Abstract
Purpose
Squamous metaplasia is a pathologic process that frequently occurs in nonkeratinized stratified ocular surface epithelia. The mechanism for this occurrence is largely unknown except for vitamin A deficiency.
Methods
Human limbal explants were cultured under airlift with or without p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 or in a submerged manner for different durations up to 2 weeks. Epithelial cell proliferation, differentiation, limbal stem cell maintenance, and expansion were studied using certain markers such as Ki67, p63, K10 and K12 keratins, filaggrin, Pax6, ABCG-2, and Musashi-1. Expression of phospho-p38 MAPK and its downstream transcription factors, C/EBPα and C/EBPβ, were studied by immunohistochemistry. Epithelial cells harvested from explants after 2 weeks of culturing under different conditions were seeded onto 3T3 feeder layers and cultured for 12 days. The differentiation of clonal epithelial cells was investigated by double staining to K12 and K10 keratins.
Results
The squamous metaplasia model was successfully created by culturing human limbal explants at an air-liquid interface (airlift) for 2 weeks. Increased stratification and hyperproliferation only happened in the limbal, but not the corneal, epithelium in airlift, but not submerged, cultures. Epithelial proliferation was associated with a transient increase of limbal epithelial stem cells. Abnormal epidermal differentiation—evidenced by positive expression of K10 keratin in suprabasal cells and filaggrin in superficial cells—ensued. Clones generated from epithelial cells harvested from airlift culture only expressed K12 keratin without K10. As early as 2 days in airlift cultures, p38 expression emerged in limbal basal epithelial cells and gradually extended to the cytoplasm and nuclei. Furthermore, addition of the p38 inhibitor SB203580 abolished abnormal epidermal differentiation without affecting limbal epithelial proliferation. Expression of C/EBPα and C/EBPβ downstream of the p38 MAPK signaling pathway, was strongly induced by airlift culture and partially was inhibited by SB203580.
Conclusions
Dryness resulting from exposure activates p38 MAPK signaling coupled with abnormal epidermal differentiation without intrinsic alteration of stem cells in the limbus. On the ocular surface, p38 inhibitors may have the potential to revert the pathologic process of squamous metaplasia induced by dryness.
Squamous metaplasia occurs when nonsquamous (keratinized) epithelium is replaced by squamous (keratinized) epithelium. It is a common pathologic process that happens in almost all epithelial tissues, including the urothelium1 and the pulmonary epithelium.2 It is also a hallmark of a variety of severe ocular surface disorders manifesting dry eye caused by the lack of lacrimal gland secretion such as Sjögren syndrome, and it can be frequently seen in Stevens-Johnson Syndrome, mucous membrane pemphigoid, chemical/thermal burns, and vitamin A deficiency.3,4 The grading of squamous metaplasia correlates well with the severity of this type of dry eye5 and may lead to severe visual loss or blindness.
Squamous metaplasia of the corneal epithelium is well correlated with the loss of cornea-specific keratin K3 and keratin K12 expression,6–8 the emergence of epidermis-specific keratin K1 and keratin K10,6,7 and such cornified envelope-specific proteins as transglutaminase I,9 involucrin, filaggrin, and loricrin.7 Aside from xerophthalmia caused by systemic vitamin A deficiency, which causes squamous metaplasia of ocular surface epithelia in experimental animals6,10 and human patients,11,12 little is known about the pathogenesis of squamous metaplasia and the signaling pathway involved in this pathologic process.
In this study, we created an ex vivo squamous metaplasia model of the corneolimbal epithelium by culturing a human limbal explant at the air-fluid interface. To our surprise, we found that abnormal epidermal differentiation emerged only from the stem cell-containing limbal, but not corneal, epithelium and that such a process could be abolished by the p38 inhibitor SB203580. The significance of this finding is further discussed.
Materials and Methods
Materials
Dulbecco modified Eagle medium (DMEM), Ham/F12 medium, HEPES buffer, amphotericin B, gentamicin, fetal bovine serum (FBS), and mouse epidermal growth factor (EGF) were purchased from Invitrogen (Carlsbad, CA). Hydrocortisone, dimethyl sulfoxide, cholera toxin, insulin-transferrin-sodium selenite media supplement, 3% hydrogen peroxide, propidium iodide (PI), Hoechst-33342 dye, acetone, Triton X-100, bovine serum albumin (BSA), and FITC-conjugated anti–mouse, anti–goat, and anti–rabbit IgGs were from Sigma (St. Louis, MO). Mouse anti–cytokeratin 10 (K10) and mouse anti–BCRP (ABCG-2) antibodies were from Chemicon (Temecula, CA). Mouse anti–Pax6 and C/EBPβ antibodies, goat anti-cytokeratin 12 (K12), filaggrin, and C/EBPα polyclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti–p63 (clone 4A4) and Ki67 antibodies and diaminobenzidine (DAB) were from DakoCytomation (Carpinteria, CA). Rabbit anti–Musashi-1 antibody was from Abcam (Cambridge, MA). Rabbit anti–phospho p38 antibody was from Cell Signaling (Danvers, MA). ABC kit (Vectastain Elite) for mouse, goat, and rabbit IgG was from Vector Laboratories (Burlingame, CA). p38 inhibitor SB203580 was from Upstate (Lake Placid, NY). Type I collagen-coated inserts were from Corning Incorporated (Corning, NY).
