Abstract
Junctional Adhesion Molecules (JAMs) that are expressed in endothelial and epithelial cells and function in tight junction assembly, also perform important roles in testis where the closely-related JAM-A, JAM-B, and JAM-C are found. Disruption of murine Jam-B and Jam-C has varying effects on sperm development and function, however deletion of Jam-A has not yet been studied. Here we show for the first time that in addition to expression in the Sertoli-Sertoli tight junctions in the seminiferous tubules, the ∼32 kDa murine JAM-A is present in elongated spermatids and in the plasma membrane of the head and flagellum of sperm. Deletion of Jam-A, using the gene trap technology, results in flagellar defects at the ultrastructural level. In Jam-A-deficient mice, which have reduced litter size, both progressive and hyperactived motility are significantly affected (P<0.0001) before and, more severely, after capacitation. The findings show that JAM-A is involved in sperm tail formation and is essential for normal motility, which may occur via its signal transduction and protein phosphorylation properties. Detection of JAM-A in human sperm protein indicates that its role may be conserved in sperm motility and that JAM-A may be a candidate gene for the analysis of idiopathic sperm motility defects resulting in male subfertility in the human population.
Keywords: spermiogenesis, elongated spermatid, progressive and hyperactivated motility, sperm flagellar defects, sperm membrane protein
INTRODUCTION
Spermiogenesis is a developmental program in which haploid round spermatids, the product of meiosis, undergo complex morphological changes and become transformed into polarized spermatozoa (Weinbauer et al., 2000). At the end of spermiogenesis sperm are morphologically mature, with nuclear condensation and well-developed acrosome and tail formation. However, functional maturity is attained after they leave the testis, and motility is not acquired until after their passage in the corpus epididymis (Yeung and Cooper, 2002). As might be expected a large number of genes are known to be haploid-expressed and to be involved in the differentiation of spermatids into mature sperm (Shima et al., 2004).
One gene that has been recently shown to be involved in spermatid differentiation is Junctional Adhesion Molecule C (Jam-C) which encodes a membrane protein, JAM-C (Gliki et al., 2004). Present at the Sertoli-spermatid junctional plaques, JAM-C anchors elongated spermatids to the Sertoli cell epithelium and is essential for the polarization of round spermatids during sperm morphogenesis (Gliki et al., 2004). The JAM protein family members belong to the immunoglobulin superfamily (IgSF) and contain two extracellular Ig-like domains and a short cytoplasmic domain which flank a single transmembrane region (Mandell and Parkos, 2005; Bazzoni, 2003; Naik and Eckfield, 2003). The extracellular Ig-like domains mediate heterophilic (Bazzoni et al., 2000; Babinska et al., 2002) and homophilic interactions (Ostermann et al., 2002; Barton et al., 2001). In general, these proteins play multifunctional roles in a variety of cellular processes and are involved in cell-cell adhesion, the assembly of tight junctions, and signal transduction (Mandell and Parkos, 2005; Bazzoni, 2003; Naik and Eckfield, 2003). Other family members of the JAM membrane proteins include JAM-A, JAM-B, JAM-D (JAM-4), and JAML. JAM-A, -B, and -C are more closely related to each other than to any other IgSF proteins, with the sequence identity among them being 32-38% (Ebnet et al., 2004).
Similar to Jam-C, Jam-A and Jam-B have been shown to be expressed in mouse spermatogenesis (Gliki et al., 2004, Mruk and Cheng, 2004). Specifically JAM-A and B are present at the Sertoli-Sertoli tight junctions, where they maintain an immunological blood-testis barrier, and JAM-B is also present at the junctional plaques connecting Sertoli cells with round and elongated spermatids (Gliki et al, 2004). This suggests that Jam-B partners with Jam-C in their involvement in Sertoli cell-spermatid communication (Gliki et al., 2004). However, while deletion of Jam-C significantly affects mouse spermatid differentiation, particularly sperm head development, leading ultimately to infertility (Gliki et al., 2004), homozygotes for Jam-B deletion have normal male and female fertility and there is an absence of detectable developmental or sperm abnormalities (Sakaguchi et al., 2006). To date, the effects of Jam-A deletion on sperm development and function have not been investigated. Thus the goal of this study was to use gene targeting to determine the effect of JAM-A on male germ cells.
MATERIALS AND METHODS
Reagents-Immunological and Non- Immunological
All non-immunological reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise specified. Four different JAM-A antibodies that were previously validated were used throughout the study. A goat anti-mouse JAM-A polyclonal antibody, JAM-A-affinity purified was obtained from R and D Systems, Inc., Cat# AF1077 and has been previously documented ((Cooke et al., 2006). A rabbit polyclonal anti-human JAM-A that is peptide-affinity purified and cross-reacts with mouse JAM-A was obtained from Zymed (ZMD #275; Zymed, South San Franscico, CA). The peptide is from the C-terminus. A monoclonal rat anti-mouse JAM-A antibody, BV12, previously validated (Martinez-Estrada et al., 2001) was obtained from Abcam, and a mouse anti-human JAM-A, M.Ab F11, validated by Naik et al, 1995, was purchased from BD Pharmingen. A monoclonal mouse PECAM-1 (Platelet endothelial cell adhesion molecule-1 antibody was obtained from BD Pharmingen). Finally, a monoclonal Heat shock cognate protein 70 (HSC 70) Ab was purchased from Santa Cruz Biotechnology (CA).
