Abstract
Extracellular type I tumor necrosis factor receptors (TNFR1) are generated by two mechanisms, proteolytic cleavage of TNFR1 ectodomains and release of full-length TNFR1 in the membranes of exosome-like vesicles. Here, we assessed whether TNFR1 exosome-like vesicles circulate in human blood. Immunoelectron microscopy of human serum demonstrated TNFR1 exosome-like vesicles, with a diameter of 27- to 36-nm, while Western blots of human plasma showed a 48-kDa TNFR1, consistent with a membrane-associated receptor. Gel filtration chromatography revealed that the 48-kDa TNFR1 in human plasma co-segregated with LDL particles by size, but segregated independently by density, demonstrating that they are distinct from LDL particles. Furthermore, the 48-kDa exosome-associated TNFR1 in human plasma contained a reduced content of N-linked carbohydrates as compared to the 55-kDa membrane-associated TNFR1 from human vascular endothelial cells. Thus, a distinct population of TNFR1 exosome-like vesicles circulate in human plasma and may modulate TNF-mediated inflammation.
Keywords: Cytokine Receptors, Cell Surface Receptors, Human, Cytokines, Inflammation
Introduction
Tumor necrosis factor (TNF) is an important regulator of inflammation, immune functions, host defense, and apoptosis. TNF signals through two distinct cell surface receptors, the 55-kDa, type I (TNFR1, TNFRSF1A) and the 75-kDa, type II (TNFR2, TNFRSF1B) TNF receptor[1]. Soluble TNF receptors function as TNF-binding proteins that may modulate TNF bioactivity. Two pathways mediate the release of TNFR1 to the extracellular compartment, the proteolytic cleavage of cell surface receptors and the release of exosome-like vesicles[2]. Exosomes are small membrane-enclosed vesicles, 30- to 200-nm in diameter, that correspond to the internal vesicles of endolysosome-related multivesicular bodies and are released from the cell via exocytic fusion with the plasma membrane[3-5]. Exosomes can interact with target cells to mediate regulatory functions in an acellular fashion, as well as facilitate the transfer of integral membrane proteins, as well as mRNAs and microRNAs[6]. Exosomes mediate several important biological functions, including antigen presentation, T cell co-stimulation, and apoptosis. Cells also release the abnormally folded prion protein scrapie (PrPsc) in exosomes, which may facilitate their spread[7], and the exosome-release pathway is utilized by retroviruses, such as HIV[8].
We have previously shown that human vascular endothelial cells (HUVEC) constitutively release a full-length, 55-kDa TNFR1 within exosome-like vesicles that are 20- to 50-nm in diameter, can be pelleted by high-speed centrifugation, sediment to a density of 1.1 g/ml, and are capable of binding TNF[2]. In contrast to exosomes derived from reticulocytes and B lymphocytes, HUVEC-derived TNFR1 exosome-like vesicles do not contain lipid raft microdomains[9, 10]. Therefore, HUVEC-derived TNFR1 exosome-like vesicles appear to be distinct from typical exosomes derived from dendritic cells or lymphocytes based upon their smaller size, lower density, and absence of lipid raft microdomains.
TNFR1 exosome-like vesicles have also been demonstrated in biological fluids, such as bronchoalveolar lavage fluid, while immunoblots have suggested that TNFR1 exosome-like vesicles may be present in human serum[2]. Therefore, we hypothesized that TNFR1 exosome-like vesicles circulate in human blood where they may modulate TNF-mediated inflammatory or immune events. Here, we report on the identification and characterization of 48-kDa TNFR1 exosome-like vesicles in human plasma that co-fractionate with, but are distinct from, LDL particles and display distinct characteristics as compared to plasma- or HUVEC-derived exosome-like vesicles. Furthermore, we demonstrate the utility of gel filtration chromatography for the isolation of exosome-like vesicles from biological fluids.
Materials and Methods
Subjects and Sample Processing
Informed consent was obtained from healthy volunteers prior to the collection of plasma and serum samples for the analysis of exosome-associated proteins, as per protocol 96-H-0100, which was approved by the National Heart, Lung, and Blood Institute Institutional Review Board.
