Abstract
Background & Aims
The ability to observe cellular and subcellular detail during routine endoscopy is a major goal in the development of new endoscopic imaging techniques. Multiphoton microscopy, which relies on nonlinear infared optical processes, has the potential to identify cellular details by excitation of endogenous fluorescent molecules. We examined the feasibility of using multiphoton microscopy to characterize mucosal histology in the human gastrointestinal tract.
Methods
A multiphoton microscope was used to determine the optimal excitation wavelength for examination of gastrointestinal mucosa. Fresh, unfixed, and unstained biopsy specimens obtained during routine endoscopy in human subjects were then examined by confocal microscopy and multiphoton microscopy. Multiphoton images also were compared to standard H&E images obtained from paired biopsy specimens. A prototype miniaturized multiphoton probe was used to examine intact rat colon.
Results
Peak multiphoton autofluorescence intensity was detected in mucosa excited at 735 nm. Multiphoton microscopic examination of unstained biopsy specimens revealed improved cellular detail relative to either unstained or stained specimens examined by confocal imaging. Resolution of structures such as epithelial nuclei, goblet cells, and interstitial fibers and cells was comparable to what was obtained using standard H&E histology. Similar findings were observed when using a prototype miniaturized multiphoton probe.
Conclusions
Multiphoton microscopy can be used to examine gastrointestinal mucosa at the cellular level, without the need for fluorescent dyes. The construction of a multiphoton endomicroscope could therefore provide a practical means of performing “virtual biopsies” during the course of routine endoscopy, with advantages over currently available endomicroscopy technologies.
Endoscopists often try to correlate gross endoscopic abnormalities with their microscopic diagnosis, but this is difficult to do with consistent reliability1,2. Endoscopic biopsies therefore remain the standard of practice for the histological diagnosis of abnormal mucosal lesions such as inflammation and neoplasia. There are, however, disadvantages to performing mucosal biopsies, including sampling error, costs, risks to the patient, and the delay in obtaining results. The ability to perform real-time diagnosis of dysplasia during surveillance exams or of suspicious lesions is therefore limited in several respects.
The recent development of a confocal endomicroscope3 provides, for the first time, the ability to perform real-time in vivo microscopy, but the usefulness of this imaging modality in patients is limited because it requires topical or systemic administration of fluorescent probes1. Multiphoton microscopy (MPM), also known as two-photon laser scanning microscopy, relies on nonlinear optical processes such as two-photon fluorescence and second harmonic generation (SHG) to achieve high resolution 3D imaging of biological tissues4,5. SHG is a coherent nonlinear scattering process in which two photons of lower energy are combined to create a single photon of exactly twice the energy of the two fundamental photons. MPM provides several advantages over single-photon, linear microscopy technologies such as confocal laser scanning microscopy (CLSM), including the generation of autofluorescence images without the need for fluorescent dyes or potentially damaging direct ultraviolet illumination and the greater capacity for deep tissue imaging. Fluorescence for multiphoton images of unstained fresh tissue derives from intrinsic molecules that are fluorescent such as NADH and flavins, and from collagen, which is an extremely effective second harmonic generator6. A wide range of tissues has been examined by MPM, but this has largely been in animal models and has not included the gastrointestinal tract7-14. Because of the presence of endogenous autofluorescent molecules and connective tissue along the gastrointestinal tract, we examined whether multiphoton microscopic examination of unfixed, unstained endoscopic biopsies was useful for detecting cellular and subcellular detail of the mucosa.
PATIENTS AND METHODS
Acquisition of human samples
Patients presenting to the Yale Gastrointestinal Procedure Center for elective outpatient endoscopy were recruited to participate in this study, which was approved by the Yale University Human Investigations Committee. Written informed consent was obtained prior to study participation. Inclusion criteria included age greater than 18 and the ability to provide informed consent. Exclusion criteria included a known or suspected bleeding disorder, INR exceeding 1.4, platelet count of less than 100,000, or aspirin use within the previous five days. Standard biopsy forceps (Olympus Medical Systems Corporation, Tokyo, Japan) were used to obtain paired mucosal “pinch” biopsies from normal areas of esophagus, stomach, duodenum, rectum, colon, and terminal ileum, as well as from abnormal areas or lesions. Each pair of biopsies was separated such that one would be examined by routine white light microscopy, and the other by multiphoton laser microscopy. The specimen sent for routine histology was placed in a cassette and submerged in 10% buffered formalin, whereas the specimen to be examined by MPM was placed directly in a container of 0.9% normal saline.
