Abstract
The symptom provocation paradigms generally used in neuroimaging studies of posttraumatic stress disorder (PTSD) have placed high demands on emotion processing but lacked cognitive processing, thereby limiting the ability to assess alterations in neural systems that subserve executive functions and their interactions with emotion processing. Thirty-nine veterans from Iraq and Afghanistan underwent functional MR imaging while exposed to emotional combat-related and neutral civilian scenes interleaved with an executive processing task. Contrast activation maps were regressed against PTSD symptoms as measured by the Davidson Trauma Scale. Activation for emotional compared to neutral stimuli was highly positively correlated with level of PTSD symptoms in ventral frontolimbic regions, notably the ventromedial prefrontal cortex, inferior frontal gyrus, and ventral anterior cingulate gyrus. Conversely, activation for the executive task was negatively correlated with PTSD symptoms in the dorsal executive network, notably the middle frontal gyrus, dorsal anterior cingulate gyrus, and inferior parietal lobule. Thus, there is a strong link between the subjectively-assessed behavioral phenomenology of PTSD and objective neurobiological markers. These findings extend the largely symptom provocation-based functional neuroanatomy to provide evidence that interrelated executive and emotional processing systems of the brain are differentially affected by PTSD symptomatology in recently deployed war veterans.
Keywords: fMRI, executive processing, emotion processing, vmPFC, dlPFC, combat stress
1. Introduction
The mental health consequences of post-9/11 military deployments to Iraq and Afghanistan have garnered much attention (Miller, 2006). Large-scale studies have found significant deployment-associated neuropsychiatric morbidity, with posttraumatic stress disorder (PTSD) rates reported at 18-20% (Hoge et al., 2004), and cognitive impairment, notably for sustained attention (Vasterling et al., 2006). PTSD symptoms include the classic triad of (i) re-experiencing or re-living symptoms (ii) emotional numbing or avoidance, and (iii) hypervigilance or hyperarousal (First et al., 1997). PTSD is also associated with several domains of cognitive impairment in executive processing, attention, verbal declarative memory, and autobiographical memory, (Buckley et al., 2000; Danckwerts and Leathem, 2003).
Symptom provocation paradigms involving script-driven imagery, or viewing trauma-related or other negatively-valenced emotional scenes, have been used to characterize brain activation patterns, particularly in frontolimbic regions often implicated in the emotion processing dysfunction of PTSD. For instance, when PTSD patients were compared to controls while viewing trauma-related and neutral pictures, patients had greater activation for combat pictures in the amygdala, cingulate cortex, occipitotemporal cortices, and inferior frontoparietal regions (Shin et al., 1997; Bremner et al., 1999b; Yang et al., 2004). In a comparison of fearful to neutral faces, PTSD patients had greater activation to fearful faces in the amygdala, dorsomedial prefrontal cortex (PFC), and posterior cingulate (Rauch et al., 2000; Shin et al., 2005; Williams et al., 2006). Studies using script-driven imagery have shown varied regional response patterns in frontolimbic regions, including increased activation in PTSD patients in the anterior temporal pole and inferior PFC but reduced activation in other regions such as the anterior cingulate, insula, and amygdala (Shin et al., 1999; Lanius et al., 2001; Lanius et al., 2002; Lanius et al., 2003a). Results in the orbitofrontal cortex show higher activity (Shin et al., 1999) whereas the ventromedial PFC shows lower activity (Lanius et al., 2001; Shin et al., 2004; Shin et al., 2005).
In addition to emotional dysfunction, PTSD is also associated with reduced performance on executive and sustained attention tasks such as the Continuous Performance, Trails B, Digit Span, and Digit Symbol, (Vasterling et al., 1998; Jenkins et al., 2000; Sachinvala et al., 2000). However, few neuroimaging studies have directly tested executive deficits in PTSD or the interplay of affective and cognitive processing. A study of the emotional counting Stroop for combat versus neutral words found lower rostral anterior cingulate activation cortex (ACC) in the PTSD group (Shin et al., 2001). A subsequent study confirmed lower ACC activation in the PTSD group for the emotional Stroop condition, and lower activation in inferior parietal lobule, visual association cortex, and precuneus for the classic Stroop condition (Bremner et al., 2004). An auditory continuous performance task study of patients with comorbid cocaine and alcohol abuse also found lower activity in rostral ACC (Semple et al., 2000). Converging support across symptom provocation studies combined with neuropsychological assessment, posit an inability of the PFC to inhibit a hyperresponsive limbic system in PTSD (Bremner et al., 1999b; Rauch et al., 2000; Shin et al., 2001).
Of particular interest is the relationship between severity of PTSD symptoms and central neural markers of executive and emotional functions. Positive correlations have been reported for a trauma script condition with amygdala and anterior hippocampus activation and negative correlations were observed in the medial frontal gyrus and the ACC activity (Shin et al., 2004). Positive correlations for fearful vs. happy faces were also found in the amygdala (Rauch et al., 2000; Shin et al., 2005). Finally, a negative correlation was reported between re-experiencing symptoms and anterior cingulate activity (Williams et al., 2006).
We have previously reported on the interplay of emotion processing and executive function in studies of healthy subjects using an emotional oddball paradigm (Yamasaki et al., 2002; Fichtenholtz et al., 2004; Wang et al., 2005) in which detection of a target geometric shape is interrupted by occasional task-irrelevant emotional and neutral distracters. Our earlier work demonstrated that executive and emotional functions are dissociated into parallel dorsal and ventral streams, respectively, that extend into the PFC (Yamasaki et al., 2002; Fichtenholtz et al., 2004; Wang et al., 2005). Reciprocal engagement of these streams showed relative deactivation of the ventral frontolimbic regions during attentional target detection and relative deactivation of the dorsal frontoparietal regions during emotional picture processing. This reciprocal relationship between dorsal and ventral processing of executive and emotional functions may be biased towards emotional processing in patients with anxiety disorders (Drevets and Raichle, 1998), similar to that found in depression (Mayberg, 1997). Thus, our earlier work supports a model of emotion and attention processing that is well suited for investigating PTSD.
The aim of the present study was to investigate the relationship of executive and emotion processing regions with severity of PTSD symptoms, and to assess whether the reciprocal relationship between activity in emotion and executive processing systems found in healthy adults (Drevets and Raichle, 1998; Yamasaki et al., 2002) would be a model that extends to subjects with PTSD symptoms. In the present study, executive function block involved making a choice response to each of a short series of geometric shapes. The symptom provocation blocks involved presentation of combat-related pictures. Control blocks of matched civilian (neutral) pictures were also included. The sensory and motor aspects of this task were relatively simple and easy to accommodate in patients during fMRI scanning.
Broadly, we expected that based on the model of dorsal-ventral segregation observed in healthy subjects, that patients with PTSD symptoms would show an imbalance towards the ventral processing of combat scenes at the expense of reduced dorsal processing of executive attention. Furthermore, we predicted that this pattern would be modulated by PTSD symptom severity such that activation for emotional stimuli in ventral frontolimbic regions during symptom provocation would be positively correlated with PTSD symptoms, whereas activation for executive processing in dorsal regions during executive processing would be negatively correlated with PTSD symptoms. Secondarily, we predicted deactivation in dorsal regions during emotion processing, and in ventral regions during executive processing. If supported, these reciprocal interactions between processing streams and their relationship to symptomatology would advance knowledge regarding the large-scale neural systems that mediate cognitive-affective symptoms of PTSD.