Cells and Reagents
Human umbilical vein endothelial cells (HUVEC) and EGM-2 medium were purchased from Cambrex Bio Science (Walkersville, MD). Peptide N-glycosidase F (PNGase F) and endoglycosidase H (Endo H) were purchased from New England Biolabs (Beverly, MA).
Immunoelectron Microscopy
Samples of human serum were centrifuged at 500 × g for 10 min and 10,000 × g for 30 min to remove cellular debris. Droplets were applied to Formvar-carbon-coated nickel electron microscopy grids and fixed in 4% paraformaldehyde for 15 min. TNFR1 was detected utilizing a rabbit polyclonal antibody (H-271, Santa Cruz Biotechnology) and a secondary donkey anti-rabbit antibody conjugated with 10 nm gold particles (Electron Microscopy Science, Hatfield, PA). A non-immune rabbit IgG was used as a negative control. Vesicular structures were visualized by negative staining with 0.5% uranyl acetate. Images were acquired with a 1200 EX transmission electron microscope (Jeol, Tokyo, Japan).
Fast Protein Liquid Chromatography (FPLC) and Plasma Analysis
Human plasma (400 ul) was filtered through a 0.45 um filter and fractionated by gel filtration chromatography utilizing two Superose 6 HR 10/30 columns (GE Biosciences, Little Chalfont, United Kingdom) connected in series[11]. Total cholesterol (TC), phospholipids, and triglycerides were analyzed using commercially-available enzyme kits (Sigma, St. Louis, MO).
Immunoblotting
Immunoblotting was performed as previously described, with minor modifications, utilizing the H-5 murine monoclonal antibody that reacts with the TNFR1 extracellular domain (Santa Cruz Biotechnology, Santa Cruz, CA)[2]. Invitrolon PVDF membranes (Invitrogen, Carlsbad, CA) were utilized for Western blots. Additional antibodies included, LAMP-1 (B-D Biosciences, San Diego, CA); LAMP-2, ICAM-1, transferrin receptor (Santa Cruz Biotechnology); apolipoprotein B-100 (ApoB-100) (Chemicon, Temecula, CA); apolipoprotein E (ApoE) and A-1 (ApoA-1) (Abcam, Cambridge, MA). For repeated blotting, membranes were stripped using the Re-blot Western Blot Recycling Kit (Chemicon International, Temecula, CA) and reacted with antibodies following the manufacturer’s instructions.
Rate Zonal Centrifugation Through Continuous Sucrose Gradients
Purified LDL fractions (1 mg) were overlaid on a continuous sucrose gradient (0.2 M to 2 M in 20 mM Tris, pH 8.0) and centrifuged at 175,000 × g for 16 hrs. Fractions (0.5 ml) were collected from the bottom of the gradient and proteins were quantified. Samples of proteins were precipitated with 10% trichloroacetic acid, as necessary, and analyzed by immunoblotting. A Palm Abbe Digital Refractometer (Misco) was used for densitometry.
Results
Human Blood Contains 48-kDa TNFR1 Exosome-like Vesicles
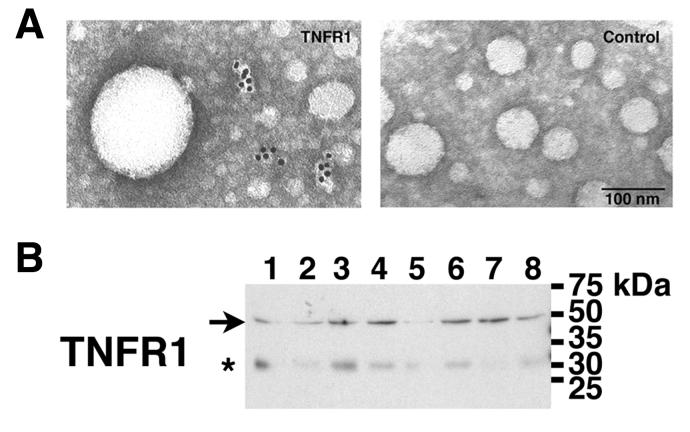
Immunoelectron microscopy was performed to assess whether TNFR1 containing vesicles are present in human blood. TNFR1 exosome-like vesicles were detected in serum as irregularly shaped particles, with a diameter of 27- to 36-nm along their short axis (Figure 1A). Western blots were next performed to characterize further TNFR1 in human blood. As shown in Figure 1B, human plasma from 8 healthy volunteers contained a 48-kDa TNFR1, which is consistent with a membrane-associated receptor, such as an exosome-like vesicle. A minor 30-kDa band, which was detected by both the H-5 anti-TNFR1 and the IgG2b isotype control antibodies, represented non-specific binding.
