Abstract
The androgen receptor (AR) is a critical effector of prostate cancer development and progression. The dependence of this tumor type on AR activity is exploited in treatment of disseminated prostate cancers, wherein ablation of AR function (achieved either through ligand depletion and/or the use of AR antagonists) is the first line of therapeutic intervention. These strategies are initially effective, and induce a mixed response of cell cycle arrest or apoptosis in prostate cancer cells. However, recurrent, incurable tumors ultimately arise as a result of inappropriately restored AR function. Based on these observations, it is imperative to define the mechanisms by which AR controls cancer cell proliferation. Mechanistic investigation has revealed that AR acts as a master regulator of G1-S phase progression, able to induce signals that promote G1 cyclin-dependent kinase (CDK) activity, induce phosphorylation/inactivation of the retinoblastoma tumor suppressor (RB), and thereby govern androgen-dependent proliferation. These functions appear to be independent of the recently identified TMPRSS2-ETS fusions. Once engaged, several components of the cell cycle machinery actively modulate AR activity throughout the cell cycle, thus indicating that crosstalk between the AR and cell cycle pathways likely modulate the mitogenic response to androgen. As will be discussed, discrete aberrations in this process can alter the proliferative response to androgen, and potentially subvert hormonal control of tumor progression.
Prostate cancer is dependent on androgen action
Prostatic adenocarcinoma is the most frequently diagnosed malignancy and second leading cause of cancer death amongst men in the United States [Jemal et al., 2005]. Localized prostate cancer can be definitively treated by surgical resection or through radiation therapy [Catalona et al., 1999; Denmeade and Isaacs, 2002; Dorff et al., 2006; Swanson, 2006]. However, invasive or even micrometastatic disease presents a clinical challenge, as these tumors respond poorly to standard cytotoxic regimens that act through genomic insult. Therefore, prostate cancers are treated based on a unique characteristic, in that they are exquisitely dependent on androgen for development, growth, and survival [Balk, 2002; Culig and Bartsch, 2006; Jenster, 1999; Klotz, 2000]. Androgen ablation triggers cell death or cell cycle arrest of prostate cancer cells [Agus et al., 1999; Denmeade et al., 1996; Huggins and Hodges, 1972; Isaacs, 1984; Knudsen et al., 1998; Kyprianou and Isaacs, 1988]. Thus, androgen ablation remains the primary course of treatment for all patients with metastatic disease [Jenster, 1999; Klotz, 2000; Loblaw et al., 2004; Sowery et al., 2007]. These therapies are initially effective, and result in disease remission. However, recurrent tumors arise within a median of 2-3 years, wherein androgen signaling has been inappropriately restored [Feldman and Feldman, 2001]. At present, few therapeutic regimens have been described to effectively manage recurrent prostate cancers, and this is considered an incurable stage of the disease. Given the dependence of prostate cancer cells on the androgen signaling axis, a concerted effort has been undertaken to determine the mechanism(s) by which androgens induce prostate cancer cell proliferation and survival.
Androgen receptor regulation in prostate cancer
Androgen exerts its biological effects through the androgen receptor (AR), a member of the nuclear receptor superfamily that acts as a ligand dependent transcription factor [Evans, 1988; Mangelsdorf et al., 1995; Shand and Gelmann, 2006; Trapman and Brinkmann, 1996]. Testosterone is the most abundant androgen in the sera, but in prostatic epithelia is converted to a more potent androgen, dihydrotestosterone (DHT) through the action of a resident enzyme, 5α-reductase [Russell and Wilson, 1994; Wilson, 1996]. Prior to ligand binding, the AR is held inactive through association with heat shock proteins and is precluded from DNA binding. Ligand binding releases the inhibitory heat shock proteins, and the receptor rapidly translocates to the nucleus, where it binds DNA as a homodimer on androgen responsive elements (AREs) within the regulatory regions of target genes [Gelmann, 2002; Marivoet et al., 1992; Trapman and Brinkmann, 1996]. Furthermore, recruitment of coactivators (which contain or recruit histone acetylases) and chromatin remodeling complexes facilitate transcriptional initiation, and AR-dependent gene expression ensues [Gnanapragasam et al., 2000; Heinlein and Chang, 2002; Heinlein and Chang, 2004]. The capacity of AR to subsequently induce a gene expression program that promotes cell cycle progression is clearly dependent on cell context. For example, during development and homeostasis it is clear that the stromal AR plays a major role in stimulating epithelial cell proliferation; by contrast, it is hypothesized that a switching mechanism arises during tumorigenesis to render the proliferative function of AR cell autonomous in prostate cancer cells. The specific combinations of cofactors recruited to AREs likely also provide a mechanism for tissue specific and ligand specific gene expression. Through these actions, it is apparent that the AR promotes prostate cancer survival and proliferation in prostate cancer cells [Balk, 2002; Feldman and Feldman, 2001].
While the subsets of AR target genes that underlie each cellular outcome have yet to be clearly defined, discovery of at least one major AR-dependent target gene, prostate specific antigen (PSA) [Riegman et al., 1991], has had a major impact on disease management. Specifically, serum PSA is monitored clinically to detect early stage disease, track tumor burden, monitor the efficacy of therapeutic intervention, and detect the emergence of recurrent tumors post-therapy [Nash and Melezinek, 2000; Ryan et al., 2006]. Thus, readouts of AR activity are critical for the assessment of disease progression and therapeutic outcome. In addition, it has been recently discovered that chromosomal translocations occur with significant frequency in prostate cancer which render the potentially pro-proliferative ETS genes (ERG, ETV1, or ETV4) under control of the AR-induced TMPRSS2 promoter/enhancer [Demichelis et al., 2007; Tomlins et al., 2005; Wang et al., 2007]. However, subsequent functional investigations suggest that the TMPRSS2-ETS fusions may principally exert their effects through alteration of tumor phenotypes other than cell proliferation. For example, overexpression of ETV1 in normal or transformed prostatic epithelia had no impact on cellular proliferation rates or cell cycle progression, but facilitated invasion of these models [Tomlins et al., 2007]. Thus, the contribution of these fusions to alterations in cell cycle control has yet to be well resolved. Lastly, it is possible that non-genomic AR signaling may influence the proliferative program. It is known that androgen stimulation in AR-positive cells can trigger rapid activation of the MAPK pathway, and thereby potentially induce a mitogenic response. Consistent with this idea, rapid androgen signaling was recently identified as a major stimulus for meiotic progression in Xenopus oocytes, and has also been linked to muscle cell proliferation [White et al., 2005; Yoshioka et al., 2006]. Thus, while the mechanisms underpinning the capacity of AR to induce a mitogenic program may be diverse and dependent on cell context, it is clear that ligand-dependent activation of AR is a limiting factor for engagement of the cell cycle machinery in prostate cancer cells.
Given these functions, inhibition of AR activity is the major therapeutic goal for management of metastatic disease, as achieved via multiple mechanisms [Balk, 2002; Feldman and Feldman, 2001; Leewansangtong and Soontrapa, 1999; Taplin and Balk, 2004]. First line treatment ablates AR function through ligand depletion, as achieved through either bilateral orchiectomy or the use of GnRH agonists. Adjuvant or second line therapies involve the use of direct AR antagonists (e.g., bicalutamide) which utilize at least two mechanisms of action [Chodak, 2005; Klotz, 2006] . First, these agents compete for DHT binding. Second, selected AR antagonists trigger the recruitment of transcriptional corepressors (e.g., NCoR) to AREs, thereby fostering active repression of AR target gene expression [Shang et al., 2002]. At the cellular level, androgen ablation induces cell death or cell cycle arrest, which underpins tumor regression [Agus et al., 1999; Denmeade et al., 1996; Huggins and Hodges, 1972; Isaacs, 1984; Knudsen et al., 1998; Kyprianou and Isaacs, 1988]. Effective AR inhibition is also observed by a loss of detectable serum PSA. However, this remission is transient, and tumor recurrence is almost invariably observed [Balk, 2002; Feldman and Feldman, 2001; Leewansangtong and Soontrapa, 1999]. Recurrence is typically preceded by a rise in PSA (also called “biochemical recurrence”) [Feldman and Feldman, 2001; Klotz, 2000; Trapman and Brinkmann, 1996], and this observation yielded some of the first evidence that tumor progression is associated with inappropriately restored AR function, despite sustained androgen ablation and/or the use of AR antagonists. AR re-activation in recurrent tumors occurs through multiple mechanisms, including AR amplification, AR mutation, ligand-independent AR activation, hypersensitivity to a low ligand environment, enhanced local production of androgens, excessive production of AR coactivators, and/or as a result of signals that disrupt AR corepressor function [Chmelar et al., 2007; Feldman and Feldman, 2001; Stanbrough et al., 2006]. Indeed, it is now well established that such “androgen independent” prostate cancer remains exquisitely dependent on AR function [Chen et al., 2004; Cheng et al., 2006; Yuan et al., 2006]. Since AR appears to be a key determinant of prostate cancer growth and progression, it is imperative to dissect the mechanisms by which AR governs cellular proliferation in prostate cancer cells.