2. Methods
2.1 Participants
Forty-six participants (n = 46) recently returning [mean ± standard deviation; 29 ± 26 months] from deployments to post-9/11 military conflicts who lacked a history of neurological disorders or major medical conditions were enrolled in the study. Thirty-nine participants (n = 39) completed the fMRI procedures and a picture rating task immediately following the scan. Seven subjects were unable to complete the fMRI procedures secondary to discomfort in the scanner but completed the picture rating task. Subjects provided written informed consent for procedures approved by the Institutional Review Boards at Duke University and the Durham VA Medical Center. Participants were recruited from a large registry of post-9/11 military service members and veterans [Durham VA Medical Center, Durham, North Carolina USA]. Subjects entering the registry completed a neuropsychiatric self-assessment battery which included the Davidson Trauma Scale (DTS) (Davidson et al., 1997), the Beck Depression Inventory (BDI) (Beck, 1961), Combat Exposure Scale (Keane, 1989), Traumatic Life Events Questionnaire (Kubany et al., 2000), Connor-Davidson Resilience Scale (Connor and Davidson, 2003), Drug Abuse Screening Test (Skinner, 1982), and the Alcohol Use Disorders Identification Test (Saunders et al., 1993). The DTS was re-administered immediately prior to scanning to allow for possible change in PTSD symptoms showed lower average DTS scores at the pre-scan assessment than registry intake [paired t(38) = 2.5, P < 0.02]. Participants ranged in age from 21 to 54 (mean ± standard deviation; 35.9 ± 9.4) with older participants tending towards lower DTS scores [r = -0.32, F(1,38) = 4.3, P = 0.04]. The DTS has a short administration time (under 10 minutes), high test-retest reliability, internal consistency, convergent and divergent validity, predictive validity, and responsiveness evidence (Davidson et al., 1997). Eighteen participants had scores at or above a cut-score of 40, previously shown to confirm a DSM-IV diagnosis of PTSD, and 23 participants met or exceeded a cut-score of 27, suggesting post-trauma psychopathology and likelihood to meet DSM-IV criteria for PTSD (Cuthbertson et al., 2004). Ten participants (mean DTS = 65.4 ± 23.7) were taking antidepressant medication that included selective serotonin reuptake inhibitors and/or norepinephrine dopamine modulators. Participants had mild to moderate depression as assessed by the BDI [17.5 ± 37.4]. Full demographic and clinical characteristics of the sample are reported in Table 1.
Table 1. Demographic and Clinical Characteristics of Subject Sample *.
| Characteristic | n = 39 | Correlation with symptom severity (DTS) |
|---|---|---|
| Age (years), [Standard Deviation] | 35.9 [9.4] | r =-0.32, F(1,38)=4.3, P=0.04 |
| Age Range [youngest : oldest] | [21: 54] | - |
| Gender, No. (%) of females | 7 (17.9) | t(38)=1.3, P=0.20 |
| Handedness, No. (%) right-handed | 37 (94.9) | - |
| Handedness, No. (%) ambidextrous | 1 (2.6) | - |
| Ethnicity, No. (%) of Caucasian subjects | 18 (46.2) | t(38)=0.29, P=0.77 |
| Education (years), [std dev] | 14.2 [2.6] | r = 0.11, F(1,38)=0.43, P=0.52 |
| Davidson Trauma Scale [std dev] | 39.4 [32.6] | - |
| Combat Exposure Scale [std dev] | 14.5 [11.2] | r = 0.30, F(1,38)=3.7, P=0.06 |
| Traumatic Life Events Questionnaire (early life sub-score) [std dev] | 5.5 [7.2] | r = 0.1, F(1,38)=0.34, P=0.56 |
| Connor-Davidson Resilience Scale, [std dev] | 74.7 [14.6] | r =-0.33, F(1,38)=4.4, P=0.04 |
| Beck Depression Inventory [std dev] | 17.5 [37.4] | r =0.53, F(1,38)=14.8, P<0.0005 |
| Alcohol Use Disorders Identification Test [std dev] | 5.9 [6.7] | r = 0.20, F(1,38)=1.5, P=0.23 |
| Drug Abuse Screening Test, [std dev] | 1.0 [2.0] | r = 0.22, F(1,38)=1.9, P=0.17 |
| Antidepressant Medication, No. (%) prescribed † | 10 (25.6) | t(38)=3.3, P<0.005 |
| DTS of Subjects taking medication, [std dev] | 65.4 [23.7] | - |
Data values represent means except where indicated otherwise.
Antidepressant medications taken were either selective serotonin reuptake inhibitors (SSRI) or norepinephrine dopamine modulators (NDM, Bupropion).
2.2 Stimulus Presentation
Stimuli compiled from internet searches and photo collections of returning soldiers (not participating in the present study) were subdivided into four categories: (i) emotional stimuli depicting combat-related scenes from Iraq and Afghanistan, (ii) neutral civilian scenes matched for overall visual complexity, luminance, presence of human figures/faces, and chromatic features, (iii) baseline images that were digitally scrambled from the emotional and neutral pictures, and (iv) executive stimuli consisting of monochromatic circles or squares. The scrambled pictures had the same average spatial frequency and luminance as the meaningful pictures. Stimuli were presented for 2 sec, with six stimuli from a single category forming a 12-sec block of that category that alternated in a repeating pattern of baseline-executive-baseline-neutral-baseline-combat. Each run lasted 6 min and 24 sec, for a total functional scan time of 52 min. Each block type (emotional, neutral, executive) occurred five times per run for eight runs for 40 total events. Subjects provided right-handed button presses during the executive condition based on a judgment of circle versus square stimuli. Subjects also responded with button presses during the emotional, neutral, and baseline conditions that did not involve a judgment but was merely an incidental task to confirm focus of attention. Subjects were asked to provide accurate responses but were not instructed to optimize response time. Stimuli were displayed using GPF software [Duke-UNC Brain Imaging and Analysis Center] on a liquid crystal display projector system.
2.3 fMRI Procedures
Scans were obtained from a General Electric 3 Tesla Signa EXCITE system equipped with high-power 40-mT/m gradients at 150 T/m/s and 8 channel head coil. Head movement was restricted using foam cushions and Velcro straps. The anterior (AC) and posterior commissures (PC) were identified in the mid-sagittal slice of a localizer series, and 34 contiguous slices were prescribed parallel to the AC-PC plane prior to obtaining 3D high-resolution anatomical images using spoiled gradient-recalled acquisition pulse sequence (TR = 500 ms; TE = 31 ms; image matrix = 256 × 256 × 68; slice thickness = 1.9 mm, in-plane resolution = 0.9375 mm2; T1 inversion time = 300 ms). Functional images were acquired using echo-planar imaging sequence (TR = 2000 ms, TE = 30 ms, FA = 60°, in-plane resolution = 3.75 mm2; slice thickness = 3.8 mm; image matrix = 64 × 64 × 34) sensitive to blood oxygen-level dependent (BOLD) contrast. Before each scan, four images were acquired and discarded to allow longitudinal magnetization to reach equilibrium.
