Abstract
Chronic stress facilitates fear conditioning in rats with hippocampal neuronal atrophy and in rats in which the atrophy is prevented with tianeptine, a serotonin re-uptake enhancer. The purpose of this study was to determine whether the lack of dissociation between fear conditioning performance and hippocampal integrity was masked by the presence of endogenous corticosteroids during training. As in previous studies, rats were stressed by daily restraint (6 h/day for 21 days), trained in the conditioning chamber (day 23), and then assessed for conditioned fear (day 25) at a time when hippocampal dendritic atrophy persists. On the training day, half of the control and stressed rats were injected with metyrapone to reduce corticosterone release. Two hours later, two paired or unpaired presentations of tone and footshock were delivered. Although metyrapone reduced conditioned fear in all rats, only stressed rats showed dissociated fear conditioning (i.e. tone conditioning was reduced while contextual conditioning was eliminated). Chronically stressed rats, regardless of metyrapone treatment displayed more rearing in the open field when tested immediately after the completion of fear conditioning. These data support the hypothesis that increased emotionality and enhanced fear conditioning exhibited by chronically stressed rats may be due to endogenous corticosterone secretion at the time of fear conditioned training. Moreover, these data suggest that chronic stress impairs hippocampal-dependent processes more robustly than hippocampal-independent processes after metyrapone to reduce corticosterone secretion during aversive training.
Keywords: Amygdala, Hippocampus, Fear conditioning, Glucocorticoid, Learning
INTRODUCTION
Chronic stress is associated with many conditions including depression, Cushing’s syndrome, schizophrenia, and Alzheimer’s disease (de Leon et al., 1988, 1993; Starkman et al., 1992; Bogerts et al., 1993; Fukuzako et al., 1996; Sheline et al., 1996). A common factor in these disorders is hippocampal shrinkage, suggesting that chronic stress may play some role in hippocampal atrophy, regardless of disease etiology. Indeed, exposure to stress for many months or years causes hippocampal cell death in rats and monkeys (Uno et al., 1989; Mizoguchi et al., 1992). Repeated psychosocial stress in tree shrews and rats produces hippocampal atrophy, assessed by reduced dendritic branching and shortened dendritic lengths of CA3 neurones (Watanabe et al., 1992c; Magariños and McEwen, 1995a; Magariños et al., 1996; Galea et al., 1997; Conrad et al., 1999b). The stress-induced hyper-secretion of glucocorticoids (i.e. corticosterone or cortisol) is responsible for these changes because glucocorticoid elevations exacerbate hippocampal atrophy (Sapolsky et al., 1985, 1990; Woolley et al., 1990; Watanabe et al., 1992a), and glucocorticoid reduction attenuates hippocampal atrophy and/or damage (Landfield et al., 1981; Magariños and McEwen, 1995b; Starkman et al., 1999).
Recent studies investigated whether hippocampal dendritic atrophy caused by repeated restraint stress compromises hippocampal function. As hypothesized, three weeks of restraint stress impaired performance on the Y-maze (Conrad et al., 1996) and radial arm maze (Luine et al., 1994). In addition, rats treated with tianeptine to prevent hippocampal dendritic atrophy demonstrated spatial learning and memory performance similar to controls (Luine et al., 1994; Conrad et al., 1996). Tianeptine enhances serotonergic re-uptake, which prevents stress-induced dendritic atrophy without inhibiting other physiological responses to chronic stress, such as reduced weight gain, shrunken thymus and enlarged adrenals (Watanabe et al., 1992b). The normal performance from chronically stressed rats treated with tianeptine and impaired performance from chronically stressed rats given vehicle strongly suggested that hippocampal atrophy was responsible for the spatial memory deficit. Spatial learning and memory were also impaired after exposure to stress levels of corticosterone for weeks or months (Luine et al., 1993; Dachir et al., 1993; Arbel et al., 1994; Bardgett et al., 1994; Bodnoff et al., 1995; Endo et al., 1996; Krugers et al., 1997, but cf. Bardgett et al., 1996; Clark et al., 1995).
Stress-induced CA3 dendritic retraction suggests that hippocampal-dependent functions may be most compromised after chronic stress. To test this hypothesis, classical fear conditioning was used to determine whether chronic stress selectively impairs hippocampal-dependent memory, such as contextual conditioning, compared to hippocampal-independent memory, such as cued conditioning (Conrad et al., 1999b). In classical conditioning, a tone (the conditioned stimulus, CS), acting as a cue, is paired with an aversive footshock (the unconditioned stimulus, US), which evokes a characteristic freezing response in rats (Blanchard and Blanchard, 1969). With repeated CS–US pairings, both the environment and tone elicit freezing in the absence of the shock. Lesions to the hippocampus or its afferents attenuate contextual fear conditioning without disrupting cued fear conditioning (Selden et al., 1991; Kim and Fanselow, 1992; Bechara et al., 1995; Phillips and LeDoux, 1992, 1994, 1995), suggesting that freezing to the environment is hippocampal-dependent, whereas freezing to tone is hippocampal-independent. Amygdala lesions impair contextual and cued fear conditioning equally (Phillips and LeDoux, 1992), and intra-amygdala injections of corticotrophin releasing factor facilitate inhibitory avoidance (Liang and Lee, 1988). Therefore, the hippocampus is implicated when contextual conditioning is most selectively impaired, whereas the amygdala plays a greater role when both contextual and cued conditioning are equally affected.
Although parameters in which hippocampal lesions impaired contextual and not cued conditioning (Phillips and LeDoux, 1992) were identical, these results were not replicated after stress-induced CA3 dendritic atrophy (Conrad et al., 1999b). Instead, chronic stress facilitated freezing to both context and cue, and rats injected daily with tianeptine to prevent dendritic atrophy performed similarly to stressed rats with atrophy (Conrad et al., 1999b). One explanation is that chronic stress influenced both hippocampal-dependent and -independent processes through mechanisms other than hippocampal dendritic atrophy. Alternatively, a hyperactive hypothalamic-pituitary-adrenal (HPA) axis in the stressed rats may have masked their hippocampal impairment. For example, hippocampal corticosteroid receptors are down-regulated after chronic stress (Sapolsky et al., 1984; Eldridge et al., 1989), preventing the hippocampus from inhibiting corticosterone secretion by the HPA axis. Since fear is potentiated by corticosterone (Corodimas et al., 1994), chronically stressed rats may have exhibited more freezing due to a hyperactive HPA axis and enhanced corticosterone secretion in response to novel stressors.
