Abstract
Streptococcus mutans, the primary etiological agent of human dental caries, possesses at least two fructose phosphotransferase systems (PTSs), encoded by fruI and fruCD. fruI is also responsible for xylitol transport. We hypothesized that fructose and xylitol transport systems do not affect virulence. Thus, colonization and cariogenicity of fruI− and fruCD− single and double mutants, their WT (UA159), and xylitol resistance (Xr) of S. mutans were studied in rats fed a high-sucrose diet. A sucrose phosphorylase (gtfA−) mutant and a reference strain (NCTC-10449S) were additional controls. Recoveries of fruI mutant from the teeth were decreased, unlike those for the other strains. The fruCD mutation was associated with a slight loss of cariogenicity on enamel, whereas mutation of fruI was associated with a loss of cariogenicity in dentin. These results also suggest why xylitol inhibition of caries is paradoxically associated with spontaneous emergence of so-called Xr S. mutans in habitual human xylitol users.
Keywords: Streptococcus mutans, PTS, xylitol, sucrose phosphorylase, caries
INTRODUCTION
Analysis of both experimental animal and human data indicates that maximal expression of dental caries is associated with both a diet rich in sucrose, consumed at high frequency, and colonization of the teeth by mutans streptococci (Mandel, 1970; Tanzer, 1979; Kuramitsu, 1993; Tanzer et al., 2001a).
The roles of sugar transport systems in expression of the virulence (cariogenicity) of Streptococcus mutans are minimally defined in vivo, except for some preliminary reports (Tanzer et al., unpublished observations), due in part to the multiplicity of those systems and their nominal functional redundancy (Slee and Tanzer, 1982; LeBlanc, 1994; Tao et al., 1993). Further complicating this is the reported generation from the disaccharide sucrose of free glucose and fructose by extracellular invertase of S. mutans (Kuramitsu, 1973), although others have reported this activity to be intracellular among the mutans streptococci (Tanzer et al., 1973, 1977). Also, extracellular fructosyltransferase and glucosyltransferases of the mutans streptococci most commonly colonizing humans, S. mutans and S. sobrinus (Coykendall, 1989; Tanzer et al., 2001a), produce free glucose and free fructose, respectively, as by-products of polymer synthesis and thus available for transport into the cells (Tanzer et al., 1985a; Kuramitsu, 1993; LeBlanc, 1994). Both of these transferases, as well as invertase, use dietary sucrose as substrate.
Two of the genetic determinants of fructose transport are now known (Wen et al., 2001); one of them also transports xylitol into S. mutans, which inhibits glycolytic metabolism (Trahan, 1995). Study of xylitol-non-transporting and thus xylitol-resistant (Xr) S. mutans thus provides opportunity for evaluation of the dimension of the role of fructose transport in virulence and the exploration of a possible basis of caries inhibition by xylitol.
Xylitol inhibition of caries has attracted considerable interest (Scheinin and Mäkinen, 1975; Mäkinen et al., 1995; Isokangas et al., 2000; Söderling et al., 2000), as has the puzzling emergence, in the mouths of habitual xylitol users, of S. mutans that are resistant to metabolic inhibition by xylitol (Xr) (Trahan and Mouton, 1987).
S. mutans has at least three fructose transport systems, two of which are phosphoenolpyruvate (PEP)-dependent, fructose-specific, phosphotransferase systems (PTSs) that are encoded by two operons. Within them, the fruI gene is inducible by both fructose and sucrose. It encodes for a protein that transports fructose and xylitol. Its deletion renders the mutant cell’s metabolism and growth resistant to xylitol inhibition (Xr). By contrast, fruCD gene is constitutive. It encodes for a protein that also transports fructose, but does not transport xylitol. fruCD deletion does not render the mutant cell’s metabolism and growth resistant to xylitol (Wen et al., 2001).