Determining the optimal two-photon excitation wavelength
A Zeiss LSM 510 NLO confocal microscope (Thornwood, NY) was equipped with a Spectra-Physics (Mountain View, CA) Tsunami Titanium:Sapphire (Ti:S) laser, with a Millenia X Argon pump laser, for multiphoton excitation. Tissue from freshly harvested rat colon, and then human colon, was excited at different excitation wavelengths ranging from 715-790 nm. Fluorescence emission was collected between 410 and 490 nm using a custom-built nondescanned (external) detector. The average intensity of selected regions was calculated by Image J software (http://rsb.info.nih.gov/ij/download.html). This was repeated over the range of excitation wavelengths, and a plot of the average fluorescence intensity produced at each wavelength was constructed.
Fluorescence imaging
In addition to the Ti:S laser, the Zeiss microscope described above was also equipped with argon and He:Ne lasers for single-photon confocal laser microscopy. Each specimen was observed using a Zeiss Plan-apochromat 20X, 0.8-NA and Neofluar 63X, 1.25-NA objective lens. Tissue was observed within four hours of biopsy to avoid structural degradation of the specimen. Specimens were excited at 735 nm for multiphoton imaging, or excited at 488 nm using an argon laser for conventional confocal imaging. Two-photon fluorescence and second harmonic generation was observed using custom-built external detectors, with emission signals collected between 510-560 nm (pseudocolored red), 410-490 nm (pseudocolored green), and 350-380 nm (pseudocolored blue). The maximum power output of the Ti:S laser was 18.6 mW, measured at the objective lens. Images were collected with a dwell time of 26 microseconds per pixel, and typical frames were 512 × 512 pixels. Optical slice thickness was 1 μm; in the z-axis, tissue was imaged in 1-μm step increments. The range of the z-axis was 0-120 μm below the surface layer. The lateral resolution was 2.53 pixels/μm (field of view 202 × 202 μm). LSM Image Browser (http://www.zeiss.com) was used to prepare images. Tissue examined by confocal microscopy was first imaged without staining, and then after the application of 0.01% fluorescein (Sigma, St. Louis, MO).
Routine Histology
Specimens collected in 10% buffered formalin (Val Tech Diagnostics, Pennsylvania, USA) were routinely processed, paraffin embedded and sectioned at 3 um. They were stained with hematoxylin and eosin (H&E) and examined using standard bright field (white light) microscopy.
Prototype miniaturized multiphoton probe
A multi-element 27x/0.7NA 3.2 mm Olympus “stick” objective lens with illumination from a Ti:S laser tuned to 740 nm was constructed and used to image intact rat colon and terminal ileum. The probe has a field of view of 220 μm. The average power output was 3 mW. Custom-built detectors were used to observe two-photon autofluorescence in the same ranges described above.
RESULTS
Optimal multiphoton excitation wavelength for mucosa
To determine the optimal multiphoton excitation wavelength for tissue autofluorescence and collagen second harmonic generation in gastrointestinal tissue, a femtosecond-pulsed, mode-locked Ti:S laser was used to examine fresh untreated segments of rat colon. The laser was tuned across a range of wavelengths between 715 and 790 nm. Fluorescence emission was collected in the 410-490 nm range, and the mean fluorescence intensity across the field of view was calculated. Figure 1 shows the relationship between excitation wavelength and average fluorescence intensity produced at each wavelength. Using our instrument and optics, the peak fluorescence intensity was detected in specimens excited at approximately 735 nm. We therefore used this excitation wavelength for subsequent multiphoton imaging of human specimens.
Figure 1.

Determination of optimal excitation wavelength for two-photon autofluorescence imaging. Unfixed colonic mucosa was excited over a range of wavelengths as fluorescence emission intensity was measured. Peak fluorescence emission intensity in the 410-490 nm range was detected in specimens excited at 735 nm.
Comparison of multiphoton and confocal imaging
We compared images of normal human colon biopsy specimens obtained with both MPM (Ti:S laser, 735 nm) and CLSM (argon laser, 488 nm). The CLSM images were obtained first from biopsy specimens without any dye application, and then after staining with 0.01% fluorescein. Confocal images without staining demonstrate the normal circular arrangement of colonic glands, with epithelial cells and interspersed goblet cells (Figure 2A). Autofluorescence was limited, and more detailed structures such as nuclei and the connective tissue between glands were not readily identifiable. Cellular detail was enhanced in colonic biopsy specimens stained with fluorescein (Figure 2B). Compared to either of these confocal images, MPM examination of unstained specimens provided good ability to distinguish between epithelial cells and their surrounding matrix, as well as subcellular detail of individual epithelial cells (Figure 2C). Two-photon excited autofluorescence and collagen SHG signal was detected along a broad wavelength spectrum, and so was collected at three non-overlapping wavelength ranges, 350-380 nm (pseduocolored blue- primarily SHG at 370 nm), 410-490 nm (colored green), and 510-560 nm (colored red).
