Abstract
To analyze the genetic alterations of pheochromocytomas and evaluate the difference among malignant, extra-adrenal, and benign pheochromocytomas. Forty-three tumor samples were tested for genetic changes using multiplex ligation-dependent probe amplification. Among them, 39 samples were available for protein expression analysis by immunohistochemistry (IHC). All 43 patients (24 women and 19 men; mean age 44.6±13.6 years; range 18–75 years; 9 with malignant, 7 extra-adrenal, and 27 benign) showed multiple copy number losses or gains. The average copy number change was 13.10 in malignant, 13.93 in benign, and 13.47 in paraganglioma patients. There is no significant difference among the three groups of pheochromocytomas. However, we discovered that in the malignant pheochromocytomas, 6 of the 9 patients (67%) showed erythroblastic leukemia viral oncogene homolog 2 (ERBB-2) oncogene gain, whereas only 12 of the 34 (35%) identified change in the benign and extra-adrenal pheochromocytomas. Further, IHC confirmed that ERBB-2-positive staining was more frequent and stronger in malignant pheochromocytomas than in benign and extra-adrenal pheochromocytomas. Our study illustrates the chromosomal changes of the whole genome of Chinese pheochromocytoma patients. The results suggest that there may be certain progression of genetic events that involves chromosomes 1p, 3p, 6p, 11q, 12q, 17q, and 19q in the development of pheochromocytomas, and the activation of ERBB-2 located on chromosome 17q is an important and early event in the malignancy development of these tumor types. The overexpression of ERBB-2 identified by IHC suggested that this oncogene could be associated with the malignancy of pheochromocytomas and paragangliomas.
Introduction
Pheochromocytomas are rare neoplasms of the chromaffin cells that derived from the embryonic neural crest. They usually occur within the adrenal medulla; however, extra-adrenal pheochromocytomas may arise in ganglia of the sympathetic nervous system. Both the clinical and biochemical features of pheochromocytomas are mostly the result of the overproduction of catecholamines. In sporadic pheochromocytomas and paragangliomas, ∼20% of them are associated with germline mutations that lead to paraganglioma syndrome (succinate dehydrogenase subunit B/C/D; SDHB, SDHC, and SDHD), von Hippel–Lindau syndrome (VHL), and multiple endocrine neoplasia type II (MEN2) and, to a much lesser extent, type 1 neurofibromatosis (NF1; Casanova et al. 1993, Maher & Kaelin 1997, Neumann et al. 2002, Bausch et al. 2006). Studies of genomic alterations in primary pheochromocytomas using comparative genomic hybridization (CGH) have shown the loss of chromosomes 1p, 3p, 3q, 11p, 17p, and 22q at various frequencies, suggesting the existence of tumor suppressor gene loci in these locations (Yokogoshi et al. 1990, Moley et al. 1992, Shin et al. 1993, Zeiger et al. 1995, Edstrom et al. 2000). Gain of chromosomes 17q and 11q13 was more frequently identified in malignant tumors (August et al. 2004, Eisenhofer et al. 2004, Cascón et al. 2005). However, it is difficult to identify which specific gene was involved in the pathogenesis of pheochromocytoma by CGH analysis. Until recently, there was lack of knowledge about the exact genes involved in the pathogenesis of sporadic pheochromocytoma. To find new candidate genes, we performed multiplex ligation-dependent probe amplification (MLPA) analysis in pheochromocytomas. MLPA is a new technique used to measure the copy number of up to 45 nucleic acid sequences in one single reaction (Schouten et al. 2002). This method relies on sequence-specific probe hybridization to genomic DNA followed by multiplex-PCR amplification of the hybridized probe and semi-quantitative analysis of the resulting PCR products. This method is easy to perform and could offer an alternative to CGH studies (Hogervorst et al. 2003, Bremmer et al. 2005, van Dijk et al. 2005, Postma et al. 2005).
