Abstract
Metastatic osteosarcoma (OS) has a very poor prognosis. New treatments are therefore wanted. The conditionally replicative adenovirus Ad5-Δ24RGD has shown promising anti-tumor effects on local cancers, including OS. The purpose of this study was to determine whether intravenous administration of Ad5-Δ24RGD could suppress growth of human OS lung metastases. Mice bearing SaOs-lm7 OS lung metastases were treated with Ad5-Δ24RGD at weeks 1, 2 and 3 or weeks 5, 6 and 7 after tumor cell injection. Virus treatment at weeks 1–3 did not cause a statistically significant effect on lung weight and total body weight. However, the number of macroscopic lung tumor nodules was reduced from a median of >158 in PBS-treated control mice to 58 in Ad5-Δ24RGD-treated mice (p = 0.15). Moreover, mice treated at weeks 5–7 showed a significantly reduced lung weight (decrease of tumor mass, p < 0.05), a significantly increased body weight gain (decrease of disease symptoms, p < 0.005) and a reduced number of macroscopic lung tumor nodules (median 60 versus > 149, p = 0.12) compared to PBS treated control animals. Adenovirus hexon expression was detected in lung tumor nodules at sacrifice three weeks after the last intravenous adenovirus administration, suggesting ongoing viral infection. These findings suggest that systemic administration of Ad5-Δ24RGD might be a promising new treatment strategy for metastatic osteosarcoma.
Findings
Osteosarcoma is the most prevalent non-hematological primary malignant bone tumor. They can be subdivided by location (medullary and surface) and by grade (high or low grade). The vast majority of high-grade osteosarcoma comprises of conventional osteosarcoma, but also includes high-grade surface, telangiectatic and small-cell osteosarcoma [1]. Patients with high-grade osteosarcoma (OS) without evident metastatic disease at presentation have 5-year survival of about 50–70% achieved by preoperative and postoperative chemotherapy with aggressive surgery [2-6]. Patients presenting with overt metastatic disease undergo the same pre- and postoperative chemotherapeutic regimen with resection of all metastases whenever feasible. The majority of osteosarcoma metastases are located in the lungs [4,7,8]. Multiple lung metastases and bilateral distribution appear to be poor prognostic factors for survival with 5-year overall survival rates of 26% and 20%, respectively; in contrast to 75% for a solitary lung lesion [8]. Moreover, OS patients that relapse after first achieving complete surgical remission during combined-modality chemotherapy have a 5-year overall survival of 23% [9]. Long-term survivors were only observed after a second surgical remission, indicating that metastastectomy of all lesions is essential. The role of second-line chemotherapeutic agents is unsure and might result in limited improved outcome [9]. Clearly, for inoperable OS patients new treatment modalities are needed.
We explore the use of conditionally replicative adenoviruses (CRAds) as a potential new treatment modality for high grade OS and its lung metastases. Virus replication will lyse tumor cells and released viral progeny can infect neighboring tumor cells leading to lateral spread and increased tumor cell kill. Previously we found that primary OS cells express low levels of the high affinity receptor for adenoviruses, the coxsackie and adenovirus receptor (CAR) [10,11]. In contrast, primary OS cells express high levels of integrins [12]. To circumvent CAR deficiency and thereby reduced tumor cell infectivity, we use the CRAd variant Ad5-Δ24RGD that carries a cyclic Arg-Gly-Asp (RGD-4C) integrin binding motif in its fiber knob domain [13]. This CRAd expresses mutant E1A that cannot bind to pRb. Consequently, E2F, essential for viral replication, is not released. Therefore, this CRAd is limited to replicate in cancer cells with constitutively active E2F and not in non-cycling normal cells with functional pRb [14]. In vivo experiments showed that this virus induced tumor regression of subcutaneous glioma, osteosarcoma and cervical cancer tumors after intra-tumoral or systemic administration [12,15-17]. Infection of CAR-deficient OS cells with this integrin-targeted virus resulted in increased infectivity and cell kill. Furthermore, we showed that intra-tumoral administration of Ad5-Δ24RGD in subcutaneous primary OS tumors resulted in a significant tumor growth delay [12].