2.4 Picture Rating Procedure
Following the scan, 41 participants viewed and rated visual stimuli for arousal and valence on a desktop computer. Five subjects who were able to complete the fMRI portion but were markedly distressed by the combat pictures and elected not to participate in the picture rating task. Ratings were provided using the Self Assessment Mannequin on a 9-point arousal (1 = lowest; 9 = highest) and valence (1 = most negative; 9 = most positive) scale (Lang et al., 2005). Subjects were provided 3 sec to view each stimulus and were permitted up to 8 sec following onset of stimulus presentation to provide responses. Subjects rated 392 pictures subdivided into seven approximately equal sets with up to a 10 minute break between sets to reduce subject fatigue.
2.5 Data Analysis
Functional data sets were analyzed using FSL version 3.3.5 [Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford University, U.K.] (Smith et al., 2004). Paradigm timing files were converted to FSL compatible format and NIFTI image data files were generated. Preprocessing (first-level analysis) was applied to individual subjects' data in the following steps: (i) brain extraction for non-brain removal (Smith, 2002), (ii) motion correction using MCFLIRT (Jenkinson et al., 2002), (iii) spatial smoothing using a Gaussian kernel of FWHM 5 mm, (iv) mean-based intensity normalization of all volumes by the same factor, and (v) high-pass filtering (Jenkinson et al., 2002; Smith et al., 2004). Functional images of each subject were co-registered to structural images in native space, and structural images were normalized to structural Talairach standard images, defined by the MNI standard brain supplied with FSL. The same transformation matrices used for structural-to-standard transformations were then used for functional-to-standard space transformations of co-registered functional images. All registrations were carried out using an intermodal registration tool based on the correlation ratio (Jenkinson and Smith, 2001). Pre-whitening or voxel-wise temporal autocorrelation was estimated and corrected using FMRIB's Improved Linear Model (FILM) (Woolrich et al., 2001). Onset times of events were used to model a signal response containing a regressor for each response type, which was convolved with a γ function to model the hemodynamic response. Model fitting generated whole brain images of parameter estimates and corresponding variance, representing average signal change above baseline (activation; positive regressor) and below baseline (deactivation; negative regressor) during each condition (emotional, neutral, executive, emotional > neutral). Contrast images were fed into a second-level statistical analysis to examine effects for the overall group as a function of symptom severity in the form of demeaned DTS scores. Given the high correlation between DTS and BDI measures, we repeated the preceding step using demeaned BDI scores. Activation and deactivation images of main effects during each condition were produced by calculating an average map based on all individual maps for each run, in a mixed effects higher level analysis using Bayesian estimation techniques, FMRIB Local Analysis of Mixed Effects (FLAME) (Beckmann et al., 2003; Woolrich et al., 2004) with conservative cluster mean threshold of Z > 2.3 and a cluster-corrected significance threshold of P < 0.05 (Worsley et al., 1992; Friston et al., 1993; Forman et al., 1995).
We assessed BOLD signal recovery by examining raw functional images thresholded at 50% of the maximal signal. This resulted in signal dropout due to susceptibility artifact throughout most of the region of the amygdala except the most superior and lateral aspect. However, there was no evidence of appreciable signal dropout in the ventromedial PFC and orbitofrontal PFC, and minimal signal dropout in the hippocampus. The analysis was conducted post hoc on the basis of expected amygdalar activation during the emotion condition.
2.6 Statistics
Mixed effects analysis as implemented in FSL feeds variance in the data and in parameter estimates from individual fMRI runs and subjects to group-level analysis leading to results that are representative of the general population. Our analysis was based on correlations of symptom severity with activation for the contrasts of interest. Voxels were identified using FSL mixed-effects analysis to regress demeaned DTS and BDI scores with parameter estimates. Cluster corrected mean Z-scores were used to generate regression plots for figures 3 and 4. This method is statistically more powerful then comparing two participant groups based on a median split or a categorical grouping such as PTSD versus non-PTSD.
Figure 3.
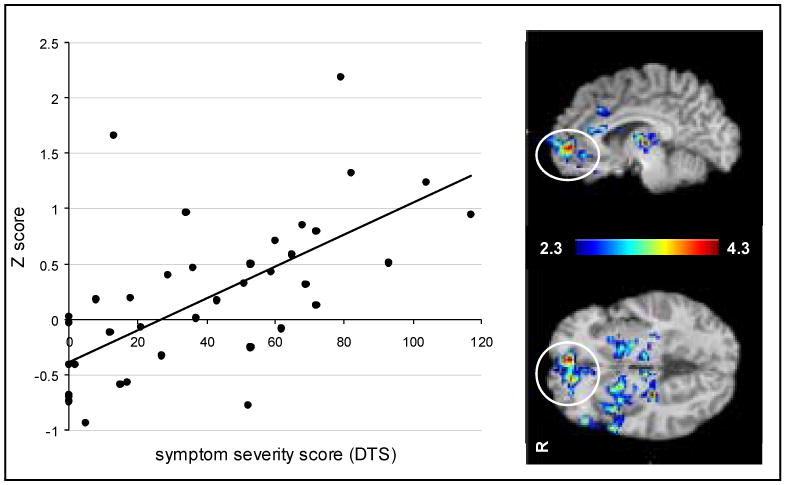
Mixed effects analysis with FSL identified clusters of voxels including the ventromedial prefrontal cortex and the ventral anterior cingulate cortex that were highly correlated with PTSD symptoms as measured by the Davidson Trauma Scale (DTS). The mean Z-score in the cluster for subjects (n=39) were regressed against DTS scores [r = .64, F(1, 40) = 25.7, p = .00001]. Activation maps represent voxel-wise correlation of Z-score with DTS score.
Figure 4.
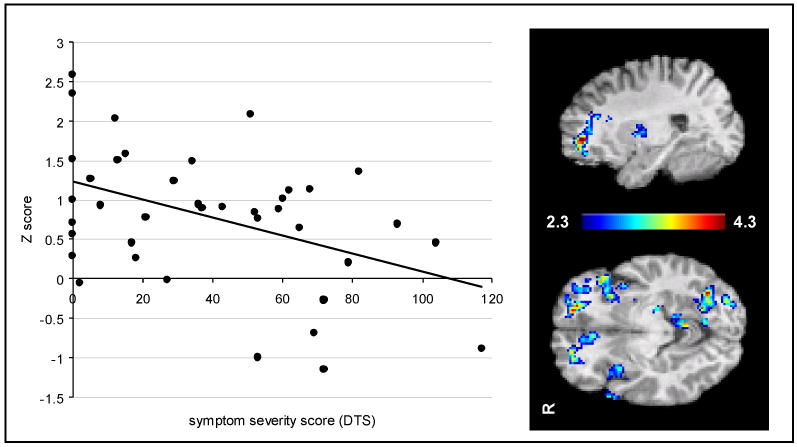
Mixed effects analysis with FSL identified clusters of voxels including the dorsolateral prefrontal cortex that were negatively correlated with severity of PTSD symptoms as measured by the Davidson Trauma Scale (DTS). Mean Z-scores in the cluster for subjects (n=39) were plotted against DTS scores (DTS) [r = -.44, F(1, 40) = 8.7, p = .005]. Activation maps represent voxel-wise correlation of Z-score with DTS score.