We have previously engineered, by allelic exchange, stable S. mutans isogenic deletion mutants of the sequenced wild-type strain UA159 (Ajdic et al., 2002) that are defective in either or both of the two PEP-dependent fructose-specific PTSs (Wen et al., 2001), and another stable isogenic mutant of UA159 that is defective in sucrose phosphorylase (gtfA−) (Wen and Burne, 2001). This, and the availability of rats free of indigenous mutans streptococci, enabled the present research to aim to characterize the colonization and cariogenicity of fructose transport mutants of S. mutans strains that are either xylitol-sensitive (Xs) or Xr in the setting of a high-sucrose diet. It also provided us with an opportunity to gain insight about the counter-intuitive emergence of Xr S. mutans within the mouths of habitual xylitol users, a condition associated with caries inhibition (Trahan, 1995). We tested the hypothesis that fructose and xylitol transport deletions have no effect on colonization or cariogenicity in sucrose-fed rats.
MATERIALS & METHODS
Micro-organisms
Stable S. mutans isogenic deletion mutants of sequenced strain UA159 defective in the two genes encoding fructose PTSs, either fruI−, fruCD−, or both fruCD−/fruI− (Wen et al., 2001), and another stable isogenic mutant of UA159 defective in sucrose phosphorylase (gtfA−) (Wen and Burne, 2001) were studied. The well-characterized virulent S. mutans strain NCTC-10449S (Tanzer, 1979; Tanzer et al., 1985b) served as a positive control, and un-inoculated status a negative control for in vivo experiments. Phenotypes and genotypes of studied UA159 and its isogenic mutants were confirmed before and after in vivo experiments. Essential traits are described in the Table.
Table.
Strains Studied
| Strains Used in This Study | Gene Defect | Progenitor | Phenotype | References |
|---|---|---|---|---|
| NCTC-10449S | Unknown | -- | Known virulent, str*, Xs | Tanzer, 1979; Tanzer et al., 1985b |
| UA159 | Unknown | -- | Known virulent, no antibiotic resistance, Xs | Ajdic et al., 2002 |
| TW17 | fruI− | UA159 | Unknown virulence, emr, Xr | Wen et al., 2001 |
| TW18 | fruCD− | UA159 | Unknown virulence, tcr, Xs | Wen et al., 2001 |
| TW20 | fruCD−/fruI− | TW18 | Unknown virulence, tcr/emr, Xr | Wen et al., 2001 |
| TW19 | gtfA− | UA159 | Unknown virulence, kmr, Xs | Wen and Burne, 2001 |
str, spontaneously streptomycin-resistant; emr, erythromycin-resistant; tcr, tetracycline-resistant; kmr, kanamycin-resistant; Xs, sensitive to inhibition of metabolism and growth by xylitol; Xr, resistant to inhibition of metabolism and growth by xylitol.
Animal Experiments
Breeders of rat strain TAN:SPFOM(OMASF)BR, maintained in a full-barrier facility, yielded progeny, born on the same day, for two in vivo experiments of identical design. The rats are free of indigenous mutans streptococci and amylase-binding streptococci (Tanzer et al., 1985b, 2001b, 2003). Each experiment had 7 groups. In the first, there were 12 rats/group, while in the second there were 10 rats/group. Weanlings (21 days old) ate diet 2000 (56% confectioner’s sugar [97% sucrose/3% cornstarch]) and drank sterile demineralized water, ad libitum. At 22 days of age, one group was inoculated with 109–1010 CFU of S. mutans wild-type UA159 or one of its isogenic mutants (Table), or with S. mutans 10449S. The infectious dose needed to colonize 100% of rats for mutans streptococci is about 105 to 106 cells. Each strain had an unambiguous antibiotic resistance phenotype. One rat group was not inoculated in each experiment. All animal procedures were approved by the IACUC of the fully accredited Univ. of Connecticut Health Center.
Animals’ teeth were swabbed at 21 days after inoculation for recovery of flora, and, immediately after euthanasia, 42 days after inoculation, molars of one hemi-mandible were removed en bloc and sonified for recoveries of total flora (trypticase soy sheep’s blood agar), total streptococci (mitis salivarius agar) (MS), and S. mutans inoculants (MS plus appropriate antibiotic). Recoveries were expressed in both relative counts (% of total recoverable flora) and absolute CFU counts/3 mandibular molars. Procedures for blinded scoring of caries lesions and statistical procedures have been detailed previously (Tanzer et al., 1985b, 2001b).
