Abstract
Co-occurring psychiatric disorders have been associated with poor prognosis among substance dependent patients, but few studies have examined this association among patients with cocaine dependence (CD). Baseline characteristics and treatment outcome were compared between cocaine-dependent patients with major depression (MDD) (N = 66), attention-deficit/hyperactivity disorder (ADHD) (N = 53), or cocaine dependence without comorbid disorders (CD alone) (N = 48), who had been randomized to the placebo arms of clinical trials of venlafaxine, methylphenidate, and gabapentin, respectively. The three groups differed significantly in racial makeup, with more Caucasians and Hispanics among patients with MDD or ADHD, and more African Americans among those with CD alone. The groups did not differ significantly in treatment retention, with retention rates ranging from 42–47%, or in the rates of achieving 2 consecutive weeks of urine-confirmed abstinence, with rates ranging from 40–50%. Using logistic regression for repeated measures with general estimating equations, modeling the likelihood of a cocaine positive week over time in treatment, diagnostic group was found to interact with the baseline level of cocaine use and time. Among cocaine dependent patients who achieved abstinence at baseline, the groups with MDD or ADHD had better outcome over time than those with CD alone. However, among patients with cocaine positive urines at baseline, MDD or ADHD were associated with poor outcome compared to patients with CD alone. The findings suggest that diagnosis and treatment of co-occurring disorders such as depression and ADHD may be an important component of treatment planning for cocaine dependence, and that baseline level of cocaine use should be included as a covariate in studies evaluating the impact of such treatment.
Keywords: ADHD, major depression, cocaine dependence, treatment retention, treatment outcome
1. Introduction
Both major depressive disorder (MDD) and attention-deficit/hyperactivity disorder (ADHD) are common psychiatric disorders in cocaine-dependent individuals seeking treatment (Rounsaville and Carroll 1991; Levin, Evans and Kleber 1998; Clure et al. 1999). The lifetime prevalence rates of major depression in the general population are notable, with rates ranging from 13–16% (Kessler et al. 2003; Hasin et al. 2005), while the rates of major depression in treatment-seeking cocaine abusers are higher, with rates ranging from 15–30% (Rounsaville and Carroll 1991; Kilbey, Breslau and Andreski 1992; Charney et al. 2005; McLean et al. 1999). Similarly, Kessler et al. (2006) found that the rate of adult ADHD in the U.S. population is 4.4%, whereas recent prevalence studies in substance dependent samples seeking treatment have obtained rates ranging from 15–24% (Levin, Evans and Kleber 1998; King et al. 1999; Schubiner et al. 2000), suggesting that these disorders merit clinical attention in substance abuse treatment settings. Despite this, there remain only limited data characterizing the impact of these co-occurring psychiatric disorders on treatment outcome in cocaine dependent patients.
A few placebo-controlled trials have examined the pharmacological treatment of depression or ADHD in adult cocaine-dependent individuals and, to date, the findings have been mixed. Some studies suggest that depressed cocaine-dependent individuals receiving an antidepressant medication have a greater improvement in their depressive symptoms than those receiving placebo, and trends toward improvement in some measures of cocaine use (Nunes et al. 1995; McDowell et al. 2005), whereas other studies have found no advantage for antidepressants (Cornelius et al. 1998; Schmitz et al. 2001). Two controlled trials of the stimulant methylphenidate among adult cocaine-dependent patients with ADHD (Schneider et al. 2001; Levin et al. epub, 2006a) found a greater improvement in at least some measures of ADHD symptoms for those receiving active medication compared to placebo. Neither trial found methylphenidate different from placebo on global measures of cocaine use outcome such as the overall proportion of cocaine-positive urines, although Levin et al. (epub, 2006a) did find a greater reduction of cocaine-positive urines over time on methylphenidate using a repeated-measures linear model.
There are even less data regarding how well psychotherapeutic approaches work for cocaine-dependent individuals with ADHD or major depression (Rounsaville 2004). Information can be gleaned from the double-blind trials referenced above in which cognitive behavioral interventions were provided, along with the medication. For most pharmacologic trials targeting cocaine-dependent individuals with psychiatric comorbidities, retention rates are below 50% (McDowell et al. 2005; Schmitz et al. 2001; Schubiner et al. 2002), demonstrating that cocaine-dependent individuals with psychiatric comorbidities are difficult to retain in treatment. In these trials less than 25% in either the active medication groups or placebo groups achieved a prolonged period of abstinence or had a proportion of negative cocaine urines exceeding greater than 50% (McDowell et al. 2005; Schmitz et al. 2001; Schubiner et al. 2002; Evans et al. Submitted). Further, these data suggest that cognitive behavioral therapy, in addition to the pharmacotherapy, is not substantially enhancing retention or abstinence. This is consistent with other studies suggesting that cognitive behavioral therapy has a modest impact on abstinence and treatment retention (Carroll 1997). It is less clear whether cocaine-dependent individuals with psychiatric comorbidity are more difficult to retain in treatment trial or have poorer treatment response than those without psychiatric comorbidity.
Longitudinal naturalistic studies examining the impact of co-occurring depression on the outcome of drug or alcohol abuse in general have also produced conflicting findings. A number of studies show major depression, or psychiatric severity in general, to be associated with poorer substance use outcome or treatment retention (Rounsaville et al. 1986; Kosten, Rounsaville and Kleber 1987; Rounsaville et al. 1987; Carroll et al. 1993; Hasin et al. 2002; Greenfield et al. 1998; Charney et al. 2005; Dodge, Sindelar and Sinha 2005), whereas other studies suggest that there is no negative impact on treatment retention or perhaps even a positive impact of depression on retention in treatment (Rounsaville et al. 1987; Agosti, Stewart and Quitkin 1991; McKay et al. 2002; Siqueland et al. 2002; Brown et al. 1998). In one review of this literature, Hasin and colleagues (2004) conclude that major depression tends to confer a poor prognosis, while the impact of depressive symptoms, measured with cross-sectional scales, is less clear. This suggests the importance of a careful clinical history and diagnosis of a depressive disorder. Rounsaville and colleagues (2004) suggest that the varied results may be explained by the fact that the low energy, impaired cognition, and anhedonia of depression may have a negative impact on the effectiveness of treatment, while on the other hand the pain of depressive symptoms may motivate patients to remain engaged in treatment. Of note, few of these naturalistic studies focused specifically on cocaine-dependent individuals.