Human Limbal Explant Cultures
Human tissue was handled in accordance with the Declaration of Helsinki. Corneoscleral tissues from human donor eyes were obtained from the Florida Lions Eye Bank (Miami, FL) immediately after the central corneal button had been used for corneal transplantation. The tissue was rinsed three times with DMEM containing 50 μg/mL gentamicin and 1.25 μg/mL amphotericin B. After careful removal of excessive sclera, conjunctiva, iris, and corneal endothelium, the corneoscleral rim was trimmed to obtain limbal tissue cubes (explants) of 2 clock hours—that is, approximately 3 × 5 mm size. After that, limbal tissue explants were placed on the center of type I collagen-coated inserts in six-well plates containing supplemented hormonal epithelial medium (SHEM) made of an equal volume of HEPES-buffered DMEM containing bicarbonate and Ham/F12, supplemented with 5% FBS, 0.5% dimethyl sulfoxide, 2 ng/mL mouse EGF, 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, 0.5 μg/mL hydrocortisone, 1 nM cholera toxin, 50 μg/mL gentamicin, and 1.25 μg/mL amphotericin B.
Limbal explants were cultured in airlift or submerge conditions (Fig. 1). In airlift cultures, 2 mL medium was added so that the explant epithelium was exposed to the air, but the remaining explant stroma was submerged in the medium. In some airlift cultures, p38 inhibitor SB203580 was added to the culture medium at a concentration of 10μM. In submerge cultures, 4 mL culture medium was added to the culture system to cover the entire limbal explant. Cultures were incubated at 37°C under 5% CO2 and 95% air, and the medium was changed every 2 days for 2 weeks before the explant was terminated. In parallel experiments, explants were terminated every 2 days for 2 weeks.
Figure 1.
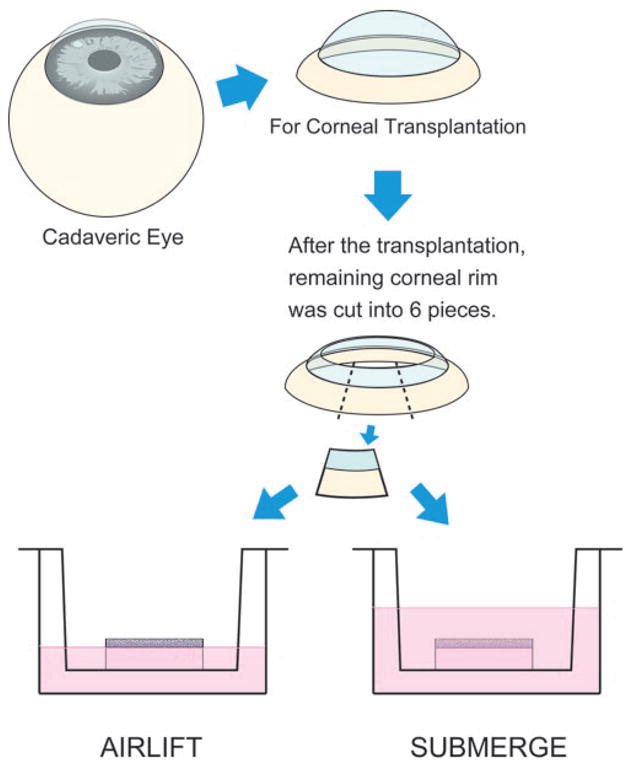
Schematic drawing of human limbal explant cultures. From a human cadaveric eye, the corneoscleral tissue was retrieved and preserved in the storage medium. After the central cornea was removed for transplantation, the remaining corneoscleral rim was subdivided into six segments, of which each was cultured under airlift or submerged mode. While in submerged mode, the culture medium was added to cover the entire limbal explant. While in airlift mode, the culture medium was added to the level at which the epithelium was at the air-medium interface.
Epithelial Colony Formation Assay on 3T3 Fibroblast Feeder Layers
After culture under different conditions for 2 weeks, epithelial sheets were harvested from the surface of limbal explants by digestion with 10 mg/mL dispase II at 4°C for 16 hours and then were rendered into single cells by 0.05% trypsin/0.53 mM EDTA at 37°C for 10 minutes. Epithelial cells were seeded at a density of 30 cells/cm2 in 60-mm dishes containing mitomycin C-treated 3T3 fibroblasts feeder layers in SHEM. Cultures were incubated at 37°C under 5% CO2 and 95% humidity, and the medium was changed every 2 to 3 days. Colonies were fixed by 4% formalin 12 days later for immunostaining or crystal violet staining and were photographed.
Histology and Immunostaining
Cryostat sections (4 μm) of the limbal explants or epithelial colonies were fixed in acetone for 10 minutes at −20°C and prepared for immunostaining by rehydration in PBS followed by incubation in 0.2% Triton X-100 for 10 minutes. After three rinses with PBS for 5 minutes each and preincubation with 2% BSA to block nonspecific staining, sections or epithelial colonies were incubated with primary antibodies for 1 hour with different dilutions (K12, K10, and filaggrin all at 1:200, ABCG-2 at 1:50, Musashi-1 at 1:100). After three washes with PBS for 15 minutes, they were incubated with an FITC-conjugated secondary antibody (goat anti–rabbit or anti–mouse IgG at 1:100) for 45 minutes. After three additional PBS washes for 15 minutes, they were counter-stained with PI (1:2000) or Hoechst 33342 (10 μg/mL) for 10 minutes and then mounted and analyzed under a fluorescence microscope. For immunohistochemical staining, the endogenous peroxidase activity was blocked by 0.6% hydrogen peroxide for 10 minutes after fixation, and nonspecific staining was blocked by 1% normal goat serum for 30 minutes. Sections were then incubated with antibodies for 1 hour with different dilutions (p63, C/EBPα, and C/EBPβ all at 1:50, Ki67 at 1:100, Pax6 at 1:200, phospho-p38 at 1:250). After three washes with PBS for 15 minutes, sections were incubated with biotinylated anti–mouse, anti–goat, and anti–rabbit IgG (1:100) for 1 hour, followed by incubation with ABC reagent for 30 minutes. The reaction product was then developed with DAB for 2 minutes.