Mitogenic signaling and the cell cycle machinery: an overview
Transitions into and within the mitotic cell cycle are dictated by the coordinate activation of cyclin-dependent kinase (CDK)/cyclin complexes, wherein cyclin binding induces the catalytic activity of the kinase [Lee and Sicinski, 2006; Malumbres and Barbacid, 2007; Sherr, 1996; Sherr and Roberts, 2004]. Mitogenic signaling pathways generally induce cell cycle progression through ordered activation of CDK-cyclin complexes, whereas anti-mitogenic signals that result from extracellular events (e.g., nutrient depletion) or intracellular insults (e.g., DNA damage) typically serve to attenuate CDK function. Although the signals that dictate commitment to the cell cycle are often cell type-specific, the core machinery that drives the cell cycle engine is well conserved. Prior to mitogenic stimulation cells can exit the cell cycle and enter into a resting stage deemed “G0”. At this stage, several key gatekeepers of cell cycle transitions are invoked to prevent unscheduled cell cycle progression. Paramount amongst these is activation of the retinoblastoma tumor suppressor protein (RB), which assembles transcriptional repressor complexes on the promoters of target genes that are required to initiate DNA replication (e.g., cyclin A, PCNA) [Knudsen and Knudsen, 2006]. Many such critical RB target genes are primed for activation by residence of activator E2F/DP1 complexes on the promoter regions, but the transcriptional repressor complexes recruited by RB (which include histone deacetylases, SWI/SNF chromatin remodeling complexes and/or polycomb group proteins) act as potent inhibitors of transcriptional activation [Johnson and Degregori, 2006]. Thus, mitogenic stimuli must act to balance RB function, and do so through activation of CDK-cyclin complexes.
Mitogenic stimuli typically trigger accumulation of D-type cyclins (cyclins D1, D2, and/or D3), which can bind and activate the early G1 kinases CDK4 or CDK6 [Lee and Sicinski, 2006; Sherr and Roberts, 2004]. D-cyclin production is a tightly regulated process, which has been most extensively studied with cyclin D1 [Alao, 2007; Gladden and Diehl, 2005; Knudsen, 2006]. The cyclin D1 transcript is controlled at the level of mRNA production, stability, splicing, and translation. Once produced, cyclin D1 action is subjected to further regulation at the level of subcellular localization, targeted degradation, and CDK4/6 binding [Alt et al., 2000; Gladden et al., 2006]. These latter processes appear to involve p21Cip1, which was originally classified as a CDK inhibitor. This classification may be overly simplistic, as while p21Cip1 can inhibit CDK2 complexes, p21Cip1 is conversely important for promoting formation, activation and nuclear enrichment of CDK4/6-cyclin D complexes [Alt et al., 2002; Cheng et al., 1999; LaBaer et al., 1997; Sherr and Roberts, 1999]. Once active, the principal cell cycle function of CDK4/6-cyclin D complexes is to initiate RB phosphorylation. The concept that this is the major cell cycle function of CDK4/6 is supported by the observation that RB-deficient tumor cells are resistant to cell cycle arrest induced by inhibition of the kinase [Lukas et al., 1995]. Thus, loss of RB in cancer is proposed to bypass the requirement for this early event in cell cycle progression.
Although RB phosphorylation/inactivation is initiated by CDK4/6, this only partially compromises the RB transcriptional repressor function [Knudsen and Knudsen, 2006]. Subsequent to this event, a downstream kinase (CDK2) is responsible for completing RB phosphorylation and thereby extinguishing its repressor function. Critically, the requisite cyclins associated with CDK2, cyclins E and A, are themselves regulated via RB-mediated transcriptional repression. Thus, CDK2-cyclin complexes constitute a feed-forward mechanism to stimulate cell cycle progression through modulation of RB. In addition, cyclin E- and cyclin A-assembled complexes impinge on additional substrates that promote activation of the DNA replication machinery, centrosome duplication, and histone gene expression. Recent findings demonstrate that these activities are associated with both catalytic (CDK2 kinase dependent) and non-catalytic functions [Geng et al., 2007; Lee and Sicinski, 2006]. Subsequently, transition through the remainder of the cell cycle (G2 and M phase) is driven by ordered activation of CDK1-cyclin A and CDK1-cyclin B complexes [Shapiro, 2006]. Cyclin B production steadily rises in G2; once reaching a threshold level, mitotic entry ensues dependent on this kinase. CDK1-cyclin B activity continues to increase throughout early mitosis until anaphase, wherein rapid degradation of cyclin B (and thereby loss of CDK1 activity) triggers mitotic exit and completion of the cell cycle [Pines, 2006; Shapiro, 2006].
To counterbalance this sophisticated coordination of CDK-cyclin activation, mechanisms exist to halt the cell cycle in the presence of cellular insult. RB typically remains hyperphosphorylated/inactivated until mitotic exit, when the “braking” action of the RB tumor suppressor is reset by phosphatase activity. However, many anti-proliferative signals result in RB dephosphorylation/activation, thus inducing RB-dependent cessation of cell cycle progression. For example, DNA damage signals that induce p53-mediated p21Cip1 induction to high levels can cause downregulation of CDK2 activity and thereby prevent RB phosphorylation [Sherr and Roberts, 2004]. Alternatively, the related CDK2 inhibitor p27Kip1 is often induced by signals such as serum deprivation, thus acting through similar pathways to halt cell cycle progression [Nickeleit et al., 2007]. From these observations, it is apparent that the G1-S cell cycle machinery plays critical roles in the response to the intra- and extracellular environments. While these general principles are conserved in the majority of cell types, it is increasingly apparent that different mitogenic cues utilize disparate mechanisms to engage the cell cycle machinery.
AR governs the cyclin D-RB axis in prostate cancer cells
Analyses of AR-dependent cell cycle progression in prostate cancer cells have shown that androgen is a critical regulator of the G1-S transition (Figure 1). Prostate cancer cells deprived of androgen arrest in early G1 phase, concomitant with loss of cyclin D1 and cyclin D3 expression, attenuated CDK4 activity (expression unchanged), and hypophosphorylated/activated retinoblastoma tumor suppressor [Knudsen et al., 1998; Xu et al., 2006]. Recent studies revealed that androgen induces D-type cyclin expression via mTOR-dependent enhancement of translation [Xu et al., 2006]. The ability of androgen to modulate cyclin D translation is distinct from mechanisms utilized by other hormones. For example, estrogen induces cyclin D1 transcription in breast cancer cells, through the ability of its cognate receptor (the estrogen receptor, ER) to directly modulate cyclin D1 regulatory regions [Eeckhoute et al., 2006; Sabbah et al., 1999]. Thus, androgen regulation of early G1 events is specific to this class of hormone.
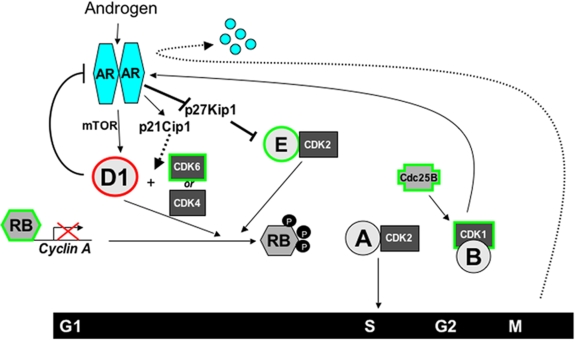
Figure 1. AR-cell cycle crosstalk.
Activated AR stimulates the accumulation of cyclin D1 (D1), through mammalian Target of Rapamycin (mTOR), to activate CDK4 and promote phosphorylation of the retinoblastoma (RB) tumor suppressor. In addition, AR-induced expression of p21Cip1 and degradation of p27Kip1 further enhance cycD1/CDK4 and cycE/CDK2-dependent inactivation of RB and allow expression of E2F target genes like cyclin A (CycA). Cyclin A in turn activates CDK2 to drive G1-S phase transition. Subsequently engaged components of the cell cycle machinery then impinge on AR to regulate the androgen response. Elevated cyclin D1 acts as in a negative feedback loop to attenuate AR activity, thereby modulating androgen action. In G2-phase, CDK1 promotes the phosphorylation and activation of AR. However, AR is degraded in M-phase and is purposed to be a “licensing factor” for DNA replication. Components that suppress AR function are outlined in red, whereas positive effectors of AR activity are outlined in green.