Figure 1. Characterization of TNFR1 Exosome-like Vesicles in Human Blood by Electron Microscopy and Western Blotting.
Panel A. TNFR1 exosome-like vesicles in human serum were visualized by immunogold electron microscopy using a polyclonal rabbit antibody directed against the TNFR1 extracellular domain. No immunogold labeling was detected when rabbit IgG was utilized as a control. Panel B. Samples (2 ul) of human plasma from 8 healthy volunteers that had been filtered through a 0.45 um filter were separated by SDS-PAGE, transferred to PVDF membranes, and reacted with the H-5 monoclonal antibody directed against the TNFR1 extracellular domain. The arrow indicates the 48-kDa TNFR1 that was only detected by the H-5 anti-TNFR1 monoclonal antibody. The asterisk indicates a 30-kDa band that was detected by both the H-5 anti-TNFR1 and the IgG2b isotype control antibodies (not shown) and represented non-specific binding. Lane numbers correspond to samples from individual patients.
The 48-kDa TNFR1 Present in Human Plasma Co-segregates with LDL Particles
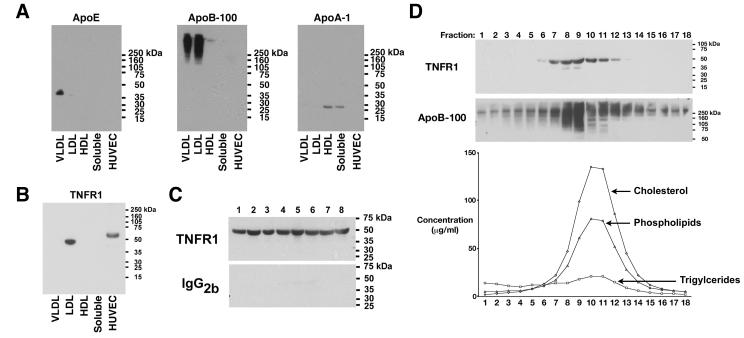
We reasoned that if the 48-kDa TNFR1 is associated with TNFR1 exosome-like vesicles in human plasma, then it should segregate in a fraction that is distinct from soluble proteins on the basis of size. Thus, plasma from healthy research volunteers was separated by gel filtration into fractions that corresponded to lipoprotein particles. As expected, apolipoprotein E (ApoE) preferentially associated with the VLDL fraction, apolipoprotein A-1 (ApoA-I) localized to the HDL fraction, and apolipoprotein B-100 (ApoB-100) was found in the VLDL and LDL fractions (Figure 2A). Interestingly, the 48-kDa TNFR1 in human plasma co-segregated with the LDL fraction (Figure 2B). TNFR1 was not detected in the VLDL or HDL fractions, or with the soluble proteins. The cell-associated 55-kDa TNFR1 present in HUVEC lysates is shown for comparison. Experiments were next performed to confirm that the 48-kDa TNFR1 co-segregated with the LDL fraction of human plasma. As shown in Figure 2C, Western blots performed on LDL fractions from 8 individual normal volunteers demonstrated the presence of the 48- kDa TNFR1 in all samples.
Figure 2. Identification of a 48-kDa TNFR1 that Co-Segregates with the LDL Fraction of Human Plasma.
