Abstract
The expression of MHC class II molecules and the invariant chain (Ii) chaperone, is coordinately regulated in professional antigen presenting cells (APC). Ii facilitates class II subunit folding as well as transit and retention in mature endosomal compartments rich in antigenic peptides in these APC. Yet, in nonprofessional APC such as tumors, fibroblasts and endocrine tissues, the expression of class II subunits and Ii may be uncoupled. Studies of nonprofessional APC indicate class II molecules access antigenic peptides by distinct, but poorly defined pathways in the absence of Ii. Here, investigations demonstrate that nonprofessional APC such as human fibroblasts lacking Ii internalize antigenic peptides prior to the binding of these ligands to recycling class II molecules. By contrast, fibroblast lines expressing Ii favor exogenous peptides binding directly to cell surface class II molecules without a need for ligand internalization. Endocytosis of class II molecules was enhanced in cells lacking Ii compared with Ii-expressing APC. These results suggest enhanced reliance on the endocytic recycling pathway for functional class II presentation in nonprofessional APC.
Keywords: Antigen presenting cells (APC), Fibroblasts, HLA class II, Invariant chain (Ii), Peptide binding, Peptide presentation, Endocytosis and recycling, CD4+ T cell recognition
1. Introduction
Major histocompatibility complex (MHC) class II molecules (HLA in humans) are expressed on professional APC such as B cells, macrophages and dendritic cells as well as nonprofessional APC including thymic epithelial cells, select endocrine tissues and tumors [1-3]. The expression of class II molecules on nonprofessional APC has been implicated in a variety of inflammatory diseases including autoimmunity, transplant rejection and infectious diseases [2,4-6]. HLA class II molecules are αβ heterodimers that associate in the endoplasmic reticulum with a non-polymorphic third glycoprotein, designated the invariant chain (Ii) [7]. Studies using cell lines as well as Ii knock-out animals, have established that Ii facilitates the proper folding of the αβ subunits as well as targeting and retention of class II complexes in mature endosomes and prelysosomes [8,9]. Within mature endosomes, Ii is sequentially degraded by endosomal proteases leaving class II-associated invariant chain peptide (CLIP) in the peptide ligand binding groove. Class II molecules with CLIP associated may transit to the cell surface where CLIP can exchange with higher affinity or more stable peptides, thus resulting in the formation of long-lived stable class II-peptide complexes [10-12]. Alternatively, in mature endosomes such as the MIIC, the HLA-encoded heterodimer DM catalyzes CLIP release and the binding of stable ligands to class II. A third pathway involves the binding of antigenic peptides to class II molecules which are actively recycling through the endosomal network [13].
While Ii directs the transit of class II molecules to mature endosomal compartments [7,14], a role for this chaperone in modulating the function of recycling class II molecules has not been addressed. HLA class II protein recycling in professional APC such as B lymphocytes, permitted the presentation of peptides or antigens requiring minimal processing within the endosomal network [13,15-18]. Nonprofessional APC expressing class II molecules alone can present peptides to T cells, but such presentation can differ from that observed in the presence of Ii [19,20]. Remarkably, immunization of animals with tumor cell vaccines lacking Ii, more effectively generate durable anti-tumor responses compared to animals treated with Ii-positive tumors [21,22]. The mechanism by which Ii modulates class II presentation in these tumors remains to be elucidated. Cell type differences in the requirement for Ii have been reported [23-25]. Thus, studies with Ii knock-out mice revealed normal class II protein folding in thymic epithelial cells in contrast to B lymphocytes which contain abundant class II aggregates in the absence of Ii [26]. In thyrocytes the expression of Ii relative to class II DR was significantly reduced in cells from patients with Hashimoto's disease [27], suggesting discordant expression of Ii and class II may be physiologically relevant in this autoimmune disorder.
In the current study, a nonprofessional APC human fibroblasts expressing either class II molecules or class II and Ii, were employed to examine the role of endocytic recycling in peptide presentation. Similar to professional APC, metabolically active or aldehyde fixed class II-positive fibroblasts expressing Ii were nearly equivalent in functionally presenting short antigenic peptides to T cells. However, class II-positive fibroblasts lacking Ii favored a different pathway for class II peptide presentation leading to T cell activation. While peptide presentation by live fibroblasts expressing class II only was efficient, aldehyde fixation of these cells reduced peptide display to T cells. Ii expression in fibroblasts did not alter the surface expression of class II DR4 molecules by cells. Yet peptide binding to class II proteins on cells lacking Ii was reduced at neutral pH compared with that observed in cells expressing Ii. The addition of a competiting antigen, bovine serum albumin (BSA) to cells did not perturb peptide presentation by Ii-positive fibroblasts, yet peptide presentation was reduced for cells lacking Ii. These results suggested a role for endocytosis in the presentation of peptides by fibroblasts lacking Ii but not those expressing Ii. Treatment of fibroblasts with an inhibitor of endocytic recycling, primiquine blocked only peptide display by cells lacking Ii but not Ii-positive fibroblasts. Finally, the endocytosis of class II molecules was quantitated using cells expressing DR alone or DR and Ii, with enhanced class II endocytosis in cells lacking Ii.