We investigated 43 pheochromocytoma tissue samples using MLPA analysis and found that several genes may contribute to the tumorigenesis of pheochromocytoma or malignancy of the disease. These results were similar to those detected by CGH analysis. The most obvious was the gain of erythroblastic leukemia viral oncogene homolog 2 (ERBB-2) oncogene located on chromosome 17q that was more common in malignant pheochromocytomas. Moreover, we analyzed the ERBB-2 oncogene products from pheochromocytomas by immunohistochemistry (IHC); the results confirmed the previous genetic analysis.
Materials and methods
Patients and samples
Forty-three patients (24 women and 19 men; mean age 44.6±13.6 years; range 18–75 years) operatively proven with sporadic pheochromocytomas were included in this study. The diagnosis of pheochromocytoma was confirmed on the basis of clinical manifestations including persistent or paroxysmal hypertension with the classic triad of severe headaches, palpitations, and diaphoresis, biochemical features including serum and urinary metanephrine (MN), nor-MN (NMN), and positive CT or 131I-MIBG scan. The diagnosis of malignant pheochromocytoma was based on the presence of distant metastases or lymph node infiltrations, that is, the presence of chromaffin tissue at sites where this tissue is normally absent (Eisenhofer et al. 1999, Weise et al. 2002, Mittendorf et al. 2007). Of the 43 patients, 9 had malignant disease, 7 were paragangliomas of the abdomen, and the rest of the 27 patients were with benign pheochromocytomas. All of the patients were operated in between 2002 and 2006 and clinically monitored at the Shanghai Clinical Center for Endocrine and Metabolic Diseases in the Ruijin Hospital. The study was approved by the Ethics Committee of the Ruijin Hospital, Shanghai JiaoTong University School of Medicine.
Tumor tissues were snap-frozen in liquid nitrogen immediately after surgery. Genomic DNA was extracted from fresh-frozen tumor specimens by standard procedures using a Qiagen DNA extraction Kit (Qiagen) according to manufacturer's guide. All pheochromocytomas were histologically analyzed before DNA extraction to ensure preparation of a population of the tumor cells without cross-contamination with the normal cells.
Multiplex ligation-dependent probe amplification
We performed MLPA analysis using SALSA P005 and P006 Chromosomal Aberration MLPA Kits (MRC-Holland, Amsterdam, The Netherlands; details available at http://www.mlpa.com) and following the manufacturer's protocol. The P005 and P006 kits include 76 genes loci, which were selected because of their reported involvement in cancer and can span all 23 chromosomes including the X and the Y. With the MLPA, DNA samples were heated at 98 °C for 5 min; after the addition of the probe mix, samples were heated for 1 min at 95 °C and then incubated for 16 h at 60 °C; ligation of the annealed oligonucleotide probes was performed for 15 min at 54 °C with Ligase-65 enzyme. Multiplex PCR amplification was carried out using Cy5-labeled primers, dNTPs, and SALSA polymerase. PCR was performed for 35 cycles of 30 s at 95 °C, 30 s at 60 °C, and 1 min at 72 °C. All the reactions were carried out in PTC-225 DNA Engine Tetrad (MJ Research Inc., San Francisco, CA, USA). PCR products were analyzed using Beckman Coulter CEQ 8800 sequencer (Beckman Coulter, Fullerton, CA, USA). Data analysis was performed with fragment analysis module. Normal control DNA of males and females was also used in the same reaction.
MLPA data analysis
The Gene Scan data of sizes and peak height of multiplex PCR products were exported to an Excel file. All the expected MLPA products were normalized by dividing each peak height by the combined peak height of all peaks in that lane (relative peak height). The relative copy number for each probe was expressed as a ratio of the relative peak height for each locus of the sample to that of the normal sex-matched control. The reference median peak heights were obtained from normal tissue samples, each of which was analyzed at least thrice independently. Male and female samples were compared with male and female control samples respectively for quantitation of X- and Y-linked probes. The ratio <0.7 and >1.35 was considered as a loss and a gain respectively.