Naturalistic studies assessing the impact of ADHD on outcome in cocaine-dependent individuals have also been limited. Two studies found that adult cocaine abusers with ADHD are less likely to complete and/or do well with substance abuse treatment (Carroll et al. 1993; Levin et al. 2002) whereas King et al. (1999) found no prognostic impact of ADHD in methadone-maintenance patients. Methadone-maintenance patients may be less likely to drop-out of treatment because of the reinforcing effects of methadone. Deficits in attention and memory have been found to be associated with high dropout rates among cocaine dependent patients without ADHD (Aharonovich et al. 2006).
Our group concurrently conducted three clinical trials of medications for patients with 1) cocaine dependence and major depression, 2) cocaine dependence and ADHD, and 3) cocaine dependence without co-occurring disorders. Other than differing co-occurring diagnoses and medications being tested, these studies were similar in inclusion/exclusion criteria and other methodologic features, including that all patients received weekly, individual manual-guided cognitive-behavioral relapse prevention therapy, a commonly used psychological platform in pharmacologic research trials. By comparing the placebo groups across these three trials, we have the opportunity to evaluate the impact of co-occurring major depression or ADHD on treatment outcome for cocaine dependent patients undergoing standard psychosocial outpatient treatment. Based on the available literature reviewed above, it was hypothesized that ADHD would be associated with a higher drop-out rate, while both major depression and ADHD would be associated with worse outcome of cocaine use.
2. Methods
2.1. Participants
This analysis compares baseline features and treatment outcome for patients randomized into the placebo arms of each of three randomized trials conducted at two university affiliated research clinics: 1) a trial of the antidepressant medication venlafaxine for cocaine dependent patients with current major depression (CD + MDD); 2) a trial of the stimulant medication methylphenidate for cocaine dependent patients with adult ADHD (CD + ADHD) (Levin et al. epub, 2006b); and 3) a trial of gabapentin for cocaine dependent patients without major depression, ADHD, or other major psychiatric comorbidity (CD alone) (Bisaga et al. 2006). There were no differences in the sampling method as all studies were run concurrently. Most patients were recruited by advertisements and none of the patients came from inpatient or other institutional settings. The studies were reviewed and approved by the Institutional Review Boards of the New York State Psychiatric Institute/Columbia University and of the Long Island Jewish Medical Center, and all participants gave written informed consent.
Participants were recruited through referrals and local advertising in the New York City metropolitan area. Prospective participants underwent a detailed medical and psychiatric assessment conducted by the same team of clinicians. The medical screening included a complete history and physical exam, an electrocardiogram and laboratory tests (including hematology, blood chemistries, liver profile, thyroid stimulating hormone, and blood pregnancy test for females). The psychiatric evaluation included the Structured Clinical Interview (SCID) for DSM-IV- Axis I disorders (First 1995) and the KSCID module for childhood and adult ADHD (Hien 1994), conducted by doctoral or masters level clinical psychologists. In order to be included in the venlafaxine trial, patients had to meet DSM-IV criteria for cocaine dependence and for current major depression that had either occurred prior to the onset of substance use disorder on a lifetime basis, had occurred during a period of prolonged abstinence in the past, or had persisted for at least 3 months in the current episode. To be included in the methylphenidate trial, patients had to meet DSM-IV criteria for cocaine dependence, to have met DSM-IV criteria for childhood ADHD, and also to meet criteria for current adult ADHD. Patients with DSM-IV cocaine dependence, but without current major depression or ADHD, were included in the gabapentin trial.
Aside from presence or absence of major depression or ADHD, inclusion/exclusion criteria for the three trials were similar. Participants had to be between the ages of 18–60 and able to give informed consent and were excluded if they: 1) met DSM-IV criteria for other current psychiatric disorders (other than ADHD, depression or substance use disorders) which required psychiatric intervention, such as bipolar illness or schizophrenia; 2) were taking any prescription psychotropic medication; 3) had an unstable medical condition that would make participation hazardous (e.g. uncontrolled diabetes); 4) had a known hypersensitivity to the study medication; 5) were nursing and/or pregnant; 6) were judged to be at significant risk for suicide; or 7) had opioid dependence, or were physiologically dependent on sedatives or alcohol such that medical detoxification was indicated. Patients with DSM-IV cannabis dependence, or alcohol dependence without risk of serious withdrawal, or meeting DSM-IV abuse criteria for other substances, could be included as long as cocaine was the main clinical problem for which the patient was presenting for treatment.
2.2. Study Procedures
Each of the trials had a one to two week single-blind, placebo lead-in phase followed by randomization to 12 weeks of active medication or placebo. For the venlafaxine trial (cocaine dependence plus major depression), patients were asked to attend the clinic twice a week. For the methylphenidate (cocaine dependence plus ADHD) and gabapentin (no comorbidity) trials, patients were asked to attend to the clinic three times a week. Patients were compensated for transportation costs. At each clinic visit, patients saw a research nurse and research assistant, gave a urine sample, and filled out research assessments. Urine samples were tested for cocaine metabolites and also for opiates, methadone, barbiturates, amphetamine and marijuana and scored as positive or negative based on the standard NIDA cutoff guidelines (e.g. 300 ng/ml for cocaine metabolite). Monthly blood pregnancy tests for women were collected. Scales assessing self-reported substance use and psychiatric symptoms were administered weekly. All participants attended weekly individual cognitive behavioral relapse prevention therapy (Carroll et al. 1994), delivered by masters and doctoral level psychologists. The therapy was manual-guided (Carroll et al. 1994), and sessions were audiotaped and reviewed during supervision with a senior clinical psychologist to insure fidelity to the treatment manual. Every week to two weeks, patients met with a research psychiatrist to review overall progress in treatment and to adjust medication.
2.3. Data Analysis
The three diagnostic groups, cocaine dependence plus major depression (CD + MDD), cocaine dependence plus ADHD (CD + ADHD), and cocaine dependence without comorbidity (CD alone)—i.e. patients randomized to the placebo arms of the venlafaxine, methylphenidate, and gabapentin trials, respectively--were compared. Baseline demographic and clinical features were compared across groups using chi-square for categorical variables and one-way ANOVA for continuous variables. Outcome measures examined were time to dropout (number of weeks remaining in treatment), the number of subjects achieving 2 or more consecutive weeks of abstinence from cocaine (all urines negative for cocaine for 2 consecutive weeks), and cocaine urine test results over time in the trial. Time-to-dropout was compared across groups using Kaplan-Meier survival curves and the log-rank test. The proportion of patients achieving two or more consecutive abstinent weeks was compared across groups with chi-square. Cocaine use over the course of the study was modeled using logistic regression for repeated measures with GEE for parameter estimation. For each subject, a week was coded as positive for cocaine use if at least one urine sample in that week tested positive for cocaine metabolites. This weekly dichotomous outcome was modeled using logistic regression (with logit link function), as a function of time, group, baseline level of cocaine use, and their interactions. The baseline level of cocaine use was dichotomous, scored, like the weekly outcome measure, as negative if all urine samples in the week prior to randomization were negative, and positive if one or more samples was positive prior to randomization. We did not have any a priori hypothesis about the interaction between the baseline level of use and the presence of comorbidity in relation to treatment outcome. However, baseline level of cocaine use is one of the strongest predictors of treatment outcome (Kampman et al. 2001; Bisaga et al. 2005) that could contribute to observed group differences to a greater extent than the presence of comorbidity. Therefore, we examined its effect in a post-hoc analysis.