Local treatment of OS with CRAds has shown promising results in animal models [12,18]. However, the major challenge to cure OS patients depends on an effective treatment for (non-resectable) metastases. Biodistribution studies in animals indicated that a considerable amount of intravenously administered Ad5-Δ24RGD is delivered to the lungs (25% of the liver dose) [17]. In addition, the RGD-modification might partially avoid reduced infectivity as result of neutralizing antibodies [19,20]. In the present study, we explore the use of intravenous administered Ad5-Δ24RGD for the treatment of OS lung metastases.
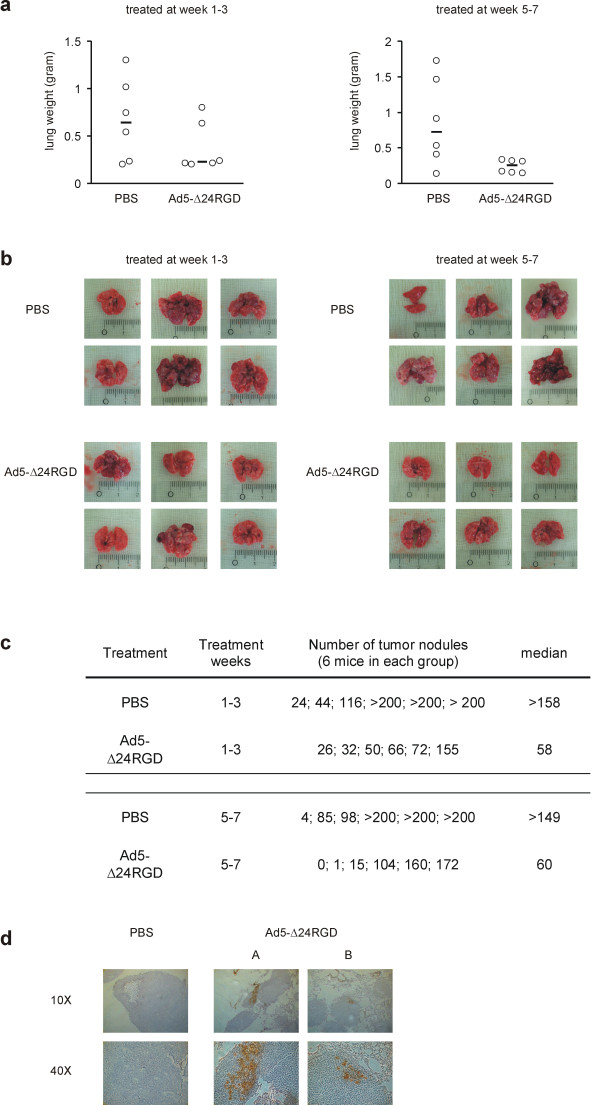
We first performed a pilot study in which mice were given 1 × 109 plaque forming units (pfu) intravenously once a week for three consecutive weeks (3 × 109 pfu total). This was the practically highest achievable dose and injection of this amount of virus did not affect body weight, suggesting that it did not induce overt toxicity. Therefore, this dose was chosen to explore the anticancer potency against OS lung metastases. SaOs-lm7 cells were injected intravenously into mice to establish OS lung metastases [21]. In a first experiment, mice bearing OS lung metastases were treated with 1 × 109 pfu Ad5-Δ24RGD or with PBS at weeks 1, 2 and 3 after SaOs-lm7 injection. As parameter of mouse well-being, their relative gain in body weight was calculated. Ten weeks after tumor cell injection, mice were sacrificed and their lungs dissected. Mouse lung weight and number of macroscopic lung tumor nodules were quantified to assess Ad5-Δ24RGD anticancer effect. Mice treated with Ad5-Δ24RGD or PBS showed no significant difference in body weight gain during the experiment (data not shown). As shown in figure 1a, average lung weight of virus treated animals appeared to be decreased in comparison to PBS treated control animals, but this difference was statistically not significant (p = 0.15). Macroscopic appearance of the lungs reflected the lung weight measurements, where a lower lung weight paralleled fewer and smaller lung metastases and a higher lung weight correlated with more and larger lung metastases (figures 1b). In the PBS treated group, lungs often contained numerous lung metastases; sometimes more than could be counted (> 200). The median number of macroscopic tumor nodules on the lungs of PBS treated animals was >158 (range 24 – >200). Lungs of Ad5-Δ24RGD treated animals carried a median number of 58 lung metastases (range 26–155; figure 1c).
Figure 1.