Panel A. Samples (120 ug) of FPLC fractions that corresponded to the size of VLDL, LDL, HDL, and soluble proteins, as well as HUVEC cell lysates (60 ug) were immunoblotted and reacted with antibodies against apolipoprotein E (ApoE), apolipoprotein B-100 (ApoB-100), or apolipoprotein A-1 (ApoA-1). Panel B. Samples (120 ug) of FPLC fractions and HUVEC cell lysates (60 ug) were immunoblotted and reacted with the H-5 murine monoclonal antibody directed against the TNFR1 extracellular domain. Panel C. LDL fractions from 8 healthy volunteers were immunoblotted and reacted with the H-5 anti-TNFR1 monoclonal antibody or an IgG2b isotype control. Lane numbers correspond to pooled LDL fractions from individual patients. Panel D. Proteins from individual FPLC fractions that corresponded to the LDL peak were precipitated with 10% trichloroacetic acid, immunoblotted, and reacted with the H-5 anti-TNFR1 monoclonal antibody. The PVDF membrane was stripped and re-probed with the ApoB-100 antibody. This blot is representative of three independent experiments that demonstrated the same result. Cholesterol, triglyceride, and phospholipids concentrations (ug/ml) in each FPLC fraction are shown below.
Additional experiments were performed to confirm that the 48-kDa TNFR1 present in human plasma co-segregates with the LDL fraction. Western blots of individual LDL fractions generated by gel filtration chromatography demonstrated that the 48-kDa TNFR1 co-segregated with ApoB-100, as well as cholesterol, phospholipids, and triglycerides (Figure 2D). The co-segregation of the 48-kDa TNFR1 and LDL by gel filtration chromatography suggested that the diameter of TNFR1 exosome-like vesicles are similar to LDL particles.
TNFR1 Exosome-like Vesicles and LDL Particles Represent Distinct Populations
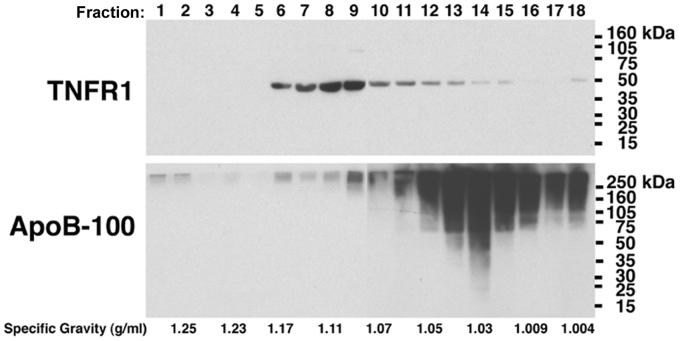
Experiments were next performed to assess whether the 48-kDa TNFR1 is a component of LDL particles or, alternatively, represents a distinct population of plasma vesicles. We reasoned that if TNFR1 exosome-like vesicles represent a unique species of circulating microvesicles that are distinct from LDL particles, then it should be possible to differentiate the two populations on the basis of density, as LDL particles typically have a density ranging from 1.02 to 1.06 g/ml, whereas TNFR1 exosome-like vesicles from human vascular endothelial cells have a peak density of 1.11 g/ml[2, 12]. As shown in Figure 3, LDL fractions from healthy volunteers were subjected to rate zonal centrifugation through continuous sucrose gradients and the density of LDL particles was determined using ApoB-100 as a marker. LDL particles sedimented to a peak density of 1.03 g/ml, which was in contrast to TNFR1 exosome-like vesicles that sedimented to a peak density of 1.09 to 1.11 g/ml. The demonstration that TNFR1 exosome-like vesicles and LDL particles segregate independently based upon density is consistent with the conclusion that TNFR1 exosome-like vesicles and LDL particles represent two distinct populations of circulating vesicular structures in human plasma.
Figure 3. Rate Zonal Centrifugation of the LDL Fraction of Human Plasma Through Continuous Sucrose Gradients.
A sample containing 1 mg of LDL proteins was layered on top of a continuous sucrose gradient (0.2 M to 2 M in 20 mM Tris, pH = 8) and centrifuged at 175,000 × g for 16 hrs. Fractions (0.5 ml) were collected from the bottom, proteins were precipitated with 10% trichloracetic acid, separated by SDS-PAGE, transferred to PVDF membranes, and reacted with antibodies against TNFR1. PVDF membranes were stripped and re-probed with antibodies against ApoB-100. Lane numbers correspond to the fractions collected. This blot is representative of two independent experiments that demonstrated the same result.