Given differential therapeutic contact across the three trials (venlafaxine required twice a week visits compared to three times a week for the other two trials), we examined the effect of therapeutic contact on the primary outcomes. We modeled the amount of therapeutic contact subjects received as a time-dependent covariate in our main model. The variable was coded to reflect the cumulative therapeutic contact each week. Thus at each week, the amount of therapy received up to that week was used as a predictor of the outcome. Therapeutic contact was defined to include therapy attendance, contact with staff when submitting urine specimen or completing other study related tasks, and was measured using the urine data as a proxy variable. There was no evidence that therapeutic contact influenced the outcome and therefore we removed the covariate from the model.
3. Results
3.1 Baseline Demographic and Clinical Characteristics
Sixty-six cocaine dependent patients with major depression (CD + MDD) were randomized onto placebo in the venlafaxine trial, 53 cocaine-dependent patients with ADHD (CD + ADHD) were randomized onto placebo in the methylphenidate treatment trial, and 48 cocaine dependent patients without other comorbidity (CD alone) were randomized onto placebo in the gabapentin trial. Table 1 compares the baseline demographic and clinical features across the three groups. The only significant difference was in racial distribution, accounted for by more Caucasians and Hispanics in the CD + MDD and CD + ADHD groups, and more African-Americans in the group without comordidity (CD alone). There were non-significant trends toward more alcohol and marijuana use disorders among the CD + ADHD patients, and less higher-income individuals (> $50K per year) among those with CD + MDD. There were no significant group differences for age, gender, education level, employment, marital status, or self-reported levels of cocaine use in the month prior to entering treatment.
Table 1.
Baseline demographic and clinical features, and treatment retention rates, for cocaine dependent patients (N = 167) with major depression, ADHD, or no psychiatric comorbidity, randomized to the placebo arms of one of three clinical trials.a
| Baseline Variable | No Comorbidity (gabapentin trial) (n=48) | ADHD (methylphenidate trial) (n=53) | Major Depression (venlafaxine trial) (n = 66) | X or F b (d.f.) | P |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 39 (8) | 37 (6) | 38 (8) | 0.85 (2,163) | 0.43 |
| Male | 39 (89%) | 44 (83%) | 48 (73%) | 2.16 (2) | 0.34 |
| Race: Caucasian | 14 (29%) | 31 (58%) | 22 (33%) | 18.11 (6) | .006 |
| Hispanic | 7 (15%) | 8 (15%) | 19 (29%) | ||
| African-American | 24 (50%) | 11 (21%) | 19 (29%) | ||
| Other | 3 (6%) | 3 (6%) | 6 (9%) | ||
| Education (College/post-Grad) | 24 (63%) | 29 (55%) | 30 (48%) | 2.36 (2) | 0.31 |
| Currently Employed | 28 (82%) | 38 (72%) | 41 (68%) | 4.20 (4) | 0.38 |
| Currently Married | 12 (31%) | 14 (26%) | 20 (31%) | 0.84 (4) | 0.93 |
| Income Under 25 K | 15 (47%) | 22 (43%) | 24 (44%) | 7.11 (4) | 0.13 |
| 25K – 50 K | 9 (28%) | 19 (37%) | 27 (49%) | ||
| Over 50 K | 8 (25%) | 10 (20%) | 4 (7%) | ||
| SCID | |||||
| Current MJ A/D | 7 (23%) | 15 (28%) | 6 (13%) | 3.24 (2) | 0.20 |
| Current Alcohol A/D | 7 (23%) | 24 (45%) | 14 (32%) | 4.42 (2) | 0.11 |
| Baseline Cocaine use | |||||
| Days of cocaine use (in last 30 days) | 14 (8) | 13 (8) | 15 (9) | 0.99 (2,161) | 0.38 |
| Total $ amount spent on cocaine per week (last 30 days) | $359 ($341) | $291 ($256) | $316 ($368) | 0.52 (2,154) | 0.60 |
| Retention in Treatment | |||||
| Weeks in Treatment | 8 (4) | 8 (5) | 9 (4) | 0.94 (2) c | 0.63 |
| Completed treatment(12 weeks) | 20 (42%) | 24 (45%) | 31 (47%) | 0.32(2) | 0.85 |
Values in the table are N (%) for categorical variables, or Mean (SD) for continuous variables.
Value of chi-square or F statistic, depending of whether the variable is categorical or continuous.
Log-Rank test.
3.2 Placebo Side Effects as a “Proxy” for Expectancy Effects
Although we did not collect data regarding the expectancies of each group to obtain a therapeutic effect from the treatment medication, placebo side effects in each group may serve as a reasonable “proxy” of expected side effects. With the exception of diarrhea (which was more common in the CD+ ADHD group), there were no differences in specific side effects. However, when total number of side effects were calculated, 23% of the CD + ADHD group and 27% of the CD+ MDD reported a moderate or severe side effect compared to 6% for the CD alone group (X2 =8.15, p=.02). However, these differences may have been due to how the side effects data were collected. Open-ended questions were used for the CD alone group whereas structured side effect questionnaires were used for the other 2 groups. This may have led to an underestimation of side effects in the CD alone group.
3.3 Treatment Retention
There were no significant group differences with respect to treatment retention (see Table 1). Thirty-one patients (47%) in the CD + MDD group, 24 (45%) in the CD + ADHD group and 20 (42%) in the CD alone group completed 12 weeks of treatment. There was also no difference in the number of weeks retained, and the survival curves representing time to dropout for the three groups were superimposable.
