Abstract
The subcortical response to peripheral somatosensory stimulation is not well studied. Prior literature suggests that somatosensory stimulation can affect dopaminergic tone. We studied the effects of electrical stimulation near the median nerve on the response to an amphetamine induced increase in synaptic dopamine. We applied the electrical stimulation close to the median nerve 20 minutes after administration of 3mg/kg amphetamine. We used fMRI and microdialysis to measure markers of DA release, together with the release of associated neurotransmitters of striatal Glutamate (Glu) and GABA.
Result
1) Changes in cerebral blood volume (CBV), a marker used in fMRI, indicate that electrical stimulation significantly attenuated increased DA release (due to AMPH) in the striatum, thalamus, medial prefrontal and cingulate cortices. 2) Microdialysis showed that electrical stimulation increased Glu and GABA release and attenuated the AMPH-enhanced DA release. The striatal DA dynamics correlated with the CBV response.
Conclusion
These results demonstrate that electrical stimulation near the median nerve activates Glu/GABA release which subsequently attenuate excess striatal DA release. These data provide evidence for physiologic modulation caused by electroacupuncture at points near the median nerve.
Keywords: amphetamine, fMRI, rCBV, microdialysis, glutamate, GABA
Introduction
Short trains of electrical or tactile pulses on the extremities are typically used for studying somatosensory responses in the brain. Applications include studying spatial and frequency perception in the somatosensory cortex [34] and evaluating cortical function after brain trauma [16, 29]. There is a large body of evidence indicating that stimulation of the median nerve leads to modulation of dopaminergic tone in normal controls and in Parkinson's disease [13, 27]. Acupuncture, an ancient healing practice using either manual or electrical manipulation (electrical acupuncture) of needles, at selective body points (acupoints), has a similar stimulation profile to the electrical stimulations discussed above. Electrical acupuncture (EA) uses a wide range of oscillation frequencies ranging from 0.5Hz to 150Hz and typically lasts more than 20 minutes clinically. Technically, EA is a prolonged and broader version of the peripheral electrical stimulation as those used in somatosensory studies. Due to the great similarity between EA and the other electrical somatosensory stimulation protocols, we wished to investigate the effect of EA in the brain from the point of view of somatosensory electrical stimulation.
Although the effect of peripheral electrical stimulation has been studied broadly, not many studies have gone beyond the level of the cerebral cortex. As demonstrated in animal and human studies, activating the corticostriatal glutamatergic (Glu) projection modulates dopamine (DA) and Gamma-aminobutyric acid (GABA) release in the striatum and subsequently affects function along the basal ganglia circuitry [15, 20]. In rodents, direct electrical stimulation in the cerebral cortex led to striatal glutamate release which subsequently facilitated the expression of immediate-early genes [28]. In humans, transcranial magnetic stimulation (TMS) over the prefrontal cortex led to dopamine release in the caudate nuclei, as measured by positron emission tomography [31]. Similar TMS studies in rats showed increases in dopamine and glutamate release in the nucleus accumbens and striatum [35]. Given these previous results, it is not a surprise that EA may arouse neuronal activity changes in the striatum and subsequently along the basal ganglia circuitry. We present in this manuscript the effect of EA in a rodent model using whole brain fMRI measurements as well as microdialysis of striatal neurotransmitter release. Previous studies of electrical forepaw stimulation with short stimulation trains (in the range of seconds) failed to show consistent modulation in the deep brain areas [16, 33]. The missing subcortical activity can be attributed to the tight regulation among neurotransmitters. In the striatum, Glu, GABA, acetylcholine, and DA modulate each other and the excitatory and inhibitory activities can be quickly nullified [1, 8]. Therefore, a short stimulation paradigm may not be sufficient to arouse activity observable by fMRI. In order to create a neurotransmitter imbalance, we pretreated animals with D-amphetamine (AMPH, a DA releaser) in order to increase DA levels well above normal. This abnormally elevated DA level is expected to magnify the detectable effect of EA on DA, whether we utilize MRI or microdialysis. In our experimental design, animals treated with AMPH alone (without EA) served as controls and the effect of EA on AMPH-induced modulation was studied.