Generation of Jam-A- deficient mice
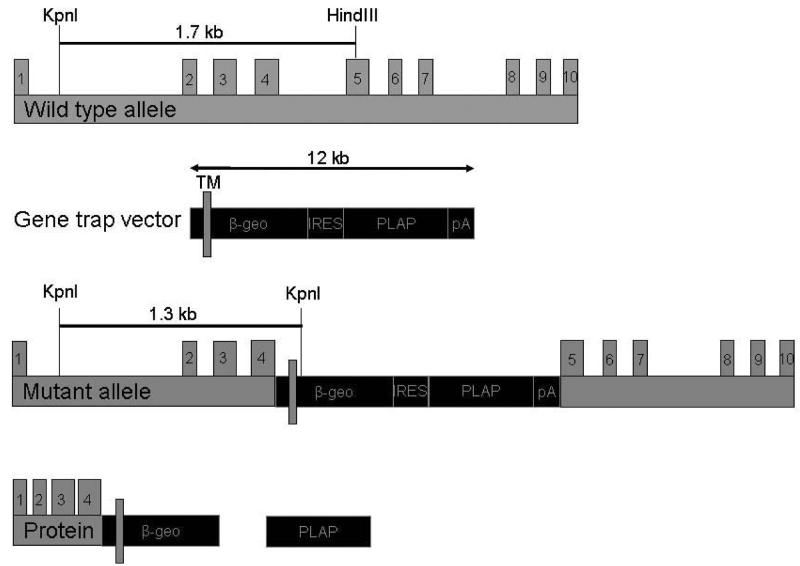
The gene trap approach (Skarnes et al., 1992; Leighton et al., 2001) was used to generate mice homozygous for the null allele of Jam-A. The 12 kb gene trap vector consisting of ß-geo (fusion between ß-galactosidase and neomycin) and the human placental alkaline phosphatase genes (Skarnes et al., 1992) was inserted between exons 4 and 5. It generated a truncated JAM-A-ß-galactosidase fusion protein containing the first Ig-like domain of the JAM-A protein (Fig.1). Jam-A null mice are on the C57BL/6 background and they were genotyped and screened as described (Cooke et al., 2006). All experimental protocols were approved by the University of Delaware Institutional Animal Care and Use Committee.
Fig. 1.
Genetic structure of murine Jam-A and the gene ablation strategy used to generate Jam-A −/− mice. The gene trap vector was inserted between exon 4 and 5. The mutant allele generated a truncated fusion protein with amino acids encoded by the first 4 exons of Jam-A and the ß-geo gene.
Developmental Reverse Transcriptase-PCR Analysis
Testes of mice at various developmental stages, ranging from day 4 to 21 and adult mice were collected. Total RNA was prepared from the tissues using the TRI reagent as previously described (Zheng et al., 2001). One microgram of total RNA was used to synthesize the first strand with Omniscript RT Kit (Qiagen) under conditions recommended by the manufacturer. The primers were designed based on the cDNA sequence: the forward (nt 66-86) is in exon 2, the reverse (nt 507-527) is in exon 5. Reaction conditions were as follows: 94°C for 2 min, followed by 35 cycles of 94°C for 1 min, 52°C for 1 min, and 72°C for 1 min. The PCR products were resolved in a 1.5% agarose gel and stained with ethidium bromide. All experiments were repeated at least twice.
Immunohistochemistry of the Testis and Confocal microscopy
Testes from 3-4 month-old sexually mature wild-type (WT) and Jam-A −/− mice males were weighed. For cryosections, testis were embedded in Tissue Tek OCT compound, snap frozen and stored at −80°C. Sections, 6μm thick, were cut on a freezing microtome and mounted on slides. For immunostaining, the sections were fixed in acetone: methanol, 1:1 for 10 min at −20°C. Non-specific binding was blocked by 3% BSA in PBS for 1.5 hr. Tissue sections were incubated in 1:100 dilution of goat anti-mouse JAM-A polyclonal antibody (R and D Systems,) in PBS containing 1% BSA followed by a 1:300 dilution of secondary antibody, Alexafluor 568-conjugated donkey anti-goat IgG (Molecular Probes, Invitrogen, Carlsbad, CA) or primary monoclonal mouse PECAM-1 (Platelet endothelial cell adhesion molecule-1, BD Pharmingen)(1:50) and Alexafluor 488-conjugated donkey anti-rat secondary body (Molecular Probes, Invitrogen,) diluted 1:200. PECAM is used as a marker for endothelial cells where JAMA is expressed. All reactions were performed in a humidified chamber at RT. Since the primary antibody is JAM-A affinity purified, control samples were treated only with the secondary antibody. Sections were viewed using LSM510 laser scanning confocal microscope.
Collection of Sperm and Assessment of Sperm Counts
Caudal epididymides from sexually mature males, WT and null, were finely minced in 1 ml PBS at 37°C and sperm were allowed to swim out into the buffer for 10 min. After sperm dispersion in the suspension, tissue fragments were separated by gravity settling. The suspension was then centrifuged at 500 rcf for 15 min to pellet the sperm without breaking their membranes. (Katkov and Mazur,1998). Sperm pellets were washed twice by centrifugation and resuspended in PBS. Prior to washing sperm counts were determined by diluting a 0.5 μl sample of each sperm suspension 1:20 and counting the number of sperm in a hemocytometer to obtain the concentration. The concentrations were used to determine the total number of sperm in the original 1 ml volume in which the sperm were suspended. The average sperm counts from the control and from the test group were then compared.
Immunocytochemistry (ICC) and Flow Cytometry
ICC and flow cytometry were performed to determine if JAM-A could be localized on the sperm surface of mature caudal sperm, and if so to quantify its presence. Sperm collected as described above were fixed in 1.5% paraformaldehyde (v/v in PBS), rinsed twice in PBS. Samples were incubated for 10 min with PBS containing 0.25% Triton X-100 for permeabilization, since the antibody that was used was raised against an amino acid sequence in the cytoplasmic tail of JAM-A. Samples were then washed in PBS three times. They were then treated with PBS + 2% BSA blocking solution for 30 min at RT, before exposure to rabbit polyclonal anti-human JAM-A that is peptide-affinity purified and cross-reacts with mouse JAM-A (ZMD #275; Zymed, South San Franscico, CA) suspended in 2% BSA block, 1:400 dilution) for 1 hr at RT. Control slides were treated with non-immune rabbit IgG. After rinsing thrice in PBS, sperm were treated with FITC-conjugated anti-rabbit IgG secondary antibody resuspended in blocking solution (1:400 dilution) for 30 min in the dark, at 4°C. They were again washed thrice in PBS, and resuspended in 20-40 μl PBS and 10-20 μl of each sample was spread on microscope slides and allowed to dry in the dark. Dried samples were stained with ρ-phenylenediamine antifade with 1.5 μg/ml of 4', 6-diamidino-2-phenylindole (DAPI), sealed under a coverslip with clear nail polish and imaged using a Zeiss Axioskop with the appropriate FITC filter set. Images were obtained with the use of a charged coupled device-cooled camera (Photometrics, Tucson, AZ) and software from IPLab Spectrum Module (Scanalytics, Fairfax, VA).
Flow Cytometry
For flow cytometry samples were prepared as described above for ICC, except that the controls were treated with BSA instead of preimmune serum. Following the PBS rinses after exposure to the secondary antibody, samples were analyzed using a FACSCalibur (Becton Dickinson, San Jose, CA) flow cytometer, which uses an argon laser at 488 nm with detectors for FITC and a Cell Quest software package. Fluorescence was measured in samples of 50,000 cells each.