Immunohistochemistry
IHC analysis was carried out to verify the gene changes detected by MLPA. To minimize sampling variability, the following steps were taken. All samples (control and experimental) were 1) run in parallel and each treated identically, 2) examinedunder identical conditions, and 3) repeated at least twice. Five micrometer sections were cut and deparaffinized. Then the sections were immersed in sodium citrate buffer adjusted to pH 6.0 and heated in a microwave oven for 5 min at 100 °C, next treated with 0.3% H2O2 for 30 min to inhibit peroxidases, then blocked with 2% normal rabbit serum for 30 min. The slides were incubated overnight in a solution of ERBB-2 monoclonal antibody (DAKO Rabbit anti-human ERBB-2 oncoprotein) diluted to 1:200 with the same buffer. The primary antibody binding was demonstrated with standard avidin–biotin-peroxidase complex technique (vector pk-7200). The slides were developed using diaminobenzidine and counterstained with hematoxylin. Breast carcinoma tissue, known to express the ERBB-2 protein, was used as the positive control. Negative controls were incubated with no primary antibody. The results were evaluated quantitatively and divided into four groups (0, negative; +, <30% positive rate; ++, 30–60% positive rate; and +++, >60% positive rate). The data were entered into a ZEISS Axioplan 2 microcomputer, and statistical analysis was carried out using KS400 3.0 statistical package. The Kruskal–Wallis test was used to evaluate the relationship among the percentage of ERBB-2 immunoreactivity (all four groups) in the benign, extra-adrenal, and malignant pheochromocytomas.
Results
Clinical characteristics
Forty-three patients were pathologically diagnosed with pheochromocytoma or abdominal paraganglioma. The median age at diagnosis was 44.6±13.6 years (range 18–75 years) with a male to female sex distribution of 56 and 44%. Tumors in 16 patients were on the left adrenal, 17 on the right, 1 with bilateral tumor, and another 9 were with paragangliomas. Of the 43 patients, 18 (42%) showed one of the classical triad, 11 (25%) showed two, and 7 (16%) showed all the three triads. However, there were seven patients with no typical symptoms of pheochromocytoma. Elevated serum MN and/or NMN were seen in 95% of the patients. Malignant tumor was seen in 21% of the patients (Table 1). In 34 benign samples, 12 showed gain of ERBB-2 with the average tumor size of 77.1 mm, while the rest 22 showed normal ERBB-2 gene with the average tumor size of 49.1 mm (T=2.729; P=0.01). The clinical characteristics of those malignant tumors were shown in Table 2. All the analyzed tumor tissues were derived from the primary lesions.
Table 1.
The clinical features of three different types of pheochromocytomas
| Malignant | Adrenal benign | Paraganglioma benign | |
|---|---|---|---|
| Number of patients | 9 | 27 | 7 |
| Sex (M/F) | 2/5 | 13/14 | 2/5 |
| Age (average±std) | 41.1±6.4 | 45.7±15.7 | 44.8±13.9 |
| Tumor size (mm) | 52.6±19.7 | 66.3±33.1 | 40.9±15.2 |
Table 2.
The clinical characteristics of malignant patients
| Case | Sex (M/F) | Age (years) | Operation times | MN/NMN (pg/ml) | Tumor size (mm) | Metastatic location |
|---|---|---|---|---|---|---|
| 5 | F | 43 | 3 | 353.4/30.9 | 48×40×43 | LN |
| 48 | F | 43 | 2 | 167.7/1414.9 | 40×30×30 | LN |
| 68 | F | 40 | 2 | 202.5/585.9 | 60×50×30 | Liver |
| 75 | M | 40 | 1 | 62.6/4669.4 | 60×50×40 | Lung |
| 49 | F | 44 | 2 | 47.2/2172.3 | 30×10×10 | Kidney |
| 67 | F | 52 | 2 | 42.5/5371.6 | 85×60×30 | Omentum |
| 25 | F | 38 | 2 | 8861.3/104.6 | 75×32×30 | Brain |
| 6 | M | 31 | 2 | 170.8/3942.2 | 50×45×40 | LN |
| 72 | M | 39 | 1 | NA | 25×10×10 | LN |
NA, not available; LN, lymph node. Reference range: NM, 14.0–90.0 pg/ml and NMN, 19–121 pg/ml.