Figure 2.

Multiphoton microscopy (MPM) is superior to confocal laser-scanning microscopy (CLSM) for imaging fresh colonic mucosa. (A) CLSM (63x) of fresh, unstained tissue demonstrated relatively homogenous autofluorescence with limited subcellular detail. (B) CLSM (63x) of tissue stained with 0.01% fluoroscein showed slightly enhanced cellular detail relative to unstained specimens. (C) MPM image (63x) of fresh, unstained tissue revealed increased cellular and subcellular detail in the 410-490 nm range (green), plus additional autofluorescence details at lower (blue) and higher (red) wavelength ranges.
Multiphoton imaging throughout the gastrointestinal tract
MPM was next used to examine mucosal biopsy specimens collected from normal areas throughout the gastrointestinal tract, as well as from several abnormal lesions. A total of 24 paired mucosal biopsy specimens from 15 human subjects were obtained from the following sites: esophagus (4), stomach (6), duodenum (3), terminal ileum (2), colon (6), and rectum (3). This included abnormal pathology, which was collected from esophageal squamous carcinoma and colonic adenoma. Figure 3 shows images of biopsies obtained from the upper gastrointestinal tract. MPM examination of esophageal biopsies (Figure 3A) revealed typical arrangement of nonkeratinized squamous epithelium plus visualization of a papilla. The intercellular space between individual cells was readily discerned, and the morphology of individual nuclei could be observed as well. Nearly all two-photon excited autofluorescence was detected in the 410-490 nm range. These same details of cellular architecture were readily correlated with hematoxylin and eosin (H&E) images (Figure 3B). In gastric specimens examined by MPM (Figure 3C), individual glands composed of cuboidal epithelial cells surrounding gastric pits were readily identified in the same wavelength range as mucosal epithelia in the esophagus. Individual epithelial cell nuclei again could be identified, but were usually located at the basal cell surface in comparison to their central location in the esophagus. Connective tissue separating individual glands could be detected at lower wavelengths (350-380 nm) via SHG of collagen. The stroma was more prominent in MPM images than in H&E images (Figure 3D), which may reflect loss of water or other tissue contents associated with fixation. The basement membrane, however, was readily identified as a thin band surrounding individual glands in both types of images. In MPM images a sparse cellular infiltrate was seen within the interstitial matrix, with peak fluorescence at a longer (510-560 nm) wavelength range; this most likely represents interstitial lymphocytes seen on H&E. Duodenal specimens (Figures 3E-F) showed columnar epithelial cells lining villi, punctuated by occasional goblet cells characterized by their absence of fluorescence. Smaller cells located within the lamina propria were also identified.
Figure 3.

Comparison of MPM and H&E light microscopic images of biopsies obtained during upper gastrointestinal endoscopy. (A) Esophagus, examined by MPM (63x), demonstrates typical arrangement of nonkeratinized, stratified squamous epithelium. Borders between cells, cell nuclei, and a single papilla are readily identifiable. (B) Esophagus, examined by H&E (40x), shows stratified squamous epithelium that corresponds to the MPM image. (C) Stomach, examined by MPM (63x), demonstrates individual fundic glands composed of cuboidal epithelial cells surrounding a central pit. The glands are separated by connective tissue bands seen via second harmonic generation (368 nm) in a lower range (350-380 nm bandpass). A sparse cellular infiltrate with a peak fluorescence in a higher range (510-560 nm) is seen within the interstitial matrix. (D) The corresponding H&E (20x), reveals a similar arrangement of fundic glands with surrounding lamina propria containing small vessels and occasional mononuclear cells. The basement membrane of cells appears as a thin pink band which corresponds to the thin blue band surrounding glands in the MPM images. (E) Duodenum, examined by MPM (63x), demonstrates columnar epithelial cells lining villi, punctuated by occasional goblet cells characterized by absence of fluorescence. Smaller cells within the lamina propria can also be seen. (F) Duodenum, examined by H&E (20x), shows the corresponding image of an intestinal villous, lined by a single layer of enterocytes with basally located nuclei and interspersed goblet cells with apically located clear mucous. The interstitium containing leukocytes and capillaries can also be seen.