Immunoelectron microscopy
Caudal sperm were washed by centrifugation at 500 rcf for 15 min in PBS. The pellet was fixed in 4% paraformaldehyde, postfixed in Osmium (1% for 1 hr) and resin (EPON 812; Electron Microscopy Sciences, Hatfield, PA) embedded, sectioned and placed on membrane coated transmission electron microscope (TEM) grids. Sections were immunostained for JAM-A using polyclonal goat anti-mouse-JAM-A primary antibody (1:40, R and D Systems, Inc. Cat# AF1077) and a 10 nm gold conjugated donkey anti-goat IgG (1:20, Electron Microscopy Science, Cat # 25805) secondary antibody. Grids were washed in filtered PBS, rinsed in filtered ddH2O and stained in phosphotungstic acid (PTA) before being subjected to the TEM (Zeiss CEM 902) analysis. Grids treated without primary antibody were used as controls.
Preparation of Protein Extracts and Western Blot Analysis
Protein extracts were prepared from the testes of sexually mature 3-month old mice, from caudal epididymal sperm, and from two human sperm samples by manually homogenizing the tissues and cells (using a mortar and pestle) in a solubilization buffer (62.5 mM Tris-HCl, 10% glycerol, 1% SDS, 1mM PMSF, pH 6.8) at 4°C. The suspensions were centrifuged at 10,000 × g for 10 min at 4°C, and the supernatant containing the proteins was collected. The protein concentration was determined by using a biocinchoninic acid protein assay kit (Pierce, Rockford, IL). An equal mass (40 μg) of each protein sample was used for all experiments. Samples were exposed to reducing conditions [99°C for 4 min in the presence of 100 mM dithiothreitol (DTT)] and processed according to standard protocols (Sambrook and Russell, 2001). Western blotting was performed with the Western Breeze Chemiluminescent Immunodetection Kit (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The membrane for the mouse proteins was probed with monoclonal rat anti-mouse JAM-A antibody, BV12 (1:1000) (Abcam); followed by a horse radish peroxidase(HRP)-conjugated secondary donkey anti-rat antibody (1:10,000) (Jackson ImmunoResearch Laboratories). Human proteins were probed with mouse anti-human JAM-A (M.Ab F11; BD Pharmingen), as the primary antibody and a HRP-conjugated anti-mouse IgG as the secondary antibody.
Sperm Motility Assay
The initial microscopic analysis of sperm using the hemocytometer for sperm counts, as described above, revealed that there were sperm motility defects in the Jam-A null mice. Sperm were thus subjected to computer-assisted sperm analysis (CASA) in PBS (uncapacitated condition) and in human tubal fluid (HTF medium, Specialty Media) (capacitated condition) using a method similar to that described by Cheng et al. (2007). After 1 hr incubation at 37°C with gassing with 5% CO2 and 95% O2 to pH 7.3 , aliquots of each sperm suspension were loaded into a 100 μm deep disposable chamber (Microcell; Conception Technologies, San Diego, CA, USA) pre-warmed at 37°C. Computer-assisted sperm motion analysis was performed using a Hamilton Thorne digital image analyzer (HTR-IVOS v 10.8 s; Hamilton Thorne Research). At least 300 spermatozoa and five fields were assessed from each animal.
Eight motion parameters were quantified in this study: (1) motility (%); (2) progressive (prog) motility (%); (3) hyperactivated (hyper) motility (%); (4) curvilinear velocity (VCL, μm/s); (5) progressive or straight-line velocity (VSL, μm/s); (6) average path velocity (VAP, μm/s); (7) straightness (STR, %) (the ratio between VSL and VAP); (8) linearity (LIN, %)(the ratio between VSL and VCL). The settings used during the analysis were: frames acquired, 30; frame rate, 60 Hz; minimum contrast, 85; minimum cell size, 4 pixels; straightness threshold, 80%; low VAP cut off, 5 μm/s; medium VAP cut off, 25 μm/s; head size – non-motile, 12 pixels; head intensity – non-motile, 130 Units(U); static head size, 0.68–2.57 pixels; static head intensity, 0.31–1.21 U; static elongation, 23–100%. The playback function was used to accurately identify motile cells. Hyperactivated motility (%) was defined as motility with starspin or high-amplitude thrashing patterns and short trajectory distances (Burkman, 1984). The criteria for detecting hyperactivated sperm were: VCL >150 μm/s; LIN <50% (Mortimer et al., 1998; Cheng et al., 2007).
RESULTS
Jam-A is expressed in the testis in both somatic and germ cells
The average testicular weights did not differ significantly (P>0.05) between WT (0.12 gm) and Jam-A−/−(0.10 gm) males. Developmental RT-PCR revealed that testicular Jam-A transcripts from WT mice are expressed in the pre-meiotic stages and as early as 4 days post-parturition (Fig.2A), a period when germ cells begin to implant into the basement membrane of the seminiferous tubules (McLean et al., 2003). The expression of testicular Jam-A RNA continues through adulthood and Fig. 2B shows its absence in Jam-A−/− mice, due to the insertion of the gene trap vector. In adults, JAM-A protein can be detected in the seminiferous tubules in the Sertoli cells as well as in spermatids in images from confocal microscopy (Fig. 2C). While the expression in Sertoli cells is very abundant, as revealed by the intense immunopositive staining, it is relatively moderate and diffuse in spermatids in the adluminal compartment of the seminiferous epithelium. Thus there appears to be different levels of expression of JAM-A in somatic and germ cells of the testis. The immunostaining in the Sertoli cells was localized in the basal compartment of the seminiferous epithelium where the tight junctions forming the blood-testis barrier are found. Fig. 3 shows that there were no histological differences in appearances of the seminiferous tubules in WT and null mice, consistent with the similar testicular weights observed.
Fig. 2.