RESULTS
Colonization and Recovery
Un-inoculated animals were again demonstrated to be free of mutans streptococci, and there was no evidence of cross-contamination among groups or of reversion of mutants to wild-type pheno- or genotype. All strains colonized well; mid-experiment tooth swab recovery data (not shown) were consistent with those at the date of death. Absolute count recoveries of inoculants after death from sonicates of 3 mandibular molar teeth (Fig. 1, panels A and C) were significantly lower (52 and 64%) for the fruI−-inoculated group than from UA159- and all other mutant-inoculated groups, in both experiments (p < 0.001). Absolute counts from the fruCD−-inoculated and the fruCD−/fruI−-inoculated groups were not consistently related to those from their WT-inoculated groups in the two experiments. Recoveries of the gtfA−-inoculated groups were like those of the UA159-inoculated groups. In the second experiment, UA159 was recovered in higher absolute numbers than 10449S (p = 0.020).
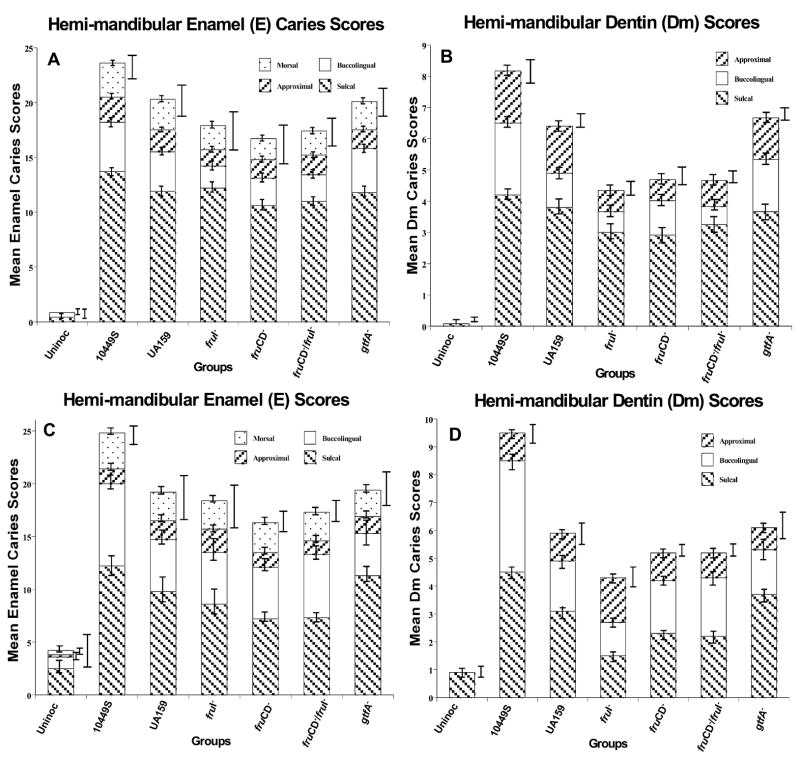
Figure 1.
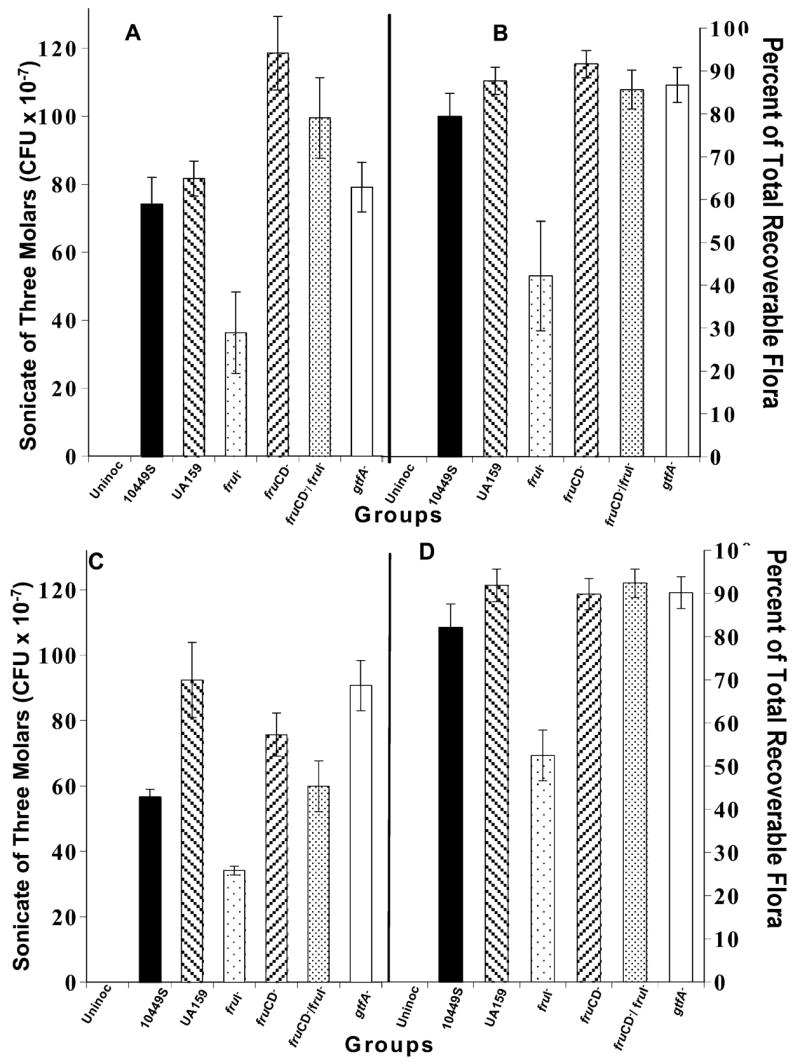
Colonization of rats’ teeth by designated S. mutans strains at 42 days post-inoculation. Strains are further described in the text and the Table. Two experiments are represented. In the first experiment (panels A and B), there were 12 rats per group; in the second experiment (panels C and D), there were 10 rats per group. Absolute counts are presented on the left, panels A and C, and percentages of total recoverable flora are on the right, panels B and D. All animals were free of indigenous mutans streptococci. The experimental design and microbiological methods are detailed in the text. Data are given as means ± SEM.
The percentages of total recoverable flora from the molars represented by the inoculants (Fig. 1, panels B and D) were very high (80–93% of total recoverable flora) and not different from one another, except for those inoculated with the fruI−mutant, which was significantly lower (42–52% of total recoverable flora) than other inoculated groups in the two experiments (both, p < 0.001).
Genotypes recovered from agar plates after sonification of molars were analyzed by PCR with gene-specific primers (Wen et al., 2001), and results showed no detectable alterations in the respective loci (data not shown).