To further investigate this issue, chronically stressed rats were tested on fear conditioning again, but the endogenous secretion of corticosterone was attenuated on the day of training with metyrapone. Metyrapone is a synthetic steroidogenesis inhibitor that blocks 11 β-hydroxylase, the enzyme responsible for converting deoxycorticosterone to corticosterone within the adrenal cortex (Schimmer and Parker, 1996). The result is attenuation of corticosterone synthesis and release in stressed animals (Strashimirov and Bohus, 1966) without disruption of basal corticosterone levels or adrenal medullary hormone release (Roozendaal et al., 1996a). The one-time use of metyrapone allows the long-term effects of prior chronic stress on hippocampal-dependent memory to be observed without interference from the temporary surge of corticosterone during fear conditioning training. Under these conditions, chronically stressed rats were hypothesized to show impaired hippocampal-dependent contextual conditioning and functional hippocampal-independent cued conditioning.
MATERIALS AND METHODS
Subjects
A total of 112 naive Sprague-Dawley male rats (200–300 g on arrival) were pair-housed in plastic cages with wire lids and in temperature controlled rooms (20–27°C) on a 12 h light-dark cycle (lights on at 0600 h). Rats arrived one week before commencement of the studies and were given rodent chow (Teklab, Harlan) and tap water ad libitum.
Apparatus
Fear Conditioning Chambers
Two rodent fear conditioning chambers were used and will be referred to as the shock and nonshock chambers (Coulbourn Instruments, E10-18TC). For both, a PC interfaced card (Coulbourn, L18-16S/C), a universal line (Coulbourn, L91-04S), and Winlinc software controlled stimulus presentation (v 1.1, Coulbourn, D91-04). The tone (CS) was produced by a frequency generator (Coulbourn, E12-01) and was presented through a speaker located in a side panel of the conditioning chamber. A camera was mounted on the ceiling (Coulbourn, E27-01) so that behavior could be videotaped and analyzed separately. A small house light (Coulbourn, E11-01) and infrared lights (Coulbourn, E27-91) were located on side panels. The infrared lights denoted the onset/offset of the tone on the non-audio videotape recordings and could not be detected by the rats.
The shock chamber was placed within a sound-attenuating cubicle (Coulbourn, E10-23, white). The shock (US) was a direct current (0.5s, Coulbourn, E13-14), equally distributed through a metal grid floor (Coulbourn, E10-18RF). The nonshock chamber was distinguished by location (different testing room), chamber wall color (black as opposed to transparent), a cross-pattern metal floor (Coulbourn, E10-18NS), lack of sound-attenuating cubicle, and scent of cleaning solvent (double distilled water as opposed to ethanol).
Open Field Arena
The open field was a square arena (100 cm × 100 cm; height: 50 cm) constructed of heavy black plastic. The maze was divided equally into 25 quadrants, and a ceiling-mounted camera allowed behavior to be videotaped and analyzed separately. This maze was located in a novel room and was cleaned with sodium bicarbonate between trials.
Restraint Stress and Pharmacological Treatment
The procedures described were approved by the Institutional Animal Care and Use Committee at Arizona State University and conformed to the applicable portions of the Animal Welfare Act and, “Guide for the Care and Use of Laboratory Animals” by DHHS.
Rats were randomly assigned to one of two treatment conditions, Stress or Control, and were maintained in two separate housing chambers. The stressed rats (n = 56) were placed in wire-mesh restraints in their home cages for 6h a day for 21 consecutive days (beginning at 0900 h; exceptions occurred on day 9 of the paired study and day 7 of the unpaired study when rats were placed in restraints at 1400 h). Controls (n = 56) were undisturbed during this time. Body weights for all rats were obtained on days 1, 7, 14 and 21.
Half of the stressed (n = 28) and control rats (n = 28) in all studies were injected with metyrapone (Aldrich, 200mg/kg, s.c.) 2h prior to training on fear conditioning (day 23) to suppress the surge of endogenous corticosterone induced by training. The remaining rats (n = 56) were injected with vehicle (1.0 ml/kg, dimethyl sulfate, s.c.).
Procedure
The day after restraint ended (day 22, adaptation), rats were transported in their home cages to the fear conditioning room and were individually placed in fear conditioning chambers for 20min. Following adaptation, rats were returned to their home cages. The chambers were cleaned with 95% ethanol between trials. On the following day (day 23, training), rats were injected with metyrapone or vehicle, creating the four conditions: Control+Vehicle (C/V); Control+Metyrapone (CM); Stress+Vehicle (S/V); Stress+Metyrapone (S/M). Two hours after injection, rats were placed into fear conditioning chambers and after 140s, rats in the paired condition (Exp. 1: n = 40,10 rats/group), were presented with a 20 s tone (75 dB, ≈3.0 kHz), immediately followed by a brief footshock (500 ms, 0.25 mA). Tone and shock were repeated 110s later. In contrast, rats in the unpaired condition (Exp. 2: n = 40, 10 rats/group) were presented with a 20s tone (75 dB, ≈3.0 kHz) after 100s. Footshock (500ms, 0.25mA) did not follow the tone but was presented after a 60 s inter-stimulus-interval. The tone-shock series was presented again 154s later. After training, rats were returned to their home cages.
Two days after training (day 25, testing), rats were returned to the training chamber for a period of 5 min with tone and shock absent. Behavior was videotaped for later assessment of contextual fear conditioning. The rats were then brought to a different testing room and placed in the nonshock chamber. In these chambers, rats were presented with tone (CS, 75 dB, ≈3.0 kHz) only. A period of 80s elapsed before three 20s tones were presented (inter-stimulus-interval = 60s). The chamber was cleaned with double distilled water between trials. Following the presentation of tones in the nonshock chambers, rats were brought to a third and final testing room where they were placed in a novel, open field maze to assess overall activity, including general arousal, anxiety, and emotionality. Rats were randomly placed along one of the arena’s four walls and allowed to explore for 5 min before being returned to their home cages. This room was moderately illuminated, and the experimenter was hidden behind a screen.