3.4 Substance Use Outcome
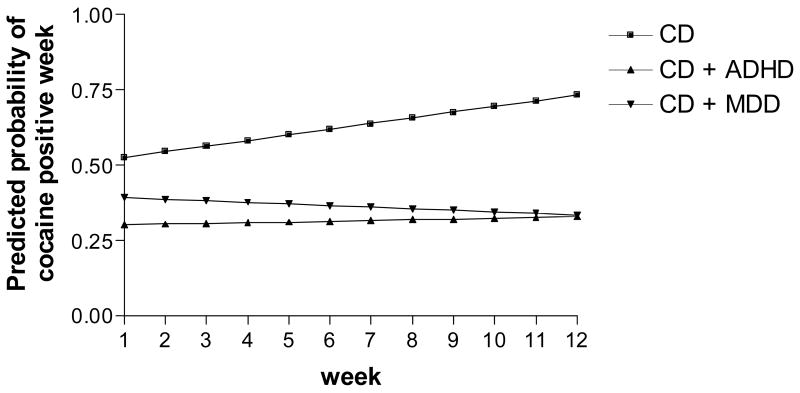
The proportion of patients achieving two or more consecutive weeks of abstinence from cocaine did not differ significantly between groups (CD + MDD 50% (33/66), CD + ADHD 40% (21/53), CD alone 40% (19/48); chi-square = 1.75, d.f. = 2, p = 0.42). In the logistic regression analysis of cocaine use over time, there was a significant main effect of baseline cocaine use (urine positive or negative) (Z = 3.74, p = 0.0002), and a significant 3-way interaction between baseline cocaine use (urine positive or negative), diagnostic group, and time (Z = −2.34, p = .02). The model is graphed in Figures 1a and 1b. As can be seen in Figure 1a, for patients with cocaine negative urines at baseline, both groups with comorbidity (CD + MDD and CD + ADHD) maintained low probabilities of cocaine use throughout the trials, while for those without comorbidity (CD alone) cocaine use increased over time. As can be seen in Figure 1b, patients with a cocaine positive week at baseline showed the opposite pattern. Both groups with comorbidity (CD + MDD and CD + ADHD) maintained high probabilities of cocaine use throughout the trials, while among the patients without comorbidity (CD alone) cocaine use decreased over time.
Figure 1.
Figure 1a. Logistic regression model of cocaine use over time for patients with cocaine negative urines during the baseline week. Groups have cocaine dependence plus major depression (CD +MDD), cocaine dependence plus ADHD (CD + ADHD), and cocaine dependence alone (CD)
Figure 1b. Logistic regression model of cocaine use over time for patients with cocaine positive urines during the baseline week. Groups have cocaine dependence plus major depression (CD +MDD), cocaine dependence plus ADHD (CD + ADHD), and cocaine dependence alone (CD).
There were no differences in the percentage of individuals who were able to achieve baseline cocaine abstinence in the 3 treatment groups. Twenty-eight percent (46/167) of the subjects were able to achieve baseline cocaine abstinence (CD 19% (9/48), CD +ADHD 25% (13/53), CD +MDD 36%(24/66); X2=4.67, f=2, p=.10). Individuals who were able to achieve baseline abstinence did not differ significantly in the amount per week and the days of use in the month prior to study entry (p >.10). Furthermore, among cocaine-dependent subjects with ADHD, those who were able to achieve baseline cocaine abstinence did not differ significantly in their baseline AARS scores from those who did not achieve cocaine abstinence (Abstainers 34.46 (10.92), Non-Abstainers 33.53 (10.03); t = .39, df = 51, p = .70). Similarly for the CD + MDD group, the two groups did not differ significantly in their baseline Ham-D scores (Abstainers 19.29 (4.43), Non-Abstainers 19.00 (5.48); t = .22, df = 64, p = .82).
4. Discussion
This analysis was undertaken to examine whether psychiatric disorders that commonly co-occur among patients with cocaine dependence, namely major depressive disorder (MDD) or attention deficit hyperactivity disorder (ADHD), affect the prognosis of cocaine dependent patients undergoing outpatient psychosocial treatment with cognitive behavioral relapse prevention. There were no differences in treatment retention between patients with cocaine dependence and major depression (CD + MDD), cocaine dependence and ADHD (CD + ACHD), or cocaine dependence without comorbidity (CD alone). When a global measure of cocaine use outcome was examined (proportion of patients achieving two or more consecutive weeks of abstinence from cocaine according to urine toxicology), there were no significant differences between groups. However, when weekly cocaine abstinence was modeled as a function of time in treatment, interesting differences emerged. For patients who were able to become abstinent from cocaine at the beginning of treatment (Figure 1a), the co-occurring disorders appear to convey a favorable prognosis. Both groups with MDD (CD + MDD) and with ADHD (CD + ADHD) maintained a relatively low probability of cocaine positive urines throughout the 12-week course of treatment, while for the group without comorbidity (CD alone) the probability of cocaine use increased over time. In contrast, among patients who were not abstinent from cocaine at the beginning of treatment (Figure 1b), both co-occurring disorders were associated with a poor prognosis. The groups with MDD (CD + MDD) and with ADHD (CD + ADHD) sustained high likelihoods of cocaine positive urines throughout the course of treatment, while the group without comorbidity improved over time.
4.1 Effect of co-occurring disorders on cocaine use
We predicted that co-occurring major depression and ADHD would have a negative impact on the outcome of psychosocial treatment for cocaine dependence. This was based on a number of studies suggesting that psychiatric comorbidity has an adverse prognostic effect on substance dependence, although few of these studies focused specifically on cocaine dependence. Also, this literature is mixed with some studies not supporting an adverse prognostic effect of comorbidity. Our findings replicate the negative prognostic effect of co-occurring disorders on cocaine use outcome, but only among patients who continued to use cocaine at the outset of treatment during the baseline week. Thus, the findings are of particular interest because they suggest that the negative prognostic effect of co-occurring disorders may be moderated by a patient’s initial response to treatment entry, and failure to account for baseline status may explain for some of mixed findings in the literature.
It is well known that many cocaine dependent patients initiate cocaine abstinence immediately upon entering treatment, and that abstinence at the outset of treatment is a strong predictor of good treatment outcome and long-term abstinence (Kampman et al. 2001; Bisaga et al. 2005). Depression may motivate substance dependent patients to seek treatment, particularly if they perceive that the substance use is making them feel worse (Rounsaville 2004). Chronic substance use and withdrawal (including cocaine and alcohol) are associated with depressive symptoms, and these improve with abstinence (Brown et al. 1995; for review see Nunes and Raby 2005). Cocaine use depletes monoamines and serotonin, and dietary depletion of serotonin of these neurotransmitters is associated with reversible worsening of depressive symptoms among individuals with mood disorders. Of particular interest, dietary serotonin depletion of serotonin has been shown to increase both depressive symptoms and craving among alcoholics with major depression (Pierucci-Lagha A 2006). Chronic cocaine use has also been associated with deficits in attention, memory, and executive functioning (Aharonovich et al. 2006) that are components of the ADHD syndrome, as well as abnormalities in blood flow and metabolism in the brain regions subserving these functions (Goldstein et al. 2004; Kosten et al. 1998; Volkow et al. 1991; Woods et al. 1991).