Caries Scores
The sum of smooth-surface and fissure scores for un-inoculated groups was much lower than for any mutans-inoculated groups (Fig. 2), indicating that the majority of caries lesions were attributable to S. mutans colonization (p < 0.001). The wild-type UA159 was less cariogenic than the internal control reference strain 10449S (p < 0.001 for the data pooled from the two studies, n = 22/group).
Figure 2.
Stacked-bar histogram of caries scores, according to tooth surface category, for the two experiments lasting 42 days post-inoculation with designated S. mutans strains. In the first experiment (panels A and B), there were 12 rats per group; in the second experiment (panels C and D), there were 10 rats per group. Mean ± SEM values are represented for each tooth surface category identified by bar shades, and the height of each stacked bar represents mean total caries score and its variance (SEM), indicated to its immediate right. Panels A and C depict the data for enamel (Keyes E) lesions, and panels B and D depict the data for dentinal (Keyes Dm) lesions, respectively. The scoring method of Keyes does not assess dentinal penetration on rat morsal tooth surfaces. The experimental design and blinded caries scoring methods are detailed in the text. The use of data pooled from the two experiments * indicates a statistically significant difference of the fruCD− mutant-infected group for enamel caries scores by comparison with those of the wild-type UA159-infected group. ‡ indicates statistically significant differences of the fruI− mutant-infected group for dentin scores by comparison with their respective wild-type UA159-infected and gtfA-infected group, as detailed in the RESULTS.
Enamel (E) caries scores for single and double fructose transport mutants were statistically lower for the fruCD−-infected group than for the UA159-infected group; this was statistically supported only after the data from the two identical experiments were pooled (p = 0.022, n = 22/group). Enamel scores for the other groups infected with mutants of UA159 were not statistically significantly different from the UA159-infected groups, with or without pooling results from the two experiments.