Pain Threshold Detection
To eliminate the possibility that chronic stress or metyrapone altered the threshold of pain detection, a separate group of rats was tested (n = 32). Previously described procedures for restraint stress, chamber adaptation and metyrapone injection were followed through day 23, creating the four conditions: C/V, CM, S/V, SM (8 rats/group). However, 2h following metyrapone injections on day 23, rats were placed into the fear conditioning chambers and a series of brief footshocks (500ms) were delivered. The footshock intensity began at 0 mA and increased by increments of approximately 0.02mA. Shocks were manually presented at varying intervals ranging from 10 to 30s, and the current (mA) eliciting the first flinch and jump was recorded. Rats received between 15 and 36 shocks, which ended when the first jump was observed.
Quantification and Statistical Analysis
Fear Conditioning
Fear conditioning to context and cue were measured during testing on day 25. Freezing was determined by the animal’s motionless posture except for movement associated with respiration (Blanchard and Blanchard, 1969). Fear conditioning to context was determined by the duration of freezing in the shock chamber in a 5 min period. Fear conditioning to cue was determined by the duration of freezing to the three tone presentations (60s total) in the nonshock chamber. Baseline freezing to context was ascertained by measuring freezing during the first minute of training on day 23 prior to the presentation of tone. Similarly, baseline freezing to cue was determined by measuring freezing to the first tone presentation in a 20s period. The amount of freezing, converted into percentages, was analyzed by a three-factor repeated measures ANOVA with two between-subject measures, Treatment (control or stress) and Drug (vehicle or metyrapone), and one within-subjects measure, day of procedure (training and testing).
The Grubb’s outlier test was applied to the data from freezing during context prior to tone and shock presentation on the day of training. Any animal that was determined to be an outlier was removed from all statistical analyses. In the first experiment, one animal was removed from the C/M group, giving rise to 9 animals in that group and 10 animals in the remaining groups (C/V, S/V, S/M). The amount of freezing to context and tone during training was not recorded for two animals in the C/V group due to a recording error, but their performance was included in all other measures. For the second experiment, the Grubb’s test eliminated an animal in the C/V group, leaving 9 animals for statistical analysis. The other groups contained 10 animals each {C/M, S/V, S/M).
Open Field
Measures of general activity and arousal were determined by the total number of quadrant crossings and rearings. The total number of crossings was determined by how often an animal’s forepaw(s) entered a quadrant from an adjacent quadrant throughout the 5 min. Rearings were noted when a rat stood on its two hind paws. The data were analyzed by a two-factor ANOVA with two between-subject measures, Treatment (control or stress) and Drug (vehicle or metyrapone), and two dependent variables, quadrant crossings and rearings.
Body Weights
Body weight data were analyzed by a two-factor repeated measures ANOVA with one between-subject measure, Treatment (control or stress), and one within-subjects measure, day (days 1, 7, 14, and 21).
All Measures
The investigator was unaware of the treatment condition until after quantification was complete. Measures of behavior analysis by two independent investigators were compared and revealed an inter-rater reliability of 96%. An analysis was considered significant if a p-value of 0.05 or less was obtained. Least significant difference (LSD) post hoc tests were conducted. Data are represented by means ±S.E.M.
RESULTS
Paired Fear Conditioning
Hippocampal-dependent contextual conditioning was most impaired after chronic stress when metyrapone was injected during training. A three-factor repeated measures ANOVA revealed a significant three-way interaction among Treatment and Drug for freezing to context by Day, F(1, 33) = 3.88, p = 0.05. Post hoc tests revealed that stressed rats given metyrapone did not increase freezing to context on testing day compared to their baseline performance on training day (Fig. 1A, open square). In contrast, all other groups showed enhanced freezing to context on the day of testing compared to their performance on training day (Fig. 1A). Post hoc tests also revealed that control and stressed rats given vehicle and control rats given metyrapone froze significantly more to context on testing day than stressed rats injected with metyrapone. Although post hoc tests for the 3-way interaction did not reveal group differences on training day, a separate 2-way ANOVA was performed for training to determine whether metyrapone influenced freezing. During training, only a main effect of drug was revealed, F(1,33) = 8.123, p<0.01, with metyrapone-treated rats freezing slightly more than vehicle-treated rats.
FIGURE 1.
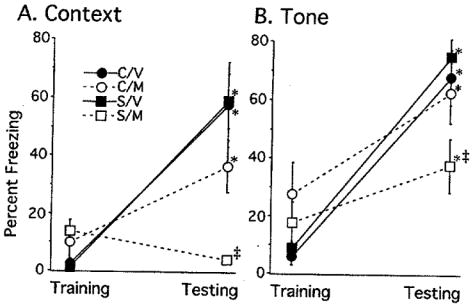
Percentage of Freezing to Context and Tone during the Paired Condition. (A) Chronically stressed rats treated with metyrapone (S/M) showed a lack of freezing to contextual conditioning, which was not significantly different from their baseline measure on training day. In contrast, control rats injected with vehicle or metyrapone (C/V and C/M) and stressed rats injected with vehicle (S/V) were not statistically different and demonstrated enhanced freezing to context compared to their baseline measures on training day (interaction among Treatment, Drug and Day, F(1, 33) = 3.88, p = 0.05. (B) For cued fear conditioning, all groups showed enhanced freezing to tone compared to their baseline measure on training day. Rats injected with vehicle (C/V, S/V) froze significantly more on the testing day compared to rats injected with metyrapone (C/M, S/M). (*) p<0.05 compared to the same group’s baseline performance during training (interaction between Drug and Day, F(1,33) = 11.91, p<0.005). (‡) p<0.05 compared to C/V, C/M, S/V on testing day. Data are represented by means ± S.E.M. with 8–10 subjects/group. C = Control, M=Metyrapone, S=Stress, V=Vehicle.
For cued conditioning, a three-factor repeated measures ANOVA did not reveal a three way interaction between Treatment, Drug and Day. However, the analysis did reveal a significant two-way interaction of Drug by Day for freezing to tone, F(1,33) = 11.91, p<0.005. Control and stressed rats given metyrapone, froze significantly less to the tone during testing day than vehicle-injected control and stressed rats (Fig. 1B). On training day, however, freezing to tone was higher for control and stressed rats given metyrapone compared to control and stressed rats given vehicle. Lastly, a significant main effect was found for freezing to tone across days, whereby all groups regardless of treatment (control, stress) or drug (metyrapone, vehicle), froze significantly more on testing day compared to their baseline measure on training day, F(1,33) = 73.89, p<0.0001 (Fig. 1B). No other main effects or interactions were significant.