Therapeutic effect of Ad5-Δ24RGD infusion 1–3 (left panel figure a & b) or 5–7 (right panel figure a & b) weeks after SaOs-lm7 injection. (a) Effect of intravenous administration of Ad5-Δ24RGD on OS pulmonary tumor burden. Lungs were removed and weighed. Open circles depict lung weight of each mouse and black lines show the median of each group. (b) Macroscopic appearance of dissected lungs. (c) Number of lung tumor nodules per mouse. (d) Immunohistochemical staining for expression of the adenovirus hexon protein in lungs of mice treated at weeks 5–7. Ten weeks after tumor cell injection and three weeks after the last virus injection lungs were isolated and analyzed for hexon expression. Representative pictures of the lungs from a PBS control mouse and two Ad5-Δ24RGD treated mice (A & B) are shown at 10× and 40× magnification.
In a next experiment, we delayed the onset of oncolytic adenovirus treatment by 4 weeks. Virus or PBS was injected once per week at 5, 6 and 7 weeks after SaOs-lm7 tumor cell injection. Mice treated with Ad5-Δ24RGD showed an average increase in weight of 18%, which was significantly higher than the average 8% weight gain observed for PBS treated control animals (P < 0.005). Moreover, intravenous administration of Ad5-Δ24RGD resulted in a significant reduction of lung weight (median 241 mg) compared to PBS treated control animals (median 723 mg; figure 1a; P < 0.05). The lung weight of virus treated mice was just above normal mouse lung weight (i.e., approximately 180 mg). The higher tumor mass in the PBS treated group suggested that the total body weight gain, after correction for increased tumor mass, differed even more between the PBS and virus treated animals. Also in this experiment, macroscopic appearance reflected lung weight measurements (figure 1b). The median number of macroscopic lung metastases (figure 1c) in PBS treated animals was >149 (range 4 – >200) and in Ad5-Δ24RGD treated animals 60 (range 0 – 172).
To strengthen the hypothesis that intravenous delivered Ad5-Δ24RGD caused an antitumor effect against OS lung metastases, the lungs of mice from the second experiment treated with Ad5-Δ24RGD at weeks 5–7 and dissected at week 10 were analyzed for adenoviral infection by hexon staining. As shown in figure 1d, foci of infected cells were present in SaOs-lm7 lung nodules, but not in normal lung tissue. Thus, adenovirus infection could still be detected in OS lung metastases 3 weeks after the last intravenous Ad5-Δ24RGD injection, supporting the assumption that ongoing virus infection contributed to the beneficial effects of systemic CRAd treatment. Adenovirus-positive foci were not surrounded by apparent fibrosis, suggesting that connective tissue barriers did not hamper intra-tumor spread of the virus. However, such efficacy-limiting physical barriers might exist in OS.
In general, Ad5-Δ24RGD treatment seemed to reduce lung weight and number of macroscopic tumor nodules in both tested treatment schedules. These parameters are commonly used for therapeutic efficacy evaluation. Treatment effects were most evident when virus treatment was applied 5–7 weeks after tumor cell injection. Treatment at week 5–7 also resulted in a significant body weight increase, suggesting improved mouse well-being. More insight into the kinetics of OS lung metastasis growth and response to treatment could be obtained if longitudinal in vivo imaging of SaOs-lm7 tumor growth would be possible. Unfortunately, a derivative reporter cell line for bioluminescent imaging is not available.
Together, our observations suggest that Ad5-Δ24RGD is active against OS lung metastases, which is in line with recent finding by Li et al [22], who showed that a transcriptional replication-competent adenovirus (AdOC-E1a) reduced the number of OS lung tumor nodules. Furthermore, our experiments suggest that timing of virus injection influences treatment outcome. A possible explanation for the more effective outcome in mice treated at weeks 5–7 might be found in a dependence on an established tumor vasculature for systemic CRAd delivery. SaOs-lm7 injected tumor cells will form microscopic lung metastases by 3–5 weeks and visible lung nodules by 6 weeks [21]. Microscopic tumors are usually in a pre-vascular state and only growth beyond 2–3 mm3 requires blood vessel formation [23]. Virus injected at week 1–3 thus presumably treats isolated tumor cells or microscopic tumor nodules, which could be reached via diffusion, whereas treatment at week 5–7 would allow vascular delivery to macroscopic tumor nodules. Although we documented significant therapeutic efficacy after three injections of Ad5-Δ24RGD, mice were not completely cured from their OS lung metastases. To increase treatment efficacy extended administration schemes could be tested. In addition, in a clinical setting in humans isolated lung perfusion to increase CRAd delivery could be considered. Several clinical trials delivering therapeutic agents to sarcoma lung metastases via isolated lung perfusion have been conducted [24]. Clinical trials with Ad5-Δ24RGD for the treatment of glioma and ovarian cancer are developed and will be started soon [25]. These studies will provide valuable data on the safety profile of this CRAd in humans important to pursue development of Ad5-Δ24RGD for OS and OS lung metastases treatment.