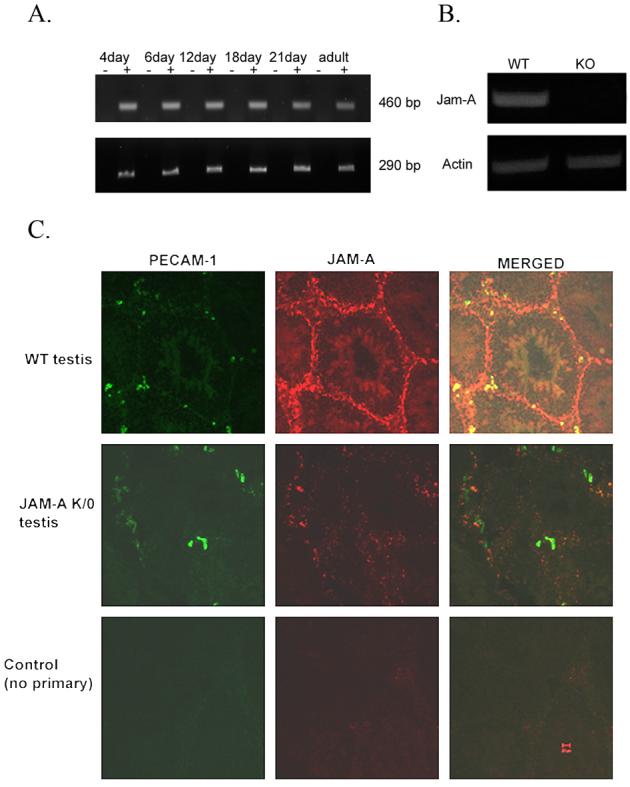
Jam-A transcription and protein localization in WT testis but not in Jam-A null mice. A. RT-PCR results of Jam-A expression in various developmental stages of WT testis. RNA was subjected to first strand synthesis with (+) or without (−) reverse transcriptase, followed by PCR amplification. The expected 460 bp fragment of Jam-A cDNA was detected in every stage (upper panel). Actin is used as control to show the same amount of RT product was used (lower panel). B. Jam-A transcription is detected in WT testis but not in Jam-A null testis. C. Confocal microscopy of JAM-A in mouse testis: Cryostat sections of testis from WT (top panel) and JAM-A null mice (middle panel) were incubated with either pAb JAM-A and mAb against PECAM-1 or no primary antibody for the control (lower panel). Primary antibodies were detected with Alexafluorconjugated secondary antibodies. The bar represents 20 μm.
Fig. 3.
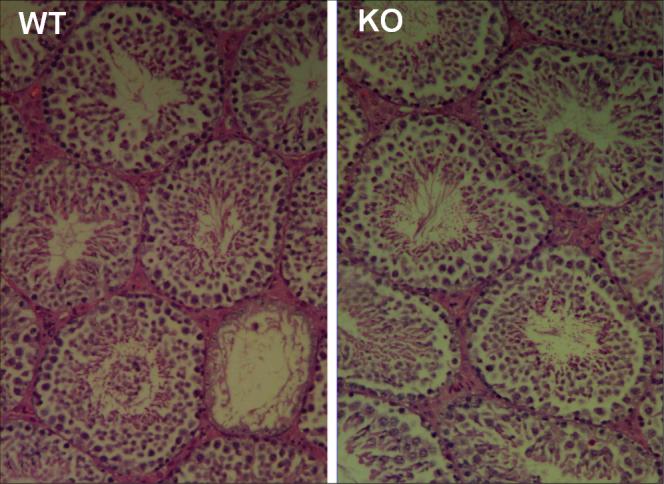
Testis cross-sections of WT and Jam-A null (KO) mice show normal seminiferous tubules in the latter after hematoxylin and eosin staining.
JAM-A is present on mature caudal epididymal sperm
To determine if JAM-A is present on mature sperm and to detect its subcellular distribution, caudal sperm from both WT and Jam-A null mice were subjected to immunocytochemistry (ICC). The protein was detected almost uniformly over the acrosome on the head, the midpiece of the flagellum, and the proximal region of the principal piece (Fig. 4A-1). No signal was detected in the control sperm treated with non-immune IgG (Fig. 4A-2), neither was it seen in Jam-A null sperm treated with either the primary antibody or the non-immume IgG (Fig. 4A-3, 4A-4).
Fig. 4.
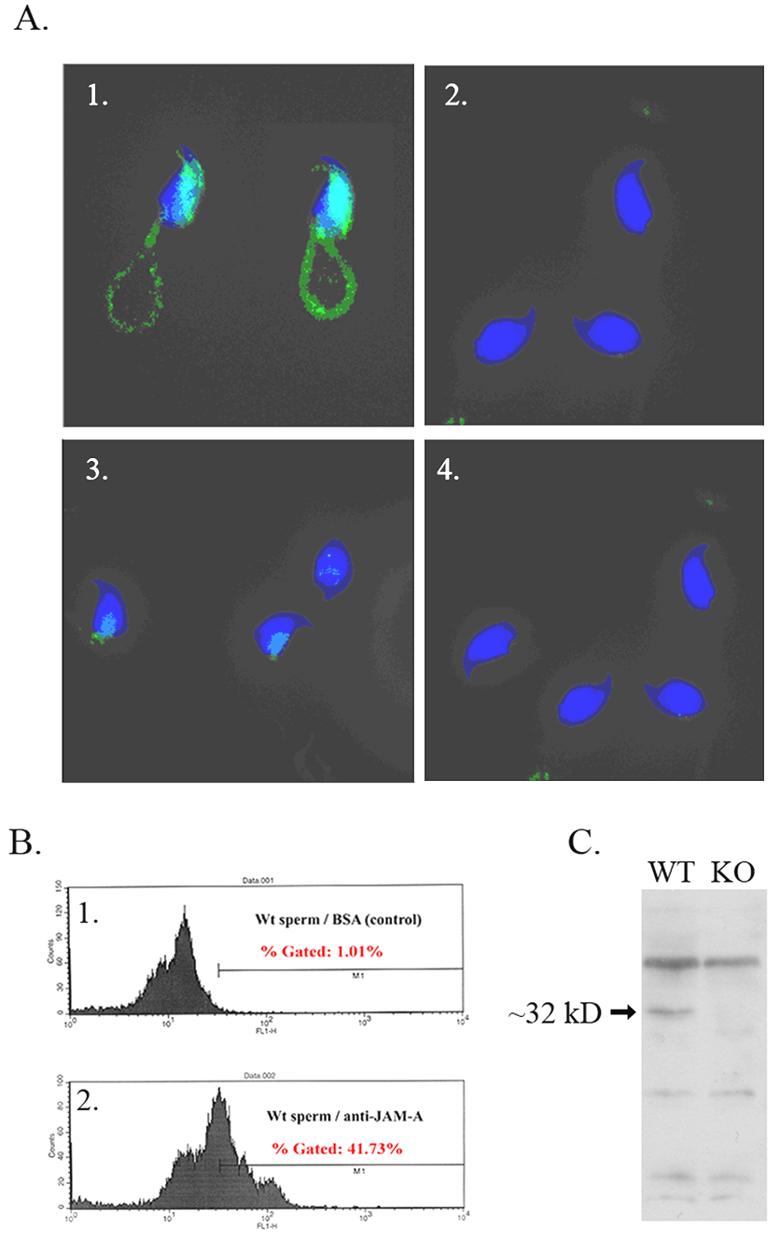
JAM-A protein is present in mature caudal mouse epididymal sperm. A. Immunocytochemistry shows JAM-A to be localized on the acrosome on the heads, and the midpiece, and proximal region of the principal piece of the flagella. Control sperm treated with non-immune IgG show no staining (A-2). Neither was staining seen in Jam-A null sperm treated with either the primary antibody or the non-immune IgG (A-3, A-4). B. Flow cytometry of 50,000 WT sperm treated with BSA as control (B-1) and JAM-A primary antibody (B-2) shows right shifts in the fluorescence peak in the latter, indicating the increased fluorescence intensity. C. Western blot shows an ∼32 kDa band that was detected in protein extracts from WT sperm, but not in Jam-A null sperm.