In contrast to the D-type cyclins, cyclin E levels remain relatively unchanged by androgen withdrawal, indicating that alteration of cyclin E expression is not a major mechanism of androgen action [Knudsen et al., 1998; Xu et al., 2006]. However, cyclin A levels and overall CDK2 activity are diminished upon androgen ablation. These data are consistent with the observation that androgen depletion invokes RB activity, as cyclin A is a well-established target of RB-mediated transcriptional repression. Furthermore, androgen depletion induces p27Kip1, which likely contributes to the observed reductions in CDK2 activity [Knudsen et al., 1998]. This supposition is consistent with more recent findings which demonstrated that low p27Kip1 expression is predictive for shorter time to disease recurrence in prostate cancer [Halvorsen et al., 2003]. Similarly, p27Kip1 loss in the context of a PTEN mutation promotes a tumorigenic phenotype in the prostate [Gao et al., 2004]. Interestingly, upon re-stimulation with androgen, p27Kip1 is degraded [Ye et al., 1999]. By contrast, p21Cip1 expression is lost upon androgen ablation in prostate cancer cells in vitro, which correlates with a higher proliferative index in human tumor specimens [Knudsen et al., 1998; Kolar et al., 2000]. Thus, p21Cip1 correlates with androgen stimulation and mitogenic proliferation in prostate cancer. Remarkably, p21Cip1 has been validated as a direct AR target gene [Lu et al., 1999], and its induction upon androgen stimulation may assist in assembling active CDK4/cyclin D1 complexes. In agreement with this, examination of p21Cip1 in prostate cancer has revealed that expression is enhanced in tumors, and correlates with a higher proliferative index and Gleason grade [Aaltomaa et al., 1999; Baretton et al., 1999].
To date, these observations culminate in a model wherein androgen induces cyclin D1 accumulation through mTOR, promotes active CDK4/cyclin D1 assembly (potentially through p21Cip1 induction), and facilitates CDK2 activation through degradation of p27Kip1. These collective events result in RB phosphorylation, de-repression of cyclin A expression, and S-phase progression. Based on this knowledge of AR function, it could be hypothesized that aberrations in the cyclin D-RB axis in cancer could supplant the requirement for androgen and contribute to disease progression. Investigations challenging this hypothesis have revealed a significant function for RB in controlling the response to androgen ablation therapy, and unique crosstalk mechanisms between the AR and cell cycle pathways that assist in coordinating and/or maintaining androgen-dependent cellular proliferation.
RB function in prostate cancer and the response to AR-directed therapeutics
As discussed above, CDK-mediated RB phosphorylation/inactivation is a key component of the proliferative response to AR. These findings suggest that RB loss may play an influential role in prostate cancer development and/or the response to AR-directed therapeutics. Consistent with this concept, RB is lost or inactivated in approximately 30-60% of prostatic adenocarcinomas through disparate mechanisms [Brooks et al., 1995; Ittmann and Wieczorek, 1996; Jarrard et al., 2002; Tricoli et al., 1996]. Accordingly, several model systems have been developed to more directly probe the importance of RB function in this tissue. Transgenic mouse models wherein RB and p53 are inactivated by SV40 large T- and small T-antigen overexpression in the luminal epithelia result in high grade PIN and/or prostate cancer (often with neuroendocrine phenotypes) and can achieve androgen independence after castration [Gingrich et al., 1997; Greenberg et al., 1995]. Tissue recombination studies showed that RB-deficient prostatic epithelia give rise to hyperplastic disease in 40% of grafted samples when recombined with wild-type rat urogenital mesenchyme [Wang et al., 2000]. Similarly, conditional RB deletion in the prostate resulted in focal hyperplasia that is potentially reminiscent of early stage disease [Maddison et al., 2004b]. These effects are exacerbated by combinatorial p53 deletion, which results in rapidly progressing metastatic carcinomas of the prostate [Zhou et al., 2006]. Together, these data are indicative that inactivation of RB may prime prostate cells to become cancerous when subjected to other insults.
In addition to these observations, emerging evidence suggests that RB inactivation may also subvert or weaken the requirement for AR-mediated cell cycle progression. Although studies are few in number, one study showed that RB mRNA expression was low in 36% of patients that failed combined androgen blockade [Mack et al., 1998]. Furthermore, by FISH analysis it was reported that RB loss is almost four times more frequent after hormone therapy [Kaltz-Wittmer et al., 2000]. Since these data indicate that RB inactivation and/or deletion may facilitate the transition to androgen independence, a recent study challenged this hypothesis in vitro, through shRNA-mediated depletion of RB in AR-dependent prostate cancer cells [Sharma et al., 2007]. In these models, RB depletion did not confer a proliferative advantage in the presence of androgen; rather RB-deficient cells failed to elicit a cytostatic response (as compared to RB-positive isogenic controls) when challenged with androgen ablation, AR antagonists, or combined androgen blockade. Not yet considered, however, is whether loss of RB or deregulation of G1-S alleviates the need for unliganded AR, and this determination is the focus of ongoing projects. However, studies examining the impact RB loss were subsequently extended to determine the impact of RB loss on the response to second line chemotherapeutic intervention, as reports in other cell systems have suggested that loss of RB-dependent DNA damage checkpoints can sensitize cells to cytotoxic agents [Harrington et al., 1998; Knudsen et al., 1998]. Indeed, RB-depleted prostate cancer cells demonstrated enhanced susceptibility to cell death induced by a select subset of chemotherapeutic agents. Combined, these data indicate that RB status may be an important determinant of the response to AR-directed therapeutic strategies against prostate cancer, and that the ability of AR to control the G1-S transition is likely a critical component for maintaining androgen dependence. While these studies highlight the importance of AR in governing the cell cycle machinery, it is becoming increasingly apparent that substantive crosstalk between the AR and cell cycle pathways may also contribute to this process.
Cell cycle regulation of AR
Only a small number of studies have directly examined the influence of cell cycle on AR activity. Nonetheless, many of the proteins found to interact with or modulate AR are also regulated during the cell cycle. These include proteins whose expression or activity are increased in G0 (RB), G1 to S phase (cyclin D1, cyclin E, Cdk6), or G2 (Cdk1). Therefore, it appears likely that AR activity is modulated during the cell cycle through interactions with one or more of these proteins. Moreover, AR may be further modulated directly during the cell cycle by transcriptional or post-transcriptional mechanisms, the latter including alterations in phosphorylation, acetylation, or ubiquitination that affect transcriptional activity or stability. The sections below first outline reported interactions between AR and cell cycle regulated proteins, and then describe studies that have directly examined AR during the cell cycle.
Retinoblastoma protein (RB)
Direct interactions between AR and RB have been reported by two groups based on GST-RB and mammalian two-hybrid protein interaction approaches [Lu and Danielsen, 1998; Yeh et al., 1998]. The interacting site on the AR was mapped to the AR N-terminal domain. Overexpression of RB enhanced AR transcriptional activity, while AR transcriptional activity was lost in cells that were RB-negative and was decreased in cells expressing RB-binding oncogenes. Interestingly, RB has also been reported to interact with the GR, and RB overexpression can similarly enhance GR transcriptional activity [Singh et al., 1995]. However, in contrast to AR, the GR remains transcriptionally active in RB deficient cells [Lu and Danielsen, 1998]. Significantly, the GR coactivation by RB is dependent on Brm, with RB and Brm forming a complex and both being required for GR coactivation [Singh et al., 1995]. It is not clear whether a similar mechanism mediates RB coactivation of AR.
AR has also been found to interact directly with an RB-associated protein, retinoblastoma-associated Kruppel protein (RbaK) [Hofman et al., 2003]. RbaK contains a Kruppel-associated box (KRAB) repressor motif at its N-terminus, in conjunction with multiple Kruppel type zinc finger domains, and contributes to the RB-dependent regulation of E2F transcription factors. RbaK interacts directly with RB and appears to interact independently with the AR LBD based on mammalian-two hybrid and coimmunoprecipitation experiments, with the interaction being androgen-independent. Although RbaK contains the KRAB repressor motif, overexpression enhances AR transcriptional activity by unclear mechanisms. Further studies are needed to determine precisely how RB and RbaK modulate AR activity, and to determine the biological significance of these interactions for AR activity, particularly in RB negative PCa [Sharma et al., 2007].
D-cyclins
Although androgen stimulates cyclin D1 accumulation and concomitant CDK4 activation [Knudsen et al., 1998; Xu et al., 2006], restoration of cyclin D1 expression under conditions of androgen ablation is insufficient to drive androgen-independent proliferation [Fribourg et al., 2000]. Moreover, it was observed that modest elevations of cyclin D1 in the presence of androgen inhibit (rather than enhance) cellular proliferation [Burd et al., 2005; Petre-Draviam et al., 2003]. This unexpected capacity of cyclin D1 to attenuate cell cycle progression is specific to AR-positive prostate cancer cells, thus suggesting a putative relationship between cyclin D1 and AR function. Detailed examination of this interaction revealed an unexpected and unique role of cyclin D1 in control of AR activity.