Materials and methods
Animals
Male Sprague Dawley rats were anesthetized using 1% halothane in a mixture gas (1:1 O2 and N2O). An intravenous catheter was placed into the tail vein percutaneously for drug administration. All procedures were conducted in accordance with the Massachusetts General Hospital Subcommittee on Research Animal Care rules and regulations and the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80−23) revised 1996.
Electrical acupuncture (EA)
EA stimulation was delivered via a pair of 36 gauge acupuncture needles at right LI4 and subcutaneously on the right forearm. LI4 is located on the dorsum of the forepaw, approximately at the midpoint of the second metacarpal bone, in the belly of the first interosseus dorsalis muscle [19]. The electrical currently oscillated at 2Hz and the current intensity was set to increase at a 0.2mA increment every 8 minutes from the on-set intensity of 1mA. The maximum current intensity was 1.42 ± 0.09mA, a level below the pain threshold [10, 11]. For fMRI study, EA was started at 10 minutes after AMPH challenge and lasted for 20 minutes. Due to the inferior temporal sampling rates of microdialysis (10 minutes per sample), EA was started at 20 minutes after AMPH in order to get sufficient data points before the start of EA.
fMRI
MR images were acquired repeatedly using a gradient echo EPI sequence with the IRON (Increased Relaxivity for Optimized Neuroimaging) technique [6, 21] to assess relative cerebral blood volume (rCBV) changes. Since the rCBV measurement is insensitive to the magnetic field strength [21], data were acquired using both 4.7T and 9.4T MRI scanners (Bruker, Billerica, MA). Basic EPI parameters were: 16 segmentations, TR/TE of 625ms/6ms (a 10s/volume temporal resolution), in-plane resolution of 0.2345mm × 0.2345mm, and 20 contiguous coronal slices, each 1 mm thick. Ten baseline points were acquired before the injection of contrast agent MION [30] (synthesized locally in our laboratory) and then 30 post-contrast baseline points were acquired before the functional task paradigm. Two groups were tested: 1. AMPH alone (3mg/kg iv, n=5, Sigma (St. Louis, MO)); and 2. AMPH (3mg/kg iv) plus EA (n=7).
Inter-animal coregistration was done by using Analysis of Functional NeuroImages (AFNI) [7]. Maps of rCBV responses were calculated on a pixel-by-pixel basis as described in [6, 21]. The rCBV effect induced by AMPH and EA were characterized by two gamma functions (t*exp(-t/τ)) using general linear model (GLM), with time constant τ representing the peak time of the gamma curve. Thus, τAMPH characterizes the effect AMPH while τEA characterizes the effect of EA. Value of τAMPH was determined by the AMPH-alone group and served as the fixed variable in the “AMPH+EA” group to obtain τEA. The degree of activation was presented by the statistical significance (p-values) of the fitting on a pixel-by-pixel basis.
Microdialysis
Preparation for microdialysis in the striatum were carried out as previously described [5] with the coordinate for the dialysate probe at [AP −0.26; ML 4.4; DV 7.0] [26]. Dialysate samples were acquired in 10 min time intervals. Assay for DA and GABA/glutamate concentrations were obtained from two separate groups of animals. Animals were challenged by either 1. AMPH-alone or 2. AMPH plus EA (AMPH+EA). DA, Glu, and GABA concentrations were assayed by HPLC with electrochemical detection (ESA, Chelmsford, MA) as described earlier [5] and in [14].