MLPA
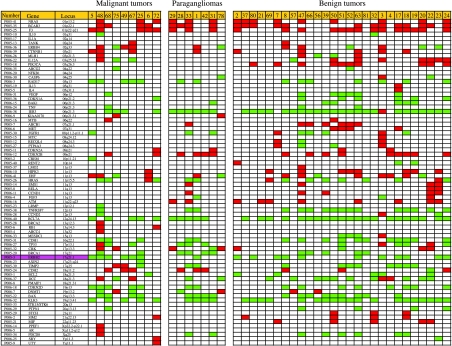
Genome-wide analysis suggested that all 43 pheochromocytoma specimens, including both malignant and benign ones, showed multiple copy number losses or gains. In the malignant, benign pheochromocytomas, and in paragangliomas, the total number of aberrations in each tumor ranged from 6 to 29, 3 to 31, and 7 to 21 respectively, and the average copy number changes were 13.10, 13.93, and 13.47 respectively. Hence, there is no significant difference among the three groups (Fig. 1).
Figure 1.
The copy number changes of all 43 analyzed tumors in a total of 76 genes spanning almost all chromosome arms that were included in the P005 and P006 probe mixes. Colors used: green, gain; red, loss; and white, no genetic alteration. ERBB-2 is emphasized with purple color.
The deletion or the duplication of the genes represents the deletion or the duplication of the chromosome where the genes are located. In our study, chromosomes 1p, 3p, 9p, and 11p showed obvious deletion, while chromosomes 6p, 12q, 17q, and 19q showed duplication. However, there is no significant difference between malignant pheochromocytomas, paragangliomas, or benign tumors.
The most obvious difference between malignant and benign tumors is the duplication of ERBB-2 gene locus on chromosome 17q21. In malignant tumors, gain of ERBB-2 gene was the most common abnormality, which was detected in six out of the nine (67%) patients; while in paragangliomas and benign pheochromocytomas, only 12 out of the 34 (35%) patients showed gain of ERBB-2 gene.
Immunohistochemistry
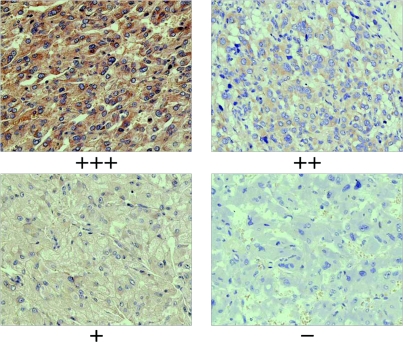
To study the expression of ERBB-2 oncogene in pheochromocytomas, we further analyzed 39 out of the 43 samples by IHC analysis. Positive ERBB-2 immunoreactivity showed a diffuse intra-cytoplasmic granular staining. The frequency of cells expressing the ERBB-2 protein differed among tumors. All the malignant pheochromocytoma samples showed positive ERBB-2 immunoreactivity, among them 86% were ++ or +++. None of the benign tumors showed +++, and 52% of the benign tumors showed negative immunoreactivity. No immunostaining was observed in the negative controls (Fig. 2). The Kruskal–Wallis test showed significantly higher ERBB-2 oncoprotein expression in the malignant pheochromocytomas (P=0.0065, K–W test; Table 3).
Figure 2.
Strong positive staining (+++) of the malignant pheochromocytoma (top left),++ of the paraganglioma (top right),+ of the benign pheochromocytoma (bottom left), and negative control (bottom right). Magnification, ×40.