In addition to its ability to modulate CDK4 kinase activity, increasing evidence has demonstrated that cyclin D1 harbors CDK-independent functions in controlling transcription factor action [Coqueret, 2002]. Cyclin D1 can directly interact with and modulate a large number of transcription factors, including v-Myb, DMP1, Sp-1, and MyoD. A landmark paper demonstrated the relevance of cyclin D1-mediated transcriptional regulation, wherein it was shown that the ability of cyclin D1 to interact with and modulate the CCAAT/enhancer binding protein (and repress a large subset of genes) has a major consequence in human tumors [Lamb et al., 2003]. In addition, it has been recently shown that “kinase independent” functions of cyclin D1 underlie mammary gland development, in that in vivo knock-in of cyclin D1 mutants that are unable to activate CDK4 can effectively reverse the mammary gland phenotype observed in cyclin D1-/- mice [Landis et al., 2006]. Thus, the CDK4-independent functions of cyclin D1 appear to serve critical cellular functions.
The largest class of transcription factors known to be modulated by cyclin D belong to the nuclear receptor superfamily, including ER (estrogen receptor α), TR (thyroid hormone receptor), PPARγ and AR [Coqueret, 2002; Ewen and Lamb, 2004]. In the case of AR, cyclin D1 binds directly to the N-terminus of the receptor and blocks conformational changes that are required for maximal AR activity upon ligand activation (N-C interaction) [Burd et al., 2005; Petre-Draviam et al., 2005]. Moreover, cyclin D1 associates with histone deacetylase 3 (HDAC3), and recruitment of HDAC activity is essential for its corepressor functions [Lin et al., 2002; Petre-Draviam et al., 2005]. These actions of cyclin D1 are independent of CDK activity, and a repressor domain within the protein (encoded by amino acids 142-253) has been identified which is capable of supporting both cyclin D1 corepressor functions [Petre-Draviam et al., 2005]. The biological consequence was shown in that even modest induction of cyclin D1 levels (at stoichiometric levels with the receptor) are sufficient to suppress both AR activity and androgen-dependent proliferation in AR-positive prostate cancer cells [Petre-Draviam et al., 2003]. As expected, AR-negative prostate cancer cells are refractory to the repressor function of cyclin D1 [Burd et al., 2006]. These data are consistent with observations that AR activity is highly regulated as a function of the cell cycle, wherein cyclin D1 levels inversely correlate with AR activity [Martinez and Danielsen, 2002]. Moreover, in a mouse model of prostate cancer, cyclin D1 levels decrease as a function of progression, whereas cyclin E levels are elevated; this observation led to the hypothesis of a putative “cyclin switch” that may occur in prostate cancer progression [Maddison et al., 2004a] , although this concept has yet to be validated in human specimens. Based on these collective observations, it is hypothesized that cyclin D1 serves as a “negative feedback switch” to modulate androgen-dependent gene expression and concomitant cellular proliferation, thereby governing the strength and duration of the androgen response. Recent analyses indicated that these “balancing” functions of cyclin D1 are disrupted in prostate cancer [Burd et al., 2006; Comstock et al., 2007; Knudsen, 2006]. In the context of normal prostatic epithelia, the role of AR is to suppress cell proliferation and drive differentiation. While not yet examined, it is possible that cyclin D1 may be important for suppressing these functions to allow for entry into the cell cycle.
Cdk6
One study found that AR could be coactivated by transfected Cdk6 [Lim et al., 2005]. Importantly, this coactivation was not due to sequestration of cyclin D1, as it was observed in cyclin D1-deficient NIH3T3 cells. Moreover, coactivation was not prevented by a mutation in Cdk6 that prevents cyclin D1 binding, or by point mutations that prevent binding of p16INK4a or inhibit catalytic activity. Cdk6 was further shown to bind AR based on coimmunoprecipitation of transfected AR and Cdk6, and this interaction was similarly not blocked by the above mutations that prevent cyclin D1 and p16INK4a binding, or catalytic activity. Stable transfection of Cdk6 into LNCaP cells could enhance androgen stimulated growth and expression of the androgen regulated PSA gene. Finally, ChIP experiments indicated that Cdk6 was part of the AR transcriptional complex that assembles on the PSA gene. Further studies are clearly needed to determine the molecular basis for this kinase independent AR coactivation by Cdk6. Interestingly, Cdk6 expression in androgen-sensitive prostate cancer cells increases in response to androgen as cells move from G1 to S phase, which may then provide a positive feedback loop to further enhance AR activity [Bai et al., 2005].
Cyclin E
Cotransfection studies carried out with AR, ARE-CAT reporter, and cyclin D1, E, and A showed that cyclin E could specifically enhance androgen-stimulated transcriptional activity [Yamamoto et al., 2000]. This coactivation was not dependent on cyclin E binding to Cdk2, was independent of cell cycle progression, and was not observed for GR or PR. Cyclin E transfection into LNCaP cells similarly enhanced expression of the endogenous PSA gene. An interaction between AR and cyclin E was demonstrated by mammalian two-hybrid protein interaction assays and by coimmunoprecipitation of transfected proteins. The interaction was mapped to the AR N-terminal domain, and GST pulldowns indicated that cyclin E was interacting with a domain at the C-terminal end of the AR N-terminal domain (amino acids 419-556). As above, further studies are needed to determine how cyclin E mediates AR coactivation and whether this interaction may contribute to PCa development or progression.
CDK-activating kinase (CAK)/Cdk7
CDK-activating kinase (CAK), composed of Cdk7, cyclin H, and MAT1, mediates the phosphorylations of both Cdk2 and Cdk1 that are required for full activation and cell cycle progression. CAK is also a component of the transcriptional machinery, and can mediate phosphorylation of ER and RAR [Rochette-Egly et al., 1997; Trowbridge et al., 1997]. Immunoblotting of anti-AR immunoprecipitates identified the TFIIH transcription factor complex, which contains CAK, and further experiments demonstrated an interaction between AR and CAK [Lee et al., 2000]. Transfection of the individual CAK proteins could weakly enhance AR transcriptional activity, with greater coactivation when all three components were cotransfected. Coprecipitation experiments in vitro with 35S-labeled CAK proteins indicated that Cdk7 and cyclin H could interact with the AR N-terminal domain. These studies support the conclusion that TFIIH, as a general transcription factor, interacts with AR. However, further studies are needed to determine whether CAK phosphorylates AR or selectively enhances its activity, or whether it directly modulates AR during the cell cycle.
Cdc25B
The Cdc25 dual function phosphatases (Cdc25A, B, and C) mediate the activation of Cdk1 by removal of inhibitory phosphates from Thr-14 and Tyr-15. The exact functions of each Cdc25 isoform are not yet clear, but recent data indicate that Cdc25B specifically dephosphorylates and activates cyclin B-Cdk1 complexes on centrosomes [Lindqvist et al., 2005]. Significantly, Cdc25B has also been identified as a steroid receptor coactivator that can enhance the activity of ER, PR, GR, and AR [Ma et al., 2001; Ngan et al., 2003]. Cdc25B can interact directly with these steroid receptors and stimulate their activity in a cell-free transcription system. Surprisingly, this stimulation is not dependent on Cdc25B phosphatase activity, and its molecular basis remains unclear. Interestingly, both Cdc25B and Cdc25C were increased in higher grade prostate cancer, with Cdc25C and a novel activated splice variant being further increased in PCa that relapses after androgen deprivation therapy [Ngan et al., 2003; Ozen and Ittmann, 2005]. The interaction with AR suggests that Cdc25B may contribute to PCa progression both through effects on AR activation and cell cycle.
Cdk1
Cdk1 is activated in the G2 phase of the cell cycle and is the critical Cdk required for mitosis. As noted above, Cdk1 associates with cyclin B and is then activated by removal of inhibitory phosphates (mediated by Cdc25 isoforms) and by an activating T loop phosphorylation mediated by Cdk7. The unliganded AR is phosphorylated primarily at one serine-proline site in the N-terminal domain (Ser-94), and is phosphorylated at multiple additional Ser-Pro sites in response to androgen [Gioeli et al., 2002; Kuiper and Brinkmann, 1995; Zhou et al., 1995]. These are candidate sites for Cdk1 and other proline-directed kinases, and studies using Cdk1 transfection and Cdk inhibitors indicate that Cdk1 phosphorylates at least one site in the AR N-terminus (Ser-81) [Chen et al., 2006]. Significantly, transfection of cells with activated Cdk1 was found to enhance the stability and transcriptional activity of AR. Conversely, Cdk1 inhibitors decreased AR expression and transcriptional activity, although these drugs are not highly specific for Cdk1 and may also effect AR by targeting other Cdks such as Cdk7 [Chen et al., 2006]. Although Cdk1 phosphorylates AR, site directed mutagenesis of Ser-81 and of additional Ser-Pro did not block the ability of Cdk1 to stabilize AR, indicating that multiple sites may mediate this effect or that Cdk1 stabilizes AR by an indirect mechanism.