Typical Exosome Membrane Proteins Co-Segregate with the HDL Fraction of Human Plasma
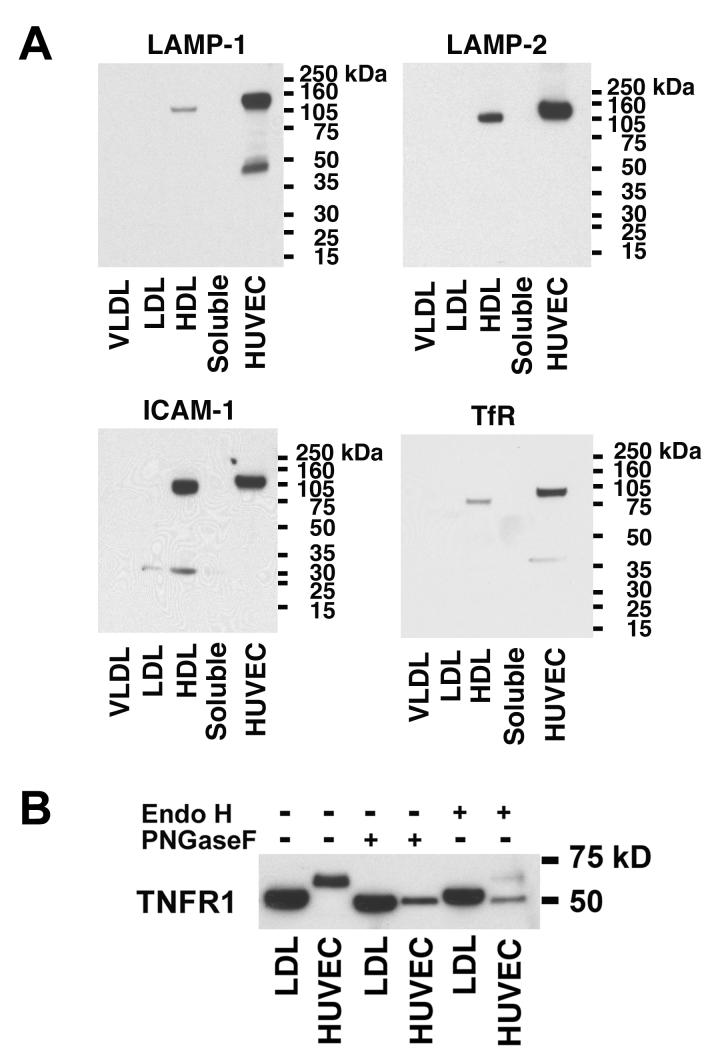
We next assessed whether membrane proteins that are known to be present in exosomes, such as LAMP-1, LAMP-2, ICAM-1, and transferrin receptor (TfR), also co-segregate with the 48-kDa TNFR1 exosome-like vesicles in the LDL fraction of human plasma[13-16]. As shown in Figure 4A, LAMP-1, LAMP-2, ICAM-1 and TfR co-segregated with the HDL fraction and were not detected in the LDL fraction. This result demonstrates that plasma-derived TNFR1 exosome-like vesicles do not co-segregate with these exosome-associated proteins and suggests that TNFR1 exosome-like vesicles represent a distinct population.
Figure 4. Panel A. Exosome Markers Co-localize with the HDL Fraction of Human Plasma.
Samples (120 ug) of FPLC fractions that corresponded to the size of VLDL, LDL, HDL, and soluble proteins, as well as HUVEC cell lysates (60 ug) were immunoblotted and reacted with antibodies against LAMP-1, LAMP-2, ICAM-1, or transferrin receptor. This blot is representative of two independent experiments that demonstrated the same result. Panel B. TNFR1 Exosome-like Vesicles Contain Reduced Quantities of N-linked Carbohydrates. Samples of LDL fractions (120 ug) and HUVEC lysates (60 ug) were incubated with 12.5 units/ul of PNGase F or Endo H for 1-h at 37° C, separated by SDS-PAGE, transferred to PVDF membranes, and reacted with antibodies against TNFR1. This blot is representative of two independent experiments that demonstrated the same result.