However, dentinal (Dm) scores for the fruI−-infected group were 30–35% lower than those of the WT- or gtfA−-infected groups (p = 0.011 and p = 0.004, respectively) when the data were pooled for the two trials (n = 22/group).
Infection by the gtfA− mutant resulted in no loss of virulence expression on either the enamel or in the dentin.
DISCUSSION
Fructose transport via the two fructose-specific PTSs, singly or in concert, is at most a weak virulence determinant of inception of enamel caries lesions of rats eating a high-sucrose diet and is more clearly associated with the constitutively expressed fruCD gene than with the fruI gene. However, deletion of the fructose-and sucrose-inducible, xylitol-transporting, fructose-PTS encoded by fruI is associated with (1) diminished colonization on/in the teeth and (2) diminished ability to contribute to penetration of lesions into dentin. We cannot with certainty explain the failure of the double mutant fruCD−/fruI− to behave in vivo as simply the sum of behaviors of the fruCD− and the fruI− mutants. The possibility of pleiotropic effects increases with double mutants (Wen et al., 2001), and it is evident that sugar metabolism is complexly regulated (Slee and Tanzer, 1982; LeBlanc, 1994; Cvitkovitch et al., 1995).
It is perhaps not surprising that, in the face of a high-sucrose diet, the condition most associated with aggressive caries in humans and rats (Mandel, 1970; Tanzer, 1979; Tanzer et al., 2001a), the impact of fructose transport per se is weak, given the recognition of multiple sucrose transport systems, transport of monosaccharides generated by extracellular glucosyl and fructosyl transferases, and putatively extracellular invertase, all producing fermentable carbohydrates that can be transported (Slee and Tanzer, 1982; LeBlanc, 1994), converted to intracellular polysaccharides available for subsequent catabolism (Birkhed and Tanzer, 1979) and directly catabolized glycolytically (Tanzer et al., 1971; LeBlanc, 1994). However, the ability to discern the contributions of fructose transport systems per se has been enabled in this study by the availability of stable mutations in two fructose-specific transporters. It is unclear from these in vivo experiments whether the fruI deletion has in some way altered the ability of the mutant to grow in plaque biofilm, to adhere to the teeth in it, or both. In vitro growth rates of UA159 and its mutants studied here were similar (data not shown) to those previously reported (Wen et al., 2001).
Notably, the now-extensive literature on xylitol inhibition of caries (Mäkinen et al., 1995; Isokangas et al., 2000), usually monitored by measure of frank cavitation of lesions into the dentin, is associated with what might seem to be paradoxical emergence among frequent users of xylitol of Xr S. mutans in the mouth (Trahan and Mouton, 1987). The present observations suggest that, in fact, Xr strains of S. mutans are of diminished virulence by virtue of compromised colonization of the teeth and compromised ability to induce lesions that penetrate dentin, i.e., to the point that they would have been scored in those clinical studies.
The present study also indicates that sucrose phosphorylase of S. mutans is not a virulence determinant in rats consuming a high-sucrose diet. This is in agreement with some authors (Barletta et al., 1988), who used mono-associated gnotobiotic rats fed diet containing only 5% sucrose but 62% cornstarch, while in disagreement with others who repeatedly inoculated specific-pathogen-free rat dams and weanlings fed the same 56% sucrose diet as used in the present study, gave 10% sucrose to drink, and removed the major salivary glands of the weanling test animals (Yamashita et al., 1993). It should also be noted, from the present data, that the now-popularly-studied sequenced strain UA159 (Ajdic et al., 2002), while a very good colonizer of rats’ teeth, nonetheless appears less cariogenic than NCTC-10449S. A cautionary note is thus in order concerning UA159’s broad use in studies of pathogenesis. It may well be that UA159 has been maintained in laboratories on the bench-top or in incubators for years, during which it has undergone untold numbers of replications, perhaps selecting for diminished virulence. The microbiological literature is replete with data on loss of virulence of laboratory strains maintained in this way. Our laboratory strain NCTC-10449S, since the mid-1970s, has been maintained in a lyophilized or deep-frozen state. It is always retrieved from −70°C stocks prior to experiments.