Interestingly, an analysis of PCa clinical samples from patients who had relapsed after androgen deprivation therapy showed that cyclin B1, cyclin B2, and Cdk1 were the most highly overexpressed cell cycle regulatory genes relative to primary untreated tumors [Stanbrough et al., 2006]. As noted above, both Cdc25B and Cdc25C were also found to be increased in higher grade prostate cancer and in tumors that relapsed after androgen deprivation therapy [Ngan et al., 2003; Ozen and Ittmann, 2005]. Cyclin B expression also increases with disease progression in the murine TRAMP model of prostate cancer [Maddison et al., 2004a]. Taken together, these observations suggest that Cdk1 may contribute to AR activation in advanced cancers. In support of this hypothesis, the AR activation mediated by low levels of DHT in C4-2 cells (a subline of LNCaP cells that is hypersensitive to low androgen levels) could be blocked by treatment with a Cdk inhibitor [Chen et al., 2006].
Direct assessment of transcriptional AR activity during the cell cycle
One study has directly examined modulation of AR transcriptional activity during the cell cycle [Martinez and Danielsen, 2002]. In this study, AR transcriptional activity in L929 cells (which express an endogenous AR) was examined using integrated ARE regulated reporter genes (MMTV-CAT and probasin-CAT). This study found that AR transcriptional activity (but not GR activity) was markedly decreased at the G1/S transition, and was regained during S-phase. AR protein level was also reduced at the G1/S transition, but this decrease was not as marked as the loss of transcriptional activity. A possible mechanism for the decreased AR expression during G1/S is increased E2F1, which has been found to suppress AR gene expression through binding to the AR promoter [Davis et al., 2006].
Significantly, an HDAC inhibitor (TSA) could partially restore AR transcriptional activity at the G1/S transition without increasing AR protein levels. One interpretation of this result is that AR recruitment of coactivator proteins with HAT activity becomes limiting at the G1/S transition, resulting in decreased histone or AR acetylation. However, while histone acetylation can enhance transcription, the role of AR acetylation in regulating AR activity is not clear [Popov et al., 2007]. Moreover, more recent data show that HDAC inhibitors decrease AR expression through inhibition of Hsp90, which must be deacetylated by HDAC6 for activity [Kovacs et al., 2005]. In any case, the multiple functions of HDACs make it difficult to clearly assess their roles in regulating AR activity during cell cycle.
One possible mediator of this loss of AR transcriptional activity at the G1/S transition is clearly cyclin D1, which increases during G1 to S progression and can function as a potent AR corepressor (see above). A second possible mediator is RB, which can function as an AR coactivator so that RB hyperphosphorylation at G1/S may decrease AR activity. Cyclin E may also function as an AR coactivator, and its increased expression as cells move into S-phase may contribute to AR reactivation. In any case, further studies are needed to confirm and extend the results of this study.
Cell cycle regulation of AR protein expression
AR protein expression during the cell cycle has been examined in one study, which used dual-color flow cytometry to assess AR expression versus binding of Hoechst dye [Litvinov et al., 2006]. Importantly, binding of the Hoechst dye is lowest in cells that have just exited mitosis, and prostate cancer cells (LNCaP, CWR22Rv1, and LAPC-4) with the lowest Hoechst dye staining had no detectable AR by flow cytometry. Sorting of this population with the lowest Hoechst dye binding, followed by AR immunoblotting, confirmed extremely low AR protein levels. Immunohistochemistry of cell lines in vitro, and of in vivo prostate cancers, further showed loss of AR protein expression in mitotic cells. The authors suggest that AR in these cells is a licensing factor for DNA replication, and that this decline in AR protein is required to license a new round of DNA replication. This decline is not observed in stromal cells, which are not androgen-sensitive and express markedly lower levels of AR, but further studies are needed to establish a link between AR degradation and DNA synthesis.
This loss of AR protein could be blocked with a proteosome inhibitor (MG132), indicating that it reflected proteosomal degradation. These observations suggest that AR may undergo posttranslational modifications, in particular ubiquitination, that enhance its degradation during mitosis. Ubiquitin ligases for AR have been identified and include E6-AP, CHIP, and MDM2, but their activities towards AR are not known to be cell cycle-regulated. Interestingly, Cdk1 has been reported to stabilize AR protein, although it is not clear whether this is due to direct AR phosphorylation or other indirect mechanisms [Chen et al., 2006]. Therefore, the decline in Cdk1 activity that occurs at the end of mitosis may contribute to the marked increase in AR degradation that is observed in early G1.
Conclusions
The clinical challenges in prostate cancer center on controlling the action of the AR, which is required for both tumor development and disease progression. Selective pressure brought on by androgen ablation typically results in a bypass mechanism to activate the receptor in the absence of ligand, and thereby restore AR-dependent cellular proliferation. Thus, dissecting the mechanisms by which AR governs cell cycle progression is instrumental for the design of new strategies to treat recurrent disease. It is apparent that activated AR governs the G1-S progression, and emerging evidence indicates that cross talk between AR and the downstream cell cycle machinery serves a critical role in modulating the androgen response. Aberrations in these processes can facilitate androgen-independent cellular proliferation, and may contribute to the development of recurrent tumors. Future investigations into the consequence of AR-cell cycle crosstalk in prostate cancer are likely to lead to new avenues of therapeutic intervention.
Acknowledgments
Acknowledgements: The authors thank Dr. Clay Comstock, Dr. Erik Knudsen, Matt Schiewer, the S.P. Balk lab, and the K. Knudsen lab for ongoing discussions and critical reading of the manuscript.
Abbreviations
- AR
androgen receptor
- ARE
androgen response element
- CAK
CDK activating kinase
- CDK
cyclin dependent kinase
- DHT
dihydrotestosterone
- ER
estrogen receptor α
- G1
gap phase 1
- G2
gap phase 2
- HDAC
histone deacetylase
- M
the phase of mitosis
- PPAR
peroxisome proliferator-activated receptor
- PSA
prostate specific antigen
- RB
retinoblastoma tumor suppressor
- S
phase of DNA replication
- TR
thyroid hormone receptor
- TSA
trichostatin A
References
- Aaltomaa S., Lipponen P., Eskelinen M., Ala-Opas M., Kosma V. M. Prognostic value and expression of p21(waf1/cip1) protein in prostate cancer. Prostate. 1999;39:8–15. doi: 10.1002/(sici)1097-0045(19990401)39:1<8::aid-pros2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Agus D. B., Cordon-Cardo C., Fox W., Drobnjak M., Koff A., Golde D. W., Scher H. I. Prostate cancer cell cycle regulators: response to androgen withdrawal and development of androgen independence. J Natl Cancer Inst. 1999;91:1869–76. doi: 10.1093/jnci/91.21.1869. [DOI] [PubMed] [Google Scholar]
- Alao J. P. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt J. R., Gladden A. B., Diehl J. A. p21(Cip1) Promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J Biol Chem. 2002;277:8517–23. doi: 10.1074/jbc.M108867200. [DOI] [PubMed] [Google Scholar]
- Alt J. R., Cleveland J. L., Hannink M., Diehl J. A. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14:3102–14. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai V. U., Cifuentes E., Menon M., Barrack E. R., Reddy G. P. Androgen receptor regulates Cdc6 in synchronized LNCaP cells progressing from G1 to S phase. J Cell Physiol. 2005;204:381–7. doi: 10.1002/jcp.20422. [DOI] [PubMed] [Google Scholar]
- Balk S. P. Androgen receptor as a target in androgen-independent prostate cancer. Urology. 2002;60:132–8; discussion 138-9. doi: 10.1016/s0090-4295(02)01593-5. [DOI] [PubMed] [Google Scholar]