Table 3.
Immunohistochemistry study of the ERBB-2 oncogene revealed that all malignant tumors showed positive of the ERBB-2 expression. Most of the benign tumors were negative
| Malignant (%) | Extra-adrenal (%) | Benign (%) | P value | |
|---|---|---|---|---|
| +++ | 2 (29) | 1 (14) | 0 | |
| ++ | 4 (57) | 2 (29) | 7 (28) | |
| + | 1 (14) | 0 | 5 (20) | 0.0065 |
| − | 0 | 4 (57) | 13 (52) | |
| N | 7 | 7 | 25 |
Discussion
The pheochromocytomas are neuronal–endocrine tumors arising from the paraganglia system found in the adrenal medulla, whereas those found along the para-vertebral and para-aortic axis, but outside the adrenals, are called paragangliomas. The pheochromocytomas can be a part of some of the hereditary syndromes, including MEN2, VHL, NF1, and paraganglioma syndrome. As the genes responsible for these hereditary cancer syndromes were identified, attention has been shifted to the identification of additional genetic alterations in sporadic pheochromocytomas, especially malignant ones (Neumann et al. 1993, Eisenhofer et al. 2004, Brouwers et al. 2005, Korpershoek et al. 2007). However, somatic genome alteration researches are very important to understand the mechanism of pheochromocytomas. Loss of chromosome 1p recently detected in pheochromocytomas (Dannenberg et al. 2000, Opocher et al. 2003, August et al. 2004, Aarts et al. 2006) was the most frequent chromosomal deletions in our study. The deletion occurred in all three types of pheochromocytomas with similar frequency; therefore, this may be indicative of early events in the tumorigenesis of sporadic pheochromocytoma. However, these lesions probably are of less importance in view of the progression to malignancy. Our study, revealed the frequent gain of chromosome 17q in malignant tumors, which indicated that this region may be associated with the malignancy of the disorders. These results were similar to that detected by CGH (Brinkschmidt et al. 2001, August et al. 2004).
The design of the MLPA analysis takes advantage of changes not only in chromosome but also in the genes themselves to demonstrate the relevant genetic changes in tumors. In the present study, ERBB-2 oncogene duplication was observed most frequently in malignant tumors. ERBB-2 oncogene, originally called NEU (neuron/glioblastoma derived oncogene homolog), was derived from the rat neuro/glioblastoma cell lines. It encodes a tumor antigen, p185, which is related to the epidermal growth factor receptor. Further studies proved that ERBB-2 gene product is a 185 kDa glycoprotein with tyrosine kinase activity (Akiyama et al. 1986). Overexpression of the ERBB-2, a breast cancer marker, is associated with rapid tumor growth, increased risk of recurrence after surgery, poor response to conventional chemotherapy, and shortened survival, and this ERBB-2 overexpression has also been implicated in the neoplastic transformation of prostate cancer (Ross & Fletcher 1998, Yu et al. 1998). Trastuzumab (Herceptin) is a human monoclonal antibody that appears to block the growth signals transmitted by ERBB-2 to the nucleus and enhances the response to chemotherapeutic agents (Pietras et al. 1998, Slamon et al. 2001). Antibody against ERBB-2 has been FDA approved for clinical treatment of cancer under the brand name Herceptin. Some recent clinical trials have found trastuzumab reduces the risk of relapse in breast cancer patients by 50% when given in the adjuvant setting (i.e., after breast cancer surgery, before the cancer has spread any further) for 1 year (Piccart-Gebhart et al. 2005, Romond et al. 2005). In our study, further IHC analysis confirmed that there was significant difference in malignant and benign pheochromocytomas, which indicated that ERBB-2 oncogene may be an important gene in the malignant development of pheochromocytomas. The effects of ERBB-2 in pheochromocytomas pathogenesis may be similar to that in breast and prostate cancers.