To determine the effect of chronic restraint stress and prior drug treatment on response to novelty, open field exploration and freezing to the nonshock chamber prior to tone onset during testing day were analyzed. For the open field measure, a two-factor ANOVA was performed on the two independent variables of Treatment (control or stress) and Drug (vehicle or metyrapone) for number of quadrant crossings and total number of rearings. There was no significant effect of chronic restraint stress or prior metyrapone injection on exploration as measured by quadrant crossings (Fig. 2A). However, previous history of repeated restraint enhanced rearing activity, regardless of prior drug treatment F(1,35) = 6.26, p<0.05 (Fig. 2B). The main effect of drug and interaction between treatment and drug were not significant, p>0.1. A similar two-way ANOVA was performed on the amount of freezing in the nonshock chamber on the testing day prior to the onset of the tone. There were no significant main effects or interaction, p>0.1 (Fig. 2C), probably because the controls showed high variability in freezing levels.
FIGURE 2.
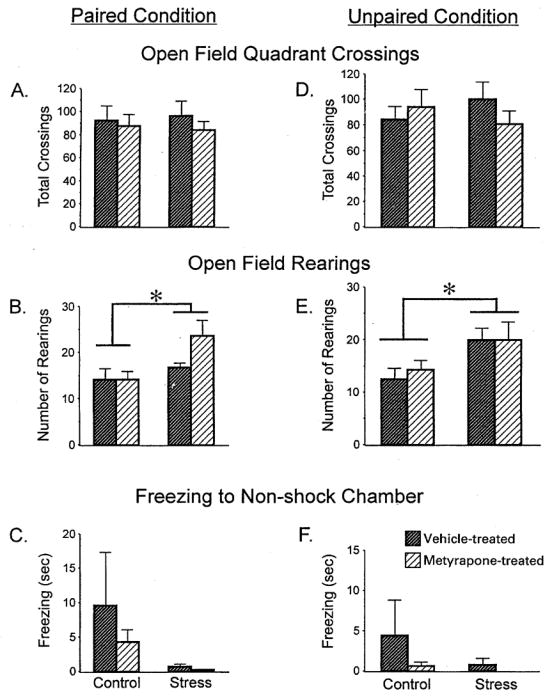
Emotionality in the Paired (A, B, C) and Unpaired (D, E, F) Conditions. (A, D) There were no significant effects of treatment or drug on total grid crossings in the open field. (B, E) Chronically stressed and control rats injected with metyrapone reared more than vehicle-injected rats in the open field (main effect of Treatment for paired, F(1, 33) = 6.26, p0 < 0.05, and unpaired, F(1, 34) = 7.43, p0< 0.01). (C, F) The amount of freezing in the non-shock chamber prior to tone presentation was not significantly different among groups. Data are represented by means±S.E.M. with 8–10 subjects/group. (*) p< 0.05.
Body weight gain measures were used to validate repeated restraint evoked physiological stress. A two-factor repeated measures ANOVA with one between-subject measure of Treatment (control or stress) and one within-subjects measure of weight across Days (1, 7, 14, and 21) showed a significant two-way interaction, F(3, 111) = 6.233, p<0.001. Post hoc analyses revealed that stressed rats had lower body weights than controls on each day of measurement and gained weight more slowly compared to control rats throughout the 21 day restraint stress treatment (Table I).
TABLE I.
Weekly body weights (g) during the 21-day restraint. Chronically stressed rats in the paired condition had lower body weights at the onset and they gained weight more slowly throughout the 21 days (interaction between Treatment and Day, F(3,111) = 6.233, p<0.001). Stressed rats in the unpaired condition also gained weight more slowly (F(3,111) = 181.48, p<0.0001)). Data are represented by means±S.E.M. with 8–10 subjects/group
| Day 1 | Day 7 | Day 14 | Day 21 | ||
|---|---|---|---|---|---|
| Paired condition | Control | 305±7 | 352±6 | 404±6 | 448±6 |
| Stress | 250±8 | 290±7 | 334±5 | 368±4 | |
| Unpaired condition | Control | 326±5 | 385±5 | 430±6 | 462±7 |
| Stress | 320±3 | 351±4 | 358±3 | 376±4 |
Unpaired Fear Conditioning
To demonstrate that freezing to tone was based on the paired presentation of tone and shock, another set of rats was fear conditioned in a situation where tone did not predict shock. In this unpaired condition, groups were hypothesized to show similar or lower freezing levels to tone compared to baseline levels because the tone did not predict shock and may even signal a “safe” non-shock period. For freezing to context, a three-factor repeated measures ANOVA did not reveal a significant three-way interaction. However, the analysis did reveal a significant two-way interaction between Drug and Day for freezing to context, F(1,35) = 49.33, p<0.0001. Post hoc tests revealed that rats given vehicle froze significantly more to context on testing day compared to their baseline measure on training day, regardless of whether they were previously stressed (Fig. 3A, closed symbols). In contrast, control and stressed rats given metyrapone did not differ in their freezing levels to context across training and testing days (Fig. 3A, open symbols). Finally, vehicle-injected rats froze significantly less than metyrapone-injected rats on training day and significantly more than them on testing day.
FIGURE 3.
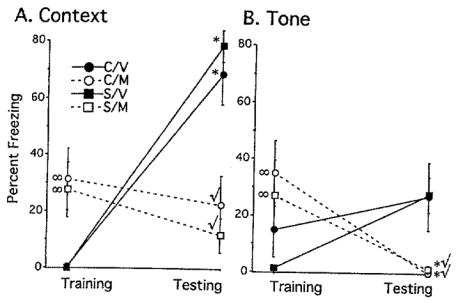
Percentage of Freezing to Context and Tone during the Unpaired Condition. For both contextual and cued conditioning, a significant interaction between Drug and Day was revealed without any chronic stress effect. (A) Control and stressed rats injected with vehicle (C/V, S/V) froze more to context compared to their baseline measure during training. Metyrapone-treated rats (C/M, S/M) showed no difference in freezing between training and testing days. (B) Rats injected with metyrapone froze significantly less to tone on testing day compared to their baseline levels on training day (C/M, S/M). Data are represented by means±S.E.M. with 9–10 subjects/group. C=Control, M=Metyrapone, S=Stress, V=Vehicle. (*) p<0.05 compared to the same group’s baseline performance during training. (∞) p<0.05 compared to C/V, S/V on training day. (✓) p<0.05 compared to C/V, S/V on testing day.