- Baretton G. B., Klenk U., Diebold J., Schmeller N., Lohrs U. Proliferation- and apoptosis-associated factors in advanced prostatic carcinomas before and after androgen deprivation therapy: prognostic significance of p21/WAF1/CIP1 expression. Br J Cancer. 1999;80:546–55. doi: 10.1038/sj.bjc.6690390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. D., Bova G. S., Isaacs W. B. Allelic loss of the retinoblastoma gene in primary human prostatic adenocarcinomas. Prostate. 1995;26:35–9. doi: 10.1002/pros.2990260108. [DOI] [PubMed] [Google Scholar]
- Burd C. J., Petre C. E., Moghadam H., Wilson E. M., Knudsen K. E. Cyclin D1 binding to the androgen receptor (AR) NH2-terminal domain inhibits activation function 2 association and reveals dual roles for AR corepression. Mol Endocrinol. 2005;19:607–20. doi: 10.1210/me.2004-0266. [DOI] [PubMed] [Google Scholar]
- Burd C. J., Petre C. E., Morey L. M., Wang Y., Revelo M. P., Haiman C. A., Lu S., Fenoglio-Preiser C. M., Li J., Knudsen E. S., Wong J., Knudsen K. E. Cyclin D1b variant influences prostate cancer growth through aberrant androgen receptor regulation. Proc Natl Acad Sci U S A. 2006;103:2190–5. doi: 10.1073/pnas.0506281103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalona W. J., Ramos C. G., Carvalhal G. F. Contemporary results of anatomic radical prostatectomy. CA Cancer J Clin. 1999;49:282–96. doi: 10.3322/canjclin.49.5.282. [DOI] [PubMed] [Google Scholar]
- Chen S., Xu Y., Yuan X., Bubley G. J., Balk S. P. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. Proc Natl Acad Sci U S A. 2006;103:15969–74. doi: 10.1073/pnas.0604193103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Snoek R., Ghaidi F., Cox M. E., Rennie P. S. Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res. 2006;66:10613–20. doi: 10.1158/0008-5472.CAN-06-0028. [DOI] [PubMed] [Google Scholar]
- Cheng M., Olivier P., Diehl J. A., Fero M., Roussel M. F., Roberts J. M., Sherr C. J. The p21(Cip1) and p27(Kip1) CDK 'inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. Embo J. 1999;18:1571–83. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. D., Welsbie D. S., Tran C., Baek S. H., Chen R., Vessella R., Rosenfeld M. G., Sawyers C. L. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Chmelar R., Buchanan G., Need E. F., Tilley W., Greenberg N. M. Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int J Cancer. 2007;120:719–33. doi: 10.1002/ijc.22365. [DOI] [PubMed] [Google Scholar]
- Chodak G. W. Maximum androgen blockade: a clinical update. Rev Urol. 2005;7 Suppl 5:S13–7. [PMC free article] [PubMed] [Google Scholar]
- Comstock C. E., Revelo M. P., Buncher C. R., Knudsen K. E. Impact of differential cyclin D1 expression and localisation in prostate cancer. Br J Cancer. 2007;96:970–9. doi: 10.1038/sj.bjc.6603615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coqueret O. Linking cyclins to transcriptional control. Gene. 2002;299:35–55. doi: 10.1016/s0378-1119(02)01055-7. [DOI] [PubMed] [Google Scholar]
- Culig Z., Bartsch G. Androgen axis in prostate cancer. J Cell Biochem. 2006;99:373–81. doi: 10.1002/jcb.20898. [DOI] [PubMed] [Google Scholar]
- Davis J. N., Wojno K. J., Daignault S., Hofer M. D., Kuefer R., Rubin M. A., Day M. L. Elevated E2F1 inhibits transcription of the androgen receptor in metastatic hormone-resistant prostate cancer. Cancer Res. 2006;66:11897–906. doi: 10.1158/0008-5472.CAN-06-2497. [DOI] [PubMed] [Google Scholar]
- Demichelis F., Fall K., Perner S., Andren O., Schmidt F., Setlur S. R., Hoshida Y., Mosquera J. M., Pawitan Y., Lee C., Adami H. O., Mucci L. A., Kantoff P. W., Andersson S. O., Chinnaiyan A. M., Johansson J. E., Rubin M. A. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:5692. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- Denmeade S. R., Isaacs J. T. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2:389–96. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denmeade S. R., Lin X. S., Isaacs J. T. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28:251–65. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Dorff T. B., Quek M. L., Daneshmand S., Pinski J. Evolving treatment paradigms for locally advanced and metastatic prostate cancer. Expert Rev Anticancer Ther. 2006;6:1639–51. doi: 10.1586/14737140.6.11.1639. [DOI] [PubMed] [Google Scholar]
- Eeckhoute J., Carroll J. S., Geistlinger T. R., Torres-Arzayus M. I., Brown M. A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev. 2006;20:2513–26. doi: 10.1101/gad.1446006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–95. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen M. E., Lamb J. The activities of cyclin D1 that drive tumorigenesis. Trends Mol Med. 2004;10:158–62. doi: 10.1016/j.molmed.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Feldman B. J., Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- Fribourg A. F., Knudsen K. E., Strobeck M. W., Lindhorst C. M., Knudsen E. S. Differential requirements for ras and the retinoblastoma tumor suppressor protein in the androgen dependence of prostatic adenocarcinoma cells. Cell Growth Differ. 2000;11:361–72. [PubMed] [Google Scholar]
- Gao H., Ouyang X., Banach-Petrosky W., Borowsky A. D., Lin Y., Kim M., Lee H., Shih W. J., Cardiff R. D., Shen M. M., Abate-Shen C. A critical role for p27kip1 gene dosage in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci U S A. 2004;101:17204–9. doi: 10.1073/pnas.0407693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmann E. P. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–15. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- Geng Y., Lee Y. M., Welcker M., Swanger J., Zagozdzon A., Winer J. D., Roberts J. M., Kaldis P., Clurman B. E., Sicinski P. Kinase-independent function of cyclin E. Mol Cell. 2007;25:127–39. doi: 10.1016/j.molcel.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Gingrich J. R., Barrios R. J., Kattan M. W., Nahm H. S., Finegold M. J., Greenberg N. M. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57:4687–91. [PubMed] [Google Scholar]
- Gioeli D., Ficarro S. B., Kwiek J. J., Aaronson D., Hancock M., Catling A. D., White F. M., Christian R. E., Settlage R. E., Shabanowitz J., Hunt D. F., Weber M. J. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J Biol Chem. 2002;277:29304–14. doi: 10.1074/jbc.M204131200. [DOI] [PubMed] [Google Scholar]
- Gladden A. B., Woolery R., Aggarwal P., Wasik M. A., Diehl J. A. Expression of constitutively nuclear cyclin D1 in murine lymphocytes induces B-cell lymphoma. Oncogene. 2006;25:998–1007. doi: 10.1038/sj.onc.1209147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden A. B., Diehl J. A. Location, location, location: the role of cyclin D1 nuclear localization in cancer. J Cell Biochem. 2005;96:906–13. doi: 10.1002/jcb.20613. [DOI] [PubMed] [Google Scholar]
- Gnanapragasam V. J., Robson C. N., Leung H. Y., Neal D. E. Androgen receptor signalling in the prostate. BJU Int. 2000;86:1001–13. doi: 10.1046/j.1464-410x.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- Greenberg N. M., DeMayo F., Finegold M. J., Medina D., Tilley W. D., Aspinall J. O., Cunha G. R., Donjacour A. A., Matusik R. J., Rosen J. M. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen O. J., Haukaas S. A., Akslen L. A. Combined loss of PTEN and p27 expression is associated with tumor cell proliferation by Ki-67 and increased risk of recurrent disease in localized prostate cancer. Clin Cancer Res. 2003;9:1474–9. [PubMed] [Google Scholar]
- Harrington E. A., Bruce J. L., Harlow E., Dyson N. pRB plays an essential role in cell cycle arrest induced by DNA damage. Proc Natl Acad Sci U S A. 1998;95:11945–50. doi: 10.1073/pnas.95.20.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinlein C. A., Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- Heinlein C. A., Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- Hofman K., Swinnen J. V., Claessens F., Verhoeven G., Heyns W. The retinoblastoma protein-associated transcription repressor RBaK interacts with the androgen receptor and enhances its transcriptional activity. J Mol Endocrinol. 2003;31:583–96. doi: 10.1677/jme.0.0310583. [DOI] [PubMed] [Google Scholar]
- Huggins C., Hodges C. V. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–40. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- Isaacs J. T. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5:545–57. doi: 10.1002/pros.2990050510. [DOI] [PubMed] [Google Scholar]
- Ittmann M. M., Wieczorek R. Alterations of the retinoblastoma gene in clinically localized, stage B prostate adenocarcinomas. Hum Pathol. 1996;27:28–34. doi: 10.1016/s0046-8177(96)90134-3. [DOI] [PubMed] [Google Scholar]