Reduced N-linked Glycosylation of TNFR1 Exosome-like Vesicles from Human Plasma as Compared to Cell-associated TNFR1
Deglycosylation experiments were performed to characterize further the plasma-derived 48-kDa TNFR1 present in exosome-like vesicles. TNFR1 has three N-glycosylation sites and is expected to be digested by N-glycosidase F (PNGase F)[17]. Consistent with this, the TNFR1 present in HUVEC lysates was sensitive to digestion by PNGase F (Figure 4B). The TNFR1 present in the LDL fraction of human plasma was also sensitive to digestion by PNGase F, but resulted in only a slight reduction in molecular weight. Furthermore, TNFR1 from both LDL and HUVEC displayed similar molecular weights following PNGase F treatment. In contrast, the TNFR1 present in LDL was resistant to digestion by endoglycosidase H (EndoH), whereas the TNFR1 present in HUVEC lysates was EndoH-sensitive. The similar molecular weights following PNGase F digestion of TNFR1 in the LDL fraction of human plasma and HUVEC lysates suggest that the 48-kDa TNFR1, which is present in plasma-derived exosome-like vesicles, contains a reduced content of N-linked carbohydrate moieties.
Discussion
Soluble forms of TNFR1, which can bind TNF and modulate TNF activity, play an important role in regulating inflammatory disease. Here we show that a distinct population of TNFR1 exosome-like vesicles circulate in human plasma. Furthermore, we identified gel filtration chromatography as a novel method for the isolation of TNFR1 exosome-like vesicles from plasma based upon the similar size of TNFR1 exosome-like vesicles and LDL particles. Consistent with this conclusion, immunoelectron microscopy revealed that TNFR1 exosome-like vesicles have a diameter of approximately 27- to 36-nm along their short axis, which is similar to, but slightly larger than LDL particles, which typically range from 18- to 23-nm size, although larger LDL particles of 27- to 28.5-nm have been described[12, 18, 19]. Despite the finding that TNFR1 exosome-like vesicles co-fractionated with LDL particles on the basis of size, they represented distinct particles based upon independent segregation characteristics on continuous sucrose gradients.
TNFR1 exosome-like vesicles in human plasma appear to have different characteristics than previously described plasma-derived exosome-like vesicles, which expressed tetraspanins (CD63 (LAMP-3), CD9, and CD81), MHC class I and II, LAMP-2, and CD41a (GPIIb), a platelet-specific marker[20]. These plasma-derived exosome-like vesicles floated to peak specific gravities of 1.21 to 1.28 g/ml on sucrose gradients and appeared to have a diameter of 50-to 90-nm by immunoelectron microscopy[20]. In contrast, we found that TNFR1 exosome-like vesicles from human plasma have a peak density of 1.09 to 1.11 g/ml and a diameter of 27- to 36-nm. In addition, TNFR1 exosome-like vesicles in plasma did not co-segregate with other proteins that are typically expressed by exosomes, such as LAMP-1, LAMP-2, ICAM-1 and TfR, which instead co-segregated with the HDL fraction of human plasma[3, 4, 16, 21]. Taken together, these data suggest that TNFR1 exosome-like vesicles represent an unique population of vesicles that is distinct from previously described plasma-derived exosome-like vesicles or exosomes derived from immunological cells, such as B cells or dendritic cells. Furthermore, this result suggests that the biogenesis of TNFR1 exosome-like vesicles may differ from that of typical exosomes.