As pheochromocytoma is a rare endocrine tumor, consequently the specimens included in our study were limited. With the increase of the tumor samples, the difference between the malignant and benign tumors may be more significant. Another limitation of our study is the difficulty in distinguishing the malignant ones from the benign, which needs long-term follow-up; and our present research has only been undertaken in a few years, there must be some malignant tumors in the ‘benign’ group under our present diagnosis.
In conclusion, the results from our study by MLPA showed that genomic alternation was identified in pheochromocytomas and paragangliomas, involving chromosomes 1p, 3p, 6p, 11q, 12q, 17q, and 19q. Gain of ERBB-2 oncogene located on chromosome 17q was an obvious difference between malignancy and benign tumors. IHC analysis of ERBB-2 further confirmed that the expression of ERBB-2 in malignant tumors was much higher than that in the benign ones, whereas no ERBB-2 protein expression was found in normal tissues. The association of ERBB-2 gene in breast cancers and prostate cancer has been widely elucidated in many studies, and the current study was only a clue to yet another possible association between ERBB-2 and pheochromocytomas, and paragangliomas, and further investigation might clarify this connection.
Acknowledgements
The present study would not have been possible without the participation of the patients. The study is supported by the grants from Shanghai Leading Academic Discipline Project (project number: Y0204) and Shanghai Committee of Science and Technology (project number: O64119629). The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Footnotes
W Yuan, W Wang and B Cui contributed equally to this work
References
- Aarts M, Dannenberg H, deLeeuw RJ, van Nederveen FH, Verhofstad AA, Lenders JW, Dinjens WN, Speel EJ, Lam WL, de Krijger RR. Microarray-based CGH, of sporadic and syndrome-related pheochromocytomas using a 0.1–0.2 Mb bacterial artificial chromosome array spanning chromosome arm 1p. Genes, Chromosomes & Cancer. 2006;45:83–93. doi: 10.1002/gcc.20268. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- August C, August K, Schroeder S, Bahn H, Hinze R, Baba HA, Kersting C, Buerger H. CGH and and CD 44/MIB-1 immunohistochemistry are helpful to distinguish metastasized from nonmetastasized sporadic pheochromocytomas. Modern Pathology. 2004;17:1119–1128. doi: 10.1038/modpathol.3800160. [DOI] [PubMed] [Google Scholar]
- Bausch B, Borozdin W, Neumann HPH, European-American Pheochromocytoma Study G Clinical and genetic characteristics of patients with neurofibromatosis type 1 and pheochromocytoma. New England Journal of Medicine. 2006;354:2729–2731. doi: 10.1056/NEJMc066006. [DOI] [PubMed] [Google Scholar]
- Bremmer JF, Braakhuis BJ, Ruijter-Schippers HJ, Brink A, Duarte HM, Kuik DJ, Bloemena E, Leemans CR, van der Waal I, Brakenhoff RH. A noninvasive genetic screening test to detect oral preneoplastic lesions. Laboratory Investigation. 2005;85:1481–1488. doi: 10.1038/labinvest.3700342. [DOI] [PubMed] [Google Scholar]
- Brinkschmidt C, Christiansen H, Terpe HJ, Simon R, Lampert F, Boecker W, Dockhorn-Dworniczak B. Distal chromosome 17 gains in neuroblastomas detected by comparative genomic hybridization (CGH) are associated with a poor clinical outcome. Medical and Pediatric Oncology. 2001;36:11–13. doi: 10.1002/1096-911X(20010101)36:1<11::AID-MPO1004>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Brouwers FM, Petricoin EF, III, Ksinantova L, Breza J, Rajapakse V, Ross S, Johann D, Mannelli M, Shulkin BL, Kvetnansky R, et al. Low molecular weight proteomic information distinguishes metastatic from benign pheochromocytoma. Endocrine-Related Cancer. 2005;12:263–272. doi: 10.1677/erc.1.00913. [DOI] [PubMed] [Google Scholar]
