations and egg activation (Fig. 7K and Table 1). To confirm that the mutant constructs were indeed translated, we selected some of the inactive constructs for transfection into Cos-7 cells. For detection of the expressed proteins, the same expression vector as above, but with the N-terminal myc-tag, was used, and western blotting was performed with anti-myc-antibody. mPLCζ ΔC2, which failed to induce Ca2+ release, was abundantly expressed in these cells (Fig. 7L). Likewise, expression of mPLCζ (1-361)D210R and mPLCζ (362-647)S405A also was clearly detected (Fig. 7L). Together, these results suggest that the absence of [Ca2+]i responses after injection of these mutants was due to inherent defects in enzymatic activity rather than to defects in protein expression or stability.
Fig. 8.
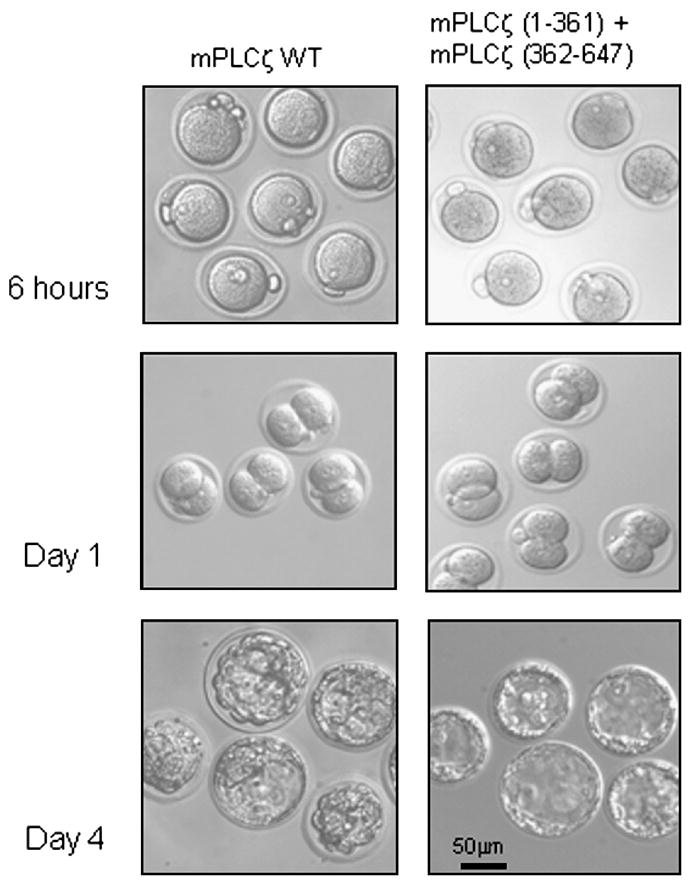
Co-expression of mPLCζ (1-361) and mPLCζ (362-647) cRNAs induces egg activation and development. Mouse eggs injected with mPLCζ WT cRNA or co-injected with cRNAs encoding mPLCζ (1-361) and mPLCζ (362-647) extruded the second polar body and formed a female PN. cRNA-injected eggs treated with cytochalasin B developed to the 2-cell stage (day 1) and blastocysts (day 4).
Finally, the developmental potential of [Ca2+]i oscillations initiated by co-expression of cRNAs encoding mPLCζ residues (1-361) and mPLCζ (362-647) was ascertained. To maintain the diploid status of parthenogenetic zygotes, following injection of the cRNAs, eggs were cultured in the presence of cytochalasin B, to prevent extrusion of the second polar body. Injection of either mPLCζ WT or co-injection of mPLCζ (1-361) and (362-647) cRNAs resulted in similar cleavage rates to the 2-cell stage (day 1), and development to the morula and blastocyst stage (day 4; Table 2 and Fig. 8).
Table 2.