- Jarrard D. F., Modder J., Fadden P., Fu V., Sebree L., Heisey D., Schwarze S. R., Friedl A. Alterations in the p16/pRb cell cycle checkpoint occur commonly in primary and metastatic human prostate cancer. Cancer Lett. 2002;185:191–9. doi: 10.1016/s0304-3835(02)00282-3. [DOI] [PubMed] [Google Scholar]
- Jemal A., Murray T., Ward E., Samuels A., Tiwari R. C., Ghafoor A., Feuer E. J., Thun M. J. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- Jenster G. The role of the androgen receptor in the development and progression of prostate cancer. Semin Oncol. 1999;26:407–21. [PubMed] [Google Scholar]
- Johnson D. G., Degregori J. Putting the Oncogenic and Tumor Suppressive Activities of E2F into Context. Curr Mol Med. 2006;6:731–8. doi: 10.2174/1566524010606070731. [DOI] [PubMed] [Google Scholar]
- Kaltz-Wittmer C., Klenk U., Glaessgen A., Aust D. E., Diebold J., Lohrs U., Baretton G. B. FISH analysis of gene aberrations (MYC, CCND1, ERBB2, RB, and AR) in advanced prostatic carcinomas before and after androgen deprivation therapy. Lab Invest. 2000;80:1455–64. doi: 10.1038/labinvest.3780152. [DOI] [PubMed] [Google Scholar]
- Klotz L. Combined androgen blockade: an update. Urol Clin North Am. 2006;33:161–6, v-vi. doi: 10.1016/j.ucl.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Klotz L. Hormone therapy for patients with prostate carcinoma. Cancer. 2000;88:3009–14. doi: 10.1002/1097-0142(20000615)88:12+<3009::aid-cncr17>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- Knudsen K. E., Arden K. C., Cavenee W. K. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J Biol Chem. 1998;273:20213–22. doi: 10.1074/jbc.273.32.20213. [DOI] [PubMed] [Google Scholar]
- Knudsen E. S., Knudsen K. E. Retinoblastoma tumor suppressor: where cancer meets the cell cycle. Exp Biol Med (Maywood) 2006a;231:1271–81. doi: 10.1177/153537020623100713. [DOI] [PubMed] [Google Scholar]
- Knudsen K. E. The cyclin D1b splice variant: an old oncogene learns new tricks. Cell Div. 2006b;1:15. doi: 10.1186/1747-1028-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar Z., Murray P. G., Scott K., Harrison A., Vojtesek B., Dusek J. Relation of Bcl-2 expression to androgen receptor, p21WAF1/CIP1, and cyclin D1 status in prostate cancer. Mol Pathol. 2000;53:15–8. doi: 10.1136/mp.53.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J. J., Murphy P. J., Gaillard S., Zhao X., Wu J. T., Nicchitta C. V., Yoshida M., Toft D. O., Pratt W. B., Yao T. P. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–7. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Kuiper G. G., Brinkmann A. O. Phosphotryptic peptide analysis of the human androgen receptor: detection of a hormone-induced phosphopeptide. Biochemistry. 1995;34:1851–7. doi: 10.1021/bi00006a005. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988;122:552–62. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- LaBaer J., Garrett M. D., Stevenson L. F., Slingerland J. M., Sandhu C., Chou H. S., Fattaey A., Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–62. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- Lamb J., Ramaswamy S., Ford H. L., Contreras B., Martinez R. V., Kittrell F. S., Zahnow C. A., Patterson N., Golub T. R., Ewen M. E. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell. 2003;114:323–34. doi: 10.1016/s0092-8674(03)00570-1. [DOI] [PubMed] [Google Scholar]
- Landis M. W., Pawlyk B. S., Li T., Sicinski P., Hinds P. W. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell. 2006;9:13–22. doi: 10.1016/j.ccr.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Lee D. K., Duan H. O., Chang C. From androgen receptor to the general transcription factor TFIIH. Identification of cdk activating kinase (CAK) as an androgen receptor NH(2)-terminal associated coactivator. J Biol Chem. 2000;275:9308–13. doi: 10.1074/jbc.275.13.9308. [DOI] [PubMed] [Google Scholar]
- Lee Y. M., Sicinski P. Targeting cyclins and cyclin-dependent kinases in cancer: lessons from mice, hopes for therapeutic applications in human. Cell Cycle. 2006;5:2110–4. doi: 10.4161/cc.5.18.3218. [DOI] [PubMed] [Google Scholar]
- Leewansangtong S., Soontrapa S. Hormonal ablation therapy for metastatic prostatic carcinoma: a review. J Med Assoc Thai. 1999;82:192–205. [PubMed] [Google Scholar]
- Lim J. T., Mansukhani M., Weinstein I. B. Cyclin-dependent kinase 6 associates with the androgen receptor and enhances its transcriptional activity in prostate cancer cells. Proc Natl Acad Sci U S A. 2005;102:5156–61. doi: 10.1073/pnas.0501203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. M., Zhao L., Cheng S. Y. Cyclin D1 Is a Ligand-independent Co-repressor for Thyroid Hormone Receptors. J Biol Chem. 2002;277:28733–41. doi: 10.1074/jbc.M203380200. [DOI] [PubMed] [Google Scholar]
- Lindqvist A., Kallstrom H., Lundgren A., Barsoum E., Rosenthal C. K. Cdc25B cooperates with Cdc25A to induce mitosis but has a unique role in activating cyclin B1-Cdk1 at the centrosome. J Cell Biol. 2005;171:35–45. doi: 10.1083/jcb.200503066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov I. V., Vander Griend D. J., Antony L., Dalrymple S., De Marzo A. M., Drake C. G., Isaacs J. T. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc Natl Acad Sci U S A. 2006;103:15085–90. doi: 10.1073/pnas.0603057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loblaw D. A., Mendelson D. S., Talcott J. A., Virgo K. S., Somerfield M. R., Ben-Josef E., Middleton R., Porterfield H., Sharp S. A., Smith T. J., Taplin M. E., Vogelzang N. J., Wade J. L., Jr., Bennett C. L., Scher H. I. American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer. J Clin Oncol. 2004;22:2927–41. doi: 10.1200/JCO.2004.04.579. [DOI] [PubMed] [Google Scholar]
- Lu S., Liu M., Epner D. E., Tsai S. Y., Tsai M. J. Androgen regulation of the cyclin-dependent kinase inhibitor p21 gene through an androgen response element in the proximal promoter. Mol Endocrinol. 1999;13:376–84. doi: 10.1210/mend.13.3.0254. [DOI] [PubMed] [Google Scholar]
- Lu J., Danielsen M. Differential regulation of androgen and glucocorticoid receptors by retinoblastoma protein. J Biol Chem. 1998;273:31528–33. doi: 10.1074/jbc.273.47.31528. [DOI] [PubMed] [Google Scholar]
- Lukas J., Bartkova J., Rohde M., Strauss M., Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995;15:2600–11. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z. Q., Liu Z., Ngan E. S., Tsai S. Y. Cdc25B functions as a novel coactivator for the steroid receptors. Mol Cell Biol. 2001;21:8056–67. doi: 10.1128/MCB.21.23.8056-8067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack P. C., χ S. G., Meyers F. J., Stewart S. L., deVere White R. W., Gumerlock P. H. Increased RB1 abnormalities in human primary prostate cancer following combined androgen blockade. Prostate. 1998;34:145–51. doi: 10.1002/(sici)1097-0045(19980201)34:2<145::aid-pros10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Maddison L. A., Sutherland B. W., Barrios R. J., Greenberg N. M. Conditional deletion of Rb causes early stage prostate cancer. Cancer Res. 2004a;64:6018–25. doi: 10.1158/0008-5472.CAN-03-2509. [DOI] [PubMed] [Google Scholar]
- Maddison L. A., Huss W. J., Barrios R. M., Greenberg N. M. Differential expression of cell cycle regulatory molecules and evidence for a "cyclin switch" during progression of prostate cancer. Prostate. 2004b;58:335–44. doi: 10.1002/pros.10341. [DOI] [PubMed] [Google Scholar]
- Malumbres M., Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007;17:60–5. doi: 10.1016/j.gde.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Thummel C., Beato M., Herrlich P., Schutz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., Evans R. M. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marivoet S., Van Dijck P., Verhoeven G., Heyns W. Interaction of the 90-kDa heat shock protein with native and in vitro translated androgen receptor and receptor fragments. Mol Cell Endocrinol. 1992;88:165–74. doi: 10.1016/0303-7207(92)90021-w. [DOI] [PubMed] [Google Scholar]
- Martinez E. D., Danielsen M. Loss of androgen receptor transcriptional activity at the G(1)/S transition. J Biol Chem. 2002;277:29719–29. doi: 10.1074/jbc.M112134200. [DOI] [PubMed] [Google Scholar]
- Nash A. F., Melezinek I. The role of prostate specific antigen measurement in the detection and management of prostate cancer. Endocr Relat Cancer. 2000;7:37–51. doi: 10.1677/erc.0.0070037. [DOI] [PubMed] [Google Scholar]
- Ngan E. S., Hashimoto Y., Ma Z. Q., Tsai M. J., Tsai S. Y. Overexpression of Cdc25B, an androgen receptor coactivator, in prostate cancer. Oncogene. 2003;22:734–9. doi: 10.1038/sj.onc.1206121. [DOI] [PubMed] [Google Scholar]