2. Materials and methods
2.1. Cell lines
Human fibroblast M1 was cultured in complete DMEM with 10% fetal bovine serum (FBS), 50 U/ml penicillin, 50 μg/ml streptomycin and supplemented with L-glutamate [28]. Retroviral vectors encoding cDNAs for DR alpha or DR beta (DRB1*401) were used to sequentially transduce M1 fibroblasts to yield constitutively class II-positive M1.DR4 cells [28, 29]. Expression of surface HLA-DR4 complexes on fibroblast cells was confirmed by flow cytometric analysis using the DR4-specific monoclonal antibody, 359-F10 [30]. M1.DR4 was also transfected with a plasmid encoding p33 and p35 forms of Ii [31,32]. These M1.DR4 and M1.DR4.Ii cells were used as APC for the study of peptide presentation by class II molecules. T cell hybridomas specific for Ig κ peptide presented in the context of HLA-DR4, was generated by immunization of DR4-transgenic mice with human IgG. The hybridoma line 1.21 responds to Ig kappa residues 145−159 [33,34]. Other T cell hybridomas used in this study include 17.9 specific for DR4 and human serum albumin (HSA) residues 64−76, and 50.84.17 specific for DR4 and human influenza hemagglutinin (HA-flu) residues 307−319 [13]. T cell hybridomas and the IL-2 dependent cell line, HT-2 were cultured in RPMI 1640 with 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin and 50 μM β-mercaptoethanol. HT-2 cell cultures were maintained using 20% Con A supernatant (T-STIM, Collaborative Biomedical Res., Bedford, MA) containing IL-2 [33,34].
2.2. Peptides
The human IgG kappa peptide κ145−159 (sequence KVQWKVDNALQSGNS) and prostate specific membrane antigen (PMSA) peptide PSMA576−596 (sequence VAQVRGGMVFELANSIVLPFD) were produced using Fmoc technology and an Applied Biosystems Synthesizer [33,34]. Peptide purity (>99%) and sequence were analyzed by reverse phase HPLC purification and mass spectroscopy. These κ145−159 and PSMA576−596 peptides were labeled as indicated at the α amino termini by the sequential addition of 2 molecules of Fmoc-6-aminohexanoic acid followed by a single biotin to yield the sequence biotin-aminohexanoic acid-aminohexanoic acid-peptide. Mass spectrometry confirmed that the peptide was tagged with a single biotin molecule at the N-terminus. The human HSA64−76 peptide (sequence VKLVNEVTEFAKTK) and HA-flu307−319 peptide (sequence PKYVKQNTLKLAT) were also synthesized using Fmoc technology as described [13].
2.3. Antigen presentation assays
APC were incubated with synthetic κ145−159, HSA64−76 and HA-flu307−319 peptide for 3−24 h at 37°C in culture media, washed, and co-cultured with T cell hybridomas 1.21, 17.9 and 50.84.17 respectively for 24 h [33,34]. In some experiments, APC were incubated with peptide in the presence or absence of a competiting antigen, BSA in Hanks' balanced salt solution (HBSS). T cell activation and cytokine production served as a measure of APC function. T cell cytokine production was measured in a bioassay based upon the IL-2 dependent proliferation of HT-2 cells [33,34]. In some cases to halt endocytosis and APC metabolic function, these cells were pre-fixed with 1% paraformaldehyde for 8 min on ice followed by extensive washing and peptide addition, or post-fixed after peptide addition and prior to coculture with T cell hybridomas. For inhibition studies, APC were pretreated with inhibitors such as chloroquine, leupeptin, bestatin, bafilomycin A1 and primaquine (Sigma, St. Louis, MO) in complete media for 30 min followed by the addition of synthetic κ145−159 (10 μM) peptide. After these treatments, APC were washed twice in PBS and fixed with 1% paraformaldehyde prior to cultivation with peptide specific T cells. All assays were repeated three to four times with the standard error for triplicate samples within a single experiment, reported. Data were corrected for isotope counting efficiency and expressed as mean ccpm ± SEM.
2.4. Peptide binding assays
Paraformaldehyde fixed M1.DR4 and M1.DR4.Ii cells were incubated overnight with biotinylated κ145−159 peptide in either HBSS or 150 mM citrate phosphate buffer CPB (pH 5.5), washed with PBS, and lysed on ice for 20 min with 50 mM Tris buffer (pH 8) containing 0.15 M NaCl and 0.5% IGEPAL CA 630 (Sigma) as described [35]. Cell lysates were centrifuged to remove intact nuclei, and the supernatants added to plates (Costar, Cambridge, MA) previously coated overnight with the anti-HLA class II antibody 37.1 [33-35]. The captured class II-biotin-peptide complexes were detected with europium-labeled streptavidin (Pharmacia Fine Chemicals, Piscataway, NJ) using a fluorescence plate reader (Delfia, Wallac, Turku, Finland). The number of total DR molecules within APC was quantitated using biotinylated L243 and the capture antibody 37.1 as described [33-35].