Embryo development following injection of mPLCζ cRNAs into mouse eggs.
| cRNA constructs | No. eggs injected | No. eggs (%) developed to
|
|||
|---|---|---|---|---|---|
| PN | 2-cell | MO+BL | BL | ||
| mPLCζ WT | 14 | 14 (100) | 14 (100) | 10 (71) | 4 (29) |
| mPLCζ (1-361) + mPLCζ (362-647) | 36 | 34 (94) | 30 (83) | 22 (61) | 14 (39) |
MO: Morula
BL: Blastocyst
DISCUSSION
The present study shows that PLCζ represents the sole Ca2+ active factor required for egg activation in porcine and most likely in all mammalian sperm. While some pSFC and all pSFpH were devoid of 72-kDa PLCζ (Kurokawa et al., 2005), all the highly Ca2+ sensitive in vitro PLC-and [Ca2+]i oscillatory activity could be accounted for the presence of PLCζ fragments, as evidenced by the obliteration of these activities by immuno/affinity depletion of 72-kDa and/or of its fragments. Further, co-expression of cRNAs encoding complementary PLCζ fragments induced [Ca2+]i oscillations, egg activation and embryo development. Together, our results point to PLCζ as the exclusive factor that underpins the fertilization-associated [Ca2+]i oscillations in mammals and our data also implies a critical regulatory role for the enzyme’s linker region during fertilization.
PLCζ is the putative sperm factor
In a previous study that characterized for the first time the complete [Ca2+]i oscillation-inducing activity of a mammalian sperm, we found that a fraction, the SFpH fraction, despite lacking 72-kDa PLCζ, was capable of inducing [Ca2+]i oscillations and egg activation of mouse eggs. Moreover, SFpH exhibited prominent in vitro PLC activity, especially at resting [Ca2+]i levels, which is a hallmark of PLCζ enzymatic activity (Kurokawa et al., 2005). Given these seemingly paradoxical results, here we examined whether fragments of PLCζ could account for the [Ca2+]i oscillatory and PLC activity in SFpH. Our present results demonstrate that although SFpH was devoid of 72-kDa PLCζ, it was endowed with several N- and C-terminal PLCζ fragments. Moreover, we observed that the 27-kDa C-terminal PLCζ fragment co-immunoprecipitated with the 33-kDa and/or 37-kDa PLCζ N-terminal fragments, and that depletion of these fragments exhausted the [Ca2+]i oscillatory and in vitro PLC activity of SFpH. Similar results were observed with depletion experiments performed with B-A7 peptides. Our results therefore demonstrate that all the [Ca2+]i oscillation-inducing activity of porcine sperm can be attributed to 72-kDa PLCζ and/or its N- and C-terminal fragments, which remain associated and form a functional complex. Our findings may also explain results from a previous report in which the ability of porcine sperm fractions to induce egg activation did not precisely coincide with the presence of 72-kDa PLCζ (Fujimoto et al., 2004).
The X-Y linker region as a regulatory domain of PLCζ function
The regulation of PLCζ function in vivo and the role of each of its domains in this process remain to be fully characterized. It is well known that both catalytic domains, the X- and Y-domains, are indispensable for the enzymatic activity of all PLC family members (Rebecchi and Pentyala, 2000). Regarding PLCζ, recent studies have shown that both the EF-hand and C2 domains are also essential for enzymatic activity, as partial deletion of the N-terminal end or of the C-terminal end of the molecule reduced the in vitro PLC activity of respective recombinant proteins, as well as abrogated the ability of corresponding cRNAs to trigger [Ca2+]i oscillations upon injection into eggs (Kouchi et al., 2005; Nomikos et al., 2005). Our results following expression of mPLCζ ΔEF or mPLCζ ΔC2 cRNAs in mouse eggs confirm those findings. In light of these results, it could be envisaged that the formation of an active complex by PLCζ fragments requires fragments with intact N- and C-terminal ends and that each fragment includes an intact X- or Y-domain; seemingly, these restrictions can be accommodated if cleavage of PLCζ takes place between the X- and Y-domains. Accordingly, co-expression of complementary PLCζ cRNAs mimicking cleavage within the X- (mPLCζ (1-247) + mPLCζ (248-647)) or the Y-domains (mPLCζ (1-453) + mPLCζ (454-647)) failed to induce [Ca2+]i oscillations or egg activation, whereas co-expression of cRNAs in which the cleavage site was within the linker region (mPLCζ (1-361) and mPLCζ (362-647)) triggered [Ca2+]i oscillations and embryo development. Furthermore, the finding that the estimated molecular weight of the polypeptides encoded by mPLCζ (1-361) and mPLCζ (362-647) cRNAs are of ~42- and 32-kDa, respectively, which are remarkably similar to those of the endogenous N-terminal (~33- and 37-kDa) and C-terminal (27-kDa) fragments in SFs, supports the notion that in vivo PLCζ cleavage takes place in the linker region. Additional supporting evidence for this possibility emanates from our in vitro proteolytic studies using the protease V8, which induced rapid disappearance of 72-kDa PLCζ from SFC while promoting concomitant accumulation of polypeptides at apparent molecular weights corresponding to the N- (33- and 37-kDa) and C-terminal (27-kDa) endogenous fragments. It should be noted that given that the X-Y linker region spans for ~70 amino acids in mouse and porcine PLCζs (residues 309-385 and 300-374, respectively), which represent ~9 kDa, it seems likely that the fragments in SFs are produced by multiple cleavages within this domain. Indeed, the total molecular weights of the complexes estimated from respective fragments (33 + 27 = 60 kDa, 37 + 27 = 64 kDa) suggest that such cleavages may remove a large portion of the X-Y linker region.