Results
Limbal Epithelial Proliferation in Airlift Cultures
Human limbal explants were cultured under submerged or airlift mode for different durations. Before culturing, the limbal epithelium remained intact (Fig. 2, d0), whereas the peripheral corneal epithelium exhibited partial desquamation probably because of postmortem storage (Fig. 2, d0C). Under the airlift mode, superficial limbal epithelial cells desquamated after 2 days of culture (Fig. 2, d2). However, limbal epithelial cell layers increased dramatically from day 4 to day 14; some areas had more than 15 cell layers (Fig. 2). As a result, the basal epithelial plane became undulated to yield an appearance resembling digital invasion of the limbal basal epithelium starting from day 4. This morphologic change became more dramatic on day 6 and was maintained until day 14. In contrast, the cell layers of the peripheral corneal epithelium decreased instantly after culturing (data not shown) and retained only 1 to 2 layers on day 14 (Fig. 2, d14C). As expected, when limbal explants were cultured under the submerged mode, the stratification of limbal epithelial and peripheral corneal epithelial cells was not increased, and there was no digital invasion of limbal epithelium (data not shown). These findings were similar to what was reported in rabbit limbal explants.13
Figure 2.

Limbal epithelial changes in airlift cultures. Hematoxylin staining showed stratified limbal (d0) and peripheral corneal (d0C) epithelium before culturing. Superficial limbal epithelial cells desquamated on day 2 (d2). Limbal epithelial cell layers increased dramatically from day 4 to day 14, resulting in an undulated basal epithelial plane and digital invasion. In contrast, the cell layers of the peripheral corneal epithelium decreased to one to two layers after 14 days of airlift culturing (d14C). Scale bar, 200 μm.
To determine whether the aforementioned increased stratification of the limbal epithelium resulted from an increase of cellular proliferation by progenitor cells, immunostaining to Ki67 and p63 was performed. Before culturing, few Ki67-positive nuclei were present in the limbal epithelium (Fig. 3A, d0), but they were absent on day 2 after culturing (Fig. 3A, d2). However, numerous Ki67-positive nuclei were present in basal and suprabasal epithelial cells on days 4 and 6; their numbers were diminished thereafter to a level still much higher than in normal control on days 10 and 14 after culturing under the airlift mode (Fig. 3A, d10 and d14, respectively). Positive p63 nuclei were noted mostly in basal and some suprabasal layers before culturing (Fig. 3B, d0). A similar pattern was noted on day 2 after culturing under the airlift mode. However, p63-positive nuclei spread to the full-thickness epithelial layers on day 4 but turned negative in superficial cell layers from day 6 to day 14 in airlift cultures (Fig. 3B). Peripheral corneal epithelial cells did not exhibit any increase of Ki67 or p63 nuclear staining (not shown). Collectively, these results indicated that increased stratification was indeed associated with hyperproliferation of limbal epithelial progenitor cells in airlift cultures.
Figure 3.

Limbal epithelial proliferation in airlift cultures. Ki67 staining (A) showed that positive nuclei were very sparse in the limbal epithelium before culturing (d0), became absent on day 2 (d2), and dramatically increased on day 4 (d4) and day 6 (d6) in mostly basal and some suprabasal epithelial cells before a gradual decline on day 10 (d10) and day 14 (d14). p63 staining (B) showed that positive nuclei were found in all basal and some suprabasal cell layers on day 0 and day 2 in airlift cultures but increased to the full-thickness cell layers on day 4 before the superficial cell layers reverted to negative from day 6 to day 14 (B). ABCG-2 staining (C, green) showed that positive staining was limited to clusters in limbal basal cells on day 0 but dramatically increased in basal and suprabasal layers on day 4 before a gradual decrease from day 6 to day 14. Musashi-1 staining (D, red) showed that positive expression was sporadic in limbal basal and very few suprabasal cells on day 0, became negligible on day 2, and dramatically increased in basal and suprabasal layers on day 4 before gradual decrease from day 6 to day 14. Scale bars, 100 μm.
To further investigate whether the hyperproliferation of limbal epithelial cells in airlift cultures involved the expansion of limbal stem cells, we performed immunostaining to ABCG-2, a recently reported limbal stem cell marker,14–17 and Musashi-1, a RNA-binding protein expressed in various epithelial stem/progenitor cells that regulate asymmetric stem cell division (for a review, see Okano18). Results showed that ABCG-2 expression by limbal basal cells was clustered before culturing. This was not significantly changed on day 2 but was dramatically increased in basal and suprabasal layers on day 4 before a steady decline occurred from day 6 to day 14 in airlift cultures (Fig. 3C, green). Similarly, Musashi-1 expression was sporadic in limbal basal and a few suprabasal cells before culturing (d0), became negligible on day 2, dramatically increased in basal and suprabasal layers on day 4, and declined steadily from days 6 to 14 in airlift cultures (Fig. 3D, red). These results indicated a transient expansion of limbal epithelial stem cells to some suprabasal layers on day 4 in an airlift manner, coinciding with the aforementioned hyperproliferation and increased stratification.
Airlift-Induced Epidermal Differentiation of Limbal Epithelial Cells
To investigate whether differentiation was also promoted in airlift cultures, immunostaining was conducted to cornea-specific differentiation K12 keratin19,20 and to epidermis-specific differentiation K10 keratin and filaggrin. As reported,21 K12 keratin expression was limited to all suprabasal cell layers of the limbal epithelium, leaving the basal cell layer negative in the control before culturing (Fig. 4A, d0). K12-negative cells dramatically increased to include up to three suprabasal layers in some areas from day 4 to day 6 in airlift cultures (Fig. 4A, d4–d6), suggesting that more nondifferentiated progenitor cells were expanded during this period. Thereafter, K12-negative cells were reverted to the basal cell layer only (Fig. 4A). As also expected, expression of K10 keratin, an epidermal keratinocyte-specific intermediate filament, was negative before culturing (Fig. 4B, d0). To our surprise, K10-positive cells started to emerge on day 4 in the suprabasal to superficial cell layers and gradually increased from day 6 to day 14 (Fig. 4B). Merged photographs of K12 and K10 double staining showed that most of the K10-positive cells maintained K12 expression (Fig. 4C). Expression of filaggrin, a marker indicative of final epidermal differentiation, was first found on the superficial cell layer on day 6 and increased dramatically until day 14 (Fig. 4D). These results indicated that abnormal epidermal differentiation emerged when limbal epithelial proliferation was promoted in airlift cultures.