2.5. Flow cytometric analysis
Cells were stained with mAbs directed against class II DR (L243), or CLIP (cerCLIP) followed by secondary antibody labeled with FITC [33-35]. For intracellular staining, cells were fixed, and permeabilized with Cytofix/Cytoperm (BD Pharmingen) and stained with antibody against Ii (Pin1.1) according to the supplied protocol. Samples were then analyzed on a FACScan using CellQuest software (BD Bioscience, Mountain View, CA). Background fluorescence was evaluated using an irrelevant isotype-matched mAb (NN4). Cells were also incubated with biotinylated PSMA576−596 peptide which does not bind to DR4 molecules on M1.DR4/M1.DR4.Ii for 60 mins at 37°C. Cells were washed, fixed to prevent internalization, permeabilized and stained with FITC-labeled streptavidin antibody (Santa Cruz Biotechnology). Samples were then analyzed on a FACScan using CellQuest software as described.
2.6. Western blot analysis
Samples of M1.DR4 and M1.DR4.Ii cells were lysed in detergent and total cell proteins were fractionated by SDS-PAGE (10%), followed by analysis by western blotting for DRαβ (mAb L243), DRα (mAb DA6.147), DRβ (mAb XD5.A11), DM (anti-sera DM-k8), Ii (mAb PIN 1.1) and GILT (Vishnu antisera) [28,29,36].
2.7. Measurement of endocytosis and class II protein recycling
Endocytosis and recycling of class II molecules were quantitatively detected as described previously [28]. Briefly, APC (samples A, B and C) were washed with cold HBSS and cell surface proteins biotinylated with sulfo-NHS-SS-biotin (Pierce, IL) for 15 min at 4°C. Sample A was immediately harvested, washed and flash frozen for subsequent analysis. Sample A, thus represents total cell surface proteins accessible to the biotin-labeling reagent in the absence of endocytosis. For sample B, cells were exposed to a glutathione solution to remove the disulfide-linked biotin from surface proteins, with typically greater than 95% release of this label. Cells in sample C, were incubated for 15 min at 37°C to allow endocytosis of biotin-labeled surface molecules, followed by exposure to the glutathione solution to remove any residual biotin-label from proteins remaining on the cell surface. Thus, sample C represents the amount of cell surface protein endocytosed in 15 min. Each set of cells were washed extensively and lysed with HBSS + 1% Triton + protease inhibitors. Biotinylated cell surface molecules were then detected by capture ELISA using plates coated with antibodies to MHC class II molecules (37.1) or transferrin receptor (TfR) (B3/25) followed by the addition of streptavidin-peroxidase and colorimetric detection of bound peroxidase. The percent endocytosis was calculated by the following formula: % cell surface protein endocytosis = (sample C-sample B) / (sample A-sample B).
3. Results
3.1. Ii expression influences peptide presentation in the context of HLA-DR4
Studies have shown that Ii expression can have both a positive and negative impact on antigen processing and presentation by class II molecules [37-40]. These investigations suggest Ii influences class II αβ folding as well as the intracellular sorting of these MHC antigens to mature endosomal compartments rich in proteases and peptide ligands. Ii has been postulated to primarily influence the maturation and ligand binding of newly synthesized class II complexes. Studies here however, using nonprofessional APC, suggest Ii may also influence peptide binding and presentation at the cell surface and within recycling endosomes. Prior work by our laboratory has shown that cell surface class II molecules on professional APC such as B cells, macrophages and dendritic cells, functionally present short synthetic class II ligands such as the Ig kappa derived, κ145−159 peptide to T cells [33,34]. Peptide presentation is observed with either live or aldehyde-fixed professional APC, suggesting direct peptide binding to surface class II and ruling out any requirement for endocytosis of this peptide or active internalization of class II molecules. By contrast, a nonprofessional APC, live M1.DR4 fibroblasts were consistently more efficient in presenting this same κ145−159 peptide, when compared with aldehyde fixed M1.DR4 cells (Fig. 1, A and B). To investigate whether Ii expression influences class II-peptide acquisition by these cells, M1.DR4 cells were stably transfected with Ii. Both live and fixed M1.DR4.Ii cells were nearly equivalent in their ability to present the synthetic κ145−159 peptide to T cells (Fig. 1.B).
Fig. 1.
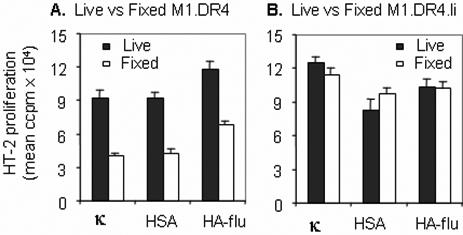
Ii expression influences peptide presentation by human fibroblasts in the context of HLA-DR4. Live or paraformaldehyde fixed fibroblast cell lines M1.DR4 (A) and M1.DR4.Ii (B) were incubated with 10 μM of κ145−159, HSA64−76 or HA-flu307−319 peptide for 4 h at 37°C. The presentation of peptides was monitored by the addition of peptide specific T cell hybridomas (1.21, 17.9 and 50.84.17 respectively) followed by IL-2 measurement using HT-2 cells. M1.DR4 and M1.DR4.Ii cells were analyzed by FACS and cell populations isolated with matched DR4 expression were used for these assays. The data is a representative of three separate experiments.