For cued fear conditioning, a three-factor repeated measures ANOVA did not reveal a significant three-way interaction. However, the analysis did reveal a significant two-way interaction between Drug and Day for freezing to tone, F(1, 35) = 20.558, p<0.0001. Post hoc tests revealed that rats injected with metyrapone froze significantly less to tone on testing day compared to their baseline measure on training day (Fig. 3B). Rats injected with vehicle showed no differences in freezing to tone between training and testing days (Fig. 3B). As observed in the context measure, metyrapone-injected rats froze more to tone than vehicle-injected rats on training day, but froze less to tone compared to vehicle-injected rats on testing day.
Open field measures were used to analyze activity levels in a novel environment and the results paralleled those for the first experiment. A two-factor ANOVA for quadrant crossings using Treatment (stress, control) and Drug (vehicle, metyrapone) as the independent variables did not reveal any significant effects (Fig. 2B, p>0.1). The analysis for the other measure of open field activity, number of rearings, revealed a main effect of Treatment, F(1,34) = 7.434, p<0.01. Stressed rats reared more than control rats, whether or not they were injected with metyrapone or vehicle (Fig. 2E). Response to novelty was also determined by measuring the amount of freezing in the nonshock chamber on the testing day prior to the presentation of tone. A two-factor ANOVA revealed no significant main effects or interactions (p<0.1), indicating that prior exposure to stress or metyrapone treatment did not influence freezing to a novel environment (Fig. 2F).
Body weight gain measures confirmed that restraint stress caused physiological stress. A two-factor ANOVA with one between-subject measure of Treatment (control or stress) and one repeated measure of weight by Day (1, 7, 14, 21) showed a significant two-way interaction, F(3,111)= 181.48, p<0.0001. Although stressed and control rats showed no significant differences in body weight on the first day of restraint, body weights were significantly different when measured on all subsequent days (7, 14, and 21). Therefore, chronically stressed rats gained weight more slowly (Table I).
Pain Threshold
An additional group of rats was tested to determine whether stress or drug treatment altered the threshold of pain detection. A two-factor ANOVA for Treatment (control or stress) and Drug (vehicle or metyrapone) revealed a near-significant main effect of treatment for flinch, F(1, 28) = 3.87, p = 0.06. Stressed rats may have detected the shock at lower currents than controls, regardless of whether they received vehicle or metyrapone (Table II). In contrast, the same analysis for jump did not demonstrate any significant main effects or interactions.
TABLE II.
Assessment of sensitivity to pain threshold. Chronically stressed rats regardless of whether they were injected with vehicle or metyrapone showed a tendency for greater sensitivity to footshock, as assessed by flinch, compared to controls (main effect of Treatment, F(1, 28) = 3.87, p = 0.06). In contrast, no significant effect for jump was revealed. Data are represented by means±S.E.M. and values represent mA
| Flinch | Jump | |
|---|---|---|
| Control+vehicle | 0.048±0.005* | 0.072±0.003 |
| Control+metyrapone | 0.046±0.002* | 0.076±0.001 |
| Stress+vehicle | 0.042±0.004 | 0.067±0.005 |
| Stress+metyrapone | 0.039±0.003 | 0.069±0.004 |
p = 0.06, controls (vehicle+metyrapone) compared to stressed rats (vehicle+metyrapone). Subjects/group=8.
DISCUSSION
The purpose of this study was to investigate the long-term consequences of restraint stress on fear conditioning when adrenal glucocorticoid secretion was attenuated during training. Rats were restrained for 6h/day for 21 days and then tested on fear conditioning within four days. On the day of training, rats were injected with metyrapone to suppress stress levels of corticosterone, thereby reducing the potential acute actions of adrenal glucocorticoid on fear conditioning and allowing the prior effects of prior chronic restraint to be observed. When metyrapone was injected on the day of training, prior chronic restraint blocked contextual conditioning, a form of learning that depends upon the hippocampus or its afferents (Selden et al., 1991; Kim and Fanselow, 1992; Phillips and LeDoux, 1992; 1995; Bechara et al., 1995). Moreover, these same animals showed attenuated, but significant conditioning to tone, which does not require an intact hippocampus (Selden et al., 1991; Kim and Fanselow, 1992; Phillips and LeDoux, 1992; Bechara et al., 1995). Therefore, metyrapone was not sufficient to eliminate fear conditioning as cued conditioning persisted in metyrapone-injected stressed rats, and both cued and contextual conditioning persisted in metyrapone-injected controls. These data suggest that hippocampal-mediated functions are more severely impaired by chronic stress when corticosterone release is reduced during training.
Control Studies
Pain sensitivity was evaluated to determine whether it contributed to performance differences. Metyrapone had no effect on shock perception, but chronically stressed rats detected the shock at a lower threshold compared to controls, as determined by flinch. However, groups did not differ when the shock may have become painful, as indicated by jump. Therefore, differences during fear conditioning are less likely to be attributed to pain perception because the current used during training more closely approximated the current that caused the animals to jump, which was not statistically different among groups.
To determine whether rats froze to tone because they learned its predictive value for shock or whether enhanced freezing was due to sensitization, rats were trained to an unpaired presentation of tone and shock. Rats were expected to freeze less to tone and more to context because tone signaled the absence of shock or a “safe” period while context indicated potential shock or a “non-safe” interval. In the paired condition, contextual cues tend to be overshadowed by phasic cues such as tone and thus, form weaker associations with the shock (Odling-Smee, 1975; Rickert et al., 1979). In the unpaired condition, context does not predict shock, but it does indicate when the shock is likely to occur and hence, the association between context and shock is stronger (Phillips and LeDoux, 1994). As expected, the groups did not show enhanced freezing to the unpaired tone, indicating that sensitization during the paired condition was not an issue (Fig. 3B, compare each group at training and testing). Metyrapone-treated rats demonstrate performance as hypothesized by not freezing during the unpaired tone presentation. In contrast, vehicle-treated rats show slightly elevated and highly variable freezing levels. For freezing to context, vehicle-treated rats performed as expected by exhibiting drastically elevated freezing levels compared to their baseline performance during training. In contrast, metyrapone-treated rats did not have elevated freezing levels compared to their baseline levels. However, the baseline levels of metyrapone-treated rats during the context measure are significantly elevated compared to vehicle-treated rats on the same day. Certainly, metyrapone enhanced freezing during training for both tone and context and attenuated performance during testing. More importantly, all groups showed enhanced freezing to context compared to tone (Fig. 3, compare freezing on testing day to context (A) and tone (B) for each group).