In summary, in the presence of a high-sucrose diet, fructose transport via either or both of two fructose PTS mechanisms is a weak determinant of virulence on enamel, but the fruI-encoded PTS does contribute to the ability to colonize the teeth and induce lesions that penetrate dentin. To our knowledge, this is the first demonstration of dissociation of the impact of a strain of S. mutans’ ability to induce decay of enamel vs. dentin.
Acknowledgments
This work was wholly funded by the University of Connecticut 4-04020 and by NIH grant DE-12236. We thank Dr. John Tsimikas for valuable statistical discussions. A preliminary report was presented at the 52nd ORCA Congress, Indianapolis, IN, USA, July, 2005 (Caries Res 39:308, Abstr 64).
References
- Ajdic D, McShan WM, McLaughlin RE, Savic G, Chang J, Carson MB, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci USA. 2002;99:14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barletta RG, Michalek SM, Curtiss R., III Analysis of the virulence of Streptococcus mutans serotype c gtfA mutants in the rat model system. Infect Immun. 1988;56:322–330. doi: 10.1128/iai.56.2.322-330.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhed D, Tanzer JM. Glycogen synthesis pathway in Streptococcus mutans NCTC 10449S and its glycogen synthesis-defective mutant 805. Arch Oral Biol. 1979;24:67–74. doi: 10.1016/0003-9969(79)90177-8. [DOI] [PubMed] [Google Scholar]
- Coykendall AL. Classification and identification of the viridans streptococci. Clin Microbiol Rev. 1989;2:315–328. doi: 10.1128/cmr.2.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvitkovitch DG, Boyd DA, Hamilton IR. Regulation of sugar transport via the multiple sugar metabolism operon of Streptococcus mutans by the phosphoenolpyruvate phosphotransferase system. J Bacteriol. 1995;177:5704–5706. doi: 10.1128/jb.177.19.5704-5706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isokangas P, Söderling E, Pienihäkkinen K, Alanen P. Occurrence of dental decay in children after maternal consumption of xylitol chewing gum, a follow-up from 0 to 5 years of age. J Dent Res. 2000;79:1885–1889. doi: 10.1177/00220345000790111201. [DOI] [PubMed] [Google Scholar]
- Kuramitsu HK. Characterization of invertase activity from cariogenic Streptococcus mutans. J Bacteriol. 1973;115:1003–1010. doi: 10.1128/jb.115.3.1003-1010.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu HK. Virulence factors of mutans streptococci: role of molecular genetics. Crit Rev Oral Biol Med. 1993;4:159–176. doi: 10.1177/10454411930040020201. [DOI] [PubMed] [Google Scholar]
- LeBlanc D. Role of sucrose metabolism in the cariogenicity of the mutans streptococci. In: Miller VL, Kaper JB, Portnoy DA, Isberg RR, editors. Molecular genetics of bacterial pathogenesis: a tribute to Stanley Falkow. Chap 31. Washington, DC: ASM Press; 1994. pp. 465–477. [Google Scholar]
- Mäkinen KK, Bennett CA, Hujoel PP, Isokangas PJ, Isotupa KP, Pape HR, Jr, et al. Xylitol chewing gums and caries rates: a 40-month cohort study. J Dent Res. 1995;74:1904–1913. doi: 10.1177/00220345950740121501. [DOI] [PubMed] [Google Scholar]
- Mandel ID. Effects of dietary modifications on caries in humans. J Dent Res. 1970;49:1201–1211. doi: 10.1177/00220345700490060501. [DOI] [PubMed] [Google Scholar]
- Scheinin A, Mäkinen KK, editors. Acta Odontol Scand. Suppl 70. Vol. 33. 1975. Turku sugar studies: I–XXI; pp. 1–348. [Google Scholar]
- Slee AM, Tanzer JM. Sucrose transport by Streptococcus mutans. Evidence for multiple transport systems. Biochim Biophys Acta. 1982;692:415–424. doi: 10.1016/0005-2736(82)90392-3. [DOI] [PubMed] [Google Scholar]
- Söderling E, Isokangas P, Pienihäkkinen K, Tenovuo J. Influence of maternal xylitol consumption on acquisition of mutans streptococci by infants. J Dent Res. 2000;79:882–887. doi: 10.1177/00220345000790031601. [DOI] [PubMed] [Google Scholar]