Methods
Cell lines
SaOs-lm7 cells are derived from SaOs-2 human OS cells by repetitive cycling through the lungs of nude mice [21]. SaOs-lm7 cells are grown in Eagle's minimal essential medium supplemented with nonessential amino acids, sodium pyruvate, L-glutamine, 10% fetal calf serum (FCS) and 50 IU/ml penicillin plus 50 μg/ml streptomycin (PS; Life Technologies, Breda, the Netherlands). Cultures are maintained and harvested at sub-confluence. A549 and 293 cells were obtained from the American Type Culture Collection (Manassas, VA) and are maintained in F12-supplemented Dulbecco's modified Eagle's medium (DMEM) with 10% FCS and PS. All cells are cultured at 37°C in a humidified, 5% carbon dioxide atmosphere.
Recombinant adenoviruses
The CRAd Ad5-Δ24RGD lacks 24 base pairs encoding 8 amino acids in the pRb binding domain of E1A and carries a cyclic RGD epitope in the HI-loop of the fiber [13]. This CRAd was propagated on A549 cells. Virus was purified using cesium chloride gradient banding. Functional titers in pfu were determined by end-point limiting dilution on 293 cells.
OS lung metastasis model in nude mice
Female athymic/nude/nude mice were purchased from Harlan (Horst, the Netherlands). Animals were maintained under pathogen-free conditions and fed a standard laboratory diet ad libitum. All experiments were approved by the local committee on animal experiments and were carried out under the conditions established by the European Community (directive 86/609/CCE). Mice were injected with 5 × 106 SaOs-lm7 cells in the lateral tail vein in 200 μl Hanks Balanced Salt Solution w/o calcium and magnesium. Intravenous injection of SaOs-lm7 cells results in microscopic lung metastases by week 3–5 and macroscopic disease at 6 weeks.
Intravenous administration of Ad5-Δ24RGD to mice bearing osteosarcoma lung metastases
In two independent experiments, 12 mice bearing SaOs-lm7 lung metastases were randomly divided into 2 groups of each 6 mice. Mice were treated three times, in weeks 1, 2, and 3 (experiment 1) or in weeks 5, 6 and 7 (experiment 2). The first group received intravenous injections in the tail vein of 1 × 109 pfu Ad5-Δ24RGD in 200 μl PBS and the second control group received 200 μl PBS.
Body weight was measured twice a week. All mice were sacrificed in week 10 after tumor cell injection. The lungs were removed in toto without difficulty and photographed. Two researchers counted tumor nodules on the outside of the lungs independently and the average of the two measurements was calculated. Lungs were snap frozen in liquid nitrogen and weighed using a Mettler H31 (± 0.1 mg; Mettler-Toledo International Inc).
Immunohistochemical analysis
Cryosections of lungs obtained at week 10 from mice treated at weeks 5, 6 and 7 after tumor cell injection were fixed in acetone. Immunohistochemical staining for adenovirus (hexon) was done using goat anti-adenovirus antibody 1056 (Chemicon International, Temecula, CA). Sections were then incubated with biotinylated goat anti-rabbit IgG antibodies. The immune reaction was visualized using the streptavidin-biotin-Horse Radish peroxidase complex method (StrepABComplex/HRP; Dako, Glostrup, Denmark) followed by diaminobenzidine solution (Vector Laboratories, Inc., Burlingame, CA). Sections were counterstained with hematoxylin (Sigma Aldrich, St. Louis, MO), dehydrated and mounted.
Statistical analysis
Differences in relative gain in mouse weight, number of lung metastases and total lung weight between treatment groups were analyzed with a two and one-tailed Mann-Whitney test, respectively, using GraphPad Instat 3.0 (GraphPad Software, Inc., San Diego, CA).
Competing interests
The author(s) declare they have no competing interests.