For freezing to tone during the unpaired condition, the stressed rats injected with vehicle showed a tendency toward enhanced freezing, probably due to sensitization and not learning (Fig. 3B). Open field measures hint that systems outside the hippocampus influence behavior in chronically stressed rats. For instance, rats reared more when chronically stressed, but showed no difference in grid crossings. The reverse pattern was observed in our previous study with chronically stressed rats crossing fewer grids and exhibiting no difference in rearing (Conrad et al., 1999b). The discrepancy between the two studies is not clear, but as a whole, open field exploration is altered by chronic stress, suggesting that emotionality is affected.
In contrast to the paired condition, the unpaired presentation of tone and shock for chronically stressed rats injected with metyrapone did not reveal a dissociation between contextual and cued fear conditioning, in which contextual conditioning was abolished and cued conditioning persisted. Similar findings have been reported for fear conditioning after hippocampal lesions (Phillips and LeDoux, 1994) with contextual conditioning being impaired after hippocampal lesions only when tone and shock are paired. Phillips and LeDoux (1994) argue that in the paired condition, context is not necessarily the best predictor of the shock and is referred to as “background,” whereas tone is highly predictive of shock and is referred to as “foreground.” When context is a background cue, hippocampal lesions disrupt contextual conditioning more readily. In the unpaired condition, the tone does not predict shock and may even signal a “safe” period, making context more salient. As a consequence, context becomes part of the foreground and hippocampal lesions do not disrupt contextual conditioning as easily. In light of this work, chronic stress- and metyrapone-treated animals performed similarly to hippocampal-impaired animals, suggesting that their performance may have been due to stress-induced CA3 dendritic atrophy.
An important concern is whether metyrapone had sedative effects, which might have interfered with fear conditioning. The 200 mg/kg dose of metyrapone chosen was based upon prior studies that used similar levels to investigate corticosterone actions on brain damage (Strashimirov and Bohus, 1966; Stein and Sapolsky, 1988; Smith-Swintosky et al., 1996; Krugers et al., 1998). However, recent reports indicate that a lower dose was successfully used to prevent corticosterone surges during stress while behaviour was measured (Roozendaal et al., 1996a; Loscertales et al., 1997; de Quervain et al., 1998; Liu et al., 1999). In the current study, metyrapone-injected rats froze slightly more during training when metyrapone was still present. While some sedative actions may have occurred, this cannot fully account for the findings because tranquilized rats would have been equally impaired on contextual and cued fear conditioning. In contrast, chronically stressed rats injected with metyrapone showed a dissociation between contextual and cued fear conditioning (Fig. 1A), with cued conditioning present and contextual conditioning absent. It is also possible that the aversive nature of the paradigm may have helped to maintain alertness during training.
Interestingly, chronic stress did not enhance performance in fear conditioning to both context and tone, which is in contrast to our previous findings (Conrad et al., 1999b). This discrepancy may have arisen from procedural changes to enhance detection of treatment differences. Specifically, training was reduced from two sessions to one because two sessions created a confound of the tone being presented in the same environment in which contextual conditioning was assessed. Consequently, tone conditioning was performed in a neutral chamber in the current study, preventing context from being incorporated into the measure of tone conditioning. Assessment of memory occurred at 48 h, as opposed to 24 h after the tone-shock training session, increasing the difficulty of the task, yet remaining in the four day period that chronic stress-induced hippocampal atrophy persists (Conrad et al., 1999b). Finally shock intensity was reduced from 0.4 to 0.25mA to increase the difficulty of associating context and tone with the shock. The change in shock intensity may have affected fear conditioning because recent evidence indicates that corticosterone is correlated with shock intensity and the establishment of contextual conditioned fear (Cordero et al., 1998). Therefore, the lower footshock current used in the present study may have been sufficiently reduced to eliminate the enhanced freezing of chronically stressed rats, which was observed previously.
Other explanations for the lack of enhanced fear conditioning in chronically stressed rats include the possibility that corticosterone levels were not sufficiently elevated in chronically stressed rats under the conditions of this experiment. In one study, metyrapone reduced learned helplessness as measured by inactivity, which was restored with corticosterone treatment (Báez et al., 1996). That study suggests that inactivity differences arise when corticosterone levels differ. In our current study, low footshock intensities may have been too low to elevate corticosterone levels in chronically stressed rats. In addition, different neural/hormonal mechanisms may account for fear conditioning enhancement induced by prior stress. Finally, the ability of metyrapone to reduce fear conditioning in chronically stressed rats may only apply to conditioning protocols with low shock intensities. Future studies will help sort through the mechanisms and conditions that underlie the effects of chronic stress on fear conditioning.
Implications
Chronic stress influences both hippocampal-dependent and -independent functions, illustrated by enhanced freezing to both context and cue during fear conditioning in previous work (Conrad et al., 1999b) and the tendency of chronically stressed rats to enhance freezing to context and tone, demonstrated in the current study. This enhancement of fear conditioning is mediated by a single episode of elevated corticosterone during fear conditioning training because attenuating corticosterone with metyrapone during training revealed a different outcome. That is, chronic stress impaired hippocampal-dependent behavior more robustly than other’ functions. The selectivity of chronic stress to impair hippocampal function cannot be conclusively linked to stress-induced CA3 dendritic atrophy because hippocampal morphology was not measured in this study. However, stress-induced changes in CA3 morphology is a strong candidate to explain this phenomenon (Conrad et al., 1999b). Consequently, these data imply that a single surge of glucocorticoid (i.e. corticosterone) during training in an aversive learning task conceals the nature of hippocampal-dependent learning in chronically stressed rats.