To quantify and confirm the expression of the JAM-A in sperm, flow cytometry was performed. The control sperm that were treated with BSA showed an arithmetic histogram with a mean of 12.44 compared to 36.42 in the antibody-treated sample. This was reflected in a right shift in the fluorescence peaks (Fig. 4B-1, 4B-2). Finally, we verified the presence of the protein in epididymal sperm by performing Western blots on extracts from both WT and Jam-A null sperm. Our results show a band of the expected size (∼32 kDa) in the WT sperm extracts but not in the null protein extracts (Fig. 4C), confirming the ablation of the gene in the Jam-A −/− mice and the expression of the protein in morphologically mature male germ cells.
Jam-A −/− mice have reduced litter sizes and sperm motility defects
In a parallel communication we showed that the mating of Jam-A +/− animals produced progeny in the ratio of 1: 1.7: 0.6 (+/+, +/−,−/−), indicating a significant deviation from the 1 :2 :1 Mendelian ratio with an approximately 40% deficiency in null mice and that litter sizes for Jam-A −/− were reduced (Cooke et al., 2006). Interestingly, there was a sex ratio distortion in the progeny of Jam-A −/− mice in favor of females (Cooke et al., 2006). To investigate the basis for these observations we assessed sperm counts and motility in WT and Jam-A −/− mice under non-capacitating and capacitating conditions using CASA. Sperm counts showed no statistically significant difference between WT and null mice: for 6 pairs of mice the average counts were respectively 6.2 ± 2.8×106 and 6.6 ± 2.1×106 respectively. At the light microscopic level there appeared to be no difference in sperm morphology in the two groups, however sperm motility appeared impaired and thus sperm were further analyzed by TEM.
CASA revealed in Table 1 that for both capacitated and uncapacitated sperm, the percentages of total motile and progressively motile spermatozoa in null mice were significantly lower than in WT mice (P<0.0001). This was also the case for hyperactivated motility where the level of significance was higher in capacitated (P<0.0001), than in uncapacitated (P<0.01) sperm; suggesting a more severe defect in the Jam-A null sperm during their final maturation period in the female tract.
Table 1.
Kinematic results obtained by CASA for cauda sperm from WT & Jam-A K/O mice
| Uncapacitated | Capacitated | |||
|---|---|---|---|---|
| Parameters |
WT |
Jam-A K/O |
WT |
Jam-A K/O |
| Sperm #s | 8111 | 7501 | 7681 | 7773 |
| % Motile | 76 ± 0.5 | 72 ± 0.5*** | 74 ± 0.5 | 56 ± 0.6▼▼▼ |
| % Prog | 76 ± 0.5 | 71 ± 0.5*** | 73 ± 0.5 | 54 ± 0.6▼▼▼ |
| % Hyper | 8 ± 0.3 | 7 ± 0.3** | 15 ± 0.4 | 11 ± 0.4▼▼▼ |
| VAP | 133.5 ± 4.0 | 117.2 ± 5.9* | 121.1 ± 10.0 | 89 ± 18.3 |
| VSL | 99.1 ± 3.6 | 93.2 ± 5.8 | 82.2 ± 7.2 | 62.1 ± 12.7 |
| VCL | 230.8 ± 9.2 | 189.8 ± 16.8 | 218.4 ± 16.8 | 157.9 ± 31.6 |
| STR | 73.7 ± 2.5 | 78.1 ± 3.9 | 65.3 ± 1.7 | 70.7 ± 4.4 |
| LIN | 45.7 ± 3.0 | 53.6 ± 7.5 | 38.6 ± 1.5 | 45.6 ± 6.1 |
P<0.0001, compared to respective WT uncapacitated controls.
P<0.01, compared to WT uncapacitated control.
P<0.0001, compared to respective WT capacitated controls.
P<0.05, compared to WT uncapacitated control sperm.
Fisher's exact test for parameters (average ± SEM): % Motile, % Prog or % Hyper.
t-test for parameters (average ± SEM): VAP, VSL, VCL, STR and LIN.
Our results for TEM and immunogold staining revealed the presence of JAM-A on the surface of the head and in the midpiece of WT mice (Fig. 5A), again confirming the ICC and the flow cytometry data. TEM also showed that there were flagellar defects present in a high proportion of Jam-A null sperm (Fig. 5B). The most remarkable defect was seen in the mitochondrial sheath of the midpiece (MP) where there was a highly significant increase of condensed mitochondria with increased electron density in the Jam-A null sperm compared to WT sperm. Similarly, in 40 sections WT sperm had no defects (0%) in the principal piece (PP) while there was 2/15 or 12% in the null sperm. It should be noted that the WT sperm and the sperm with the Jam-A disruption are of the same C57BL/6 background.
Fig.5.