Figure 4.

Abnormal epidermal differentiation of limbal epithelial cells in airlift cultures. Double immunostaining to K12 keratin (A) and K10 keratin (B) and merged images (C) show that the expression of K12 keratin was limited to suprabasal cell layers of the limbal epithelium before culturing (d0). K12-negative basal cells expanded to suprabasal cell layers from day 2 to day 6 and then reverted to the basal cell layer again on day 10 and day 14. Expression of K10 keratin was negative before culturing and on day 2 in airlift cultures but became positive mostly in superficial cell layers on day 4 and gradually increased from day 6 to day 14. Merged images showed nearly all K10-positive cells maintained K12 expression. Filaggrin-positive cells were first found in superficial cells on day 6 and gradually increased in superficial cells until day 14 (D). Pax6 (E, F) was expressed by almost all epithelial cells throughout the culture duration, except some desquamated cells on the superficial limbus on day 2, day 10, and day 14 and some cells in the basal epithelium on day 0 and day 14 (arrows). Scale bars, 100 μm.
As opposed to the epidermal epithelium, one distinguished characteristic of the ocular surface epithelium is the expression of Pax6, a transcription factor playing a key role in eye morphogenesis.22,23 To determine whether the aforementioned abnormal epidermal differentiation was associated with the loss of differentiation into the ocular surface lineage in airlift cultures, we performed immunohistochemical staining to Pax6 with nuclear counterstaining by Hoechst 33342, of which the latter helped highlight the Pax6-negative nuclei. The results showed that Pax6-positive nuclei were present throughout the full thickness of the limbal epithelium except for some small patches in the limbal basal layer before culturing (Figs. 4E, 4F, d0). Such Pax6 positivity was noted in all epithelial cells throughout the culture duration except for some desquamated cells on the superficial limbus on days 2, 10, and 14 and for a notable number of limbal basal cells on day 14. Altogether, these data indicated that limbal epithelial cells still maintained the ocular surface lineage even when there was abnormal epidermal differentiation during 2 weeks of airlift culturing.
p38 MAPK Signaling Pathway Activation in Airlift Cultures
The p38 signaling transduction pathway, a mitogen-activated protein kinase (MAPK) pathway, plays an essential role in regulating many cellular processes, including inflammation, cell proliferation, cell differentiation, migration, and cell survival (for a review, see Ono and Han24). To determine whether the p38 signaling pathway was activated in airlift culture, phospho-p38 (P-p38) expression was investigated by immunohistochemical staining at different stages of the airlift culture (Fig. 5). The results showed that P-p38 was negative in limbal epithelial and stromal cells on day 0 and in the normal control without culturing. On day 2, positive staining was noted in few epithelial cells in limbal basal and suprabasal layers. Positive staining was increased in the basal layer on day 4, whereas stromal cells maintained negative staining. On day 6, when epithelial stratification increased significantly, cytoplasmic expression of P-p38 increased accordingly without a concomitant increase in nuclear staining. However, at this time, stromal cells started to show weak positive nuclear staining. On days 10 and 14, cytoplasmic expression of P-p38 increased dramatically, and nuclear staining spread from the basal layer to the superficial layer; stromal expression also increased. These results indicated that the p38 signaling pathway is indeed activated from the early stage of airlift cultures.
Figure 5.
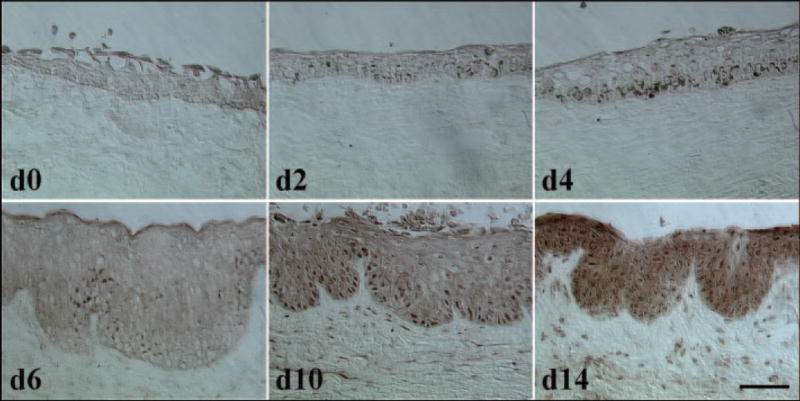
p38 activation of limbal epithelial cells and stromal cells in airlift cultures. Immunohistochemical staining of P-p38 at different stages of airlift cultures were performed. P-p38 staining was negative in limbal epithelial and stromal cells on day 0. On day 2, positive epithelial cells were present in limbal basal and suprabasal layers. Positive staining was increased in the basal layer on day 4, whereas stromal cells continued to stain negatively. On day 6, cytoplasmic expression of P-p38 increased without a concomitant increase in nuclear staining, whereas stromal cells started to show weak positive nuclear staining. On day 10 and day 14, cytoplasmic expression of P-p38 increased dramatically, and nuclear staining spread from the basal layer to the superficial layer. Stromal expression also increased. Scale bar, 200 μm.