We have previously reported that HUVEC constitutively release a 55-kDa TNFR1 within the membranes of exosome-like vesicles, which is in contrast to the 48-kDa TNFR1 exosome-like vesicles that we identified in human plasma[2]. We reasoned that the difference in size between HUVEC-associated TNFR1 and TNFR1 exosome-like vesicles in human plasma might be a result of differential glycosylation, as TNFR1 contains 3 potential N-glycosylation sites[17]. As expected, TNFR1 present in HUVEC lysates was sensitive to digestion with either PNGase F or Endo H, whereas the TNFR1 present in the LDL fraction of human plasma was only sensitive to PNGase F. This is consistent with the ability of EndoH to digest proteins residing in the endoplasmic reticulum and cis-Golgi apparatus that contain high mannose N-linked oligosaccharides[17]. Furthermore, the molecular weight of the deglycosylated TNFR1 from HUVEC and the LDL fraction of human plasma was similar following PNGase F digestion. This suggests that the reduced size of the 48-kDa TNFR1 exosome-like vesicles from human plasma as compared with the 55-kDa TNFR1 from HUVEC cell lysates represents a reduced content of N-linked carbohydrates.
In conclusion, we have demonstrated that plasma from healthy human volunteers contains 48-kDa TNFR1 exosome-like vesicles that fractionate with, but are distinct from, LDL particles, and display unique characteristics as compared to plasma- or HUVEC-derived exosome-like vesicles. These results show that TNFR1 exosome-like vesicles circulate in human plasma where they may modulate TNF-mediated inflammatory or immune events.
Acknowledgements
The authors thank Drs. Joel Moss, Martha Vaughan, and Vincent Manganiello for their helpful discussions and critical review of the manuscript. We acknowledge Dr. Zu-Xi Yu of the Pathology Core Facility, NHLBI for his valuable expertise regarding the immunoelectron microscopy experiments. This research was funded by the Division of Intramural Research, NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- [2].Schall TJ, Lewis M, Koller KJ, Lee A, Rice GC, Wong GHW, Gatanaga T, Granger GA, Lentz R, Raab H, Kohr WJ, Goeddel DV. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990;61:361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- [3].Hawari FI, Rouhani FN, Cui X, Yu ZX, Buckley C, Kaler M, Levine SJ. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci U S A. 2004;101:1297–1302. doi: 10.1073/pnas.0307981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(Pt 19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- [5].Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, Biogenesis and Function. Nature Reviews Immunology. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- [6].van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem (Tokyo) 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- [7].Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- [8].Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, Raposo G. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid raft-associated protein sorting in exosomes. Blood. 2003;102:4336–4344. doi: 10.1182/blood-2003-03-0871. [DOI] [PubMed] [Google Scholar]
- [10].Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278:10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- [11].Applebaum-Bowden D, Kobayashi J, Kashyap VS, Brown DR, Berard A, Meyn S, Parrott C, Maeda N, Shamburek R, Brewer HB, Jr., Santamarina-Fojo S. Hepatic lipase gene therapy in hepatic lipase-deficient mice. Adenovirus-mediated replacement of a lipolytic enzyme to the vascular endothelium. J Clin Invest. 1996;97:799–805. doi: 10.1172/JCI118479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–1379. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- [13].Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD, Jr., Thomson AW. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- [14].Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- [15].Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- [16].Kohne C, Johnson A, Tom S, Peers DH, Gehant RL, Hotaling TA, Brousseau D, Ryll T, Fox JA, Chamow SM, Berman PW. Secretion of glycosylation site mutants can be rescued by the signal/pro sequence of tissue plasminogen activator. J Cell Biochem. 1999;75:446–461. [PubMed] [Google Scholar]
- [17].Barzilai N, Atzmon G, Schechter C, Schaefer EJ, Cupples AL, Lipton R, Cheng S, Shuldiner AR. Unique lipoprotein phenotype and genotype associated with exceptional longevity. Jama. 2003;290:2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- [18].Teerlink T, Scheffer PG, Bakker SJ, Heine RJ. Combined data from LDL composition and size measurement are compatible with a discoid particle shape. J Lipid Res. 2004;45:954–966. doi: 10.1194/jlr.M300521-JLR200. [DOI] [PubMed] [Google Scholar]
- [19].Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- [20].Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- [21].Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- [22].Islam A, Adamik B, Hawari FI, Ma G, Rouhani FN, Zhang J, Levine SJ. Extracellular TNFR1 release requires the calcium-dependent formation of a nucleobindin 2-ARTS-1 complex. J Biol Chem. 2006;281:6860–6873. doi: 10.1074/jbc.M509397200. [DOI] [PubMed] [Google Scholar]