- Nickeleit I., Zender S., Kossatz U., Malek N. P. p27kip1: a target for tumor therapies? Cell Div. 2007;2:13. doi: 10.1186/1747-1028-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen M., Ittmann M. Increased expression and activity of CDC25C phosphatase and an alternatively spliced variant in prostate cancer. Clin Cancer Res. 2005;11:4701–6. doi: 10.1158/1078-0432.CCR-04-2551. [DOI] [PubMed] [Google Scholar]
- Petre-Draviam C. E., Williams E. B., Burd C. J., Gladden A., Moghadam H., Meller J., Diehl J. A., Knudsen K. E. A central domain of cyclin D1 mediates nuclear receptor corepressor activity. Oncogene. 2005;24:431–44. doi: 10.1038/sj.onc.1208200. [DOI] [PubMed] [Google Scholar]
- Petre-Draviam C. E., Cook S. L., Burd C. J., Marshall T. W., Wetherill Y. B., Knudsen K. E. Specificity of cyclin D1 for androgen receptor regulation. Cancer Res. 2003;63:4903–13. [PubMed] [Google Scholar]
- Pines J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 2006;16:55–63. doi: 10.1016/j.tcb.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Popov V. M., Wang C., Shirley L. A., Rosenberg A., Li S., Nevalainen M., Fu M., Pestell R. G. The functional significance of nuclear receptor acetylation. Steroids. 2007;72:221–30. doi: 10.1016/j.steroids.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegman P. H., Vlietstra R. J., van der Korput J. A., Brinkmann A. O., Trapman J. The promoter of the prostate-specific antigen gene contains a functional androgen responsive element. Mol Endocrinol. 1991;5:1921–30. doi: 10.1210/mend-5-12-1921. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C., Adam S., Rossignol M., Egly J. M., Chambon P. Stimulation of RAR α activation function AF-1 through binding to the general transcription factor TFIIH and phosphorylation by CDK7. Cell. 1997;90:97–107. doi: 10.1016/s0092-8674(00)80317-7. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Wilson J. D. Steroid 5 α-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- Ryan C. J., Smith A., Lal P., Satagopan J., Reuter V., Scardino P., Gerald W., Scher H. I. Persistent prostate-specific antigen expression after neoadjuvant androgen depletion: an early predictor of relapse or incomplete androgen suppression. Urology. 2006;68:834–9. doi: 10.1016/j.urology.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Sabbah M., Courilleau D., Mester J., Redeuilh G. Estrogen induction of the cyclin D1 promoter: involvement of a cAMP response-like element. Proc Natl Acad Sci U S A. 1999;96:11217–22. doi: 10.1073/pnas.96.20.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand R. L., Gelmann E. P. Molecular biology of prostate-cancer pathogenesis. Curr Opin Urol. 2006;16:123–31. doi: 10.1097/01.mou.0000193384.39351.64. [DOI] [PubMed] [Google Scholar]
- Shang Y., Myers M., Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–10. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- Shapiro G. I. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–83. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- Sharma A., Comstock C. E., Knudsen E. S., Cao K. H., Hess-Wilson J. K., Morey L. M., Barrera J., Knudsen K. E. Retinoblastoma tumor suppressor status is a critical determinant of therapeutic response in prostate cancer cells. Cancer Res. 2007;67:6192–203. doi: 10.1158/0008-5472.CAN-06-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J. Cancer cell cycles. Science. 1996;274:1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- Singh P., Coe J., Hong W. A role for retinoblastoma protein in potentiating transcriptional activation by the glucocorticoid receptor. Nature. 1995;374:562–5. doi: 10.1038/374562a0. [DOI] [PubMed] [Google Scholar]
- Sowery R. D., So A. I., Gleave M. E. Therapeutic options in advanced prostate cancer: present and future. Curr Urol Rep. 2007;8:53–9. doi: 10.1007/s11934-007-0021-9. [DOI] [PubMed] [Google Scholar]
- Stanbrough M., Bubley G. J., Ross K., Golub T. R., Rubin M. A., Penning T. M., Febbo P. G., Balk S. P. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- Swanson G. P. Management of locally advanced prostate cancer: past, present, future. J Urol. 2006;176:S34–41. doi: 10.1016/j.juro.2006.06.079. [DOI] [PubMed] [Google Scholar]
- Taplin M. E., Balk S. P. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91:483–90. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- Tomlins S. A., Laxman B., Dhanasekaran S. M., Helgeson B. E., Cao X., Morris D. S., Menon A., Jing X., Cao Q., Han B., Yu J., Wang L., Montie J. E., Rubin M. A., Pienta K. J., Roulston D., Shah R. B., Varambally S., Mehra R., Chinnaiyan A. M. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–9. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- Tomlins S. A., Rhodes D. R., Perner S., Dhanasekaran S. M., Mehra R., Sun X. W., Varambally S., Cao X., Tchinda J., Kuefer R., Lee C., Montie J. E., Shah R. B., Pienta K. J., Rubin M. A., Chinnaiyan A. M. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- Trapman J., Brinkmann A. O. The androgen receptor in prostate cancer. Pathol Res Pract. 1996;192:752–60. doi: 10.1016/S0344-0338(96)80097-5. [DOI] [PubMed] [Google Scholar]
- Tricoli J. V., Gumerlock P. H., Yao J. L., χ S. G., D'Souza S. A., Nestok B. R., deVere White R. W. Alterations of the retinoblastoma gene in human prostate adenocarcinoma. Genes Chromosomes Cancer. 1996;15:108–14. doi: 10.1002/(SICI)1098-2264(199602)15:2<108::AID-GCC5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Trowbridge J. M., Rogatsky I., Garabedian M. J. Regulation of estrogen receptor transcriptional enhancement by the cyclin A/Cdk2 complex. Proc Natl Acad Sci U S A. 1997;94:10132–7. doi: 10.1073/pnas.94.19.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Li W., Liu X. S., Carroll J. S., Janne O. A., Keeton E. K., Chinnaiyan A. M., Pienta K. J., Brown M. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27:380–92. doi: 10.1016/j.molcel.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hayward S. W., Donjacour A. A., Young P., Jacks T., Sage J., Dahiya R., Cardiff R. D., Day M. L., Cunha G. R. Sex hormone-induced carcinogenesis in Rb-deficient prostate tissue. Cancer Res. 2000;60:6008–17. [PubMed] [Google Scholar]
- White S. N., Jamnongjit M., Gill A., Lutz L. B., Hammes S. R. Specific modulation of nongenomic androgen signaling in the ovary. Steroids. 2005;70:352–60. doi: 10.1016/j.steroids.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. Role of dihydrotestosterone in androgen action. Prostate Suppl. 1996;6:88–92. [PubMed] [Google Scholar]
- Xu Y., Chen S. Y., Ross K. N., Balk S. P. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–92. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Hashimoto Y., Kohri K., Ogata E., Kato S., Ikeda K., Nakanishi M. Cyclin E as a coactivator of the androgen receptor. J Cell Biol. 2000;150:873–80. doi: 10.1083/jcb.150.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D., Mendelsohn J., Fan Z. Androgen and epidermal growth factor down-regulate cyclin-dependent kinase inhibitor p27Kip1 and costimulate proliferation of MDA PCa 2a and MDA PCa 2b prostate cancer cells. Clin Cancer Res. 1999;5:2171–7. [PubMed] [Google Scholar]
- Yeh S., Miyamoto H., Nishimura K., Kang H., Ludlow J., Hsiao P., Wang C., Su C., Chang C. Retinoblastoma, a tumor suppressor, is a coactivator for the androgen receptor in human prostate cancer DU145 cells. Biochem Biophys Res Commun. 1998;248:361–7. doi: 10.1006/bbrc.1998.8974. [DOI] [PubMed] [Google Scholar]
- Yoshioka M., Boivin A., Ye P., Labrie F., St-Amand J. Effects of dihydrotestosterone on skeletal muscle transcriptome in mice measured by serial analysis of gene expression. J Mol Endocrinol. 2006;36:247–59. doi: 10.1677/jme.1.01964. [DOI] [PubMed] [Google Scholar]
- Yuan X., Li T., Wang H., Zhang T., Barua M., Borgesi R. A., Bubley G. J., Lu M. L., Balk S. P. Androgen receptor remains critical for cell-cycle progression in androgen-independent CWR22 prostate cancer cells. Am J Pathol. 2006;169:682–96. doi: 10.2353/ajpath.2006.051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z. X., Kemppainen J. A., Wilson E. M. Identification of three proline-directed phosphorylation sites in the human androgen receptor. Mol Endocrinol. 1995;9:605–15. doi: 10.1210/mend.9.5.7565807. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Flesken-Nikitin A., Corney D. C., Wang W., Goodrich D. W., Roy-Burman P., Nikitin A. Y. Synergy of p53 and Rb Deficiency in a Conditional Mouse Model for Metastatic Prostate Cancer. Cancer Res. 2006;66:7889–98. doi: 10.1158/0008-5472.CAN-06-0486. [DOI] [PubMed] [Google Scholar]