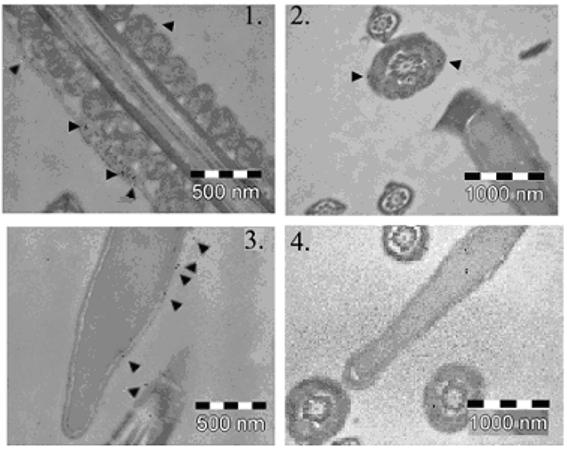
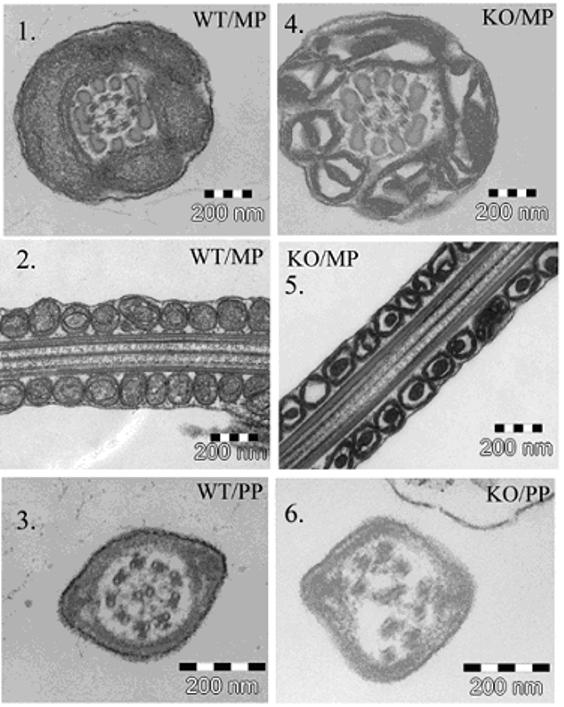
Fig. 5A. Immunoelectron microscopic analysis of JAM-A protein in WT sperm confirms the immunocytochemistry data. Immunogold labeling (arrowheads) of WT sperm demonstrates the presence of JAM-A on the surface of the midpiece (1, 2) and on the surface of the head and on the principal piece (3). The control in 4, with no primary antibody, shows the gold particles randomly distributed.
Fig. 5B. TEM images of the midpiece (MP) of the flagellum of WT (1-2) and Jam-A −/− (4-5) sperm show pronounced defect in the mitochondrial sheath of the latter. In 145 sections from two pairs of males studied there was a significant increase in electron dense condensed mitochondria (χ2 = 15.76; P<0.001) in null sperm compared to controls. Null sperm (6) also showed defects in the principal piece (PP) in a higher frequency compared to WT (3).
To determine if human JAM-A might also be involved in sperm function, we investigated if JAM-A protein is present in human sperm extracts, using Western analysis. Protein extracts of sperm donations from two males showed a band of ∼32 kDa, indicating the presence of JAM-A (Fig. 6).
Fig.6.
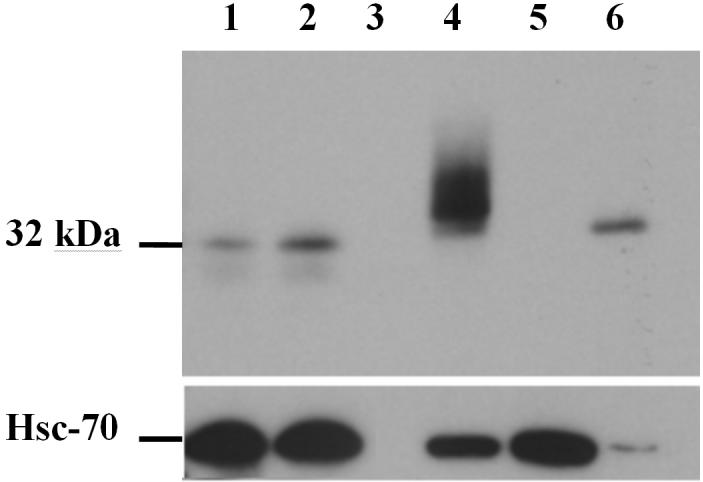
JAM-A is conserved in human sperm. Western blot analysis shows the ∼32 kDa JAM-A band from sperm protein extracts of 2 males in Lanes 1 and 2. Lane 3 is blank and Lane 4, a positive control, shows recombinant human JAM-A from Chinese hamster cells (CHO) transfected with human JAM-A cDNA containing a hemagglutinin (HA) tag. Lane 5, a negative control, shows the absence of JAM-A from mock-transfected CHO cells while Lane 6 is a positive control showing endogenous JAM-A from human umbilical vein endothelial cells (HUVEC) which are known to express high levels of the protein. The loading control in the lower panel shows heat shock cognate protein 70 (HSC 70) present for all samples, including the mock transfected negative control.
DISCUSSION
Testis size and sperm numbers are not affected by disruption of Jam-A
Our results from confocal microscopy (Fig. 2) and in vitro culture of Sertoli cells (data not shown) revealed that JAM-A is localized in Sertoli cells at the basal compartment where the Sertoli-Sertoli tight junctions are found, confirming previous findings (Gliki et al, 2004; Mruk and Cheng, 2004). Despite the role of Sertoli cells in providing structural support and nourishment for germ cells via their secretory products, creating an impermeable and immunological barrier in the testis, and in participating in germ cell movement and spermiation (Weinbauer et al., 2000), disruption of Jam-A did not appear to affect testis size, sperm numbers, or the gross sperm morphological appearance. This finding is similar to that for Jam-B deletion where mice were shown to have normal spermatogenesis and testicular and sperm morphology (Sakaguchi et al., 2002). Since normal spermatogenesis depends on functional interactions between reproductive germ cells and somatic Sertoli cells where both JAM-A and JAM-B are localized, it appears that these two JAM proteins compensate for the loss of each other with respect to Sertoli cell function and that the interactions are homophilic. The importance of the role of Sertoli-Sertoli cell interaction in spermatogenesis is underscored by the functional redundancy of these two JAM protein family members.