Thus, substance dependent patients with co-occurring disorders such as depression or ADHD, who are able to rapidly achieve abstinence, may be encouraged to maintain abstinence by an improvement in their psychiatric symptoms and/or may avoid further use to avoid worsening of depression or cognitive functioning. Therefore in cocaine dependent patients with co-occurring psychiatric disorders early abstinence may strongly reinforce drug-avoiding behavior, and may be associated with a better long-term prognosis, producing a pattern of outcome such as that in Figure 1a. On the other hand, for substance dependent patients who are unable to rapidly achieve abstinence upon treatment entry, continuing presence of psychiatric symptoms may interfere with motivation and ability to quit substances, or with capacity to benefit from psychosocial treatment to a greater extent than in patients without additional psychiatric symptoms. producing the pattern observed in Figure 1b.
Alternatively, cocaine-dependent individuals with additional comorbidities who were placed on placebo medication may have had a higher expectancy of therapeutic efficacy from the medication than those without psychiatric comorbidity. This attribution may explain why there was a better treatment response for those who achieved baseline abstinence but still does not adequately explain why the psychiatric samples that used cocaine at treatment entry did worse than those with psychiatric comorbidity. In any event, the overall observations suggest that if initial abstinence can be fostered, either through inpatient treatment or salient incentives, clinical improvement might be more likely to occur in those with psychiatric comorbidity. Further, cognitive-behavioral therapy may be better able to ‘”take hold” with early initial abstinence. However, poor motivation or environmental factors will also need to be addressed if abstinence is to be maintained.
4.2 Effect of co-occurring disorders on treatment retention
We had predicted that cocaine dependent patients with ADHD would have a higher dropout rate than the other groups. This was based on substantial dropout rates in a published trials of treatment of ADHD among cocaine dependent patients (Schubiner et al. 2002), and evidence that impairment in neuropsychological functioning, including attention and memory, is associated with high dropout from cognitive-behavioral treatment among cocaine dependent patients without ADHD (Aharonovich et al. 2006). Thus, the similarity in dropout rates across the diagnostic groups was contrary to our prediction. This may reflect a leveling effect of cognitive-behavioral relapse prevention therapy, which emphasizes sticking with treatment despite setbacks. Also, the overall milieu of the research clinics where these patients were treated is likely to have had an impact on retention, since patients have regular contact with research assistants, nurses, physicians and other staff, all of whom are trained to encourage retention, and deliberate efforts are made to contact patients who miss visits.
The equivalent retention rate in patients with major depression is consistent with what may be offsetting influences (Rounsaville 2004). On the one hand depressive symptoms of low interest, energy, and motivation, and pessimism, among others, may make treatment engagement more difficult. On the other hand, depression has been associated with treatment seeking and better treatment retention in some other samples of substance dependent patients (Rounsaville et al. 1987; Agosti, Stewart and Quitkin 1991; McKay et al. 2002; Siqueland et al. 2002; Brown et al. 1998). Depressed patients may respond well to the supportive milieu of a clinic. Further, the cognitive-behavioral relapse prevention therapy, received by the patients studied here (Carroll et al. 1994), addresses symptoms of depression through modules on coping with dysphoric moods and their role in drug relapse, and this would likely help maintain treatment engagement for such patients.
4.3 Strengths and limitations
This is one of the few studies to examine the impact of co-occurring psychiatric disorders on treatment outcome among cocaine dependent patients, and it is, to our knowledge, the first to simultaneously examine the impact of major depression and ADHD in cocaine dependence. Other strengths include rigorous psychiatric diagnostic methods, similar inclusion and exclusion criteria (other than the presence/absence of MDD or ADHD), and comparable methodology across all three clinical trials.
There are also important limitations that need to be considered in interpreting the findings. Findings from a sample of patients recruited to university-based research clinics may not generalize to community-based treatments. Individuals accessing treatment research may have been more motivated or resourceful and those with additional comorbidities, such as severe alcohol dependence, bipolar disorder or medically unstable were excluded from the trials. Further, assessing treatment outcome based on baseline abstinence was a post-hoc analysis and although the findings have potential clinical relevance, this limitation requires replication in future studies.
There were some differences in methodology between studies, which might have influenced outcome across the three diagnostic groups. For example, patients with major depression were asked to attend the clinic twice per week, while those in the other studies were asked to attend three visits per week. However, there was no evidence that cumulative therapeutic contact had an impact on substance use. The groups also differed significantly in racial/ethnic distribution, and there were trends toward other baseline differences, for example in other substance use and in income levels (see Table 1). There is no evidence that rates of major depression (Kessler et al. 1994) or ADHD (Szatmari P 1989) differ across ethnic groups in the general population, thus the difference seen here likely relates to recruitment methods or other factors that may influence treatment-seeking. Our data suggest that African-American individuals with cocaine dependence are less likely to seek treatment if they have co-occurring disorders as compared to Caucasians or Hispanics. The role that psychiatric co-morbidity plays as a risk factor for the development or maintenance of addictions may also differ across ethnic groups (Nunes et al. 2000).
By including only the placebo groups from the three studies, this analysis does not examine the potential impact of specific treatment for co-occurring disorders on outcome. Since patients in the venlafaxine and methylphenidate studies received a medication for their co-occurring disorders, while those in the gabapentin study received instead a putative medication treatment for cocaine dependence, comparison of the medication groups would have been difficult to interpret. Finally, although findings were analyzed based on whether patients were able to achieve baseline cocaine abstinence, a substantial percentage of this group met criteria for alcohol abuse/dependence (50%) or cannabis abuse/dependence (22%) and a minority of patients (11–46% for the 3 treatment groups) had a positive urine for marijuana. Thus, it is likely that a substantial number of individuals in the cocaine-abstinent group were using alcohol or marijuana during the initial week of treatment. However, this does not minimize the importance of individuals being able to achieve early cocaine abstinence since cocaine is the drug that brought these patients into treatment and achieving early cocaine abstinence may have prognostic significance.