Authors' contributions
HCAG: designed and carried out the experiments and drafted the manuscript
VWB: designed experiments, analyzed data and critically commented on final manuscript
FHES: counted OS lung tumor metastases and corrected manuscript
MAW: optimized mouse model and corrected manuscript
ESK: optimization of mouse model, and commented on manuscript
MNH: designed experiments and corrected manuscript
WRG: supervised project and commented on manuscript
GJLK: obtained funding, supervised project and corrected final manuscript
PIJMW: obtained funding, supervised project and corrected final manucript
All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
In memory of professor Paul I.J.M. Wuisman.
Contributor Information
Harm CA Graat, Email: hca.graat@vumc.nl.
Victor W van Beusechem, Email: vw.vanbeusechem@vumc.nl.
Frederik HE Schagen, Email: e.schagen@vumc.nl.
M Adhiambo Witlox, Email: ma.witlox@vumc.nl.
Eugenie S Kleinerman, Email: ekleiner@mdanderson.org.
Marco N Helder, Email: m.helder@vumc.nl.
Winald R Gerritsen, Email: winald.gerritsen@vumc.nl.
Gertjan JL Kaspers, Email: gjl.kaspers@vumc.nl.
Paul IJM Wuisman, Email: orthop@vumc.nl.
References
- Fletcher CDM, Unni KK, Mertens F. WHO Classification of tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, IARC Press; 2002. [Google Scholar]
- Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- Goorin AM, Schwartzentruber DJ, Devidas M, Gebhardt MC, Ayala AG, Harris MB, Helman LJ, Grier HE, Link MP. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21:1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- Bacci G, Ferrari S, Longhi A, Perin S, Forni C, Fabbri N, Salduca N, Versari M, Smith KV. Pattern of relapse in patients with osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Eur J Cancer. 2001;37:32–38. doi: 10.1016/S0959-8049(00)00361-0. [DOI] [PubMed] [Google Scholar]
- Lewis IJ, Nooij MA, Whelan J, Sydes MR, Grimer R, Hogendoorn PC, Memon MA, Weeden S, Uscinska BM, van Glabbeke M, Kirkpatrick A, Hauben EI, Craft AW, Taminiau AH. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst. 2007;99:112–128. doi: 10.1093/jnci/djk015. [DOI] [PubMed] [Google Scholar]
- Eselgrim M, Grunert H, Kuhne T, Zoubek A, Kevric M, Burger H, Jurgens H, Mayer-Steinacker R, Gosheger G, Bielack SS. Dose intensity of chemotherapy for osteosarcoma and outcome in the Cooperative Osteosarcoma Study Group (COSS) trials. Pediatr Blood Cancer. 2006;47:42–50. doi: 10.1002/pbc.20608. [DOI] [PubMed] [Google Scholar]
- Voute PA, Souhami RL, Nooij M, Somers R, Cortes-Funes H, van der Eijken JW, Pringle J, Hogendoorn PC, Kirkpatrick A, Uscinska BM, van Glabbeke M, Machin D, Weeden S. A phase II study of cisplatin, ifosfamide and doxorubicin in operable primary, axial skeletal and metastatic osteosarcoma. European Osteosarcoma Intergroup (EOI) Ann Oncol. 1999;10:1211–1218. doi: 10.1023/A:1008361612767. [DOI] [PubMed] [Google Scholar]
- Kager L, Zoubek A, Potschger U, Kastner U, Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M, Winkelmann W, Jundt G, Kabisch H, Reichardt P, Jurgens H, Gadner H, Bielack SS. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21:2011–2018. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- Kempf-Bielack B, Bielack SS, Jurgens H, Branscheid D, Berdel WE, Exner GU, Gobel U, Helmke K, Jundt G, Kabisch H, Kevric M, Klingebiel T, Kotz R, Maas R, Schwarz R, Semik M, Treuner J, Zoubek A, Winkler K. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23:559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- Witlox MA, Van Beusechem VW, Grill J, Haisma HJ, Schaap G, Bras J, Van Diest P, De Gast A, Curiel DT, Pinedo HM, Gerritsen WR, Wuisman PI. Epidermal growth factor receptor targeting enhances adenoviral vector based suicide gene therapy of osteosarcoma. J Gene Med. 2002;4:510–516. doi: 10.1002/jgm.308. [DOI] [PubMed] [Google Scholar]