To investigate whether this observation held true for other class II peptide ligands, two additional DR4-restricted peptides, HSA64−76 and HA-flu307−319 were tested. Similar to results with the κ145−159 peptide, live M1.DR4 and M1.DR4.Ii cells functionally presented these peptides, whereas fixed M1.DR4 cells showed reduced presentation of both the peptides as assessed by T cell activation (Fig. 1). Similar results were observed using live or fixed APC incubated with a range of κ145−159 peptide concentrations (data not shown). The nearly equivalent presentation of these peptides by live APC, cast doubt as to whether the observed differences could be due to differential co-stimulation of T cells by live vs. aldehyde-fixed APC. To directly test this question, a strong costimulatory signal was delivered to T cells via antibody cross-linking of CD28 prior to incubation with APC. Again, T cell responses to fixed M1.DR4 and variable concentrations of the κ145−159 peptide were reduced compared with that observed using live M1.DR4 and these same amounts of peptide (data not shown), confirming that the reduced peptide presentation by cells lacking Ii was not due to a lack of co-stimulation. Similar differences in peptide presentation using live and aldehyde-fixed APC was observed using murine fibroblast lines L.DR4 and L.DR4.Ii expressing human class II DR4 in the presence and absence of human Ii (data not shown). Together these studies suggest a role for Ii in regulating direct peptide presentation in the context of class II molecules at the cell surface.
3.2. Class II αβ surface expression is similar in human nonprofessional APC +/− Ii
Occupancy of the class II-peptide binding site by Ii plays an important role in the class II assembly, preventing the aggregation of class II chains and facilitating the formation of class II αβ-peptide complexes [41]. Although surface expression of class II molecules in most transfected cell lines is minimally affected by Ii co-expression, we analyzed both the cell lines to investigate any differences in surface class II expression. Flow cytometric analysis of fibroblast lines showed that both M1.DR4 and M1.DR4.Ii cells expressed similar levels of surface class II molecules (Fig. 2A), suggesting Ii did not alter surface class II expression in M1.DR4 cells. The level of intracellular HLA-DM molecules which act as a peptide editor was also tested in both fibroblasts by Western blotting. No DM molecules were detected in those cell lines (data not shown). A lysosomal reductase, interferon-gamma-inducible lysosomal thiol reductase (GILT), has been identified in professional APC which can enhance Ag presentation [36,42,43]. We could not detect any GILT in fibroblast cells (data not shown), suggesting that this enzyme did not have any role in this differential peptide presentation.
Fig. 2.
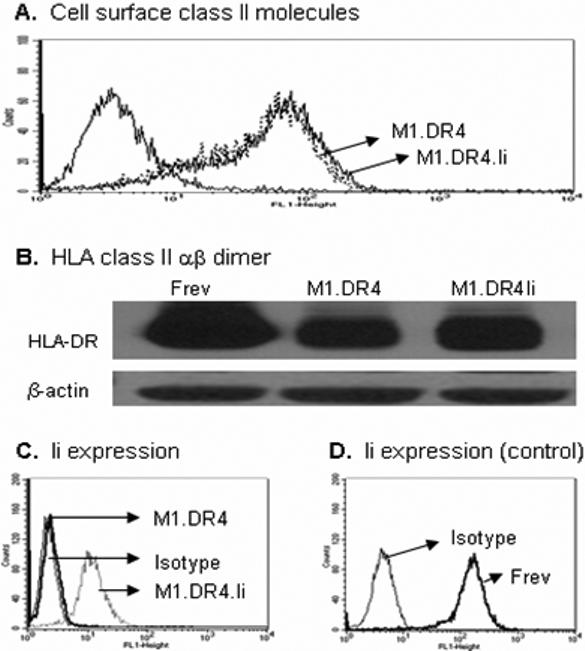
Analysis of class II molecules and Ii expression in fibroblast lines M1.DR4 and M1.DR4.Ii. Cells were stained with antibody against DR (L243) and Ii (Pin 1.1) for surface DR4 and intracellular Ii respectively. Cells samples were also analyzed by western blot analysis for stable class II dimer. (A) Expression of cell surface class II molecules on M1.DR4 and M1.DR4.Ii. (B) Class II dimers in fibroblast cells plus or minus Ii. Equivalent levels of total cell protein, was analyzed by SDS-PAGE and immunoblotting. β-actin was used as a loading control. (C-D) Ii expression in M1.DR4.Ii and M1.DR4 cells and a control B-cell line Frev was determined by intracellular staining. Staining patterns with specific Abs (thick line) and isotype-matched control (thin line) are shown. Data are representative of at least three separate experiments.
We also investigated the stability of class II dimers in M1 fibroblast line with or without Ii (Fig. 2B). Immunoblotting confirmed the presence of more stable dimers in Ii-transfected fibroblasts (M1.DR4.Ii) compared to those of lacking Ii (M1.DR4). In a parallel assay, Ii expression in M1.DR4.Ii cells was also confirmed by intracellular staining and flow cytometric analysis using Pin 1.1 antibody (Fig. 2C). These data suggest class II αβ complex folding is partly perturbed in cells lacking Ii as previously reported [7,44]. This data also suggests class II molecules may be loaded with unstable endogenous ligands in cells lacking Ii. A fragment of Ii, CLIP fills the binding groove of class II molecules and can influence folding and peptide acquisition by αβ. Fibroblasts were tested for the presence of CLIP-class II complexes, yet no increase in cell surface staining using the CLIP-specific mAb, cer-CLIP was detected in cells plus or minus Ii (data not shown). Studies have suggested DM-independent mechanisms exist for releasing CLIP potentially related to differential class II sorting or intracellular pH [45-48]. Such pathways may account for the lack of DR-CLIP complex accumulation in these human fibroblasts.