Jam-A is expressed in spermatids and mature spermatozoa of Wild-type Mice
Based on the findings by Gliki et al. (2004), JAM-A was reported to be localized in the testis only at the Sertoli-Sertoli tight junctions. However, our immunohisto-chemical analysis clearly shows that spermatids, both round and elongated, are immunopositive for JAM-A. Thus this is the first report of the presence of JAM-A in germ cells, implicating a role in spermiogenesis, as is the case for JAM-C (Gliki et al., 2004), and in sperm function. The presence of JAM-A in germ cells is supported by the finding of the protein in epididymal caudal sperm by Western blotting and confirmed by immunocytochemistry, flow cytometry, and immunoelectron microscopy. Importantly, the detection of JAM-A in human sperm reveals that it is conserved and therefore suggests an important role for this protein in mammalian male germ cells. Our detection of JAM-A in human sperm has been recently confirmed by another group which also showed the protein to be present in the human testis (Gye et al., 2007)
JAM-A is required for normal sperm motility
Although JAM-A and JAM-B appear to play redundant roles in maintaining the blood-testis barrier, that is conducive to producing normal spermatogenesis; they have different roles with respect to sperm function. Disruption of Jam-A results in severe motility defects, particularly after capacitation: while there is no functional impairment with Jam-B deletion (Sakaguchi et al., 2006). The lack of impairment of Jam-B deletion is not surprising since, unlike Jam-A, it does not appear to be present in spermatids or spermatozoa (Gliki et al., 2004, Sakaguchi et al., 2006); although it is present with Jam-C in the Sertoli-spermatid junctional plaques (Gliki et al., 2004). This redundancy of Jam-B with both Jam-A and Jam-C may lead to functional compensation when Jam-B is inactivated. It is interesting that while deletion of Jam-C leads to complete infertility due to impairment in the differentiation of round spermatids into spermatozoa, deletion of Jam-A which results in sperm motility defects is associated with reduced litter size and subfertility (Cooke at al., 2006). In human populations as well as in mice, fertility is dependent not only on sperm numbers, but also on their morphology and motility.
Our CASA results revealed that the percentages of motile spermatozoa and progressive and hyperactivated motility were significantly (P<0.01; P<0.0001) lower in null mice, in both capacitated and uncapacitated groups. The mean VAP value for uncapacitated sperm from Jam-A −/− mice was significantly lower than for WT. Interestingly, in the Jam-A null sperm after capacitation all three velocity parameters and the ratios (STR, and LIN) had very large standard errors of the means (Table 1) compared to those of the WT sperm (18.3 vs 10, 12.7 vs 7.2, 31.6 vs 16.8, 4.4 vs 1.7, 6.1 vs 1.5). This suggests dramatic variations among individual Jam-A null sperm. Since hyperactivated motility is a characteristic of capacitated sperm and the differences were more severe after capacitation (P<0.0001 vs P<0.01), it appears that JAM-A plays a more important role in motility after the final maturation of sperm in the oviduct than in their progressive motility in the uterus. Hyperactivated motility seen after capacitation is characterized by the development of high velocity, large amplitude and asymmetrical flagellar waves (Cooper and Yeung, 2000). Thus it plays important physiological roles including: prevention of entrapment, improvement of the chances of oocyte contact, assistance in cumulus penetration, and in zona penetration (Suarez and Dai, 1992). Reduction of these activities in Jam-A null sperm could lead to reduced numbers of fertilized oocytes and ultimately reduced litter sizes. Thus future studies will focus on determining if the subfertility seen in Jam-A −/− mice is male- or female-derived.
Our observations of the ultrastructure of WT and Jam-A null sperm show significantly (P<0.001) higher percentages of dysplasia of the mitochondrial sheaths and the principal piece in sperm from the latter, indicating a role for JAM-A in tail formation. They also suggest that defects in the flagellum, in both the mitochondrial sheath in the midpiece and the proximal principal piece, may be responsible for the motility defects. Although the large majority of the ATP used in basal sperm motility is obtained by glycolysis (Mukai and Okuno, 2004), hyperactivated motility may be dependent on ATP obtained by oxidative phosphorylation occurring in the mitochondria in the midpiece of the flagellum.
Interestingly, the condensed electron dense mitochondria seen in high frequency in Jam-A null sperm bear a striking resemblance to those seen in hyperactivated motility defects of sterile mice with the Plasma membrane Ca2+-ATPase 4 (Pmca4) gene disrupted (Schuh et al., 2004; Okunade et al., 2004). The condensed mitochondria reflects Ca2+ overload which occurs when efflux is inhibited in the absence of PMCA4 (the protein encoded by Pmca4), which is present on the principal piece (Schuh et al., 2004; Okunade et al., 2004). We proposed that JAM-A and PMCA4 which co-localize on the principal piece may interact in regulating Ca2+ efflux or may be on the same signal transduction pathway, and will test this hypothesis in future experiments.
In the principal piece AKAP4 (A kinase Anchoring Protein 4) functions as a scaffolding protein for a variety of signal transducing molecules. It anchors PKA (protein kinase A) to the fibrous sheath and directs signal transduction pathways that control motility (Miki et al., 2002). The importance of tyrosine phosphorylation of flagellar proteins for sperm motility and its correlation with hyperactivation (Si and Okuno, 1999) suggest that JAM-A, which has a tyrosine phosphorylation site within the PDZ domain-binding sequence at the cytoplasmic tail and which binds to scaffolding proteins (Ebnet et al., 2004), may be involved in the signal transduction pathway for hyperactivated motility. Due to the presence of JAM-A on the sperm head it is also possible that it might be involved in the protein phosphorylation associated with capacitation (Galantino-Homer et al., 1997). These possibilities will also be investigated in future studies.