The present demonstration that PLCζ fragments in sperm and in SFs retain activity and are capable of inducing [Ca2+]i oscillations is the first example that spontaneous proteolysis of PLCs may play a physiological role in cellular function. Importantly, earlier studies have demonstrated that the X-Y linker domain of PLCβ, γ, and δ isoforms was susceptible to in vitro proteolysis, and those studies also found that the two resulting PLC fragments, which remained associated and formed a functional heterodimer, exhibited enhanced rather than reduced PLC catalytic activity compared to the holoenzymes (Ellis et al., 1993; Fernald et al., 1994; Schnabel and Camps, 1998). As a corollary, it was suggested that cleavage in this domain might cause a conformational change in the tertiary association of the X- and Y-domains, thereby facilitating interaction and hydrolysis with the lipid substrates, or that it might result in removal of part of the X-Y liker domain, thereby facilitating the access of the catalytic site to PIP2 (Jones and Wu, 2000). Consistent with these hypotheses, the in vitro PLC activity of SFpH is 4 to 5-fold higher than that of SFC (data not shown; also see Kurokawa et al., 2005). In addition, in vitro assays revealed that the Ca2+ sensitivity of PLC activity of SFC fractions was greatly enhanced by the presence of PLCζ fragments (Kurokawa et al., 2005). Lastly, our previous observation that after FPLC fractionation one of the SFC fractions was mostly devoid of PLC- and [Ca2+]i oscillation-inducing activity despite that 72-kDa PLCζ was readily detectable (Kurokawa et al., 2005), supports the view that modification of the X-Y linker domain affects PLCζ activity.
Besides affording modulation of PLCζ function by undergoing proteolysis, the X-Y linker domain may regulate PLCζ function via other mechanisms. For instance, the presence of extended charged amino acid repeats within this region of PLCζ may facilitate interaction with other proteins (Boehning et al., 2005), which may serve to further modulate/protect PLCζ; these acidic repeats may also serve as additional motifs for Ca2+ regulation (Haber-Pohlmeier et al., 2007). Lastly, the presence in this domain of a conserved stretch of positively charged amino acids, which serve as a nuclear localization signal, is reportedly also required to attain maximal enzymatic activity (Kuroda et al., 2006), possibly by regulating the cellular distribution of the enzyme and/or by favoring the interaction of the substrate with PLCζ (Heo et al., 2006; Nomikos et al., 2007). Importantly, it is this last property that may be interfered with by the binding of peptide A7 to PLCζ and that results in decrease in vitro PLC activity of the enzyme. While presently we do not know the site(s) of interaction between pPLCζ and peptide A7, given that their interaction is likely underpinned by complementary electrostatic charges and that the X-Y linker region is enriched in charged residues, it is logical to envision that peptide A7 is binding to this domain and that this might interfere with the association/processing of the substrate by PLCζ. Collectively, our results therefore support the possibility that in addition to being a target of proteolysis, the X-Y linker domain may play other important regulatory roles in the activity of PLCζ.
Does proteolytic cleavage of PLCζ play a role in mammalian fertilization?
Whether or not proteolytic processing of PLCζ X-Y linker domain plays a role in mammalian fertilization remains to be determined. However, the finding that PLCζ fragments consistent with enzyme cleavage in this region are observed in fresh intact sperm and in sperm extracts, together with the evidence that this cleavage may enhance the catalytic activity of PLCζ, strengthens the possibility that processing of this domain may play a role during mammalian fertilization. It is important to note that when whole sperm samples were prepared for immunoblotting, the samples were handled such that sperm retain motility until addition of the sample buffer, greatly diminishing the possibility that the detected PLCζ fragments were generated by our manipulations. To the best of our knowledge, this is the first demonstration that functional cleavage of a PLC actually takes place at the endogenous levels.