3.3. HLA class II molecules on fibroblasts bind κ145−159 peptide at acidic and neutral pH
Peptide binding to class II molecules can occur at the cell surface or within acidic compartments of the endocytic network. To determine if the differences in peptide presentation by fibroblasts +/− Ii was linked to alterations in peptide binding, the association of κ145−159 peptide with DR4 was assessed using these cells. Data showed that κ145−159 peptide bound to DR4 molecules on fibroblasts at acidic and neutral pH (Fig. 3). The κ145−159 peptide bound with a somewhat higher affinity at neutral pH to class II αβ on M1.DR4.Ii cells compared with M1.DR4, while this trend was reversed at acidic pH (Figs. 3A and 3B). Overall, peptide binding to DR4 was significantly enhanced at acidic pH regardless of cellular Ii expression (Fig. 3B). To further study the effect of low pH on κ145−159 peptide binding, fixed cells were pretreated with low pH buffer (citrate-phosphate buffer, pH 5.5) and tested for peptide binding at neutral and low pH. M1.DR4 cells lacking Ii again bound to κ145−159 peptide better than M1.DR4.Ii cells at low pH (data not shown). However, low pH buffer pretreated fibroblasts lines with or without Ii now bound this peptide with somewhat similar affinity at neutral pH (data not shown). Taken together, these data suggest that at acidic pH, class II αβ on M1.DR4 and M1.DR4.Ii cells undergo changes which favor the binding of exogenous peptides.
Fig. 3.

Differences in cell surface peptide binding to class II molecules with Ii expression. Fibroblast cell lines M1.DR4 and M1.DR4.Ii were fixed with paraformaldehyde and incubated overnight with biotin labeled κ145−159 peptide in either neutral buffer HBSS, pH 7.2 (A) or acidic citrate phosphate buffer CPB, pH 5.5 (B). Cells were washed, lysed, and the extent of binding of peptide to DR4 molecules was examined in a capture ELISA using europium-labeled streptavidine. Data are representative of mean fluorescence ± SEM for at least four separate experiments.
3.4. Requirement for peptide internalization in cells lacking Ii for functional presentation via class II molecules
Antigenic peptides can be loaded onto class II molecules in the endosomal and lysosomal compartments as well as on the cell surface. While fibroblast cell lines M1.DR4 and M1.DR4.Ii expressed similar levels of surface class II molecules (Fig. 2), fixed M1.DR4 cells in the absence of Ii showed reduced peptide presentation and T cell activation. To investigate the requirement of peptide internalization by cells lacking Ii, M1.DR4 and M1.DR4.Ii cells were fed bovine serum albumin (BSA) together with the κ145−159 peptide. Data showed that κ145−159 peptide presentation by M1.DR4 was reduced in the presence of BSA whereas the peptide presentation by M1.DR4.Ii was not affected by feeding BSA (Fig. 4A). To determine whether the uptake of peptides varies in cells +/−Ii, we incubated M1.DR4 and M1.DR4.Ii cells with a biotinylated PSMA576−596 peptide which does not bind to cell surface DR4, followed by intracellular staining. Flow cytometric analysis showed that the Ii expression did not influence the uptake of PSMA576−596 peptide by M1.DR4 and M1.DR4.Ii cells after 60 mins of incubation (Fig. 4B). These data suggest that the differences in peptide presentation observed (Fig. 4A), is not the result of differential uptake of antigenic peptides by fibroblast cells plus or minus Ii. In prior studies, we showed high concentrations of exogenous antigens such as BSA yield peptides that can compete within APC for binding to class II molecules [49]. Thus, in M1.DR4 cells, the κ145−159 peptide may compete for binding to class II molecules in endocytic compartments where antigens such as BSA are processed. Native BSA does not readily bind to surface class II molecules but rather requires intracellular processing within endosomal and lysosomal compartments to yield peptides capable of loading onto class II molecules [49]. These results suggest that APC expressing Ii may bind antigenic peptides directly with little competition from exogenous antigens. By contrast, in APC lacking Ii, peptides such as κ145−159 appear to bind class II molecules in endocytic compartments where exogenous antigens are also processed.
Fig. 4.
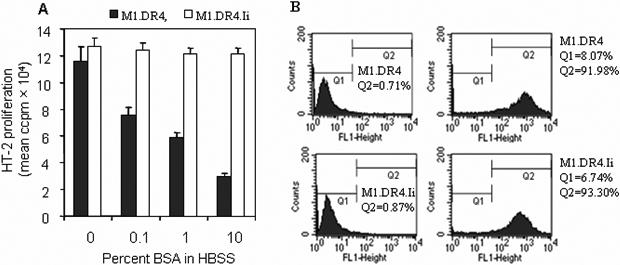
Ii expression influences the site of peptide association with class II molecules, but not peptide internalization. (A) Live fibroblasts M1.DR4 and M1.DR4.Ii were incubated with 20 μM of κ145−159 peptide in the presence or absence of 10% BSA in HBSS. Cells were washed and co-cultured with DR4: κ145−159 specific T cell hybridoma (1.21) followed by quantitation of IL-2 production. Filled bars, assay conditions HBSS; and open bars, assay conditions HBSS+10%BSA. Data is expressed as mean ccpm ± SEM, and representative of three separate experiments. (B) Peptide internalization in M1.DR4 and M1.DR4.Ii cells was determined using the biotin-labeled PSMA576−596 peptide as described in methods. Staining patterns at 60 mins with streptavidin Abs and isotype-control are shown. Data are representative of at least three separate experiments.