- Casanova S, Rosenberg-Bourgin M, Farkas D, Calmettes C, Feingold N, Heshmati HM, Cohen R, Conte-Devolx B, Guillausseau PJ, Houdent C, et al. Pheochromocytoma in multiple endocrine neoplasia type 2A: survey of 100 cases. Clinical Endocrinology. 1993;38:531–537. doi: 10.1111/j.1365-2265.1993.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Cascón A, Ruiz-Llorente S, Rodríguez-Perales S, Honrado E, Martínez-Ramírez A, Letón R, Montero-Conde C, Benítez J, Dopazo J, Cigudosa JC, et al. A novel candidate region linked to development of both pheochromocytoma and head/neck paraganglioma. Genes, Chromosomes and Cancer. 2005;42:260–268. doi: 10.1002/gcc.20139. [DOI] [PubMed] [Google Scholar]
- Dannenberg H, Speel EJ, Zhao J, Saremaslani P, van Der Harst E, Roth J, Heitz PU, Bonjer HJ, Dinjens WN, Mooi WJ, et al. Losses of chromosomes 1p and 3q are early genetic events in the development of sporadic pheochromocytomas. American Journal of Pathology. 2000;57:353–359. doi: 10.1016/S0002-9440(10)64547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk MC, Rombout PD, Boots-Sprenger SH, Straatman H, Bernsen MR, Ruiter DJ, Jeuken JW. Multiplex ligation-dependent probe amplification for the detection of chromosomal gains and losses in formalin-fixed tissue. Diagnostic Molecular Pathology. 2005;14:9–16. doi: 10.1097/01.pas.0000146701.98954.47. [DOI] [PubMed] [Google Scholar]
- Edstrom E, Mahlamaki E, Nord B, Kjellman M, Karhu R, Hoog A, Goncharov N, Teh BT, Backdahl M, Larsson C. Comparative genomic hybridization reveals frequent losses of chromosomes 1p and 3q in pheochromocytomas and abdominal paragangliomas, suggesting a common genetic etiology. American Journal of Pathology. 2000;156:651–659. doi: 10.1016/S0002-9440(10)64769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Lenders JW, Linehan WM, Walther MM, Goldstein DS, Keiser HR. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel–Lindau disease and multiple endocrine neoplasia type 2. New England Journal of Medicine. 1999;340:1872–1879. doi: 10.1056/NEJM199906173402404. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NK, Dahia PL, de Krijger RR, Giordano TJ, Greene LA, Goldstein DS. Malignant pheochromocytoma: current status and initiatives for future progress. Endocrine-Related Cancer. 2004;11:423–436. doi: 10.1677/erc.1.00829. [DOI] [PubMed] [Google Scholar]
- Hogervorst FB, Nederlof PM, Gille JJ, McElgunn CJ, Grippeling M, Pruntel R, Regnerus R, van Welsem T, van Spaendonk R, Menko FH, et al. Large genomic deletions and duplications in the BRCA1 gene identified by a novel quantitative method. Cancer Research. 2003;63:1449–1453. [PubMed] [Google Scholar]
- Korpershoek E, Petri BJ, van Nederveen FH, Dinjens WN, Verhofstad AA, de Herder WW, Schmid S, Perren A, Komminoth P, de Krijger RR. Candidate gene mutation analysis in bilateral adrenal pheochromocytoma and sympathetic paraganglioma. Endocrine-Related Cancer. 2007;14:453–462. doi: 10.1677/ERC-06-0044. [DOI] [PubMed] [Google Scholar]
- Maher ER, Kaelin WG., Jr von Hippel–Lindau disease. Medicine. 1997;76:381–391. doi: 10.1097/00005792-199711000-00001. [DOI] [PubMed] [Google Scholar]