3. Results
3.1 Picture Rating Task
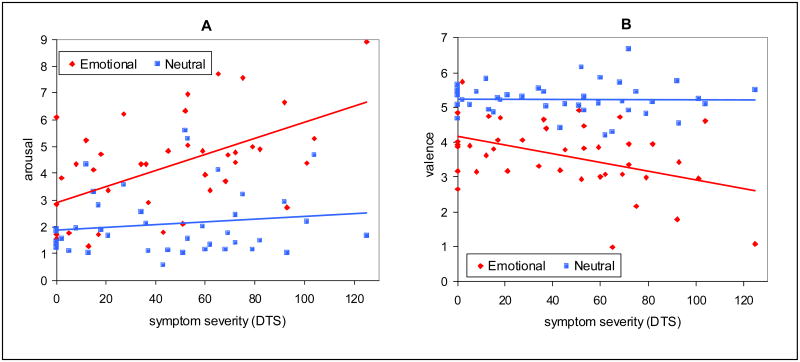
Regression analysis of picture ratings (see Fig. 1) showed that symptom severity, as measured by the Davidson Trauma scale, was predictive of arousal ratings for emotional pictures [r = 0.55, F(1, 40) = 16.6, P < 0.001] but not neutral pictures [r = 0.12, F(1, 40) = 0.74, P = 0.39]. Analysis of Covariance (ANCOVA) showed an interaction of symptom severity * picture type for arousal ratings [F(1, 78) = 7.3, P < 0.01]. Likewise, valence was negatively correlated with symptom severity for emotional pictures [r = 0.43, F(1, 40) = 8.8, P = 0.005] but not neutral pictures [r = 0.02, F(1, 40) = 0.02, P = 0.90] and an interaction of symptom severity * picture type for valence rating [F(1, 78) = 6.5, P = 0.01].
Figure 1.
A) Arousal ratings for emotional pictures were correlated with severity of PTSD symptoms measured with the Davidson Trauma Scale (DTS) [r =.55, F(1, 40) = 16.6, p = .0002] but not for neutral pictures [r =.12, F(1, 40) = .74, p = .39], with a significant interaction of symptom severity * picture type [F(1, 78) = 7.3, p < .01]. B) Valence ratings for emotional pictures were negatively correlated with DTS [r =.43, F(1, 40) = 8.8, p = .005] but not for neutral pictures [r = .02, F(1, 40) = .02, p = .90], with a significant interaction of symptom severity * picture type [F(1, 78) = 6.5, p = .01].
3.2 Behavioral Performance
Correlational analysis between the rate of correct responses in the executive condition and symptom severity scores did not reveal a significant relationship [r = -0.26, F(1,36) = 2.8, P = 0.10]. Analysis of variance (ANOVA) of response latency revealed a main effect for condition (emotional, neutral, executive) [F(2,72) = 14.2, P < 0.001]. Post hoc analysis showed a longer latency for the emotional condition than for neutral [t(74) = 1.8, P < 0.04] and executive [t(74) = 2.6, P < 0.005] conditions. Regression analysis did not reveal a relationship of latency with symptom severity for executive [r = 0.12, F(1,36) = 0.55, P = 0.46], emotional [r = 0.02, F(1,36) = 0.02, P = 0.89], and neutral [r = 0.05, F(1,36) = 0.11, P = 0.74] conditions.
3.3 Analysis of Functional MRI Data
Main contrasts for FSL mixed effects analysis were performed for emotional > neutral pictures and the executive task (relative to pre-task baseline) (see Table 2). Corresponding analyses of BOLD deactivation were also conducted for these contrasts (see Table 3). Regression analyses yielded areas of BOLD activation (see Table 4) and deactivation (see Table 5) significantly correlated with PTSD and BDI symptoms.
Table 2. Regions of activation for experimental contrasts.
| Talairach coordinates | Max Z values of contrasts
(P < 0.05 cluster corrected) |
||||||
|---|---|---|---|---|---|---|---|
| Neural system | Brodmann Area | Side | x | y | z | executive | emotional > neutral |
| dorsomedial prefrontal cortex | 10 | B | 4 | 58 | 6 | - | 4.2 |
| superior/middle temporal gyrus | 22/37/39 | L>R | -46 | -60 | 26 | - | 6.0 |
| precuneus | 7/31 | L | -2 | -54 | 42 | - | 6.1 |
| inferior parietal lobe | - | R | 34 | -46 | 50 | - | 4.2 |
| caudate | - | L | -10 | 2 | 12 | - | 4.4 |
| inferior frontal gyrus | 44 | R | 54 | 20 | 20 | - | 3.1 |
| precentral gyrus | 6 | L | -42 | -4 | 34 | - | 3.6 |
| posterior cingulate | 23/30 | B | 2 | -44 | 24 | - | 5.0 |
| dorsal anterior cingulate cortex | 32 | B | -6 | 8 | 40 | 3.9 | - |
| inferior frontal gyrus | 9 | B | -50 | 6 | 26 | 5.4 | - |
| inferior parietal lobule | 40 | B | 48 | -32 | 54 | 7.9 | - |
| superior parietal lobule | 7 | B | 30 | -54 | 54 | 6.7 | - |
| precentral gyrus | 6 | R | 34 | -10 | 64 | 5.3 | - |
| postcentral gyrus | 5 | B | -40 | -40 | 58 | 6.6 | - |
| superior frontal gyrus | 6 | B | 6 | 8 | 48 | 6.4 | - |
| middle frontal gyrus | 10/9 | B | -40 | 40 | 22 | 5.3 | - |
| insula | 13 | B | -34 | 20 | 0 | 6.6 | - |
| cerebellum | - | L >R | -20 | -52 | -18 | 6.6 | - |
| putamen | - | B | -18 | 0 | 8 | 6.7 | - |
| thalamus | - | B | -16 | -8 | 12 | 8.0 | - |
Table 3. Regions of deactivation for experimental contrasts.