Results
The insertion of EA needles alone did not cause the rCBV changes observed in animals receiving AMPH
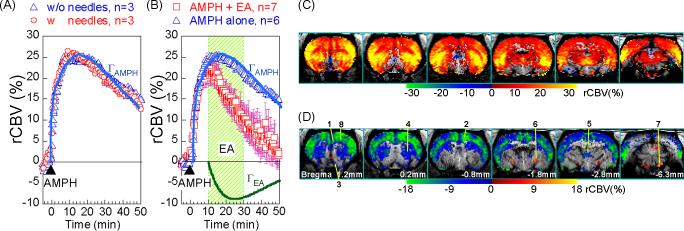
In order to assure that the insertion of EA needles on the forepaw didn't introduce any additional modulation on the AMPH-induced hemodynamic changes, the AMPH-only (i.e. no-EA) animals were subdivided into two groups: 3 rats with EA needles inserted but no current passing and the other 3 rats had no needle insertion at all. This pair of data showed that rats with no needle insertion do not differ from rats with needles inserted but without current passing in terms of rCBV time courses (figure 1A). The time courses and rCBV maps from the whole AMPH-only group were similar to those published previously [5] .
Figure 1.
rCBV time course from the AMPH+EA group (n=7) was decomposed to AMPH (ΓAMPH ) and EA (ΓEA ) components using gamma fit. (A) Rats with and without needle insertion had similar rCBV time courses in response to AMPH challenge. (B) EA at LI4 significant attenuated the “AMPH-induced rCBV increase”. Dots represent data points and solid lines show the GLM fittings of ΓAMPH and ΓEA. Timecourses were from CPu. (C) Map of ΓAMPH shows that brain areas with copious amount of DA release, in response to AMPH challenge. (D) Map of ΓEA shows brain areas effected by EA, with attenuation in blue tone and enhancement in red tone. 1. mPFC, 2. Cing, 3. NAc, 4. CPu, 5. thalamus, 6. LGP, 7. SNR, 8. M1.
Effect of EA on the AMPH-induced rCBV changes
Compared to the AMPH-only group, EA significantly attenuated the increased rCBV (due to massive dopamine release induced by AMPH) in the nucleus accumbens (NAc), medial prefrontal cortex (mPFC), cingulate cortex (cing), thalamus, and part of the caudate/putamen (CPu) (figure 1B). The AMPH-only groups showed that a gamma function with τAMPH =18 minutes well characterized the AMPH timecourse in the CPu and other major dopaminergic brain areas (p-value < 10−10). For the AMPH+EA group, τEA of 15 minutes gave a good fit for the EA effect (p-value < 10−10) when τAMPH was fixed at 18 minutes as determined by the AMPH-only study. The statistical significances of the GLM fitting were presented as maps ΓAMPH and ΓEA for effect of AMPH and EA, respectively (Figure 1C & 1D). Interestingly, in addition to the attenuation phenomenon, EA appeared to enhance rCBV increases in areas around the lateral globus pallidus (LGP) and substantial nigra pars reticulata (SNR). The meaning of rCBV increase in those areas remains to be investigated due to the densely packed structures and the lack of anatomical boundary in the MR images.
Dopamine
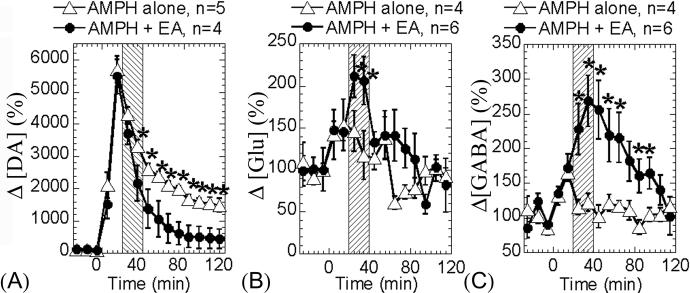
AMPH, a DA release agent, significantly elevated synaptic DA level [5]. In the “AMPH-only” group, the DA level peaked at the second dialysate (10−20 minutes) post AMPH challenge and then slowly attenuated toward baseline level afterward. In the “AMPH+EA” group, AMPH induced a similar raising slope (the first two dialysates post AMPH challenge) as in the “AMPH-only” group. Then, EA induced a significantly faster attenuation (p < 0.05) to bring the elevated DA level back to baseline, compared to the natural pharmacological time course (Figure 2A) induced by AMPH. Half-life of the DA attenuation curves: “AMPH-only” is 63.38 min, “AMPH+EA” 24.82 min.