To determine whether the p38 MAPK signaling pathway was involved in the aforementioned epidermal differentiation, we added SB203580, a p38 inhibitor, to airlift cultures and compared to that without adding SB203580 or under the submerge mode. Immunostaining performed at the end of 2 weeks showed that K12 keratin was expressed in suprabasal and superficial epithelial cells in submerged cultures, similar to the normal control, whereas many suprabasal and superficial epithelial cells lost K12 expression in the airlift culture (Fig. 6A). In contrast, adding SB203580 caused K12-negative cells to revert to the basal cell layer (Fig. 6A). Similar to what is depicted in Figure 4B, K10 keratin was absent in the normal control and the submerged cultures but was expressed in superficial and suprabasal cell layers in airlift cultures. Adding SB203580 to the airlift culture completely abolished such K10-positive expression (Fig. 6B). Filaggrin was not expressed in the normal control or the submerged culture but was found in superficial cell layers in airlift cultures, similar to what was previously noted. In contrast, filaggrin-positive cells were absent after SB203580 was added (Fig. 6C). Composites of micrographs of these cultures taken at lower magnification clearly showed that the loss of K12 expression (Fig. 6D) and the gain of K10 expression (Fig. 6E) were confined to the limbal epithelium in airlift cultures. There was no difference in the number of cell layers in the limbal epithelium in airlift cultures, with or without SB203580. Furthermore, immunostaining to Ki67 did not reveal any difference in the frequency of Ki67-positive nuclei in airlift cultures with or without the addition of SB203580 (not shown). These data collectively indicated that epidermal differentiation induced in airlift cultures occurred through activation of the p38 signaling pathway. This abnormal differentiation could be abolished by SB203580 without affecting cellular proliferation.
Figure 6.
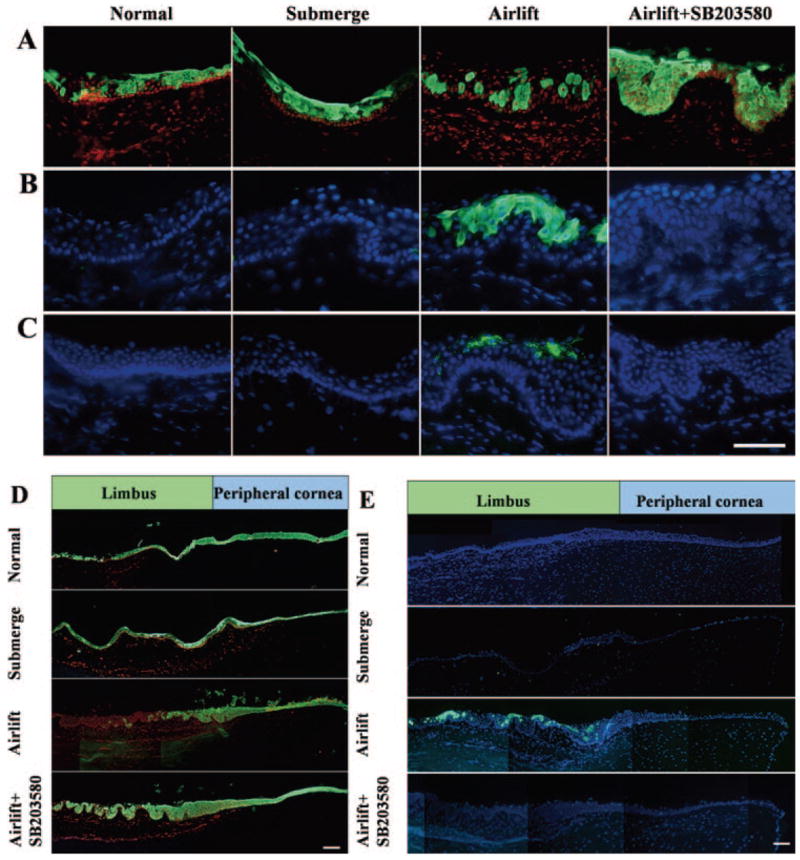
Suppression of abnormal epidermal differentiation by p38 inhibitor SB203580. Immunostaining to K12 keratin (A), K10 keratin (B), and filaggrin (C) was performed in the normal control (before culturing), submerged cultures, and airlift cultures, of which the latter was also added with SB203580 for 2 weeks. K12 keratin was expressed by suprabasal and superficial cells in the normal control and the submerged culture, decreased to single cells and patches of cells in the airlift culture, and reverted to the normal pattern after the addition of SB203580. K10 keratin-positive cells were absent in the normal control and the submerged cultures but was found in superficial and suprabasal cell layers in airlift cultures. They disappeared after SB203580 was added. Filaggrin-positive cells were found only in superficial cell layers in airlift cultures but disappeared after SB203580 was added. Composites of micrographs at lower magnification confirmed the loss of K12 keratin expression (D). The gain of K10 keratin expression (E) was confined to the limbal epithelium in airlift cultures and was reverted by SB203580. Scale bars: (A–C) 100 μm; (D, E) 200 μm.
Stem Cell Status in Abnormal Epidermal Differentiation
To determine whether abnormal epidermal differentiation of the limbal epithelium induced in airlift cultures resulted from intrinsic alteration of limbal epithelial progenitor cells, we harvested the surface epithelium from human limbal explants cultured under submerged or airlift mode, of which the latter was treated with or without SB203580 for 2 weeks, and seeded them on mitomycin C-treated 3T3 fibroblast feeder layers for 12 days. Epithelial colonies were double stained with antibodies against K12 and K10 keratins and were compared with those derived from human oral mucosal epithelium serving as the control (Fig. 7). The results showed that all epithelial colonies generated from different culturing conditions still expressed K12 keratin but not K10 keratin. As a comparison, those from the oral mucosal epithelium, as expected, did not express K10 or K12 keratin. Taken together, these results indicated that abnormal epidermal differentiation induced in airlift cultures did not result from intrinsic alteration of limbal progenitor cells.
Figure 7.