The aversive nature of fear conditioning and the resulting corticosterone increase during training may have masked the effects of prior chronic stress on hippocampal-dependent learning. For example, corticosterone during training is necessary for footshock-induced inactivity in rats (Báez et al., 1996). Injections of metyrapone to reduce corticosterone cause amnesia on passive avoidance in chicks (Loscertales et al., 1997) and prevent stress-induced facilitation of inhibitory avoidance in rats (Liu et al., 1999). Moreover, corticosterone elevations are correlated with better contextual conditioning (Cordero et al., 1998). Glucocorticoid agonist infusion into the hippocampus facilitates inhibitory avoidance, suggesting that the hippocampus participates in aversive training through direct glucocorticoid actions (Roozendaal and McGaugh, 1997). In contrast, glucocorticoid agonists impair spatial learning on the Y-maze, a task that does not require fear to learn (Conrad et al., 1997, 1999a). Consequently, a single surge of corticosterone is a fundamental component in aversive learning, indicating that metyrapone was effective in revealing the effects of prior chronic stress on fear conditioning. The water maze data do not completely fit with these findings because placing rats in water is stressful and could evoke fear (Roozendaal et al., 1996b; Park et al., in press). However, corticosterone is not solely responsible for performance of the rats in the present study or both control and metyrapone-treated rats would have shown similar fear conditioning to context and cue. Therefore, chronic stress impairs hippocampal-dependent functions, which may be masked when the learning paradigm is aversive and corticosterone levels are elevated during training.
The amygdala may serve a critical role in modulating memory during aversive training when corticosterone levels are enhanced. Glucocorticoids enhance inhibitory avoidance, an effect that is prevented by basolateral amygdala lesions (Roozendaal and McGaugh, 1997). Although lesions of the basolateral amygdala do not alter performance alone, they prevent the modulatory actions of glucocorticoids (for review, see Roozendaal, 2000). Moreover, amygdala lesions block both contextual and cued fear conditioning, whereas hippocampal lesions block contextual conditioning only (Selden et al., 1991; Kim and Fanselow, 1992; Phillips and LeDoux, 1992; Bechara et al., 1995). These data suggest that the enhanced emotionality on open field exploration and enhanced freezing on fear conditioning observed in chronically stressed rats injected with vehicle were caused by modulatory actions of glucocorticoid on the amygdala.
To conclude, chronic stress can enhance or impair memory depending upon the situation. In fear conditioning, chronic stress enhances performance through elevated emotionality (Conrad et al., 1999b), which may be caused in part by the aversive nature of the task and subsequent elevation of corticosterone during training. This enhancement of emotionality may occur through glucocorticoids acting on the amygdala to modulate memory (Cahill and McGaugh, 1998; Roozendaal, 2000). When corticosterone levels are prevented from increasing during fear conditioning training, contextual conditioning is more dramatically impaired than cued conditioning after a history of chronic stress. This dissociation of memory impairment suggests that when low corticosterone levels exist during training, hippocampal-dependent memory is more susceptible to chronic stress than hippocampal-independent memory. Similarly, previous studies have shown that chronic stress impairs spatial memory (Luine et al., 1994; Conrad et al., 1996), which depends heavily upon the hippocampus. Hippocampal-dependent memories may be more susceptible to disruption because hippocampal CA3 neurones exhibit dendritic atrophy after chronic stress (Watanabe et al., 1992c; Magariños et al., 1996; Conrad et al., 1999b), which is not expressed in other hippocampal neurones (Woolley et al., 1990). Therefore, chronic stress and the subsequent dendritic atrophy can be detrimental to memory processes associated with the hippocampus.
Preliminary results were presented at the 1999 Society for Neuroscience Conference in Miami, Florida. This research was supported by the Arizona State University College of Liberal Arts and Sciences (CDC). We gratefully acknowledge Dr Janet Neisewander, Dr Miles Orchinik, Dr Edward Castañeda, and Melanie Paquette’s critique of the experiments and manuscript.
References
- Arbel I, Kadar T, Silbermann M, Levy A. The effects of long-term corticosterone administration on hippocampal morphology and cognitive performance of middle-aged rats. Brain Res. 1994;657:227–235. doi: 10.1016/0006-8993(94)90972-5. [DOI] [PubMed] [Google Scholar]
- Báez M, Siriczman I, Volosin M. Corticosterone is involved in foot shock-induced inactivity in rats. Physiol Behav. 1996;60:795–801. doi: 10.1016/0031-9384(96)00025-x. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Taylor GT, Csernansky JG, Newcomer JW, Nock B. Chronic corticosterone treatment impairs spontaneous alternation behavior in rats. Behav Neural Biol. 1994;61:186–190. doi: 10.1016/s0163-1047(05)80074-3. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Newcomer JW, Taylor GT. The effects of chronic corticosterone on memory performance in the platform maze task. Physiol Behav. 1996;59:1111–1115. doi: 10.1016/0031-9384(95)02172-8. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1120. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard R. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerts B, Lieberman JA, Ashtair M, Bilder RM, De Greef G, Lerner G, Johns C, Masiar S. Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry. 1993;33:236–246. doi: 10.1016/0006-3223(93)90289-p. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasing declarative memory. TINS. 1998;21:273–313. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Clark AS, Mitre MC, Brinck-Johnsen T. Anabolic-androgenic steroid and adrenal steroid effects on hippocampal plasticity. Brain Res. 1995;679:64–71. doi: 10.1016/0006-8993(95)00202-2. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LAM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y-Maze and this effect is blocked by tianeptine pre-treatment. Behav Neurosci. 1996;110:1–14. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, Thanasoulis LC, McEwen BS. The effects of Type I and II corticosteroid receptor agonists on exploratory behavior and spatial memory in the Y-Maze. Brain Res. 1997;759:76–83. doi: 10.1016/s0006-8993(97)00236-9. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, McEwen BS. Support for a bimodal role for Type II adrenal steroid receptors in hippocampus-dependent spatial recognition memory. Neurobiol Learn Mem. 1999a;72:39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Magariños AM, LeDoux JE, McEwen BS. Repeated restraint stress increases fear conditioning, independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999b;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Merino JJ, Sandi C. Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextual fear conditioning. Behav Neurosci. 1998;112:885–891. doi: 10.1037//0735-7044.112.4.885. [DOI] [PubMed] [Google Scholar]