Figure 2.
Microdialysis measurements of striatal dopamine, glutamate, and GABA release. (A) DA release was significantly attenuated by EA, compared to the AMPH-alone group. (B) Glu release was significantly enhanced during the period of EA treatment. (C) GABA release was significantly but gradually increased by EA. However, the maximum enhancement occurred later than that of Glu. After EA was turned off, the GABA level remained significantly high, compared to the AMPH-alone group. Arrowheads indicate the AMPH challenge and shaded areas indicate EA. Mean ± std errors. * p<0.05
Glutamate and GABA
Analysis of the Glu/GABA dialysates showed that AMPH slightly increased synaptic Glu and GABA concentration in both “AMPH-only” and “AMPH+EA” groups. The Glu concentration was significantly increased and plateaued (P<0.05) during the EA treatment period, but right after EA was turned “off”, the elevated Glu level immediately returned back to the same levels as those in the “AMPH-only” group (figure 2B). The GABA concentration was gradually but significantly increased (p<0.05) during the EA treatment. After EA was turned off, the GABA level remained significantly higher than those in the “AMPH-only” group for the next 50 minutes with a slow attenuation back to the baseline level. The degree of GABA increases correlated to the degree of DA attenuation induced by EA (P<0.006).
Discussion
We showed in this manuscript that electroacupuncture modulates the dopaminergic function in the brain pre-loaded with AMPH. Before interpreting our results in the context of acupuncture, it is instructive to examine the prior literature on the stimulation of the median nerve. There is a large body of evidence suggesting that electrical stimulation of the median nerve can lead to pre-synaptic inhibition in the muscle [32]. This inhibition can also extend to the motor cortex and hence decrease motor input to the basal ganglia, and has been found to be abnormal in diseases with primary dopamine deficiencies such as Parkinson's disease [3]. Electrical stimulation of the median nerve, which includes the stimulation of the acupoint LI4, changed somatosensory evoked potentials (SEP) [18] and striatal dopamine release [25]. Thus, the EA results obtained here are not inconsistent with a direct stimulation of the median nerve.
In the striatum, release of glutamate enhances DA release via innervating N-methyl-D-aspartate (NMDA) or DL-alpha-amino-3-hydroxy-5-methyl-4-isoxazole-4-propionate (AMPH) receptors on the DA terminals [37]. Although the majority of the glutamate released is confined within the synapse and quickly uptaken by the high-affinity glutamate transporters, some glutamate escapes the synapse (“spill over”) and can bind to metabotropic glutamate receptors to suppress dopaminergic neurotransmission [36]. Glutamate also indirectly inhibits DA release via activation of the “GABA” inter-neurons [12]. Glutamate thus possesses a dual role in modulating dopaminergic and GABAergic activity. DA and GABA subsequently regulate glutamatergic activity via activation of their receptors on the glutamatergic terminals [8, 17]. This forms a self-contained regulation mechanism which efficiently checks the activity level among the Glu/DA/GABA neurons in the striatum. However, this kind of autoregulation makes it harder to detect the causality of effects among neurotransmitters. We thus used AMPH to control the release of DA (maximized by AMPH) in order to observe how EA modulates the glutamatergic and GABAergic activity, which in turn affect the DA release. Indeed, the rCBV measurement, which is strongly sensitive to the synaptic DA concentration and neuronal activity [5], suggests that the excess DA release (induced by AMPH) was returned back to the drug naïve level at a rate faster than the nature AMPH pharmacological time course. Direct measurement of the synaptic Glu/GABA/DA concentration showed that the striatal glutamatergic activity was enhanced by EA and subsequently led to a prolonged GABA innervation and thus an inhibition of DA release. If the primary effect of EA while DA is at an excess state is to produce a persistent increase in striatal GABA levels, then one expects the DA release to be diminished and this is indeed what we observed. These data are consistent with prior literature showing the effects of GABA and GABA agonists on DA release [9, 22]. Our hypothesis is also in line with the finding that high-frequency activation of corticostriatal fibers led to either long-term depression or long-term potentiation of excitatory transmission (LTD or LTP) depending on the subclass of glutamate receptor and neurons activated [4]. We thus have a plausible first order explanation for the return-to-baseline phenomenon of acupuncture on the central nervous system. Note that, with AMPH on board, we could only examine the EA modulation in the brain areas affected by AMPH (figure 1C), although EA might have broader influence in the brain. We thus are not comparing the drug effect induced by AMPH, but the effect induced by EA on the deviant DA system in this study.