| Talairach coordinates | Max Z values of contrasts
(P < 0.05 cluster corrected) |
||||||
|---|---|---|---|---|---|---|---|
| Neural system | Brodmann Area | Side | x | y | z | executive | emotional > neutral |
| superior temporal gyrus | 22 | B | 58 | 2 | -6 | - | 4.5 |
| insula | 13 | R | 38 | -18 | 18 | - | 3.8 |
| medial frontal gyrus | 6 | B | 4 | -16 | 58 | - | 3.8 |
| dorsal anterior cingulate | 24 | R > L | 4 | 2 | 40 | - | 3.2 |
| precentral gyrus | 4 | B | 64 | -10 | 28 | - | 3.9 |
| postcentral gyrus | 43 | B | -58 | -8 | 20 | - | 4.4 |
| hippocampus | - | B | 30 | -12 | -20 | 5.1 | - |
| orbitofrontal cortex | 11 | B | 4 | 40 | -22 | 6.5 | - |
| ventromedial prefrontal | 10 | R > L | 4 | 62 | 4 | 5.2 | - |
| superior frontal gyrus | 9 | R | 26 | 26 | 40 | 6.5 | - |
| subgenual cortex | 25 | B | 4 | 8 | -12 | 6.5 | - |
| inferior frontal gyrus | 47 | R | 54 | 28 | 2 | 4.1 | - |
| precuneus | 31 | B | 2 | -48 | 32 | 6.5 | - |
| inferior parietal lobule | 40 | R | 54 | -50 | 30 | 5.5 | - |
| insula | 13 | L | -40 | -18 | 16 | 5.1 | - |
| middle temporal gyrus | 20 | R > L | 58 | -18 | -12 | 5.5 | - |
| parahippocampal gyrus | - | R > L | 28 | -30 | -12 | 6.6 | - |
| caudate | - | B | 4 | 8 | 0 | 6.6 | - |
Table 4. Correlations of activation with symptom severity (DTS).
| Talairach coordinates | Max Z values of contrasts
(P < 0.05 cluster corrected) |
||||||
|---|---|---|---|---|---|---|---|
| Neural system | Brodmann Area | Side | x | y | z | executive | emotional > neutral |
| orbitofrontal cortex | 11/25 | B | -10 | 50 | 0 | - | 4.4 |
| ventromedial prefrontal | 10 | B | -4 | 50 | 12 | - | 3.4 |
| ventral anterior cingulate | 24 | L | -2 | 36 | -2 | - | 4.2 |
| inferior frontal gyrus | 45 | L | -50 | 36 | 2 | - | 3.5 |
| caudate | - | R | 10 | 2 | 12 | - | 4.4 |
| inferior parietal lobule | 40 | L | -60 | -36 | 42 | - | 3.4 |
| insula | - | L | -36 | 8 | 2 | - | 3.5 |
| superior temporal gyrus | 39/22 | L | -50 | -60 | 26 | - | 5.4 |
| middle frontal gyrus | - | R | 36 | 40 | 22 | - | 3.5 |
Table 5. Negative correlations of activation with symptom severity (DTS).
| Talairach coordinates | Max Z values of contrasts
(P < 0.05 cluster corrected) |
||||||
|---|---|---|---|---|---|---|---|
| Neural system | Brodmann Area | Side | x | y | z | executive | emotional |
| middle frontal gyrus | 45/46 | B | 26 | 46 | -6 | 4.2 | - |
| medial frontal gyrus | 9 | B | -2 | 36 | 30 | 3.9 | - |
| dorsal anterior cingulate | 24 | B | 2 | 30 | 20 | 2.7 | - |
| ventral anterior cingulate | 24 | B | -2 | 34 | 16 | 4.0 | - |
| superior frontal gyrus | 10 | L | -24 | 50 | 0 | 4.2 | - |
| inferior frontal gyrus | 44 | B | -54 | 12 | 12 | 4.0 | - |
| thalamus | - | R | 20 | -18 | 6 | 4.2 | - |
| inferior occipital gyrus | 19 | R | 30 | -86 | -4 | 4.0 | - |
| superior parietal lobule | 7 | R | 30 | -52 | 60 | 2.9 | - |
| inferior parietal lobule | 40 | R | 46 | -46 | 40 | 4.0 | - |
| cerebellum | - | R | 6 | -66 | -28 | 3.3 | - |
| insula | 13 | B | 38 | 16 | 2 | 3.9 | - |
3.4 Regions of Activation and Deactivation
As predicted, activation for the emotional > neutral condition (see Table 2) was prominent in ventral brain regions including dorsomedial PFC, inferior frontal gyrus, superior temporal gyrus (posterior portion), and precuneus. Activation for the executive task was prominent in dorsal frontoparietal regions (see Fig. 2B) including dorsal ACC, superior and middle frontal gyri, inferior and superior parietal lobules, and subcortical regions (see Table 2). Some ventral PFC activation was seen in the inferior frontal gyrus.
Figure 2.
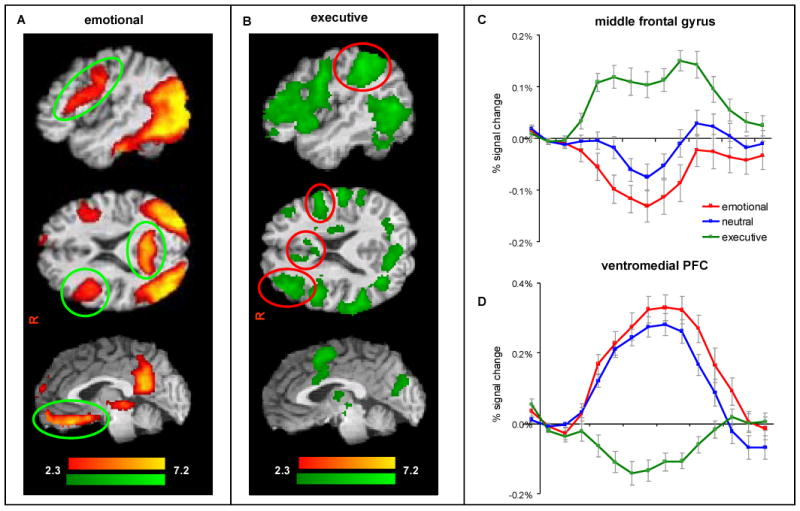
Participants (n=39) showed A) regions of activation (red) are prominent in ventromedial prefrontal cortex, inferior frontal gyrus, and precuneus. B) regions of activation (green) for the executive task are prominent in middle frontal gyrus, superior parietal cortex, and dorsal anterior cingulate gyrus C) time course of BOLD signal for emotional (red), neutral (blue) and executive (green) for the middle frontal gyrus and D) the ventromedial prefrontal gyrus. Each task block represented by the activation time courses was preceded by a block of scrambled images that constituted our baseline condition.
Regions of deactivation were identified where the BOLD signal was significantly below the baseline condition (scrambled picture). Deactivation for the emotional task overlapped significantly with positive activation for the executive task. For instance, while there was robust activation in the middle frontal gyrus during the executive condition, this region was deactivated during the neutral condition and further deactivated during the emotional condition as seen in Figure 2C. Deactivation for the emotional condition was greater (more negative) than the neutral condition in the superior temporal gyrus and insula (see Table 3). In a reciprocal fashion, regions of deactivation for the executive task overlapped significantly with positive activation for the emotional task. For instance, while the ventromedial PFC showed positive activation during the emotional condition, this region was deactivated during the executive condition as seen in Fig. 2D. Deactivated areas for the executive condition included the ventromedial and dorsomedial PFC, orbitofrontal cortex, inferior parietal lobule, and precuneus (see Table 3).