Acknowledgements
The study was supported by NIH- COBRE grant # 5P20RR015588-07. We are grateful to Dr. George Gerton at the University of Pennsylvania Medical Center for allowing us the use of his CASA system. We are also thankful to Dr. Deni S. Galileo for assistance with the flow cytometric analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Babinska A, Kedees MH, Athar H, Ahmed T, Batuman O, Ehrlich YH, Hussain MM, Kornecki E. F11-receptor (F11R/JAM) mediates platelet adhesion to endothelial cells: role in inflammatory thrombosis. Thromb. Haemost. 2002;8:843–850. [PubMed] [Google Scholar]
- Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, Nusrat A, Parkos CA, Dermody TS. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104:441–451. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- Bazzoni G. The JAM family of Junctional adhesion molecules. Curr. Opin. Cell Biol. 2003;15:525–30. doi: 10.1016/s0955-0674(03)00104-2. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Martinez-Estrada OM, Mueller F, Nelboeck P, Schmid G, Bartfai T, Dejana E, Brockhaus M. Homophilic interaction of Junctional adhesion molecule. J. Biol. Chem. 2000;275:30970–30976. doi: 10.1074/jbc.M003946200. [DOI] [PubMed] [Google Scholar]
- Burkman LJ. Characterization of hyperactivated motility by human spermatozoa during capacitation: comparison of fertile and oligozoospermic sperm populations. Archives of Andrology. 1984;13:153–165. doi: 10.3109/01485018408987514. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Buffone MG, Kouadio M, Goodheart M, Page DC, Gerton GL, Davidson I, Wang PJ. Abnormal Sperm in Mice Lacking the Taf7l Gene. Mol. Cellular Biol. 2007;27:2582–2588. doi: 10.1128/MCB.01722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke VG, Naik MU, Naik UP. Fibroblast Growth Factor-2 Failed to Induce Angiogenesis in Junctional Adhesion Molecule-A-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2006;26:2005–2011. doi: 10.1161/01.ATV.0000234923.79173.99. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Yeung C-H, Yeung . Physiology of Sperm maturation and fertilization. In: Nieschlag E, Behre HM, editors. Andrology: Male Reproductive Health and Dysfunction. 2nd ed. Springer Verlag; Berlin, Germany: 2000. pp. 63–82. [Google Scholar]
- Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J. Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- Galantino-Homer HL, Visconti PE, Kopf GS. Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3′5′-monophosphate-dependent pathway. Biol. Reprod. 1997;56:707–19. doi: 10.1095/biolreprod56.3.707. [DOI] [PubMed] [Google Scholar]
- Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–324. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- Gye MC, Park HJ, Kim HJ, Park NC. Junctional adhesion molecules in mouse and human testis and spermatozoa. Biol Reprod 2007 Special Issue, Abstract #77; 40th Annual Meeting of the Society for the Study of Reproduction; San Antonio,Texas. July 21-25th.2007. [Google Scholar]
- Katkov I, Mazur P. Influence of Centrifugation Regimes on Motility, Yield and Cell Associations of Mouse Spermatozoa. J. Androl. 1998;19:232–241. [PubMed] [Google Scholar]
- Leighton PA, Mitchell KJ, Goodrich LV, Lu X, Pinson K, Scherz P, Skarnes WC, Tessier-Lavigne M. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410:174–179. doi: 10.1038/35065539. [DOI] [PubMed] [Google Scholar]
- Mandell KJ, Parkos CA. The JAM family of proteins. Adv. Drug Deliv. Rev. 2005;57:857–867. doi: 10.1016/j.addr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- McLean DJ, Friel PJ, Johnston DS, Griswold MD. Characterization of Spermatogonial Stem Cell Maturation and Differentiation in Neonatal Mice. Biol. Reprod. 2003;69:2085–2091. doi: 10.1095/biolreprod.103.017020. [DOI] [PubMed] [Google Scholar]
- Miki K, Willis WD, Brown PR, Goulding EH, Fulcher KD, Eddy EM. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev. Biol. 2002;248:331–342. doi: 10.1006/dbio.2002.0728. [DOI] [PubMed] [Google Scholar]
- Mortimer ST, Swan MA, Mortimer D. Effect of seminal plasma on capacitation and hyperactivation in human spermatozoa. Hum. Reprod. 1998;13:2139–2146. doi: 10.1093/humrep/13.8.2139. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mukai C, Okuno M. Glycolysis plays a major role for Adenosine Triphosphate Supplementation in mouse sperm flagellar movement. Biol. Reprod. 2004;71:540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- Naik UP, Eckfield K. Junctional adhesion molecule 1 (JAM-1) J. Biol. Regul. Homeost Agents. 2003;17:341–347. [PubMed] [Google Scholar]
- Okunade GW, Miller ML, Pyne GJ, Sutliff RI, O'Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Schull GE. Targeted Ablation of Plasma membrane Ca2+-ATPase (PMCA) 1 an 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol. Chem. 2004;279:33742–33750. doi: 10.1074/jbc.M404628200. [DOI] [PubMed] [Google Scholar]
- Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Nishimoto M, Miyagi S, Iwama A, Morita Y, Iwamori N, Nakauchi H, Kiyonari H, Muramatsu M, Okuda A. Putative “Stemness” Gene Jam-B is not required for maintenance of stem cell state in embryonic, neural, or hematopoietic stem cells. Mol. Cell Biol. 2006;26:6557–70. doi: 10.1128/MCB.00729-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual; v.1. 3rd ed. Cold Spring Harbor laboratory Press; Cold Spring Harbor, N.Y: [Google Scholar]
- Schuh K, Cartwright EJ, Jankevics E, Bundschu K, Liebermann J, Williams JC, Armesilla AL, Emerson M, Oceandy D, Knobeloch KP, Neyes L. Plasma membrane Ca 2+-ATPase 4 is required for sperm motility and male fertility. J. Biol Chem. 2004;279:28220–28226. doi: 10.1074/jbc.M312599200. [DOI] [PubMed] [Google Scholar]
- Shima JE, McLean DJ, McCarrey JR, Griswold MD. The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biol. Reprod. 2004;71:319–330. doi: 10.1095/biolreprod.103.026880. [DOI] [PubMed] [Google Scholar]
- Si Y, Okuno M. Role of tyrosine phosphorylation of flagellar proteins in hamster sperm hyperactivation. Biol. Reprod. 1999;61:240–246. doi: 10.1095/biolreprod61.1.240. [DOI] [PubMed] [Google Scholar]
- Skarnes WC, Auerbach BA, Joyner AL. A gene trap approach in mouse embryonic stem cells: the lacZ reported is activated by splicing, reflects endogenous gene expression, and is mutagenic in mice. Genes Dev. 1992;6:903–918. doi: 10.1101/gad.6.6.903. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Dai X. Hyperactivation enhances mouse sperm capacity by penetrating viscoelastic media. Biol. Reprod. 1992;46:686–691. doi: 10.1095/biolreprod46.4.686. [DOI] [PubMed] [Google Scholar]
- Weinbauer GF, Gromoll J, Simoni M, Nieschlag E. Physiology of testicular function. In: Nieschlag E, Behre HM, editors. Andrology: Male Reproductive Health and Dysfunction. 2nd ed. Springer Verlag; Berlin, Germany: 2000. pp. 23–61. [Google Scholar]
- Yeung CH, Cooper TG. Acquisition and development of sperm motility upon maturation in the epididymis. In: Robaire B, Hinton BT, editors. The epididymis from molecules to clinical practice. Kluwer Academic / Plenum Publishers; New York: 2002. pp. 417–434. [Google Scholar]
- Zheng Y, Deng XD, Martin-DeLeon PA. Lack of Sharing of Spam1 (Ph-20) among Mouse Spermatids and Transmission Ratio Distortion. Biol. Reprod. 2001;64:1730–1738. doi: 10.1095/biolreprod64.6.1730. [DOI] [PubMed] [Google Scholar]