LI4 is clinically one of the most used and versatile acupoints. On an empirical investigation performed in two primarily acupuncture hospital clinics in Beijing, China, of a total of 796 consecutive treatments performed on a wide variety of conditions by senior acupuncturists, LI4 was used in >65 percent of the treatments [23, 24]. The stimulation of LI4 appears to be similar in location to the median nerve stimulation carried out routinely in the western medicine research. This allows us to associate some of the acupuncture mechanism with practices based on the rationales of western science. Further test on the effect of acupoints along the same meridian and along the median nerve territory, such as acupoint PC6, will add our understanding of the acupuncture mechanism from the view of somatosensory stimulation. With regard to our work on the controls, our prior fMRI studies have demonstrated that AMPH provoked a long lasting hemodynamic response (half-life > 40 minutes) [5]. In the striatum, the hemodynamic changes correlated tightly to the dopamine released measured by microdialysis. The tight link between DA function and the hemodynamic response in the striatum was further validated in animals with DA neurons ablated (with missing hemodynamic response) and later restored by fetal/stem cell transplantation (with the restoration of hemodynamic response) [2]. We characterized the hemodynamic timecourse using a mathematical model and the effect of EA could be separated from the effect AMPH in the brain. Combined the results of fMRI with microdialysis, we demonstrated that EA induced Glu release in the striatum, leading to the elevation of GABA activity which subsequently caused a decrease in DA concentration and thus the hemodynamic changes observed by fMRI. These results suggest a mechanism by which acupuncture might provide some benefit in the clinical treatment of dopaminergic disorders.
Acknowledgements
Funding agency: sponsored by NIH/NCCAM grant PO1 AT002048
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- 2.Bjorklund LM, Sanchez-Pernaute R, Chung S, Andersson T, Chen YI, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhmann C, Gorsler A, Baumer T, Hidding U, Demiralay C, Hinkelmann K, Weiller C, Siebner HR, Munchau A. Abnormal excitability of premotor-motor connections in de novo Parkinson's disease. Brain. 2004;127:2732–2746. doi: 10.1093/brain/awh321. [DOI] [PubMed] [Google Scholar]
- 4.Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 1996;19:19–24. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- 5.Chen YI, Choi JK, Andersen SL, Rosen BR, Jenkins BG. Mapping dopamine D2/D3 receptor function using pharmacological magnetic resonance imaging. Psychopharmacology (Berl) 2004;180:705–715. doi: 10.1007/s00213-004-2034-0. [DOI] [PubMed] [Google Scholar]
- 6.Chen YI, Mandeville JB, Nguyen TV, Talele A, Cavagna F, Jenkins BG. Improved mapping of pharmacologically induced neuronal activation using the IRON technique with superparamagnetic blood pool agents. J Magn Reson Imaging. 2001;14:517–524. doi: 10.1002/jmri.1215. [DOI] [PubMed] [Google Scholar]