3.5. Endocytic recycling is required for peptide presentation by fibroblasts lacking Ii
The observations that metabolically active fibroblasts function better in peptide presentation, suggested peptide processing or intracellular transport might be important for ligand binding by class II. To address this issue M1.DR4 and M1.DR4.Ii cells were treated first with inhibitors of antigen processing and proteolysis such as chloroquine, bestatin or leupeptin and monitored for their ability to present the κ145−159 peptide to T cells. Chloroquine, a lysosomotrophic agent disrupts lysosomal pH and affects proteolysis and processing reactions within APC [13,16]. Chloroquine treatment of fibroblast lines with or without Ii, had no significant effect on κ145−159 peptide presentation (Fig. 5). Leupeptin is a cysteine protease inhibitor, and treatment of APC with this inhibitor can modulate antigen presentation by reducing Ii degradation and antigen processing [13,16]. We treated both M1.DR4 and M1.DR4.Ii cells with leupeptin and did not see any difference in functional κ145−159 presentation (Fig. 5). A previous study had shown that aminopeptidases expressed by dendritic cells can degrade the κ145−159 peptide, preventing T cell recognition [50]. APC were treated with bestatin which inhibits aminopeptidases. Blocking this enzyme had no effect on functional presentation of κ145−159 peptide (Fig. 5). Bafilomycin A1 blocks the transportation of ligands from early to late endosomes, and inhibition by this compound indicates a requirement for late endosomes or lysosomes [13,16]. Treating fibroblast cells plus or minus Ii, with bafilomycin did not have any effect on peptide presentation (Fig. 5). In addition to transiting from early to late endosomes, some Ags/peptides are dependent upon delivery into recycling endosomes for processing and presentation. Treatment of M1.DR4.Ii cells with primaquine, a drug which blocks the endosomal recycling pathway [13,16], had no significant effect on the presentation of κ145−159 (Fig. 5). Yet primaquine treatment of M1.DR4 cells lacking Ii, inhibited the functional presentation of the κ145−159 peptide (Fig. 5). Primaquine did not inhibit endocytosis of the κ145−159 peptide, but rather blocked the recycling of HLA-DR4 molecules back to the plasma membrane of M1.DR4 cells (data not shown). These results suggest that in cells lacking Ii, endosomal recycling may facilitate peptide loading and presentation by class II molecules.
Fig. 5.
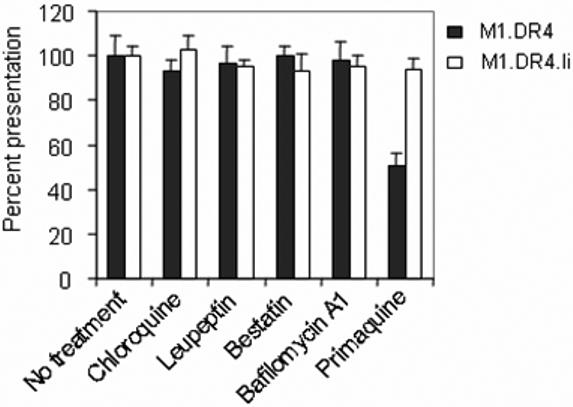
Fibroblasts lacking Ii utilizes the recycling pathway for peptide presentation by class II molecules. Fibroblast cell lines M1.DR4 and M1.DR4.Ii were pretreated with chloroquine, leupeptin, bestatin, bafilomycin A1 and primaquine in complete media for 30 min followed by the addition of κ145−159 peptide (10 μM) for 4 h. Cells were then washed, fixed with paraformaldehyde and cocultured with the κ145−159 peptide specific T cell hybridoma (1.21) for 24 h. T cell production of IL-2 was measured using HT-2 cells. Data is a representative of at least three separate experiments.
3.6. Ii expression influences endocytosis and class II protein recycling
To study further how Ii influences peptide presentation in nonprofessional APC, the endocytosis of class II DR was measured in fibroblast lines M1.DR4 and M1.DR4.Ii (Fig. 6). In this assay, the endocytosis of class II molecules is detected by biotin labeling molecules at the cell surface and monitoring their transit into the endosomal network. The data show that endocytosis of class II proteins in M1.DR4 cells, is measurably enhanced (15.6% vs 8.2%) compared with M1.DR4.Ii cells (Fig. 6). Experiments using TfR as a control did not show any significant differences in endocytosis (4.8% vs 4.2%) by the fibroblast lines M1.DR4 and M1.DR4.Ii. Together with previous experiments, these results suggest enhanced endocytosis of class II molecules in the absence of Ii, may facilitate peptide presentation. There are reports that multiple processing pathways exist for class II antigen and peptide presentation to T cells [13-18]. In M1.DR4.Ii cells, newly synthesized class II molecules, which likely route through acidic compartments until Ii release, prompts the sorting of these class II molecules to the cell surface. By contrast, in M1.DR4 cells lacking Ii, class II molecules are sorted to the cell surface, prior to transit through endosomal compartments for peptide acquisition.