4.4 Future Directions
The findings suggest that diagnosis and treatment of co-occurring depression and ADHD should be an important component of an overall treatment plan for cocaine dependent patients. The adverse impact, observed here, of these co-occurring disorders on cocaine use outcome among patients who do not rapidly achieve abstinence on entry into treatment suggests the hypothesis that the diagnosis and treatment of comorbid psychiatric disorders has an impact on the cocaine use. A meta-analysis (Nunes and Levin 2004) has shown that mood improvement in response to antidepressant medication is associated with improvement in self-reported levels of substance use among substance dependent patients, although only a few such placebo-controlled trials have targeted cocaine dependent samples (Nunes et al. 1995; Schmitz et al. 2001; McDowell et al. 2005) with mixed results. Placebo-controlled trials that examined the impact of medication treatment for ADHD in substance dependent patients, are also scarce and their results are mixed (Schubiner et al. 2002; Levin et al. epub, 2006a). Analysis of the methylphenidate trial, from which one of the placebo groups was drawn for the present study, suggests a modest benefit of treatment of ADHD on cocaine urine toxicologies over time (Levin et al. epub, 2006a). More studies examining the impact of treatment of co-occurring disorders in cocaine dependent patients are needed. While studies to date have focused on medications, effective psychotherapeutic approaches exist for depression, and behavioral approaches may offer some benefit for ADHD as well (Wells et al. 2000). The findings also suggest that placebo-controlled trials of treatments for co-occurring disorders should include baseline level of cocaine use as a covariate in the principal analyses of outcome. Consistent with this, a study of the tricyclic antidepressant desipramine for cocaine dependent patients with depressive disorders found some evidence that medication was effective in reducing cocaine use, but mainly among patients with high levels of cocaine use at baseline (McDowell et al. 2005). Among patients with low levels of cocaine use at treatment entry, the data suggest that levels of cocaine use may remain low over the short term without specific treatment for co-occurring disorders. Such treatment should be important for improving overall level of functioning, perhaps reducing risk of relapse to drug use over the long term. In the short term there is a theoretical possibility that alleviating symptoms of the co-occurring disorder might remove some of the motivation to avoid cocaine, although we know of no evidence to support such a phenomenon, and the preponderance of evidence suggests improvements in comorbidity correlate with improvements in substance use.
Although not reaching statistical significance, the trends toward greater rates of alcohol and cannabis use disorders among the patients with ADHD warrant attention. Anecdotally, we observed that patients in the ADHD group who were regular users of marijuana reported that it helped them “feel calm” or “relax.” This suggests the potential complexity of relationships between substance use and psychiatric disorders, and that future research should attend to patterns of multiple substance use.
Finally, while the retention rates observed here were respectable compared to other studies of cocaine dependent patients in treatment, the overall treatment completion rates, in the 40% range at 12 weeks, still leave much room for improvement. Similarly, examination of Figures 1a and 1b shows that rates of cocaine use remain substantial across all the groups, again with the lowest rates among patients with co-occurring disorders and negative urines at baseline. Dropout from treatment and ongoing cocaine use remain significant challenges in regard to efforts to develop better treatments for cocaine dependence. However, treatment of co-occurring disorders is likely to be just one piece of this larger puzzle. The effect sizes of medication treatments for depression are in the small to medium range among depressed outpatients (Walsh et al. 2002) and substance dependent patients (Nunes and Levin 2004). Effect sizes for stimulant treatment of ADHD are large among both children and adults, but as yet unclear, and perhaps smaller, among those with substance use disorders (Schubiner et al. 2002; Levin et al. epub, 2006a). Thus, larger sample sizes and multisite trials will be needed to better characterize the impact of treatment of comorbidity on outcome of treatment for drug dependence. Efforts to improve retention and drug use outcome will also need to simultaneously address other known prognostic factors such as motivation, neuropsychological functioning, and treatment alliance, and to seek further advances in our understanding of treatment outcome for cocaine dependence.
Acknowledgments
This research was supported by NIDA grants RO1 DA11755, P50 DA12761, P50 DA09236, KO2 DA00465, KO2 DA00288. Also, we gratefully acknowledge the nursing support provided by Gary Pagliaro, R.N., the clinical support of the Substance Treatment and Research Service of Columbia University/NYSPI and the Project Outreach Staff of the Long Island Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agosti V, Stewart JW, Quitkin FM. Life Satisfaction and Psychosocial Functioning in Chronic Depression: Effect of Acute Treatment with Antidepressants. J Affect Disord. 1991;23(1):35–41. doi: 10.1016/0165-0327(91)90033-o. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive Deficits Predict Low Treatment Retention in Cocaine Dependent Patients. Drug Alcohol Depend. 2006;81(3):313–22. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Nunes EV. A Randomized Placebo-Controlled Trial of Gabapentin for Cocaine Dependence. Drug Alcohol Depend. 2006;81(3):267–74. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Vosburg SK, Nunes EV. Utility of Lead-in Period in Cocaine Dependence Pharmacotherapy Trials. Drug Alcohol Depend. 2005;77(1):7–11. doi: 10.1016/j.drugalcdep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Brown RA, Monti PM, Myers MG, Martin RA, Rivinus T, Dubreuil ME, Rohsenow DJ. Depression among Cocaine Abusers in Treatment: Relation to Cocaine and Alcohol Use and Treatment Outcome. Am J Psychiatry. 1998;155(2):220–5. doi: 10.1176/ajp.155.2.220. [DOI] [PubMed] [Google Scholar]
- Brown SA, Inaba RK, Gillin JC, Schuckit MA, Stewart MA, Irwin MR. Alcoholism and Affective Disorder: Clinical Course of Depressive Symptoms. Am J Psychiatry. 1995;152(1):45–52. doi: 10.1176/ajp.152.1.45. [DOI] [PubMed] [Google Scholar]