Figure 4 shows images of biopsies obtained from the lower gastrointestinal tract. MPM examination of biopsies from the terminal ileum (Figure 4A) revealed details similar to those of the duodenum, though the nuclei were more elongated and a brush border could be seen as a thin band of low-intensity fluorescence along the apical surface of the villous. These features corresponded to the H&E stained images (Figure 4B). In colon (Figure 4C-D) and rectum (Figure 4E-F), MPM revealed cellular and subcellular detail of individual glands, including a typical foveolar pattern with central, round crypt openings. Goblet cells and epithelial nuclei could be identified, as could an interstitial cellular infiltrate which fluoresced at a longer wavelength.
Figure 4.
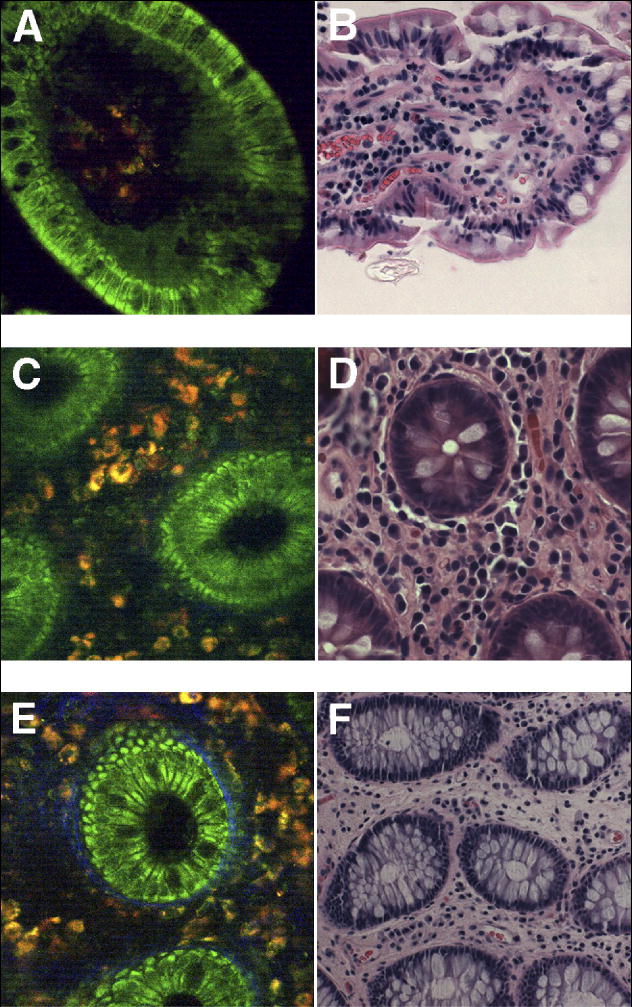
Comparison of MPM and H&E light microscopic images of biopsies obtained during colonoscopy. (A) Terminal ileum, examined by MPM (63x), demonstrates columnar epithelial cells interspersed with goblet cells, lining a single villous. The nuclei are elongated and arranged along the basal surface of the cells. The lamina propria contains a cellular infiltrate that fluoresces at a longer wavelength range. A faint band of lower fluorescence lines the apical aspect of the epithelium, which most likely represents the microvilli that comprise the brush border. (B) Terminal ileum, examined by H&E (20x), shows findings that correlate readily with MPM images, although the interstitial space appears more dense on H&E. (C) Colon, examined by MPM (63x), demonstrates typical glandular pattern with central, round crypt openings. A dense interstitial space separates the glands and contains cellular infiltrate with autofluorescence at longer wavelengths. (D) Colon, examined by H&E (40x), shows cross sections of crypts that correlate well with MPM images. (E) Rectum, examined by MPM (63x), demonstrates features similar to colonic mucosa, including the presence of interspersed goblet cells. Additionally, a thin blue band surrounds individual glands, which likely represents the basement membrane and portions of myofibroblastic sheath. (F) Rectum, examined by H&E (20x), has a similar appearance to colon, with an increased number of goblet cells. The basement membrane is difficult to visualize at this magnification.
Figure 5 demonstrates the examination of pathologic lesions by MPM. Biopsies of squamous cell carcinoma of the esophagus (Figure 5A) revealed nonkeratinized squamous epithelium in which most autofluorescence was detected in the 410-490 nm range, similar to what was observed in normal esophagus (Figure 3A). Unlike what was observed in normal tissue, though, the intercellular space between individual cells was not readily discerned, individual nuclei were enlarged and varied in size, and there was an increased nuclear to cytoplasmic ratio. These same details of cellular architecture were readily correlated with H&E images (Figure 5B). In a colonic adenoma (Figure 5C), MPM revealed individual glands, many of which included a typical foveolar pattern with central, round crypt openings, similar to what was observed in normal colonic mucosa (Figure 4C). Unlike what was observed in normal colonic mucosa, both the glands and their nuclei were enlarged and irregular in size and shape, and the glands were only rarely separated by collagen bands. These same details of cellular architecture were readily correlated with H&E images (Figure 5D). Together, these findings suggest that MPM may be useful for identifying not only normal but also abnormal cellular architecture and features within the gastrointestinal tract.