- Corodimas KP, LeDoux JE, Gold PW, Schulkin J. Corticosterone potentiation of conditioned fear in rats. Ann NY Acad Sci. 1994;746:392–393. doi: 10.1111/j.1749-6632.1994.tb39264.x. [DOI] [PubMed] [Google Scholar]
- Dachir S, Kadar T, Robinzon B, Levy A. Cognitive deficits induced in young rats by long-term corticosterone administration. Behav Neural Biol. 1993;60:103–109. doi: 10.1016/0163-1047(93)90173-f. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, McRae T, Tsai JR, George AE, Marcus DL, Freedman M, Wolf AP, McEwen B. Abnormal cortisol response in Alzheimer’s disease linked to hippocampal atrophy. Lancet. 1988;2:391–392. doi: 10.1016/s0140-6736(88)92855-3. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Golomb J, George AE, Convit A, Tarshish CY, McRae T, De Santi S, Smith G, Ferris SH, Noz M, Rusinek H. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. Am J Neuroradiol. 1993;14:897–906. [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJF, Roosendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Eldridge JC, Brodish A, Kute TE, Landfield PW. Apparent age-related resistance of type n hippocampal corticosteroid receptors to down-regulation during chronic escape training. J Neurosci. 1989;9:3237–3242. doi: 10.1523/JNEUROSCI.09-09-03237.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Nishimura J, Kimura F. Impairment of maze learning in rats following long-term glucocorticoid treatments. Neurosci Lett. 1996;203:199–202. doi: 10.1016/0304-3940(95)12296-6. [DOI] [PubMed] [Google Scholar]
- Fukuzako H, Fukuzako T, Hashiguchi T, Hokazono Y, Takeuchi K, Hirakawa K, Ueyama K, Takigawa M, Kajiya Y, Nakajo M, Fujimoto T. Reduction in hippocampal formation volume is caused mainly by its shortening in chronic schizophrenia: assessment by MRI. Biol Psychiatry. 1996;39:938–945. doi: 10.1016/0006-3223(95)00251-0. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–676. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Douma BRK, Andringa G, Bohus B, Korf J, Luiten PGM. Exposure to chronic psychosocial stress and corticosterone in the rat: effects on spatial discrimination learning and hippocampal protein kinase Cγ immunoreactivity. Hippocampus. 1997;7:427–436. doi: 10.1002/(SICI)1098-1063(1997)7:4<427::AID-HIPO8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Kemper HA, Korf J, Ter Horst GJ, Knollema S. Metyrapone reduces rat brain damage and seizures after hypoxia-ischemia: An effect independent of modulation of plasma corticosterone levels. J Cereb Blood Flow Metab. 1998;18:386–390. doi: 10.1097/00004647-199804000-00006. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Baskin RK, Pitler TA. Brain aging correlates: Retardation by hormonal-pharmacological treatments. Science. 1981;214:581–584. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- Liang KC, Lee EHY. Intra-amygdala injections of corticotropin releasing factor facilitate inhibitory avoidance learning and reduce exploratory behavior in rats. Psychopharmacology. 1988;96:232–236. doi: 10.1007/BF00177566. [DOI] [PubMed] [Google Scholar]
- Liu L, Tsuji M, Takeda H, Takada K, Matsumiya T. Adrenocortical suppression blocks the enhancement of memory storage produced by exposure to psychological stress in rats. Brain Res. 1999;821:134–140. doi: 10.1016/s0006-8993(99)01085-9. [DOI] [PubMed] [Google Scholar]
- Loscertales M, Rose SPR, Sandi C. The corticosteroid synthesis inhibitors metyrapone and aminoglutethimide impair long-term memory for a passive avoidance task in day-old chicks. Brain Res. 1997;769:357–361. doi: 10.1016/s0006-8993(97)00735-x. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine VN, Spencer RL, McEwen BS. Effects of chronic corticosterone ingestion on spatial memory performance and hippocampal serotonergic function. Brain Res. 1993;616:65–70. doi: 10.1016/0006-8993(93)90193-q. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors. Neuroscience. 1995a;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory ammo acid receptors. Neuroscience. 1995b;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Kunishita T, Chui DH, Tabira T. Stress induces neuronal death in the hippocampus of castrated rats. Neurosci Lett. 1992;138:157–160. doi: 10.1016/0304-3940(92)90495-s. [DOI] [PubMed] [Google Scholar]
- Odling-Smee FJ. The role of background stimuli during Pavolvian conditioning. Q J Exp Psychol. 1975;27:161–169. doi: 10.1080/14640747508400480. [DOI] [PubMed] [Google Scholar]
- Park CR, Campbell AM, Diamond DM. Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in rats. Biol Psychiatry. doi: 10.1016/s0006-3223(01)01255-0. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1:34–44. [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. J Neurosci. 1995;15:5308–5315. doi: 10.1523/JNEUROSCI.15-07-05308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert EJ, Lorden JF, Dawson R, Jr, Smyly E, Jr, Callahan MF., Jr Stimulus processing and stimulus selection in rats with hippocampal lesions. Behav Neural Biol. 1979;27:454–465. doi: 10.1016/s0163-1047(79)92040-5. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: effects on emotion and memory. Psychoneuroendocrinology. 1996a;21:681–693. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Portillo-Marquez G, McGaugh JL. Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behav Neurosci. 1996b;110:1074–1083. doi: 10.1037//0735-7044.110.5.1074. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur J Neurosci. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984;114:287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmer BP, Parker KL. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 1996. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones; pp. 1459–1485. [Google Scholar]
- Selden NRW, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JC, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Swintosky VL, Pettigrew LC, Sapolsky RM, Phares C, Craddock SD, Brooke SM, Mattson MP. Metyrapone, an inhibitor of glucocorticoid production, reduces brain injury induced by focal and global ischemia and seizures. J Cereb Blood Flow Metab. 1996;16:585–598. doi: 10.1097/00004647-199607000-00008. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s Syndrome. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s Disease. Biol Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- Stein BA, Sapolsky RM. Chemical adrenalectomy reduces hippocampal damage induced by kainic acid. Brain Res. 1988;473:175–180. doi: 10.1016/0006-8993(88)90332-0. [DOI] [PubMed] [Google Scholar]
- Strashimirov D, Bohus B. Effect of 2-methyl-1,2-bis-(3-pyridyl)-1-propanone (SU-4885) on adrenocortical secretion in normal and hypophysectomized rats. Steroids. 1966;7:171–180. doi: 10.1016/0039-128x(66)90024-9. [DOI] [PubMed] [Google Scholar]
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992a;2:431–436. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992b;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992c;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]