- Graat HC, Wuisman PI, van Beusechem VW, Carette JE, Gerritsen WR, Bras J, Schaap GR, Kaspers GJ, Ogose A, Gu W, Kawashima H, Hotta T. Coxsackievirus and adenovirus receptor expression on primary osteosarcoma specimens and implications for gene therapy with recombinant adenoviruses. Clin Cancer Res. 2005;11:2445–7; author reply 2447-8. doi: 10.1158/1078-0432.CCR-04-2375. [DOI] [PubMed] [Google Scholar]
- Witlox AM, Van Beusechem VW, Molenaar B, Bras H, Schaap GR, Alemany R, Curiel DT, Pinedo HM, Wuisman PI, Gerritsen WR. Conditionally replicative adenovirus with tropism expanded towards integrins inhibits osteosarcoma tumor growth in vitro and in vivo. Clin Cancer Res. 2004;10:61–67. doi: 10.1158/1078-0432.CCR-0609-03. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Fueyo J, Krasnykh V, Reynolds PN, Curiel DT, Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res. 2001;7:120–126. [PubMed] [Google Scholar]
- Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Lamfers ML, Grill J, Dirven CM, Van Beusechem VW, Geoerger B, Van Den Berg J, Alemany R, Fueyo J, Curiel DT, Vassal G, Pinedo HM, Vandertop WP, Gerritsen WR. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62:5736–5742. [PubMed] [Google Scholar]
- Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, Liu TJ, Jiang H, Lemoine MG, Suzuki K, Sawaya R, Curiel DT, Yung WK, Lang FF. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- Bauerschmitz GJ, Kanerva A, Wang M, Herrmann I, Shaw DR, Strong TV, Desmond R, Rein DT, Dall P, Curiel DT, Hemminki A. Evaluation of a selectively oncolytic adenovirus for local and systemic treatment of cervical cancer. Int J Cancer. 2004;111:303–309. doi: 10.1002/ijc.20217. [DOI] [PubMed] [Google Scholar]
- Hemminki A, Kanerva A, Kremer EJ, Bauerschmitz GJ, Smith BF, Liu B, Wang M, Desmond RA, Keriel A, Barnett B, Baker HJ, Siegal GP, Curiel DT. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther. 2003;7:163–173. doi: 10.1016/S1525-0016(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Blackwell JL, Li H, Gomez-Navarro J, Dmitriev I, Krasnykh V, Richter CA, Shaw DR, Alvarez RD, Curiel DT, Strong TV. Using a tropism-modified adenoviral vector to circumvent inhibitory factors in ascites fluid. Hum Gene Ther. 2000;11:1657–1669. doi: 10.1089/10430340050111313. [DOI] [PubMed] [Google Scholar]
- Hemminki A, Belousova N, Zinn KR, Liu B, Wang M, Chaudhuri TR, Rogers BE, Buchsbaum DJ, Siegal GP, Barnes MN, Gomez-Navarro J, Curiel DT, Alvarez RD. An adenovirus with enhanced infectivity mediates molecular chemotherapy of ovarian cancer cells and allows imaging of gene expression. Mol Ther. 2001;4:223–231. doi: 10.1006/mthe.2001.0446. [DOI] [PubMed] [Google Scholar]
- Jia SF, Worth LL, Turan M, Duan Xp XP, Kleinerman ES. Eradication of osteosarcoma lung metastasis using intranasal gemcitabine. Anticancer Drugs. 2002;13:155–161. doi: 10.1097/00001813-200202000-00007. [DOI] [PubMed] [Google Scholar]
- Li X, Jung C, Liu YH, Bae KH, Zhang YP, Zhang HJ, Vanderputten D, Jeng MH, Gardner TA, Kao C. Anti-tumor efficacy of a transcriptional replication-competent adenovirus, Ad-OC-E1a, for osteosarcoma pulmonary metastasis. J Gene Med. 2006;8:679–689. doi: 10.1002/jgm.904. [DOI] [PubMed] [Google Scholar]
- Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Grootenboers MJ, Heeren J, van Putte BP, Hendriks JM, van Boven WJ, Van Schil PE, Schramel FM. Isolated lung perfusion for pulmonary metastases, a review and work in progress. Perfusion. 2006;21:267–276. doi: 10.1177/0267659106073984. [DOI] [PubMed] [Google Scholar]
- developmental and therapeutics program NCI/NIH; rapid access to intervention development http://dtpncinihgov/branches/brb/bdp/bdpraidhtml#bdp_project_445