Fig. 6.
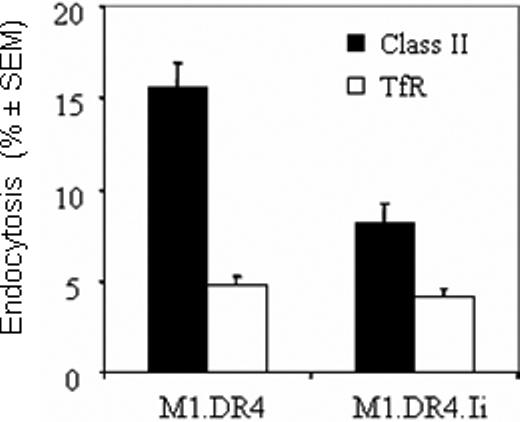
Ii expression influences the endocytosis of class II molecules. Cell surface proteins expressed on the fibroblast cell lines M1.DR4 and M1.DR4.Ii, were biotinylated with sulfo-NHS-SS-biotin. The cells were warmed to allow internalization of these biotin-labeled surface molecules, followed by cleavage of residual biotin from cell surface proteins with a glutathione solution. The biotin-labeled endocytosed class II molecules and transferrin receptors (TfR) were then detected in a capture ELISA, and the percent endocytosis was calculated as described in Materials and Methods. Data is a representative of at least three separate experiments and expressed as percent class II endocytosis ± SEM.
4. Discussion
Evidence is offered here that Ii expression influences HLA class II-restricted peptide presentation by nonprofessional APC. Fibroblasts lacking Ii acquired peptides within the endosomal network, while the expression of Ii in these cells favored a direct peptide binding to cell surface class II molecules. Live fibroblasts expressing class II molecules were able to present a variety of antigenic peptides to stimulate T cells, demonstrating that nonprofessional APC have the machinery required for class II-restricted antigen presentation and T cell activation. Yet, these studies show for the first time that class II endocytosis and peptide acquisition can be modulated by Ii expression in nonprofessional APC.
While Ii expression is typically co-regulated with class II proteins in many cell types, there are circumstances where class II is expressed in cells with little to no Ii [21-27]. Regulation of Ii expression may provide a novel means to modulate Ag/peptide presentation; reduced or no expression would shift presentation towards peptide ligands acquired in endosomal compartments. Understanding these different pathways involved in the presentation of class II ligands, may thus be important for development of novel immunotherapeutics against malignant diseases.
While no differences in surface class II expression in fibroblasts plus or minus Ii was observed, the intracellular pools of stable class II complexes were slightly different in these cells (Fig. 2). Expression of stable class II complexes was slightly reduced in fibroblasts without Ii. In M1.DR4 and M1.DR4.Ii cells, DM molecules were not detected nor was there a significant presence of cell surface CLIP (data not shown). Thus, DM-independent mechanisms in peptide presentation appear to function in these nonprofessional APC. In the absence of Ii, metabolically active fibroblasts function better in exogenous presentation of class II ligands, suggesting peptide processing or intracellular transport might be important in these cells. Treatment of cells with a number of inhibitors that interfere with the processing or transport of peptides demonstrated that peptide processing was not required, but rather that differential transport or delivery of ligands to T cells played dominant role in cells lacking Ii. This was also supported by studies suggesting class II molecules in APC lacking Ii favor ligand binding at acidic pH values similar to that found in endosomes and lysosomes. At acidic pH, increased class II-peptide binding was observed for M1.DR4 cells lacking Ii compared to M1.DR4.Ii cells. Acidic pH may facilitate the release or exchange of unstable ligands from the binding groove of class II αβ in Ii-negative cells or favor subtle changes in class II conformation. Studies revealed that blocking endosomal recycling pathways significantly inhibited class II peptide presentation by fibroblasts lacking Ii (Fig. 5). Finally, direct measurements of class II protein endocytosis in fibroblasts plus and minus Ii indicated enhanced trafficking of class II molecules into endosomes in cells lacking Ii. Together, these results suggest that Ii expression in nonprofessional APC modulates the pathways of peptide presentation by class II molecules.
Studies in tumor cells suggest the absence of Ii may influence class II presentation and the recognition of tumor cells by host CD4+ T cells [23-25, 51]. Studies have also shown that class II-positive APC lacking Ii may differentially acquire peptides from diverse subcellular compartments and activate CD4+ T cells [52], whereas Ii-expressing APC do so by the classical class II pathway [7,8]. Thus, it has been suggested that Ii-negative nonprofessional APC may prove more effective as reagents for tumor immunotherapy (51,53). In nonprofessional APC and tumors, the spectrum of peptides loaded may also vary with more unstable ligands binding to class II in compartments such as the ER (51,53). Exchange or release of these peptides, may require the delivery of unstable class II-peptide complexes into acidic endosomes. Studies have also suggested that unstable class II complexes may be internatlized by APC (54), thus providing an explanation of the observed enhanced endocytosis and recycling of class II in the absence of Ii. Here, a mechanistic basis is offered in that cells lacking Ii acquire peptide ligands during class II endocytosis. Studies with dendritic cells suggest that class II molecules are endocytosed and retained within these APC, due to ubiquitin-modification of the HLA class II beta chain. Disruption of this ubiquitin moiety promoted class II transit to the cell surface. In fibroblasts, we were unable to observe this ubiquitin modification, thus this pathway does not appear to control class II endocytosis in these nonprofessional APC. Other factors such as cytoplasmic adaptor proteins, may regulate HLA class II transit in these nonprofessional APC.