Progenitor cell status after explant cultures. Human limbal explants cultured under submerge, airlift, and airlift+SB203580 modes for 2 weeks. Single cells were harvested from the explant surface by dispase and subsequent trypsin/EDTA and were seeded onto mitomycin C-treated 3T3 fibroblast feeder layers for 12 days. The resultant epithelial clones were double stained with antibodies against K12 (top) and K10 (bottom) keratins and were counterstained to DAPI. All clones remained K12 keratin positive but K10 keratin negative, and those derived from oral mucosal epithelial cells were K12 keratin negative and K10 keratin negative. Scale bar, 200 μm.
Involvement of C/EBPα and C/EBPβ in Abnormal Epidermal Differentiation Induced by Airlift
Because the p38 signaling pathway plays an important role in mediating keratinocyte differentiation,24 we sought to determine whether it operated by downstream differentiation-associated transcription factors C/EBPα and C/EBPβ in the upregulation of K1/K10 keratin genes, as reported.25–28 Immunohistochemical staining to P-p38, C/EBPα and C/EBPβ was thus performed (Fig. 8). The results showed that P-p38 was not expressed in the limbal epithelium or stroma of the control before culturing, as shown in Figure 5, was weakly positive in limbal basal epithelial cells and stromal cells in submerged cultures, and was strongly expressed in the cytoplasm and nuclei of limbal epithelial cells and in the nuclei of stromal cells in airlift cultures for 2 weeks. P-p38 staining decreased in epithelial and stromal cells in airlift cultures treated with SB203580. C/EBPα was weakly expressed in normal limbal epithelial nuclei before culturing but was markedly upregulated in the nuclei of limbal epithelial cells in submerged and airlift cultures. Such nuclear staining decreased dramatically when SB203580 was added in airlift cultures. C/EBPβ was also weakly expressed in the nuclei of limbal epithelial cells before culturing but was strongly expressed in almost all nuclei of epithelial and stromal cells in submerged and airlift cultures. However, such nuclear staining was suppressed when SB203580 was added in airlift cultures. These results indicated that the p38 signaling pathway was indeed involved in airlift-induced abnormal epidermal differentiation of limbal epithelial cells. C/EBPα and C/EBPβ were upregulated in submerged and airlift cultures, suggesting that these two transcription factors were not directly linked to abnormal epidermal differentiation, despite their presumed downstream scenario after SB203580 was added. These results suggest that the p38 inhibitor blocked abnormal differentiation partially through the down-regulation of C/EBPα and C/EBPβ.
Figure 8.

Immunohistochemistry to P-p38, C/EBPα, and C/EBPβ. Immunohistochemical staining to P-p38 was negative in the normal limbal epithelium and stroma, weakly positive in the limbal basal epithelium and stromal cells in submerged cultures, and strongly positive in cytoplasms and nuclei of the limbal epithelium and in the nuclei of stromal cells in airlift cultures. P-p38 staining was decreased in epithelial and stromal cells in airlift cultures treated with SB203580. C/EBPα was weakly expressed in the normal limbal epithelium but was strongly positive in the nuclei of limbal epithelial cells in submerged and airlift cultures. Such positive nuclear staining was dramatically decreased when SB203580 was added to airlift cultures. C/EBPβ was also weakly expressed in the nuclei of limbal epithelial cells but was strongly expressed in the nuclei of almost all epithelial and stromal cells in submerged and airlift cultures. However, such nuclear staining was decreased by SB203580 in airlift cultures. Scale bar, 100 μm.
Discussion
This study showed for the first time that an ex vivo model of squamous metaplasia can be developed in the corneal/limbal epithelium using limbal explants cultured under an airlift mode without altering the level of vitamin A in the medium. Because squamous metaplasia is commonly found in many ocular surface diseases manifesting dry eye where ocular surface epithelia are exposed to air because of the lack of preocular tear film, our model might be used to dissect the pathogenesis of pathologic squamous metaplasia of the ocular surface and other tissues.
Exposure to the air-fluid interface by airlift is a common maneuver to induce epithelial stratification in organotypic cultures of epidermal keratinocytes. In skin organotypic cultures, such an increase of epithelial stratification is thought to be caused by the upregulation of keratinocyte growth factor expression by cocultured fibroblasts under the upregulation of IL-1 released by keratinocytes.29–31 Although we did not know whether a similar mechanism also operated in our model system, we noted that airlift induced the proliferation of limbal epithelial progenitor cells, judged by an increase of nuclear staining to Ki67 and p63 by basal and suprabasal cell layers as early as day 4. Such hyperproliferation was restricted to the limbal epithelium (Fig. 2), which is known to contain the stem cell population for the corneal epithelium.21 There was concomitant increased expression of such putative stem cell marker as ABCG-2 and Musashi-1 extending to suprabasal layers on day 4 only (Figs. 3C, 3D, respectively). In addition, K12(–) nondifferentiated cells expanded from the basal to the suprabasal layers. These results collectively supported the notion that transient expansion of the limbal stem cell pool was most likely induced by airlift.