3.5 Correlation of Activity with PTSD Symptom Severity
Several ventral frontolimbic regions were highly correlated with PTSD symptoms for the emotional > neutral condition (see Fig. 3), including orbitofrontal cortex, ventromedial PFC, ventral anterior cingulate, and inferior frontal gyrus (see Table 4). There were no regions where activation during the emotional > neutral decreased with symptom severity. However, activation during the executive condition decreased with symptom severity (see Fig. 4) primarily in the in the middle and inferior frontal gyri, anterior cingulate, and inferior parietal lobule (see Table 5). There were no regions where activation during the executive task increased with symptom severity.
3.6 Correlation of Activity with Depression Symptom Severity
Correlations of activation with depression symptom severity (BDI) were performed based on our observation that DTS scores were highly correlated with BDI scores [r = 0.53, F(1,38) = 14.8, P < 0.001], and consistent with earlier reports (Breslau et al., 1997; North et al., 1999). There were no regions for which emotional > neutral condition was correlated to BDI. The executive condition was negatively correlated with BDI scores in the dorsal anterior cingulate, and superior parietal lobule. Thus, regions of activation correlated with BDI were quite different and relatively circumscribed than for DTS as reported previously (Shin et al., 2005).
4. Discussion
The present study examined the neural correlates of emotional and executive processing and their relationship with PTSD symptomatology in war veterans who recently returned from deployment to Iraq and Afghanistan. We found that processing of combat-related stimuli largely activated ventral frontolimbic regions whereas executive processing generally activated dorsal frontoparietal regions. Importantly, we found that activation for combat-related stimuli, in contrast to neutral stimuli, was positively correlated with PTSD symptom severity in a number of key regions of the ventral emotional processing stream, including ventromedial PFC, inferior frontal gyrus and ventral ACC. These results were consistent with higher arousal and lower valence ratings for combat-related pictures in subjects with higher symptom severity. Conversely, activation for the executive task was strongly correlated with lower symptom severity in several key regions of the dorsal frontoparietal network, including middle frontal gyrus, dorsal ACC, and inferior parietal lobule. We also found that PTSD severity was correlated with activation for combat-related (> neutral) stimuli outside the expected ventral system, in regions such as the middle frontal gyrus, which suggests that affective processing is usurping putative executive regions.
As predicted, we found that subjects' overall response times were slowest for the emotional condition and fastest overall for the executive task. We hypothesized that during the executive task, subjects' accuracy would decrease with increasing PTSD symptoms, and that subjects' response times for emotional pictures would be slower with increasing PTSD symptoms. Our data did not support either of these. However, we note that functional brain measures distinguished subjects on the basis of PTSD severity, but were not discernable from behavioral performance measures.
The present findings provide empirical support to a neuroanatomical model of PTSD (Bremner et al., 1999b; Rauch et al., 2000; Shin et al., 2001) that posits a hyperresponsive limbic system that in turn places greater processing demands on fronto-cingulo-parietal regulatory resources. Thus, our results suggest a cognitive-affective imbalance where a dysregulation of a ventral affective processing network is ostensibly responsible for interference of processing in the dorsal executive network.
4.1 Integration with Symptom Provocation Literature
The regions consistently reported in functional neuroimaging studies of symptom provocation in PTSD include amygdala, orbitofrontal cortex, ventromedial PFC, and ACC (Liberzon and Martis, 2006). Our findings show activation in ventral ACC for the emotional condition was correlated with greater symptom severity and dorsal ACC was negatively correlated with symptom severity in the executive condition. However, it is difficult to compare and interpret the present study relative to earlier findings given the heterogeneity of past stimulus presentation paradigms and subject samples. It has generally been reported that ACC has lower activation associated with PTSD (Shin et al., 1997; Lanius et al., 2001; Shin et al., 2001; Gilboa et al., 2004; Shin et al., 2004; Yang et al., 2004; Shin et al., 2005; Williams et al., 2006). Higher activation was found for the combat imagery condition (but not perception condition) in Vietnam veterans (Rauch et al., 1996; Shin et al., 1997) and with script-driven provocation in a dissociative state (Lanius et al., 2002). The conflicting findings may be explained by differential involvement across studies of the dorsal (“cognitive”) versus ventral-rostral (“affective”) subdivisions of the ACC (Bush, 2000; Shin et al., 2001). Our findings are consistent with affective versus cognitive subdivisions, with ventral ACC activation for emotional stimuli correlated with higher PTSD symptoms and dorsal ACC activity correlated with lower PTSD symptoms in the executive condition.
We found activation in the ventromedial PFC for the emotion processing was highly correlated with PTSD symptoms. Correspondingly, we found deactivation for the executive task in laterally situated ventral PFC was correlated with PTSD symptoms. These findings are consistent with the findings of (Northoff et al., 2000) who propose that negative emotional states engage ventromedial PFC whereas positive emotional states engage the ventrolateral PFC. The ventromedial PFC area has been implicated in emotion, motivation, and social behavior (Kringelbach, 2005; Heimer and Van Hoesen, 2006) as well as regulation of the peripheral glucocorticoid and sympathetic response to stress (Bremner et al., 1999a; Erickson et al., 2003) and emotion modulation through inhibitory connections to the amygdala (Bremner et al., 1999a; Shin et al., 2004). Dysfunction of this area may represent a neural correlate of the failure of extinction to fear responding, as well as heightened state of arousal in PTSD.
The literature contains conflicting data regarding activation of ventromedial PFC, with higher activation found using script driven imagery of childhood trauma (Shin et al., 1999), and during dissociative states induced in young adult PTSD patients (Lanius et al., 2002). On the other hand, generally older Vietnam era veterans with chronic PTSD showed lower activation using script driven imagery (Shin et al., 2004), fearful faces (Shin et al., 2005), trauma pictures and sounds (Bremner et al., 1999b) and emotional pictures (Phan et al., 2006). Given that our findings, and those of some earlier groups who also found increased ventromedial prefrontal activation for emotional stimuli, were obtained in subjects with recent-onset PTSD, suggests that illness chronicity may account for discrepant findings. Chronicity of PTSD has been shown to accelerate age-associated cognitive decline (Golier et al., 2006). Furthermore, chronic PTSD patients may represent a vulnerable subset of early onset PTSD typified by our sample (Perkonigg et al., 2005).
We found activation for emotional (> neutral) was correlated with PTSD severity in subcortical regions including the caudate, while activation for the executive task was negatively correlated in the thalamus. The basolateral nucleus of the amygdala has strong connections with the stritaum and thalamus, which in turn projects to the PFC (Davis and Whalen, 2001). Previous studies show lower thalamic activation in PTSD patients for combat sights and sounds as well as script-driven symptom provocation (Bremner et al., 1999b; Lanius et al., 2001; Lanius et al., 2003b). Our findings suggest that in PTSD the role of the thalamus is impaired in executive processing and striatal regions generally are impaired in affective processing.