In summary, our results show that PLCζ and/or its complexed fragments account for all the [Ca2+]i oscillation-inducing activity of porcine sperm/SFs as well as for all the high-Ca2+ sensitive in vitro PLC activity of these fractions. Our data also suggest that proteolytic processing of PLCζ may play a role during fertilization and that the linker region of PLCζ, which is likely the site of cleavage, may have several other important regulatory functions. Lastly, given that the amino acid sequence of the X-Y linker domain of PLCζ is poorly conserved among species except for the presence of charged amino acids, it is tempting to speculate that the different motifs in this region may underlie the well-established species-specific patterns of [Ca2+]i oscillations among mammals.
Acknowledgments
We thank Ms. Changli He for technical assistance. This work was supported in part by a National Research Initiative Competitive Grant no. 2007-35203-17840 from the USDA Cooperative State Research, Education, and Extension Service and by an RO3 grant from the NIH to R.A.F. M.K was also supported by a Lalor Foundation Fellowship. D.A. was supported by a DE 016289 grant from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boehning D, van Rossum DB, Patterson RL, Snyder SH. A peptide inhibitor of cytochrome c/inositol 1,4,5-trisphosphate receptor binding blocks intrinsic and extrinsic cell death pathways. Proc Natl Acad Sci USA. 2005;102:1466–1471. doi: 10.1073/pnas.0409650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP. Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev Biol. 2002;250:280–291. [PubMed] [Google Scholar]
- Dupont G, McGuinness OM, Johnson MH, Berridge MJ, Borgese F. Phospholipase C in mouse oocytes: characterization of beta and gamma isoforms and their possible involvement in sperm-induced Ca2+ spiking. Biochem J. 1996;316:583–591. doi: 10.1042/bj3160583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MV, Carne A, Katan M. Structural requirements of phosphatidylinositol-specific phospholipase Cδ1 for enzyme activity. Eur J Biochem. 1993;213:339–347. doi: 10.1111/j.1432-1033.1993.tb17767.x. [DOI] [PubMed] [Google Scholar]
- Ellis MV, James SR, Perisic O, Downes CP, Williams RL, Katan M. Catalytic domain of phosphoinositide-specific phospholipase C (PLC). Mutational analysis of residues within the active site and hydrophobic ridge of PLCδ1. J Biol Chem. 1998;273:11650–11659. doi: 10.1074/jbc.273.19.11650. [DOI] [PubMed] [Google Scholar]
- Fernald AW, Jones GA, Carpenter G. Limited proteolysis of phospholipase C-γ1 indicates stable association of X and Y domains with enhanced catalytic activity. Biochem J. 1994;302:503–509. doi: 10.1042/bj3020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fissore RA, He CL, Vande Woude GF. Potential role of mitogen-activated protein kinase during meiosis resumption in bovine oocytes. Biol Reprod. 1996;55:1261–1270. doi: 10.1095/biolreprod55.6.1261. [DOI] [PubMed] [Google Scholar]
- Fukami Y, Sato K, Ikeda K, Kamisango K, Koizumi K, Matsuno T. Evidence for autoinhibitory regulation of the c-src gene product. A possible interaction between the src homology 2 domain and autophosphorylation site. J Biol Chem. 1993;268:1132–1140. [PubMed] [Google Scholar]
- Fujimoto S, Yoshida N, Fukui T, Amanai M, Isobe T, Itagaki C, Izumi T, Perry AC. Mammalian phospholipase Cζ induces oocyte activation from the sperm perinuclear matrix. Dev Biol. 2004;274:370–383. doi: 10.1016/j.ydbio.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber-Pohlmeier S, Abarca Heidemann K, Korschen HG, Kaur Dhiman H, Heberle J, Schwalbe H, Klein-Seetharaman J, Kaupp UB, Pohlmeier A. Binding of Ca2+ to glutamic acid-rich polypeptides from the rod outer segment. Biophys J. 2007;92:3207–3214. doi: 10.1529/biophysj.106.094847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Cruttwell C, Parrington J, Swann K. A mammalian sperm cytosolic phospholipase C activity generates inositol trisphosphate and causes Ca2+ release in sea urchin egg homogenates. FEBS Lett. 1998;437:297–300. doi: 10.1016/s0014-5793(98)01254-x. [DOI] [PubMed] [Google Scholar]
- Jones GA, Wu Y. Effect of limited proteolysis on phospholipase C-γ1 kinetics. Arch Biochem Biophys. 2000;375:229–239. doi: 10.1006/abbi.1999.1670. [DOI] [PubMed] [Google Scholar]
- Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S. Recombinant phospholipase Cζ has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem. 2004;279:10408–10412. doi: 10.1074/jbc.M313801200. [DOI] [PubMed] [Google Scholar]
- Kouchi Z, Shikano T, Nakamura Y, Shirakawa H, Fukami K, Miyazaki S. The role of EF-hand domains and C2 domain in regulation of enzymatic activity of phospholipase Cζ. J Biol Chem. 2005;280:21015–21021. doi: 10.1074/jbc.M412123200. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Ito M, Shikano T, Awaji T, Yoda A, Takeuchi H, Kinoshita K, Miyazaki S. The role of X/Y linker region and N-terminal EF-hand domain in nuclear translocation and Ca2+ oscillation-inducing activities of phospholipase Cζ, a mammalian egg-activating factor. J Biol Chem. 2006;281:27794–27805. doi: 10.1074/jbc.M603473200. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Fissore RA. Mammalian fertilization: from sperm factor to phospholipase Cζ. Biol Cell. 2004a;96:37–45. doi: 10.1016/j.biolcel.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Smyth J, Wu H, Fukami K, Takenawa T, Fissore RA. Evidence that activation of Src family kinase is not required for fertilization-associated [Ca2+]i oscillations in mouse eggs. Reproduction. 2004b;127:441–454. doi: 10.1530/rep.1.00128. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Wu H, He C, Malcuit C, Black SJ, Fukami K, Fissore RA. Functional, biochemical, and chromatographic characterization of the complete [Ca2+]i oscillation-inducing activity of porcine sperm. Dev Biol. 2005;285:376–392. doi: 10.1016/j.ydbio.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Liu N, Fukami K, Yu H, Takenawa T. A new phospholipase Cδ4 is induced at S-phase of the cell cycle and appears in the nucleus. J Biol Chem. 1996;271:355–360. doi: 10.1074/jbc.271.1.355. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992;257:251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Blayney LM, Larman MG, Campbell K, Rossbach A, Saunders CM, Swann K, Lai FA. Role of phospholipase Cζ domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J Biol Chem. 2005;280:31011–31018. doi: 10.1074/jbc.M500629200. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Mulgrew-Nesbitt A, Pallavi P, Mihalyne G, Zaitseva I, Swann K, Lai FA, Murray D, McLaughlin S. Binding of Phosphoinositide-specific Phospholipase C-ζ (PLC-ζ) to Phospholipid Membranes: Potential role of an unstructured cluster of basic residues. J Biol Chem. 2007;282:16644–16653. doi: 10.1074/jbc.M701072200. [DOI] [PubMed] [Google Scholar]
- Parrish JJ, Susko-Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod. 1988;38:1171–1180. doi: 10.1095/biolreprod38.5.1171. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- Rice A, Parrington J, Jones KT, Swann K. Mammalian sperm contain a Ca2+-sensitive phospholipase C activity that can generate InsP3 from PIP2 associated with intracellular organelles. Dev Biol. 2000;228:125–135. doi: 10.1006/dbio.2000.9929. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLCζ: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- Schnabel P, Camps M. Activation of a phospholipase Cβ2 deletion mutant by limited proteolysis. Biochem J. 1998;330:461–468. doi: 10.1042/bj3300461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr Top Dev Biol. 1995;30:21–62. doi: 10.1016/s0070-2153(08)60563-3. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Wu H, He CL, Fissore RA. Injection of a porcine sperm factor triggers calcium oscillations in mouse oocytes and bovine eggs. Mol Reprod Dev. 1997;46:176–189. doi: 10.1002/(SICI)1098-2795(199702)46:2<176::AID-MRD8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Wu H, Smyth J, Luzzi V, Fukami K, Takenawa T, Black SL, Allbritton NL, Fissore RA. Sperm factor induces intracellular free calcium oscillations by stimulating the phosphoinositide pathway. Biol Reprod. 2001;64:1338–1349. doi: 10.1095/biolreprod64.5.1338. [DOI] [PubMed] [Google Scholar]
- Yoneda A, Kashima M, Yoshida S, Terada K, Nakagawa S, Sakamoto A, Hayakawa K, Suzuki K, Ueda J, Watanabe T. Molecular cloning, testicular postnatal expression, and oocyte-activating potential of porcine phospholipase Cζ. Reproduction. 2006;132:393–401. doi: 10.1530/rep.1.01018. [DOI] [PubMed] [Google Scholar]