Epithelial hyperproliferation can be coupled by aberrant epithelial differentiation. In the epidermis, hyperproliferation triggers the alternative pathway of K6/K16 keratin expression.32 Herein, we noted that limbal epithelial hyperproliferation preceded squamous metaplasia, as evidenced by positive expression of K10 keratin and filaggrin, and started as soon as day 4 and day 6, respectively (Fig. 4). Of note, cells expressing K10 keratin were suprabasally located and still expressing K12 keratin, suggesting that abnormal epidermal differentiation took place in lineage-committed cells. This result favored the interpretation that this model of squamous metaplasia represented aberrant transdifferentiation of terminally differentiated cells, mimicking one of the models raised by Liang et al.33 in their urothelial squamous metaplasia study. Although there was a progressive increase of filaggrin in superficial cells toward the end of 2 weeks of culturing, these cells retained positive nuclear expression of Pax6 (Fig. 4E), indicating that they were still committed to the lineage of ocular surface epithelial cells. Moreover, the clones generated from epithelial cells harvested from 2-week airlift-cultured explants still expressed K12 but not K10 keratin when seeded onto 3T3 feeder layers for 12 days (Fig. 7), further confirming that progenitor cells expanded in this culture system still stayed in the corneal lineage. Our findings differed from a previous study in which epidermal transdifferentiation of the rabbit corneal epithelium was induced by engrafting into the embryonic dermis, where Pax6 expression was downregulated by a mouse embryonic dermal signal.34
The p38 signaling pathway has recently emerged as a key modulator of cell differentiation processes of various cell types, including adipocytes,35 intestinal epithelial cells,36 and myocytes.37 The p38 MAPK signaling pathway was also activated in pathologic proliferation and squamous metaplasia of the rat lung epithelium induced by tobacco smoke.2 For ocular surface epithelia, activation of the p38 MAPK signaling pathway was induced by desiccation in the pathologic state of the mouse dry eye model.38 We noted that activation of the p38 signaling pathway was also involved in our squamous metaplasia model. P-p38 expression emerged in limbal basal epithelial cells as early as 2 days in airlift cultures and gradually extended to the cytoplasm and nuclei with time (Fig. 5), whereas it was only weakly expressed in the cytoplasm of epithelial cells in submerge cultures (Fig. 8). Moreover, adding SB203580, a specific p38 inhibitor, to airlift cultures abolished the expression of K10 keratin and filaggrin by limbal epithelial cells without affecting cell stratification and proliferation (Fig. 6), suggesting a potential therapeutic effect of p38 inhibitors in preventing or reverting pathologic squamous metaplasia.
In normal epidermal epithelial differentiation, K10 gene expression requires transcription factors C/EBPα and C/EBPβ,27 which are downstream molecules of the p38 signaling pathway. Our study showed that these two transcription factors were strongly expressed in airlift cultures and were suppressed by SB203580 (Fig. 8). However, expression of C/EBPα and C/EBPβ was also upregulated in submerge cultures, indicating that these two transcription factors may not be critical in the abnormal differentiation of limbal epithelial cells. Further study is needed to elucidate other downstream molecule(s) of p38 that may play a specific role in inducing squamous metaplasia. Another unexpected finding is that P-p38 and C/EBPβ were also activated in limbal stromal cells in submerge and airlift explant cultures, suggesting that mesenchymal cells in the limbal stroma might also be activated. Previously, the maneuver of airlift promoted the proliferation of fibroblasts cultured in collagen gel39 and in rabbit limbal explants.13 Future studies are warranted to investigate the interaction between limbal basal epithelial progenitor cells and limbal stromal cells in modulating the pathologic state of squamous metaplasia.
Acknowledgments
Supported by National Eye Institute, National Institutes of Health Grants EY06819 and EY15735 (SCGT), a research grant from Tissue-Tech, Inc., and an unrestricted grant from Ocular Surface Research and Education Foundation. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure: W. Li, None; Y. Hayashida, None; Y.-T. Chen, None; H. He, None; D.Y. Tseng, None; M. Alonso, None; S.-Y. Chen, None; X. Xi, None; S.C.G. Tseng, None
Presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2007.
References
- 1.Varley CL, Stahlschmidt J, Smith B, et al. Activation of peroxisome proliferator-activated receptor-gamma reverses squamous metaplasia and induces transitional differentiation in normal human urothelial cells. Am J Pathol. 2004;164:1789–1798. doi: 10.1016/s0002-9440(10)63737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong CY, Zhou YM, Douglas GC, et al. MAPK/AP-1 signal pathway in tobacco smoke-induced cell proliferation and squamous metaplasia in the lungs of rats. Carcinogenesis. 2005;26:2187–2195. doi: 10.1093/carcin/bgi189. [DOI] [PubMed] [Google Scholar]
- 3.Beitch I. The induction of keratinization in the corneal epithelium: a comparison of the “dry” and vitamin A-deficient eyes. Invest Ophthalmol. 1970;9:827–843. [PubMed] [Google Scholar]
- 4.Tseng SCG. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology. 1985;92:728–733. doi: 10.1016/s0161-6420(85)33967-2. [DOI] [PubMed] [Google Scholar]
- 5.Murube J, Rivas L. Impression cytology on conjunctiva and cornea in dry eye patients establishes a correlation between squamous metaplasia and dry eye clinical severity. Eur J Ophthalmol. 2003;13:115–127. doi: 10.1177/112067210301300201. [DOI] [PubMed] [Google Scholar]
- 6.Tseng SCG, Hatchell D, Tierney N, et al. Expression of specific keratin markers by rabbit corneal, conjunctival, and esophageal epithelia during vitamin A deficiency. J Cell Biol. 1984;99:2279–2286. doi: 10.1083/jcb.99.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura T, Nishida K, Dota A, et al. Elevated expression of transglutaminase 1 and keratinization-related proteins in conjunctiva in severe ocular surface disease. Invest Ophthalmol Vis Sci. 2001;42:549–556. [PubMed] [Google Scholar]