4.2 Deactivation
As expected, we found that during activation of dorsal frontoparietal regions (executive task) there was a concomitant deactivation of ventral fronto-limbic regions. One the other hand, during activation of ventral frontolimbic regions (emotional task) there was a concomitant deactivation of dorsal frontoparietal regions (Drevets and Raichle, 1998, Yamasaki, 2002 #2). Explorations of deactivation are largely missing from the PTSD literature. A study of women participants listening to scripts of childhood trauma found deactivation in subcallosal region of ACC, hippocampus, fusiform gyrus, supramarginal gyrus, and visual association cortex (Bremner et al., 1999a), and a study of Vietnam veterans presented with combat sights and sounds found deactivation in medial PFC, subcallosal gyrus and middle temporal gyrus (Bremner et al., 1999b). The present study provides a preliminary model for understanding deactivation patterns in PTSD by systematically evaluating the reciprocal system of activation and deactivation in dorsal executive and ventral affective brain networks.
4.3 Interpretation of Unexpected Findings
Unexpectedly, we found that activation in the inferior frontal gyrus during the executive task was negatively correlated with PTSD severity. The role of the inferior frontal cortex is considered vital to inhibitory processes which are considered to be an essential component of a selection task (reviewed in Aron et al., 2004). The executive task our study is primarily a selection task and consistent with strong IFG activation. Our findings suggest that IFG dysfunction during executive processing is associated with greater severity of PTSD symptoms. Thus, IFG dysfunction seems to play a role in both emotion and executive processing in PTSD.
4.4 Strengths and Limitations
Three major strengths of the present study improve upon previous efforts to identify functional neuroanatomy of PTSD. First, the experimental design incorporated both symptom provocation and executive decision-making tasks that were interleaved to provide separate analyses of both of these functions. Second, we used a relatively large sample, compared to other neuroimaging studies, and a homogeneous sample of fairly young post-9/11 war veterans with recent trauma and relatively low level of comorbid substance use. Third, a more powerful analytic approach was applied to the neuroimaging data that exploited illness severity as a dimensional variable in favor of the usual binary categorical variable (i.e. PTSD or non-PTSD) and subtraction approach. Our approach enhances statistical power and permits characterization of the relationship between a continuous endophenotype measure with a continuous behavioral measure. Previous studies that performed similar correlations between activation measures and illness severity limited their analyses to subjects that met full diagnostic criteria for PTSD (Rauch et al., 2000; Shin et al., 2004; Shin et al., 2005), therefore lack the ability to characterize neural findings associated with sub-threshold PTSD symptomatology (Grubaugh et al., 2005).
We used brain imaging data to distinguish PTSD and depression symptom severity in a way that is not possible using standard behavioral measures. Our analyses showed no correlations of activation for emotional > neutral with BDI and a relatively limited regions that correlated for the executive condition. This analysis provides evidence that the DTS correlations we observed may be uniquely associated with PTSD symptoms and not related to the comorbid depressive symptoms.
Several participants in the present sample are being treated with psychotropic medication. It is alleged, but yet unsubstantiated, that psychotropic agents reduce the BOLD signal (Goldman et al., 1996; Rose et al., 2006). It is unlikely that the present results are fully explained by medication effects given that highly symptomatic participants had greater activity for one condition and simultaneously lower activity for another condition and vice-versa, depending on the brain region. Moreover, the regional patterns of activation and their relationship with illness severity were largely consistent with our a priori hypotheses.
It is possible the observed negative correlation of activation for the executive task with DTS scores was a result of a persisting state produced by the preceding emotional block. However it appears that this observed state effect (trauma related thought) is being modulated by a trait effect (symptom) severity. Broadly, this is consistent with dissociative effects of PTSD on attention reported in neuropsychological studies (Kaufman, 2002; Roca et al., 2006). A correlation between behavioral performance on the executive task and DTS may have been observed by increasing the cognitive demands of the task.
Our study was hampered by poor BOLD signal recovery in the amygdala region due to differences in magnetic susceptibility. Previous findings for the amygdala are discordant. Most studies report greater amygdalar activation associated with PTSD (Shin et al., 1997; Rauch et al., 2000; Semple et al., 2000; Hendler et al., 2003; Shin et al., 2004; Shin et al., 2005; Williams et al., 2006), but there are contradictory reports of greater activation in controls (Britton et al., 2005; Phan et al., 2006), and numerous studies lack amygdalar findings entirely (Bremner et al., 1999a; Bremner et al., 1999b; Lanius et al., 2001; Shin et al., 2001; Lanius et al., 2002; Lanius et al., 2003b; Sakamoto et al., 2005). Quantitative analysis of signal-to-noise in this region showed poor signal quality using our standard echoplanar imaging sequence at 3T. For future studies, a change in fMRI sequence such as inverse spiral imaging, spiral in/out, or a shortened TE with EPI is likely to enhance our ability to discern amygdalar activity (Glover and Law, 2001).
Finally, SCID assessment of comorbid psychiatric disorders was lacking. However, we collected important information on a number comorbid disorders that often accompany PTSD using self assessment measures for depression, substance and alcohol use disorders.
4.5 Conclusions
To our knowledge, this is the first neuroimaging study of recent-onset combat-related PTSD from post-9/11 military conflicts. We emphasize that the brain-behavior relationships are inherently correlational and are retrospective in nature, and as such they do not indicate whether the alterations in brain function were caused by deployment or were a precipitating factor. Determining the plasticity in these brain regions following treatment will be of keen interest. The present findings lend further credence to mounting evidence establishing a clear link between the subjectively-assessed behavioral phenomenology of PTSD and objective neurobiological markers. Our findings extend the current symptom provocation-based functional neuroanatomy of PTSD by providing evidence that reciprocally functioning dorsal executive and ventral emotion processing systems are differentially affected by severity of PTSD symptoms. We used brain imaging data to distinguish PTSD and depression symptom severity in a way that is not possible using standard behavioral measures. Our results support a model of PTSD emphasizing a cognitive-affective dysmetria that can promote further study on the effects of dysregulation of emotion processing on cognitive brain regions.