- Tanzer JM. Essential dependence of smooth surface caries on, and augmentation of fissure caries by, sucrose and Streptococcus mutans infection. Infect Immun. 1979;25:526–531. doi: 10.1128/iai.25.2.526-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer JM, Chassy BM, Krichevsky MI. Sucrose metabolism by Streptococcus mutans, SL-I. Biochim Biophys Acta. 1971;261:379–387. doi: 10.1016/0304-4165(72)90062-1. [DOI] [PubMed] [Google Scholar]
- Tanzer JM, Brown AT, McInerney MF. Identification, preliminary characterization, and evidence for the regulation of invertase in Streptococcus mutans. J Bacteriol. 1973;116:192–202. doi: 10.1128/jb.116.1.192-202.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer JM, Brown AT, McInerney MF, Woodiel FN. Comparative study of invertases of Streptococcus mutans. Infect Immun. 1977;16:318–327. doi: 10.1128/iai.16.1.318-327.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer JM, Freedman ML, Fitzgerald RJ. Virulence of mutants defective in glucosyl transferase, dextran-mediated aggregation, or dextranase activity. In: Mergenhagen SE, Rosan B, editors. Molecular basis of oral microbial adhesion. Washington, DC: American Society for Microbiology; 1985a. pp. 204–211. [Google Scholar]
- Tanzer JM, Kurasz AB, Clive J. Competitive displacement of mutans streptococci and inhibition of tooth decay by Streptococcus salivarius TOVE-R. Infect Immun. 1985b;48:44–50. doi: 10.1128/iai.48.1.44-50.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer JM, Livingston J, Thompson AM. Consensus development conference on the diagnosis & management of dental caries throughout life. National Institute of Dental and Craniofacial Research and Office of Medical Applications of Research, NIH; 2001a. The microbiology of primary dental caries. Full Paper with Evidence Tables. http://www.nidcr.nih.gov/news/consensus/jason_tanzer.pdf. [Google Scholar]
- Tanzer JM, Baranowski LK, Rogers JD, Haase EM, Scannapieco FA. Oral colonization and cariogenicity of Streptococcus gordonii in specific pathogen-free TAN:SPFOM(OM)BR rats consuming starch or sucrose diets. Arch Oral Biol. 2001b;46:323–333. doi: 10.1016/s0003-9969(00)00126-6. [DOI] [PubMed] [Google Scholar]
- Tanzer JM, Grant L, Thompson A, Li L, Rogers JD, Haase EM, et al. Amylase-binding proteins A (AbpA) and B (AbpB) differentially affect colonization of rats’ teeth by Streptococcus gordonii. Microbiology. 2003;149:2653–2660. doi: 10.1099/mic.0.26022-0. [DOI] [PubMed] [Google Scholar]
- Tao L, Sutcliffe IC, Russell RR, Ferretti JJ. Transport of sugars, including sucrose, by the msm transport system of Streptococcus mutans. J Dent Res. 1993;72:1386–1390. doi: 10.1177/00220345930720100701. [DOI] [PubMed] [Google Scholar]
- Trahan L. Xylitol: a review of its action on mutans streptococci and dental plaque—its significance. Int Dent J. 1995;45(1 Suppl 1):77–92. [PubMed] [Google Scholar]
- Trahan L, Mouton C. Selection for Streptococcus mutans with an altered xylitol transport capacity in chronic xylitol consumers. J Dent Res. 1987;66:982–988. doi: 10.1177/00220345870660052301. [DOI] [PubMed] [Google Scholar]
- Wen ZT, Burne RA. Construction of a new integration vector for use in Streptococcus mutans. Plasmid. 2001;45:31–36. doi: 10.1006/plas.2000.1498. [DOI] [PubMed] [Google Scholar]
- Wen ZT, Browngardt C, Burne RA. Characterization of two operons that encode components of fructose-specific enzyme II of the sugar:phosphotransferase system of Streptococcus mutans. FEMS Microbiol Lett. 2001;205:337–342. doi: 10.1111/j.1574-6968.2001.tb10969.x. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Bowen WH, Burne RA, Kuramitsu HK. Role of Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]