Figure 5. Comparison of MPM and H&E light microscopic images of pathologic gastrointestinal lesions.

(A) Squamous carcinoma of the esophagus, examined by MPM (63x), demonstrates nonkeratinized, stratified squamous epithelium. In contrast to normal squamous epithelia, borders between cells are less distinct, cell nuclei are larger and more heterogenous in size, and the nuclear to cytoplasmic ratio is much greater. (B) Squamous carcinoma of the esophagus, examined by H&E (40x), shows features that corresponds to the MPM image. (C) Colonic adenoma, examined by MPM (63x), demonstrates glandular pattern similar to what was observed in normal colon, but with heterogeneous gland sizes, elongated and irregular nuclei, much sparser and less regular interstitial fibers, and little to no cellular infiltrate in the interstitium. (D) Colonic adenoma, examined by H&E (40x), correlates well with the MPM image.
Serial sections of stomach (Figure 6A), ileum (Figure 6B), and rectum (Figure 6C) were obtained, from which cross-sectional images greater than 100 μm in depth were constructed. This allowed visualization of the three-dimensional architecture of gastric pits, intestinal villi, and rectal glands. Video images of z-stacks through the gastric and rectal mucosa could be constructed as well (See Supplementary Videos 1 and 2, respectively, online at www.cghjournal.org). Collection of these three-dimensional images took approximately two minutes each, which is similar to the time that would be needed to collect similar data using confocal endomicroscopy. Figure 7 demonstrates the use of a prototype miniaturized multiphoton probe. A small diameter (3.2 mm) multi-element 0.7 NA “stick” objective lens (Figure 7A) was used to collect a multiphoton image of rat colon (Figure 7B), which showed subcellular detail similar to what was observed in human colon using our conventional two-photon microscope and objective lenses (Figure 4). This demonstrates the potential feasibility of collecting multiphoton images endoscopically, although additional work will be needed to adapt this type of miniaturized probe for use through the accessory channel of a standard endoscope, or else incorporated directly into the tip of an endoscope.
Figure 6.

Three-dimensional reconstruction of gastrointestinal mucosa examined by MPM. Serial optical sections of unfixed biopsies were obtained in order to create cross-sectional images in the x-y, y-z, and x-z planes. (A) Stomach, 63x. Serial images through a depth of 128 μm were collected to reveal cross-sections through several gastric glands. (B) Terminal ileum, 63x. Serial images through a depth of 102 μm were collected to reveal cross-sections through a single villous structure. (C) Rectum, 63x. Serial images through a depth of 104 μm were collected to reveal opposing sides of a typical rectal gland with its central crypt.
Figure 7.

Rat colon imaged using a miniaturized multiphoton probe. (A) The 27x/0.7NA 3.2 mm Olympus “stick” objective lens has a field of view of 220 μm, and two-photon excitation was achieved using a Ti:S laser tuned to 740 nm, with average power 3 mW. (B) Multiphoton image of rat colon collected through the stick objective. As in the multiphoton images of Figure 4, mucosal epithelial cells of the circular colonic glands show peak autofluorescence in the 410-490 nm range (green), while cells in the interstitial space are detected at longer wavelengths (510-560 nm; red). Collagen second harmonic generation again appears as blue (detection at ~370 nm) and is in close proximity to the colonic glands.
DISCUSSION
Initial reports using confocal laser scanning microscopy to examine gastrointestinal mucosa ex vivo demonstrated limited image detail in specimens that were not treated with fluorescent dyes15-16. Our results show that multiphoton laser microscopy provides the ability to detect cellular and subcellular details in unfixed, unstained gastrointestinal mucosa, with image quality that is superior to confocal imaging with or without fluorescent staining. Throughout the gastrointestinal tract, mucosal epithelial cells showed peak autofluorescence in the 410-490 nm range, and nuclei were characterized by their absence of fluorescence. This fluorescence most likely is primarily due to intracellular NADH, which is thought to be the principal endogenous fluorophore in mucosal epithelia that emits in this wavelength range6. Cells within the interstitial space fluoresced mostly at longer wavelengths, and these cells were most prominent in the gastric, colonic, and rectal mucosa. This fluorescence most likely is due to FAD and other flavin proteins, and possibly lipofuscin, which are found in white blood cells within the lamina propria, and which are known to autofluoresce in this wavelength range6, 17. Connective tissue in the lamina propria was also most prominent in the stomach, colon, and rectum, and fluoresced at lower wavelengths. This fluorescence most likely reflects collagen, which fluoresces in this wavelength range through second harmonic generation when examined by two photon microscopy6, 18. Examination of squamous carcinoma revealed characteristic subcellular changes, while changes in glandular structure and the surrounding connective tissue were observed in an adenoma.