- Carroll KM. Manual-Guided Psychosocial Treatment. A New Virtual Requirement for Pharmacotherapy Trials? Arch Gen Psychiatry. 1997;54(10):923–8. doi: 10.1001/archpsyc.1997.01830220041007. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Power ME, Bryant K, Rounsaville BJ. One-Year Follow-up Status of Treatment-Seeking Cocaine Abusers. Psychopathology and Dependence Severity as Predictors of Outcome. J Nerv Ment Dis. 1993;181(2):71–9. doi: 10.1097/00005053-199302000-00001. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Gordon LT, Nich C, Jatlow P, Bisighini RM, Gawin FH. Psychotherapy and Pharmacotherapy for Ambulatory Cocaine Abusers. Arch Gen Psychiatry. 1994;51(3):177–87. doi: 10.1001/archpsyc.1994.03950030013002. [DOI] [PubMed] [Google Scholar]
- Charney DA, Palacios-Boix J, Negrete JC, Dobkin PL, Gill KJ. Association between Concurrent Depression and Anxiety and Six-Month Outcome of Addiction Treatment. Psychiatr Serv. 2005;56(8):927–33. doi: 10.1176/appi.ps.56.8.927. [DOI] [PubMed] [Google Scholar]
- Clure C, Brady KT, Saladin ME, Johnson D, Waid R, Rittenbury M. Attention-Deficit/Hyperactivity Disorder and Substance Use: Symptom Pattern and Drug Choice. Am J Drug Alcohol Abuse. 1999;25(3):441–8. doi: 10.1081/ada-100101871. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Thase ME, Haskett RF, Daley DC, Jones-Barlock A, Upsher C, Perel JM. Fluoxetine Versus Placebo in Depressed Alcoholic Cocaine Abusers. Psychopharmacol Bull. 1998;34(1):117–21. [PubMed] [Google Scholar]
- Dodge R, Sindelar J, Sinha R. The Role of Depression Symptoms in Predicting Drug Abstinence in Outpatient Substance Abuse Treatment. J Subst Abuse Treat. 2005;28(2):189–96. doi: 10.1016/j.jsat.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR, Brooks DJ, Garawi F. Memantine Treatment for Alcohol Dependence: Implementation of Contingency Management Procedures to Enhance Clinical Trials. Submitted. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, William JBW. Structured Clinical Interview for Dsm-Iv Axis I Disorders- Patient Edition (Scid-I/P, Patient Verision 2.0) Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of Neuropsychological Impairment in Cocaine and Alcohol Addiction: Association with Metabolism in the Prefrontal Cortex. Neuropsychologia. 2004;42(11):1447–58. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Weiss RD, Muenz LR, Vagge LM, Kelly JF, Bello LR, Michael J. The Effect of Depression on Return to Drinking: A Prospective Study. Arch Gen Psychiatry. 1998;55(3):259–65. doi: 10.1001/archpsyc.55.3.259. [DOI] [PubMed] [Google Scholar]
- Hasin D, Liu X, Nunes E, McCloud S, Samet S, Endicott J. Effects of Major Depression on Remission and Relapse of Substance Dependence. Arch Gen Psychiatry. 2002;59(4):375–80. doi: 10.1001/archpsyc.59.4.375. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of Major Depressive Disorder: Results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62(10):1097–106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Nunes EV, Meydan J. Comorbidity of Alcohol, Drug and Psychiatric Disorders: Epidemiolgy. In: Kranzler HR, Tinsley JA, editors. Dual Diagnosis and Treatment: Substance Abuse and Comordid Disorders. New York: Marcel Dekker; 2004. [Google Scholar]
- Hien D, Matzner F, First M, Spitzer R, William J. The Structured Clinical Interview for Dsm-Iv, Childhood Version (Kid-Sci) - Unpublished Document. 1994 [Google Scholar]
- Kampman KM, Alterman AI, Volpicelli JR, Maany I, Muller ES, Luce DD, Mulholland EM, Jawad AF, Parikh GA, Mulvaney FD, Weinrieb RM, O’Brien CP. Cocaine Withdrawal Symptoms and Initial Urine Toxicology Results Predict Treatment Attrition in Outpatient Cocaine Dependence Treatment. Psychol Addict Behav. 2001;15(1):52–9. doi: 10.1037/0893-164x.15.1.52. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The Prevalence and Correlates of Adult Adhd in the United States: Results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–23. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The Epidemiology of Major Depressive Disorder: Results from the National Comorbidity Survey Replication (Ncs-R) Jama. 2003;289(23):3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-Month Prevalence of Dsm-Iii-R Psychiatric Disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kilbey MM, Breslau N, Andreski P. Cocaine Use and Dependence in Young Adults: Associated Psychiatric Disorders and Personality Traits. Drug Alcohol Depend. 1992;29(3):283–90. doi: 10.1016/0376-8716(92)90103-j. [DOI] [PubMed] [Google Scholar]
- King VL, Brooner RK, Kidorf MS, Stoller KB, Mirsky AF. Attention Deficit Hyperactivity Disorder and Treatment Outcome in Opioid Abusers Entering Treatment. J Nerv Ment Dis. 1999;187(8):487–95. doi: 10.1097/00005053-199908000-00005. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Cheeves C, Palumbo J, Seibyl JP, Price LH, Woods SW. Regional Cerebral Blood Flow During Acute and Chronic Abstinence from Combined Cocaine-Alcohol Abuse. Drug Alcohol Depend. 1998;50(3):187–95. doi: 10.1016/s0376-8716(98)00038-6. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Markou A, Koob GF. Depression and Stimulant Dependence: Neurobiology and Pharmacotherapy. J Nerv Ment Dis. 1998;186(12):737–45. doi: 10.1097/00005053-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. A 2.5-Year Follow-up of Cocaine Use among Treated Opioid Addicts. Have Our Treatments Helped? Arch Gen Psychiatry. 1987;44(3):281–4. doi: 10.1001/archpsyc.1987.01800150101012. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of Cocaine Dependent Treatment Seekers with Adult Adhd: Double-Blind Comparison of Methylphenidate and Placebo. Drug Alcohol Depend. 2006a doi: 10.1016/j.drugalcdep.2006.07.004. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Brooks DJ, Kalbag AS, Garawi F, Nunes EV. Treatment of Methadone-Maintained Patients with Adult Adhd: Double-Blind Comparison of Methylphenidate, Bupropion and Placebo. Drug Alcohol Depend. 2006b;81(2):137–48. doi: 10.1016/j.drugalcdep.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Kleber HD. Prevalence of Adult Attention-Deficit Hyperactivity Disorder among Cocaine Abusers Seeking Treatment. Drug Alcohol Depend. 1998;52(1):15–25. doi: 10.1016/s0376-8716(98)00049-0. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, McDowell DM, Brooks DJ, Nunes E. Bupropion Treatment for Cocaine Abuse and Adult Attention-Deficit/Hyperactivity Disorder. J Addict Dis. 2002;21(2):1–16. doi: 10.1300/J069v21n02_01. [DOI] [PubMed] [Google Scholar]