- Mittendorf EA, Evans DB, Lee JE, Perrier ND. Pheochromocytoma: advances in genetics, diagnosis, localization, and treatment. Hematology/Oncology Clinics of North America. 2007;21:509–525. doi: 10.1016/j.hoc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Moley JF, Brother MB, Fong CT, White PS, Baylin SB, Nelkin B, Wells SA, Brodeur GM. Consistent association of 1p loss of heterozygosity with pheochromocytomas from patients with multiple endocrine neoplasia type 2 syndromes. Cancer Research. 1992;52:770–774. [PubMed] [Google Scholar]
- Neumann HP, Berger DP, Sigmund G, Blum U, Schmidt D, Parmer RJ, Volk B, Kirste G. Pheochromocytomas, multiple endocrine neoplasia type 2, and von Hippel–Lindau disease. New England Journal of Medicine. 1993;329:1531–1538. doi: 10.1056/NEJM199311183292103. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, Schipper J, Klisch J, Altehoefer C, Zerres K, et al. Germ-line mutations in nonsyndromic pheochromocytoma. New England Journal of Medicine. 2002;346:1459–1466. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- Opocher G, Schiavi F, Vettori A, Pampinella F, Vitiello L, Calderan A, Vianello B, Murgia A, Martella M, Taccaliti A, et al. Fine analysis of the short arm of chromosome 1 in sporadic and familial pheochromocytoma. Clinical Endocrinology. 2003;59:707–715. doi: 10.1046/j.1365-2265.2003.01910.x. [DOI] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. New England Journal of Medicine. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Pegram MD, Finn RS, Maneval DA, Slamon DJ. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene. 1998;17:2235–2249. doi: 10.1038/sj.onc.1202132. [DOI] [PubMed] [Google Scholar]
- Postma C, Hermsen MA, Coffa J, Baak JP, Mueller JD, Mueller E, Bethke B, Schouten JP, Stolte M, Meijer GA. Chromosomal instability in flat adenomas and carcinomas of the colon. Journal of Pathology. 2005;205:514–521. doi: 10.1002/path.1733. [DOI] [PubMed] [Google Scholar]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-posititive breast caner. New England Journal of Medicine. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16:413–428. doi: 10.1002/stem.160413. [DOI] [PubMed] [Google Scholar]
- Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by mutiplex ligation-dependent probe amplification. Nucleic Acids Research. 2002;30:e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E, Fujita S, Takami K, Kurahashi H, Kurita Y, Kobayashi T, Mori T, Nishisho I, Takai S. Deletion mapping of chromosome 1p and 22q in pheochromocytoma. Japanese Journal of Cancer Research. 1993;84:402–408. doi: 10.1111/j.1349-7006.1993.tb00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New England Journal of Medicine. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Weise M, Merke DP, Pacak K, Walther MM, Eisenhofer G. Utility of plasma free metanephrines for detecting childhood pheochromocytoma. Journal of Clinical Endocrinology and Metabolism. 2002;87:1955–1960. doi: 10.1210/jcem.87.5.8446. [DOI] [PubMed] [Google Scholar]
- Yokogoshi Y, Yoshimoto K, Saito S. Loss of heterozygosity on chromosomes 1 and 11 in sporadic pheochromocytomas. Japanese Journal of Cancer Research. 1990;81:632–638. doi: 10.1111/j.1349-7006.1990.tb02620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Jing T, Liu B, Yao J, Tan M, McDonnell TJ, Hung MC. Overexpression of ErbB2 blocks Taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Molecular Cell. 1998;2:581–591. doi: 10.1016/s1097-2765(00)80157-4. [DOI] [PubMed] [Google Scholar]
- Zeiger MA, Zbar B, Keiser H, Linehan WM, Gnarra JR. Loss of heterozygosity on the short arm of chromosome 3 in sporadic, von Hippel–Lindau disease-associated, and familial pheochromocytoma. Genes, Chromosomes and Cancer. 1995;13:151–156. doi: 10.1002/gcc.2870130303. [DOI] [PubMed] [Google Scholar]