Confocal laser endomicroscopy has recently been used to perform in vivo histological examination of a variety of mucosal abnormalities including colonic neoplasia, inflammatory bowel disease, microscopic colitis, early gastric cancer, and Barrett’s esophagus1,19-21. The limitations of CLSM, however, invite the development of improved endomicroscopic techniques. Because MPM relies on nonlinear optical processes typically carried out using near-infared excitation, it has the ability to excite autoflourescent molecules normally found in biological tissues6,18, including within the gastrointestinal mucosa22-24. This can obviate the need to administer potentially toxic fluorescent dyes, most commonly intravenous fluorescein sodium and topical acriflavine hydrochloride, which must be used for in vivo confocal laser endomicroscopy1,25,26. Additionally, multiphoton absorption occurs in the near-infared wavelength and so allows for deeper imaging of biological tissue with less light scattering than CLSM. While CLSM imaging is limited to a depth of ~250 μm 1, MPM has been used to image as deep as 1 mm 27. In this initial proof-of-principle study, we showed examples of cross-sectional images demonstrating depths of greater than 100 μm in gastrointestinal mucosa. Pathologic features of a number of gastrointestinal disorders are characterized by changes in the architecture of mucosal glands or villi, rather than or in addition to changes that can be detected in individual two-dimensional images, so this capability of multiphoton imaging may provide additional diagnostic benefit. MPM may also be useful to evaluate the histology of organs outside of the gastrointestinal tract, such as the liver, pancreas, or ovary, by utilizing transluminal endoscopic approaches28,29. MPM should be an even safer technique than CLSM for imaging gastrointestinal mucosa, because it is much less phototoxic than CLSM despite similar amounts of power delivery to cells and tissues; this is in part because of the longer excitation wavelengths used, the fact that effective excitation occurs only within the focal plane, and because MPM laser illumination is femtosecond-pulsed rather than continuous4.
Several groups are currently developing laser-scanning imaging endoscopes30, some of which employ multiphoton excitation. However, the majority of designs that use nonlinear excitation are instruments for neurobiology research rather than clinical use31,32. There are a number of engineering challenges in building a useful multiphoton laser-scanning endoscope, such as ensuring efficient propagation of femtosecond pulses through optical fibers and design of miniature scanning mechanisms. Micro- and nanotechnology have provided solutions for the short pulse delivery in the form of photonic crystal optical fibers33 and for miniaturization in the use of Micro-Electro-Mechanical Systems (MEMS) technology30. Although several of the current multiphoton endoscope implementations use graded-index (GRIN) lenses which typically suffer from severe spherical aberration, it is possible to design miniature optics with performance equal to modern objective lenses. In this report, we have shown a prototype design of a miniaturized 3.2 mm multiphoton probe34 that is capable of providing high resolution microscopic images of gastrointestinal mucosa with cellular and subcellular detail similar to what we observed using conventional optics. Well designed miniature optics can now be fabricated as small as ~1 mm in diameter and can achieve a nearly aberration-free diffraction-limited focus. Technology is now available to construct a miniature laser scanning multiphoton endoscope that will have the imaging performance nearly equivalent to what can now be obtained using multiphoton microscopy on ex vivo samples. This study demonstrates that development of such a device could provide a major advance in our ability to diagnose and characterize various gastrointestinal disorders during the course of routine endoscopy.
Supplementary Material
Serial cross-sectional imaging of gastric mucosa (20x magnification) using MPM, with z-stacks displayed as a three-dimensional video. The architecture and depth of gastric pits can be appreciated.
Serial cross-sectional imaging of rectal mucosa (20x magnification) using MPM, with z-stacks displayed as a three-dimensional video. A regular arrangement of rectal glands can be visualized.
Acknowledgments
The authors thank Drs. Priya Jamidar, Deborah Proctor, and Uzma Siddiqui for their assistance in patient enrollment and acquisition of biopsy specimens.
Funding Sources: This work was supported by NIH grants P30 DK34989 and R01 DK45710 to MHN and R01 CA116583, P41 EB01976, and P41 RR04224 to WRZ. We also acknowledge a pilot grant from the Pentax Corporation to support the cost of processing biopsy specimens for H&E staining.