- McDowell D, Nunes EV, Seracini AM, Rothenberg J, Vosburg SK, Ma GJ, Petkova E. Desipramine Treatment of Cocaine-Dependent Patients with Depression: A Placebo-Controlled Trial. Drug Alcohol Depend. 2005;80(2):209–21. doi: 10.1016/j.drugalcdep.2005.03.026. [DOI] [PubMed] [Google Scholar]
- McKay JR, Pettinati HM, Morrison R, Feeley M, Mulvaney FD, Gallop R. Relation of Depression Diagnoses to 2-Year Outcomes in Cocaine-Dependent Patients in a Randomized Continuing Care Study. Psychol Addict Behav. 2002;16(3):225–35. [PubMed] [Google Scholar]
- McLean MJ, Morrell MJ, Willmore LJ, Privitera MD, Faught RE, Holmes GL, Magnus-Miller L, Bernstein P, Rose-Legatt A. Safety and Tolerability of Gabapentin as Adjunctive Therapy in a Large, Multicenter Study. Epilepsia. 1999;40(7):965–72. doi: 10.1111/j.1528-1157.1999.tb00804.x. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Levin FR. Treatment of Depression in Patients with Alcohol or Other Drug Dependence: A Meta-Analysis. Jama. 2004;291(15):1887–96. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- Nunes EV, McGrath PJ, Quitkin FM, Ocepek-Welikson K, Stewart JW, Koenig T, Wager S, Klein DF. Imipramine Treatment of Cocaine Abuse: Possible Boundaries of Efficacy. Drug Alcohol Depend. 1995;39(3):185–95. doi: 10.1016/0376-8716(95)01161-6. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Raby WN. Comorbidity of Depression and Substance Abuse. In: Licinio J, Wong ML, editors. Biology of Depression. Vol. 1. Wiley-VCH; 2005. pp. 341–64. [Google Scholar]
- Nunes EV, Weissman MM, Goldstein R, McAvay G, Beckford C, Seracini A, Verdeli H, Wickramaratne P. Psychiatric Disorders and Impairment in the Children of Opiate Addicts: Prevalances and Distribution by Ethnicity. Am J Addict. 2000;9(3):232–41. doi: 10.1080/10550490050148062. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha AFR, Modesto-Lowe V, Swift R, Nellissery M, Covault J, Kranzler HR. Effects of Rapid Tryptophan Depletion on Mood and Urge to Drink in Patients with Co-Morbid Major Depression and Alcohol Dependence. Psychopharmacology (Berl) 2006;171(3):340–8. doi: 10.1007/s00213-003-1588-6. [DOI] [PubMed] [Google Scholar]
- Rounsaville B, Carroll K. Psychiatric Disorders in Treatment-Entering Cocaine Abusers. NIDA Res Monogr. 1991;110:227–51. [PubMed] [Google Scholar]
- Rounsaville BJ. Treatment of Cocaine Dependence and Depression. Biol Psychiatry. 2004;56(10):803–9. doi: 10.1016/j.biopsych.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Dolinsky ZS, Babor TF, Meyer RE. Psychopathology as a Predictor of Treatment Outcome in Alcoholics. Arch Gen Psychiatry. 1987;44(6):505–13. doi: 10.1001/archpsyc.1987.01800180015002. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Kosten TR, Weissman MM, Kleber HD. Prognostic Significance of Psychopathology in Treated Opiate Addicts. A 2.5-Year Follow-up Study. Arch Gen Psychiatry. 1986;43(8):739–45. doi: 10.1001/archpsyc.1986.01800080025004. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Averill P, Stotts AL, Moeller FG, Rhoades HM, Grabowski J. Fluoxetine Treatment of Cocaine-Dependent Patients with Major Depressive Disorder. Drug Alcohol Depend. 2001;63(3):207–14. doi: 10.1016/s0376-8716(00)00208-8. [DOI] [PubMed] [Google Scholar]
- Schneider U, Altmann A, Baumann M, Bernzen J, Bertz B, Bimber U, Broese T, Broocks A, Burtscheidt W, Cimander KF, Degkwitz P, Driessen M, Ehrenreich H, Fischbach E, Folkerts H, Frank H, Gurth D, Havemann-Reinecke U, Heber W, Heuer J, Hingsammer A, Jacobs S, Krampe H, Lange W, Lay T, Leimbach M, Lemke MR, Leweke M, Mangholz A, Massing W, Meyenberg R, Porzig J, Quattert T, Redner C, Ritzel G, Rollnik JD, Sauvageoll R, Schlafke D, Schmid G, Schroder H, Schwichtenberg U, Schwoon D, Seifert J, Sickelmann I, Sieveking CF, Spiess C, Stiegemann HH, Stracke R, Straetgen HD, Subkowski P, Thomasius R, Tretzel H, Verner LJ, Vitens J, Wagner T, Weirich S, Weiss I, Wendorff T, Wetterling T, Wiese B, Wittfoot J. Comorbid Anxiety and Affective Disorder in Alcohol-Dependent Patients Seeking Treatment: The First Multicentre Study in Germany. Alcohol Alcohol. 2001;36(3):219–23. doi: 10.1093/alcalc/36.3.219. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N, Edwards A, Donlin J, Pihlgren E. Double-Blind Placebo-Controlled Trial of Methylphenidate in the Treatment of Adult Adhd Patients with Comorbid Cocaine Dependence. Exp Clin Psychopharmacol. 2002;10(3):286–94. doi: 10.1037//1064-1297.10.3.286. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Tzelepis A, Milberger S, Lockhart N, Kruger M, Kelley BJ, Schoener EP. Prevalence of Attention-Deficit/Hyperactivity Disorder and Conduct Disorder among Substance Abusers. J Clin Psychiatry. 2000;61(4):244–51. doi: 10.4088/jcp.v61n0402. [DOI] [PubMed] [Google Scholar]
- Siqueland L, Crits-Christoph P, Gallop R, Barber JP, Griffin ML, Thase ME, Daley D, Frank A, Gastfriend DR, Blaine J, Connolly MB, Gladis M. Retention in Psychosocial Treatment of Cocaine Dependence: Predictors and Impact on Outcome. Am J Addict. 2002;11(1):24–40. doi: 10.1080/10550490252801611. [DOI] [PubMed] [Google Scholar]
- Szatmari POD, Boyle MH. Ontario Child Health Study: Prevalence of Attention Deficit Disorder with Hyperactivity. J Child Psychol Psychiatry. 1989;30(2):219–30. doi: 10.1111/j.1469-7610.1989.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in Brain Glucose Metabolism in Cocaine Dependence and Withdrawal. Am J Psychiatry. 1991;148(5):621–6. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo Response in Studies of Major Depression: Variable, Substantial, and Growing. Jama. 2002;287(14):1840–7. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- Wells KC, Pelham WE, Kotkin RA, Hoza B, Abikoff HB, Abramowitz A, Arnold LE, Cantwell DP, Conners CK, Del Carmen R, Elliott G, Greenhill LL, Hechtman L, Hibbs E, Hinshaw SP, Jensen PS, March JS, Swanson JM, Schiller E. Psychosocial Treatment Strategies in the Mta Study: Rationale, Methods, and Critical Issues in Design and Implementation. J Abnorm Child Psychol. 2000;28(6):483–505. doi: 10.1023/a:1005174913412. [DOI] [PubMed] [Google Scholar]
- Woods SW, O’Malley SS, Martini BL, McDougle CJ, Price LH, Krystal JH, Hoffer PB, Kosten TR. Spect Regional Cerebral Blood Flow and Neuropsychological Testing in Non-Demented Hiv-Positive Drug Abusers: Preliminary Results. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15(5):649–62. doi: 10.1016/0278-5846(91)90055-6. [DOI] [PubMed] [Google Scholar]