- 7.Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Brain Res Rev. 2005;50:336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Dewey SL, Smith GS, Logan J, Brodie JD, Yu DW, Ferrieri RA, King PT, MacGregor RR, Martin TP, Wolf AP, et al. GABAergic inhibition of endogenous dopamine release measured in vivo with 11C-raclopride and positron emission tomography. J Neurosci. 1992;12:3773–3780. doi: 10.1523/JNEUROSCI.12-10-03773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gsell W, Burke M, Wiedermann D, Bonvento G, Silva AC, Dauphin F, Buhrle C, Hoehn M, Schwindt W. Differential effects of NMDA and AMPA glutamate receptors on functional magnetic resonance imaging signals and evoked neuronal activity during forepaw stimulation of the rat. J Neurosci. 2006;26:8409–8416. doi: 10.1523/JNEUROSCI.4615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gyngell ML, Bock C, Schmitz B, Hoehn-Berlage M, Hossmann KA. Variation of functional MRI signal in response to frequency of somatosensory stimulation in alpha-chloralose anesthetized rats. Magn Reson Med. 1996;36:13–15. doi: 10.1002/mrm.1910360104. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez LF, Segovia G, Mora F. Effects of activation of NMDA and AMPA glutamate receptors on the extracellular concentrations of dopamine, acetylcholine, and GABA in striatum of the awake rat: a microdialysis study. Neurochem Res. 2003;28:1819–1827. doi: 10.1023/a:1026115607216. [DOI] [PubMed] [Google Scholar]
- 13.Huttunen J, Kahkonen S, Kaakkola S, Ahveninen J, Pekkonen E. Effects of an acute D2-dopaminergic blockade on the somatosensory cortical responses in healthy humans: evidence from evoked magnetic fields. Neuroreport. 2003;14:1609–1612. doi: 10.1097/00001756-200308260-00013. [DOI] [PubMed] [Google Scholar]
- 14.Jayaram P, Steketee JD. Cocaine-induced increases in medial prefrontal cortical GABA transmission involves glutamatergic receptors. Eur J Pharmacol. 2006;531:74–79. doi: 10.1016/j.ejphar.2005.11.056. [DOI] [PubMed] [Google Scholar]
- 15.Kelley AE, Andrzejewski ME, Baldwin AE, Hernandez PJ, Pratt WE. Glutamate-mediated plasticity in corticostriatal networks: role in adaptive motor learning. Ann N Y Acad Sci. 2003;1003:159–168. doi: 10.1196/annals.1300.061. [DOI] [PubMed] [Google Scholar]
- 16.Kim YR, Huang IJ, Lee SR, Tejima E, Mandeville JB, van Meer MP, Dai G, Choi YW, Dijkhuizen RM, Lo EH, Rosen BR. Measurements of BOLD/CBV ratio show altered fMRI hemodynamics during stroke recovery in rats. J Cereb Blood Flow Metab. 2005;25:820–829. doi: 10.1038/sj.jcbfm.9600084. [DOI] [PubMed] [Google Scholar]
- 17.Lacey CJ, Boyes J, Gerlach O, Chen L, Magill PJ, Bolam JP. GABA(B) receptors at glutamatergic synapses in the rat striatum. Neurosci. 2005;136:1083–1095. doi: 10.1016/j.neuroscience.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Lee CH, Jung HS, Lee TY, Lee SR, Yuk SW, Lee KG, Lee BH. Studies of the central neural pathways to the stomach and Zusanli (ST36) Am J Chin Med. 2001;29:211–220. doi: 10.1142/S0192415X01000241. [DOI] [PubMed] [Google Scholar]
- 19.Li Z. Experimental acupuncture, Chinese tradictional medicine press. 2003. p. 351.