Acknowledgments
This research was supported by the Department of Veterans Affairs, Mental Illness Research Education and Clinical Center Grant for Post-Deployment Mental Health and by the National Institute of Mental Health Grant K23 MH073091. Special thanks to Jonathan Davidson M.D., Syam Gadde, Jeffrey Hoerle, Steven Green, Jacquelynn Price, Larry Tupler Ph.D., and Lihong Wang Ph.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bremner J, Vermetter E, Vythilingam M, Afzal N, Schmahl C, Elzinga, et al. Neural Correlates of the Classic Color and Emotional Stroop in Women with Abuse-Related Posttraumatic Stress Disoder. Biological Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999a;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological Psychiatry. 1999b;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Archives of General Psychiatry. 1997;54:81–87. doi: 10.1001/archpsyc.1997.01830130087016. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biological Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD: a review of the empirical literature. Clinical Psychology Review. 2000;20:1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Connor KM, Davidson JRT. Development of a new resilience scale: The Connor-Davidson Resilience Scale (CD-RISC) Depression & Anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- Cuthbertson BH, Hull A, Strachan M, Scott J. Post-traumatic stress disorder after critical illness requiring general intensive care. Intensive Care Medicine. 2004;30:450–455. doi: 10.1007/s00134-003-2004-8. [DOI] [PubMed] [Google Scholar]
- Danckwerts A, Leathem J. Questioning the link between PTSD and cognitive dysfunction. Neuropsychology Review. 2003;13:221–235. doi: 10.1023/b:nerv.0000009485.76839.b7. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Book SW, Colket JT, Tupler LA, Roth S, David D, et al. Assessment of a new self-rating scale for post-traumatic stress disorder. Psychological Medicine. 1997;27:153–160. doi: 10.1017/s0033291796004229. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition & Emotion. 1998;12:353–385. [Google Scholar]
- Erickson K, Drevets W, Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neuroscience & Biobehavioral Reviews. 2003;27:233–246. doi: 10.1016/s0149-7634(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS. Emotion-attention network interactions during a visual oddball task. Cognitive Brain Research. 2004;20:67–80. doi: 10.1016/j.cogbrainres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- First MBS, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV for Axis I Disorders (SCID) New York, NY: Psychiatric Institute, Biometrics Research; 1997. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston K, KJ W, RSJ F, JC M, AC E. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1993;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O. Functional Connectivity of the Prefrontal Cortex and the Amydala in Posttraumatic Stress Disorder. Biological Psychiatry. 2004;55:263–272. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Goldman RG, Alexander GE, Zemishlany Z, Mukherjee S, Sackeim H, Prohovnik I. Acute effects of haloperidol on cerebral cortex blood flow in normal and schizophrenic subjects. Biological Psychiatry. 1996;40:604–608. doi: 10.1016/0006-3223(95)00391-6. [DOI] [PubMed] [Google Scholar]
- Golier JA, Harvey PD, Legge J, Yehuda R. Memory performance in older trauma survivors: implications for the longitudinal course of PTSD. Annals of the New York Academy of Sciences. 2006;1071:54–66. doi: 10.1196/annals.1364.006. [DOI] [PubMed] [Google Scholar]
- Grubaugh AL, Magruder KM, Waldrop AE, Elhai JD, Knapp RG, Frueh BC. Subthreshold PTSD in primary care: prevalence, psychiatric disorders, healthcare use, and functional status. Journal of Nervous & Mental Disease. 2005;193:658–664. doi: 10.1097/01.nmd.0000180740.02644.ab. [DOI] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neuroscience & Biobehavioral Reviews. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben-Bashat D, et al. Sensing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage. 2003;19:587–600. doi: 10.1016/s1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine. 2004;351:13–22. doi: 10.1056/NEJMoa040603. see comment. [DOI] [PubMed] [Google Scholar]
- Jenkins MA, Langlais PJ, Delis DA, Cohen RA. Attentional dysfunction associated with posttraumatic stress disorder among rape survivors. Clinical Neuropsychologist. 2000;14:7–12. doi: 10.1076/1385-4046(200002)14:1;1-8;FT007. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kaufman ML. Dissertation Abstracts International: Section B: The Sciences and Engineering. Vol. 63 2002. Dissociation status and attentional allocation in male Vietnam combat veterans with posttraumatic stress disorder. [Google Scholar]
- Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, Mora CA. Clinical evaluation of a measure to assess combat exposure. Psychological Assessment. 1989;1:53–55. [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychological Assessment. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. Gainesville, Florida: The Center for Research in Psychophysiology, University of Florida; 2005. International Affective Picutre System (IAPS): Digitized photogrphs, instruction manual and affective ratings. [Google Scholar]
- Lanius RA, Hopper JW, Menon RS. Individual differences in a husband and wife who developed PTSD after a motor vehicle accident: a functional MRI case study. American Journal of Psychiatry. 2003a;160:667–669. doi: 10.1176/appi.ajp.160.4.667. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biological Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. American Journal of Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biological Psychiatry. 2003b;53:204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Annals of the New York Academy of Sciences. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry & Clinical Neurosciences. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Miller G. Mental health. Widening the attack on combat-related mental health problems. Science. 2006;313:908–909. doi: 10.1126/science.313.5789.908. [DOI] [PubMed] [Google Scholar]
- North CS, Nixon SJ, Shariat S, Mallonee S, McMillen JC, Spitznagel EL, Smith EM. Psychiatric disorders among survivors of the Oklahoma City bombing. JAMA. 1999;282:755–762. doi: 10.1001/jama.282.8.755. [DOI] [PubMed] [Google Scholar]
- Northoff G, Richter A, Gessner M, Schlagenhauf F, Fell J, Baumgart F, et al. Functional dissociation between medial and lateral prefrontal cortical spatiotemporal activation in negative and positive emotions: a combined fMRI/MEG study. Cerebral Cortex. 2000;10:93–107. doi: 10.1093/cercor/10.1.93. [DOI] [PubMed] [Google Scholar]
- Perkonigg A, Pfister H, Stein MB, Hofler M, Lieb R, Maercker A, Wittchen HU. Longitudinal course of posttraumatic stress disorder and posttraumatic stress disorder symptoms in a community sample of adolescents and young adults. American Journal of Psychiatry. 2005;162:1320–1327. doi: 10.1176/appi.ajp.162.7.1320. [DOI] [PubMed] [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Archives of General Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Archives of General Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Roca V, Hart J, Kimbrell T, Freeman T. Cognitive function and dissociative disorder status among veteran subjects with chronic posttraumatic stress disorder: a preliminary study. Journal of Neuropsychiatry & Clinical Neurosciences. 2006;18:226–230. doi: 10.1176/jnp.2006.18.2.226. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Simonotto E, Spencer EP, Ebmeier KP. The effects of escitalopram on working memory and brain activity in healthy adults during performance of the n-back task. Psychopharmacology. 2006;185:339–347. doi: 10.1007/s00213-006-0334-2. [DOI] [PubMed] [Google Scholar]
- Sachinvala N, von Scotti H, McGuire M, Fairbanks L, Bakst K, McGuire M, et al. Memory, attention, function, and mood among patients with chronic posttraumatic stress disorder. Journal of Nervous & Mental Disease. 2000;188:818–823. doi: 10.1097/00005053-200012000-00005. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, et al. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. Neuroimage. 2005;26:813–821. doi: 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Semple WE, Goyer PF, McCormick R, Donovan B, Muzic RF, Jr, Rugle L, et al. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared with normals. Psychiatry. 2000;63:65–74. doi: 10.1080/00332747.2000.11024895. [DOI] [PubMed] [Google Scholar]
- Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, et al. Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Archives of General Psychiatry. 1997;54:233–241. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. American Journal of Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addictive Behaviors. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 1:S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Proctor SP, Amoroso P, Kane R, Heeren T, White RF. Neuropsychological outcomes of army personnel following deployment to the Iraq war. JAMA. 2006;296:519–529. doi: 10.1001/jama.296.5.519. see comment. [DOI] [PubMed] [Google Scholar]
- Wang L, McCarthy G, Song AW, Labar KS. Amygdala activation to sad pictures during high-field (4 tesla) functional magnetic resonance imaging. Emotion. 2005;5:12–22. doi: 10.1037/1528-3542.5.1.12. [DOI] [PubMed] [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, et al. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow & Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. see comment. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Wu MT, Hsu CC, Ker JH. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neuroscience Letters. 2004;370:13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]