- 8.Espana EM, Di Pascuale MA, He H, et al. Characterization of corneal pannus removed from patients with total limbal stem cell deficiency. Invest Ophthalmol Vis Sci. 2004;45:2961–2966. doi: 10.1167/iovs.03-1397. [DOI] [PubMed] [Google Scholar]
- 9.Nishida K, Yamanishi K, Yamada K, et al. Epithelial hyperproliferation and transglutaminase 1 gene expression in Stevens-Johnson syndrome conjunctiva. Am J Pathol. 1999;154:331–336. doi: 10.1016/S0002-9440(10)65279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatchell DL, Sommer A. Detection of ocular surface abnormalities in experimental vitamin A deficiency. Arch Ophthalmol. 1989;102:1389–393. doi: 10.1001/archopht.1984.01040031131040. [DOI] [PubMed] [Google Scholar]
- 11.Wittpenn JR, Tseng SCG, Sommer A. Detection of early xerophthalmia by impression cytology. Arch Ophthalmol. 1986;104:237–239. doi: 10.1001/archopht.1986.01050140091027. [DOI] [PubMed] [Google Scholar]
- 12.Singh M, Singh G, Dwevedi S, et al. Conjunctival impression cytology in xerophthalmia among rural children. Indian J Ophthalmol. 1997;45:25–29. [PubMed] [Google Scholar]
- 13.Kawakita T, Espana EM, He H, et al. Intrastromal invasion by limbal epithelial progenitor cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am J Pathol. 2005;167:381–393. doi: 10.1016/S0002-9440(10)62983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, De Paiva CS, Luo L, et al. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe K, Nishida K, Yamato M, et al. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6–10. doi: 10.1016/j.febslet.2004.03.064. [DOI] [PubMed] [Google Scholar]
- 16.Budak MT, Alpdogan OS, Zhou M, et al. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005;118:1715–1724. doi: 10.1242/jcs.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YT, Li W, Hayashida Y, et al. Human amniotic epithelial cells as novel feeder layers for promoting ex vivo expansion of limbal epithelial progenitor cells. Stem Cells. 2007;25:1995–2005. doi: 10.1634/stemcells.2006-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okano H, Kawahara H, Toriya M, et al. Function of RNA-binding protein Musashi-1 in stem cells. Exp Cell Res. 2005;306:349–356. doi: 10.1016/j.yexcr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Kurpakus MA, Maniaci MT, Esco M. Expression of keratins K12, K4, and K14 during development of ocular surface epithelium. Curr Eye Res. 1994;13:805–814. doi: 10.3109/02713689409025135. [DOI] [PubMed] [Google Scholar]
- 20.Liu C-Y, Zhu G, Converse R, et al. Characterization and chromosomal localization of the cornea-specific murine keratin gene Krt1.12. J Biol Chem. 1994;260:24627–24636. [PubMed] [Google Scholar]
- 21.Schermer A, Galvin S, Sun T-T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- 23.Koroma BM, Yang J-M, Sundin OH. The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1997;38:108–120. [PubMed] [Google Scholar]
- 24.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 25.Maytin EV, Habener JF. Transcription factors C/EBP alpha, C/EBP beta, and CHOP (Gadd153) expressed during the differentiation program of keratinocytes in vitro and in vivo. J Invest Dermatol. 1998;110:238–246. doi: 10.1046/j.1523-1747.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhu S, Oh HS, Shim M, et al. C/EBPbeta modulates the early events of keratinocyte differentiation involving growth arrest and keratin 1 and keratin 10 expression. Mol Cell Biol. 1999;19:7181–7190. doi: 10.1128/mcb.19.10.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maytin EV, Lin JC, Krishnamurthy R, et al. Keratin 10 gene expression during differentiation of mouse epidermis requires transcription factors C/EBP and AP-2. Dev Biol. 1999;216:164–181. doi: 10.1006/dbio.1999.9460. [DOI] [PubMed] [Google Scholar]
- 28.Krieg TM, Schafer MP, Cheng CK, et al. Organization of a type I keratin gene: evidence for evolution of intermediate filaments from a common ancestral gene. J Biol Chem. 1985;260:5867–5870. [PubMed] [Google Scholar]
- 29.Maas-Szabowski N, Szabowski A, Stark HJ, et al. Organotypic co-cultures with genetically modified mouse fibroblasts as a tool to dissect molecular mechanisms regulating keratinocyte growth and differentiation. J Invest Dermatol. 2001;116:816–820. doi: 10.1046/j.1523-1747.2001.01349.x. [DOI] [PubMed] [Google Scholar]
- 30.Smola H, Thiekotter G, Fusenig NE. Mutual induction of growth factor gene expression by epidermaldermal cell interaction. J Cell Biol. 1993;122:417–429. doi: 10.1083/jcb.122.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maas-Szabowski N, Stark HJ, Fusenig NE. Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in resting fibroblasts. J Invest Dermatol. 2000;114:1075–1084. doi: 10.1046/j.1523-1747.2000.00987.x. [DOI] [PubMed] [Google Scholar]
- 32.Jiang CK, Magnaldo T, Ohtsuki M, et al. Epidermal growth factor and transforming growth factor alpha specifically induce the activation- and hyperproliferation-associated keratins 6 and 16. Proc Natl Acad Sci USA. 1993;90:6786–6790. doi: 10.1073/pnas.90.14.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang FX, Bosland MC, Huang H, et al. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J Cell Biol. 2005;171:835–844. doi: 10.1083/jcb.200505035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearton DJ, Yang Y, Dhouailly D. Transdifferentiation of corneal epithelium into epidermis occurs by means of a multistep process triggered by dermal developmental signals. Proc Natl Acad Sci USA. 2005;102:3714–3719. doi: 10.1073/pnas.0500344102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engelman JA, Lisanti MP, Scherer PE. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3–L1 adipogenesis. J Biol Chem. 1998;273:32111–32120. doi: 10.1074/jbc.273.48.32111. [DOI] [PubMed] [Google Scholar]
- 36.Houde M, Laprise P, Jean D, et al. Intestinal epithelial cell differentiation involves activation of p38 mitogen-activated protein kinase that regulates the homeobox transcription factor CDX2. J Biol Chem. 2001;276:21885–21894. doi: 10.1074/jbc.M100236200. [DOI] [PubMed] [Google Scholar]
- 37.Wu Z, Woodring PJ, Bhakta KS, et al. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol. 2000;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 39.Toda S, Yokoi F, Yamada S, et al. Air exposure promotes fibroblast growth with increased expression of mitogen-activated protein kinase cascade. Biochem Biophys Res Commun. 2000;270:961–966. doi: 10.1006/bbrc.2000.2466. [DOI] [PubMed] [Google Scholar]