Abbreviations
- CLSM
Confocal laser scanning microscopy
- H&E
Hematoxylin and Eosin
- MPM
Multiphoton microscopy
- SHG
Second harmonic generation
- Ti:S
Titanium:Sapphire
Footnotes
Competing Interest Statement: The authors have no competing financial interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffman A, Goetz M, Vieth M, Galle PR, Neurath MF, Kiesslich R. Confocal laser endomicroscopy: technical status and current indications. Endoscopy. 2006;38:1275–1283. doi: 10.1055/s-2006-944813. [DOI] [PubMed] [Google Scholar]
- 2.Norfleet R, Ryan M, Wyman J. Adenomatous and hyperplastic polyps cannot be reliably distinguished by their appearance through the fiberoptic sigmoidscope. Dig Dis Sci. 1988;33:1175–1177. doi: 10.1007/BF01535796. [DOI] [PubMed] [Google Scholar]
- 3.Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S, Nafe B, Galle PR, Neurath MF. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–713. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 4.Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- 5.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 6.Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci. 2003;100:7075–7080. doi: 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtz R, Fricke M, Kalb J, Tinnefeld P, Sauer M. Application of multiline two-photon microscopy to functional in vivo imaging. J Neurosci Meth. 2006;151:276–286. doi: 10.1016/j.jneumeth.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Laiho L, Pelet S, Hancewicz T, Kaplan P, So P. Two-photon 3-D mapping of ex vivo human skin endogenous fluorescence species based on fluorescence emission spectra. J Biomed Opt. 2005;10:024016. doi: 10.1117/1.1891370. [DOI] [PubMed] [Google Scholar]
- 9.Molitoris B, Sandoval R. Intravital multiphoton microscopy of dynamic renal processes. Am J Physiol Renal Physiol. 2005;288:F1084–F1089. doi: 10.1152/ajprenal.00473.2004. [DOI] [PubMed] [Google Scholar]
- 10.Rubart M. Two-photon microscopy of cells and tissue. Circ Res. 2004;95:1154–1166. doi: 10.1161/01.RES.0000150593.30324.42. [DOI] [PubMed] [Google Scholar]
- 11.Calahan M, Parker I, Wei S, Miller M. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat Rev Immunol. 2002;2:872–880. doi: 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helmchen F, Denk W. New developments in multiphoton microscopy. Curr Opin Neurobiol. 2002;12:593–601. doi: 10.1016/s0959-4388(02)00362-8. [DOI] [PubMed] [Google Scholar]
- 13.Malone J, Hood AF, Conley T, Nurnberger J, Baldridge LA, Clendenon JL, Dunn KW, Phillips CL. Three-dimensional imaging of human skin and mucosa by two-photon laser scanning microscopy. J Cutan Pathol. 2002;29:453–458. doi: 10.1034/j.1600-0560.2002.290802.x. [DOI] [PubMed] [Google Scholar]
- 14.Christie RH, Bacskai BJ, Zipfel WR, Williams RM, Kajdasz ST, Webb WW, Hyman BT. Growth arrest of individual senile plaques in a model of Alzheimer’s disease observed by in vivo multiphoton microscopy. J Neurosci. 2001;21:858–864. doi: 10.1523/JNEUROSCI.21-03-00858.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakashita M, Inoue H, Kashida H, Tanaka J, Cho JY, Satodate H, Hidaka E, Yoshida T, Fukami N, Tamegai Y, Shiokawa A, Kudo S. Virtual histology of colorectal lesions using laser-scanning confocal microscopy. Endoscopy. 2003;35:1033–1038. doi: 10.1055/s-2003-44595. [DOI] [PubMed] [Google Scholar]
- 16.Inoue H, Igari T, Nishikage T, Ami K, Yoshida T, Iwai T. A novel method of virtual histopathology using laser-scanning confocal microscopy in-vitro with untreated fresh specimens from the gastrointestinal mucosa. Endoscopy. 2000;32:439–443. doi: 10.1055/s-2000-654. [DOI] [PubMed] [Google Scholar]
- 17.Mochizuki Y, Park MK, Mori T, Ogura A, Kawashima S. Formation of lipofuscin-like autofluorescent materials in NG108-15 cells: involvement of lysosomal protein degradation. Gerontology. 1998;44:1–8. doi: 10.1159/000021975. [DOI] [PubMed] [Google Scholar]