- 20.Lovinger DM, Tyler E. Synaptic transmission and modulation in the neostriatum. Int Rev Neurobiol. 1996;39:77–111. doi: 10.1016/s0074-7742(08)60664-9. [DOI] [PubMed] [Google Scholar]
- 21.Mandeville JB, Jenkins BG, Chen YI, Choi JK, Kim YR, Belen D, Liu C, Kosofsky BE, Marota JJ. Exogenous contrast agent improves sensitivity of gradient-echo functional magnetic resonance imaging at 9.4 T. Magn Reson Med. 2004;52:1272–1281. doi: 10.1002/mrm.20278. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto M, Kanno M, Togashi H, Ueno K, Otani H, Mano Y, Yoshioka M. Involvement of GABAA receptors in the regulation of the prefrontal cortex on dopamine release in the rat dorsolateral striatum. Eur J Pharmacol. 2003;482:177–184. doi: 10.1016/j.ejphar.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Napadow V, Kaptchuk TJ. Patient characteristics for outpatient acupuncture in Beijing, China. J Altern Complement Med. 2004;10:565–572. doi: 10.1089/1075553041323849. [DOI] [PubMed] [Google Scholar]
- 24.Napadow V, Liu J, Kaptchuk TJ. A systematic study of acupuncture practice: acupoint usage in an outpatient setting in Beijing, China. Complement Ther Med. 2004;12:209–216. doi: 10.1016/j.ctim.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Nieoullon A, Cheramy A, Glowinski J. Nigral and striatal dopamine release under sensory stimuli. Nature. 1977;269:340–342. doi: 10.1038/269340a0. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- 27.Rossini PM, Bassetti MA, Pasqualetti P. Median nerve somatosensory evoked potentials. Apomorphine-induced transient potentiation of frontal components in Parkinson's disease and in parkinsonism. Electroencephalogr Clin Neurophysiol. 1995;96:236–247. doi: 10.1016/0168-5597(94)00292-m. [DOI] [PubMed] [Google Scholar]
- 28.Sgambato V, Maurice N, Besson MJ, Thierry AM, Deniau JM. Effect of a functional impairment of corticostriatal transmission on cortically evoked expression of c-Fos and zif 268 in the rat basal ganglia. Neurosci. 1999;93:1313–1321. doi: 10.1016/s0306-4522(99)00267-5. [DOI] [PubMed] [Google Scholar]
- 29.Shen Q, Ren H, Cheng H, Fisher M, Duong TQ. Functional, perfusion and diffusion MRI of acute focal ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:1265–1279. doi: 10.1038/sj.jcbfm.9600132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen T, Weissleder R, Papisov M, Bogdanov A, Jr., Brady TJ. Monocrystalline iron oxide nanocompounds (MION): physicochemical properties. Magn Reson Med. 1993;29:599–604. doi: 10.1002/mrm.1910290504. [DOI] [PubMed] [Google Scholar]
- 31.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strassburg HM, Thoden U, Mundinger F. Mesencephalic chronic electrodes in pain patients. An electrophysiological study. Appl Neurophysiol. 1979;42:284–293. doi: 10.1159/000102375. [DOI] [PubMed] [Google Scholar]
- 33.Thobois S, Hassoun W, Ginovart N, Garcia-Larrea L, Le Cavorsin M, Guillouet S, Bonnefoi F, Costes N, Lavenne F, Broussolle E, Leviel V. Effect of sensory stimulus on striatal dopamine release in humans and cats: a [(11)C]raclopride PET study. Neurosci Lett. 2004;368:46–51. doi: 10.1016/j.neulet.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 34.Ureshi M, Kershaw J, Kanno I. Nonlinear correlation between field potential and local cerebral blood flow in rat somatosensory cortex evoked by changing the stimulus current. Neurosci Res. 2005;51:139–145. doi: 10.1016/j.neures.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Zangen A, Hyodo K. Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport. 2002;13:2401–2405. doi: 10.1097/00001756-200212200-00005. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Sulzer D. Glutamate spillover in the striatum depresses dopaminergic transmission by activating group I metabotropic glutamate receptors. J Neurosci. 2003;23:10585–10592. doi: 10.1523/JNEUROSCI.23-33-10585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zigmond MJ, Castro SL, Keefe KA, Abercrombie ED, Sved AF. Role of excitatory amino acids in the regulation of dopamine synthesis and release in the neostriatum. Amino Acids. 1998;14:57–62. doi: 10.1007/BF01345243. [DOI] [PubMed] [Google Scholar]