- 18.Williams R, Zipfel W, Webb W. Interpreting second-harmonic generation images of collagen I fibrils. Biophys J. 2005;88:1377–1386. doi: 10.1529/biophysj.104.047308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiesslich R, Hoffman A, Goetz M, Biesterfeld S, Vieth M, Galle PR, Neurath MF. In vivo diagnosis of collagenous colitis by confocal endomicroscopy. Gut. 2006;55:591–592. doi: 10.1136/gut.2005.084970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, Hoffman A, Jung M, Nafe B, Galle PR, Neurath MF. In vivo histology of Barrett’s esophagus and associated neoplasias by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;8:979–987. doi: 10.1016/j.cgh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Kiesslich R, Goetz M, Lammersdorf K, Schneider C, Burg J, Stolte M, Vieth M, Nafe B, Galle PR, Neurath MF. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874–82. doi: 10.1053/j.gastro.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 22.Fiarman GS, Nathanson MH, West B, Deckelbaum LI, Kelly L, Kapadia CR. Differences in laser-induced autofluorescence between adenomatous and hyperplastic polyps and normal colonic muocsa by confocal microscopy. Dig Dis Sci. 1995;40:1261–1268. doi: 10.1007/BF02065535. [DOI] [PubMed] [Google Scholar]
- 23.Schomacker KT, Frisoli JK, Compton CC, Flotte TJ, Richter JM, Nishioka NS, Deutsch TF. Ultraviolet laser induced fluorescence of colonic tissue: basic biology and diagnostic potential. Lasers Surg Med. 1992;12:63–78. doi: 10.1002/lsm.1900120111. [DOI] [PubMed] [Google Scholar]
- 24.Kapadia CR, Cutruzzola FW, O’Brien KM, Stetz ML, Enriquez R, Deckelbaum LI. Laser-induced fluorescence spectroscopy of human colonic mucosa. Detection of adenomatous transformation. Gastroenterology. 1990;99:150–7. doi: 10.1016/0016-5085(90)91242-x. [DOI] [PubMed] [Google Scholar]
- 25.Lipson B, Yannuzzi L. Complications of intravenous fluorescein injections. Int Opthalmol Clin. 1989;29:200–205. doi: 10.1097/00004397-198902930-00011. [DOI] [PubMed] [Google Scholar]
- 26.Kim SG, Cho JY, Chung YS, Ahn E, Lee K, Han Y. Suppression of xenobiotic-metabolizing enzyme expression in rats by acriflavine, a protein kinase C inhibitor. Drug Metab Dispos. 1998;26:66–72. [PubMed] [Google Scholar]
- 27.Theer P, Hasan M, Denk W. Two-photon imaging of 1000 microns in living brains by use of a Ti:A1203 regenerative amplifier. Opt Lett. 2003;28:1022–1024. doi: 10.1364/ol.28.001022. [DOI] [PubMed] [Google Scholar]
- 28.ASGE/SAGES Working Group on Natural Orifice Translumenal Endoscopic Surgery. White Paper October 2005. Gastrointest Endosc. 2006;63:199–203. doi: 10.1016/j.gie.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114–117. doi: 10.1016/s0016-5107(04)01309-4. [DOI] [PubMed] [Google Scholar]
- 30.Flusberg BA, Cocker ED, Piyawattanametha W, Jung JC, Cheung ELM, Schnitzer MJ. Fiberoptic fluorescence imaging. Nat Methods. 2005;2:941–950. doi: 10.1038/nmeth820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung JC, Schnitzer MJ. Multiphoton endoscopy. Opt Lett. 2003;28:902–904. doi: 10.1364/ol.28.000902. [DOI] [PubMed] [Google Scholar]
- 32.Helmchen F, Fee MS, Tank DW, Denk W. A miniature head-mounted two-photon microscope: high resolution brain imaging in freely moving animals. Neuron. 2001;31:903–912. doi: 10.1016/s0896-6273(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 33.Ouzounov DG, Moll KD, Foster MA, Zipfel WR, Webb WW, Gaeta AL. Delivery of nanojoule femtosecond pulses through large-core microstructured fibers. Opt Lett. 2002;27:1513–1515. doi: 10.1364/ol.27.001513. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda Y, Kawano Y, Tanikawa Y, Oba M, Koyama M, Takagi H, Matsumoto M, Nagayama K, Setou M. In vivo imaging of the dendritic arbors of layer V pyramidal cells in the cerebral cortex using a laser scanning microscope with a stick-type objective lens. Neuroscience Letters. 2006;400:53–57. doi: 10.1016/j.neulet.2006.02.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serial cross-sectional imaging of gastric mucosa (20x magnification) using MPM, with z-stacks displayed as a three-dimensional video. The architecture and depth of gastric pits can be appreciated.
Serial cross-sectional imaging of rectal mucosa (20x magnification) using MPM, with z-stacks displayed as a three-dimensional video. A regular arrangement of rectal glands can be visualized.