Acknowledgments
This work was supported by grants from the Leukemia and Lymphoma Society (#3024), ACS-IRG (#85241) and American Lung Association (RG-10435) to A.H., and National Institutes of Health Grants AI49589 and AI056097 to J.S.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bryant PW, Lennon-Dumenil AM, Fiebiger E, Lagaudriere-Gesbert C, Ploegh HL. Proteolysis and antigen presentation by MHC class II molecules. Adv. Immunol. 2002;80:71–114. doi: 10.1016/S0065-2776(02)80013-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haque A, Blum JS. New insights in antigen processing and epitope selection: development of novel immunotherapeutic strategies for cancer, autoimmunity and infectious diseases. J. Biol. Regul. Homeost. Agents. 2005;19:93–104. [PubMed] [Google Scholar]
- 3.Li P, Gregg JL, Wang N, Zhou D, O'Donnell P, Blum JS, Crotzer VL. Compartmentalization of class II antigen presentation: contribution of cytoplasmic and endosomal processing. Immunol. Rev. 2005;207:206–217. doi: 10.1111/j.0105-2896.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 4.Jones EY, Fugger L, Strominger JL, Siebold C. MHC class II proteins and disease: a structural perspective. Nat. Rev. Immunol. 2006;6:271–282. doi: 10.1038/nri1805. [DOI] [PubMed] [Google Scholar]
- 5.Daar AS, Fuggle SV, Fabre JW, Ting A, Morris PJ. The detailed distribution of MHC Class II antigens in normal human organs. Transplant. 1984;38:293–298. doi: 10.1097/00007890-198409000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa TY, Brissette WH, Lira PD, Griffiths RJ, Petrushova N, Stock J, McNeish JD, Eastman SE, Howard ED, Clarke SR, Rosloniec EF, Elliott EA, Rudensky AY. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity. 1999;10:207–217. doi: 10.1016/s1074-7613(00)80021-7. [DOI] [PubMed] [Google Scholar]
- 7.Dixon AM, Stanley BJ, Matthews EE, Dawson JP, Engelman DM. Invariant chain transmembrane domain trimerization: a step in MHC class II assembly. Biochemistry. 2006;45:5228–5234. doi: 10.1021/bi052112e. [DOI] [PubMed] [Google Scholar]
- 8.Stern LJ, Potolicchio I, Santambrogio L. MHC class II compartment subtypes: structure and function. Curr. Opin. Immunol. 2006;18:64–69. doi: 10.1016/j.coi.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Maehr R, Kraus M, Ploegh HL. Mice deficient in I-chain and MHC class II exhibit a normal mature B2 cell compartment. Eur. J. Immunol. 2004;34:2230. doi: 10.1002/eji.200425246. [DOI] [PubMed] [Google Scholar]
- 10.Busch R, Rinderknecht CH, Roh S, Lee AW, Harding JJ, Burster T, Hornell TM, Mellins ED. Achieving stability through editing and chaperoning: regulation of MHC class II peptide binding and expression. Immunol. Rev. 2005;207:242–260. doi: 10.1111/j.0105-2896.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 11.Sant AJ, Chaves FA, Jenks SA, Richards KA, Menges P, Weaver JM, Lazarski CA. The relationship between immunodominance, DM editing, and the kinetic stability of MHC class II:peptide complexes. Immunol. Rev. 2005;207:261–278. doi: 10.1111/j.0105-2896.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 12.Honey K, Forbush K, Jensen PE, Rudensky AY. Effect of decreasing the affinity of the class II-associated invariant chain peptide on the MHC class II peptide repertoire in the presence or absence of H-2M. J. Immunol. 2004;172:4142–4150. doi: 10.4049/jimmunol.172.7.4142. [DOI] [PubMed] [Google Scholar]
- 13.Pathak SS, Blum JS. Endocytic recycling is required for the presentation of an exogenous peptide via MHC class II molecules. Traffic. 2000;1:561–569. doi: 10.1034/j.1600-0854.2000.010706.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhong G, Castellino F, Romagnoli P, Germain RN. Evidence that binding site occupancy is necessary and sufficient for effective major histocompatibility complex (MHC) class II transport through the secretory pathway redefines the primary function of class II-associated invariant chain peptides (CLIP) J. Exp. Med. 1996;184:2061–2066. doi: 10.1084/jem.184.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinnathamby G, Eisenlohr LC. Presentation by recycling MHC class II molecules of an influenza hemagglutinin-derived epitope that is revealed in the early endosome by acidification. J. Immunol. 2003;170:3504–3513. doi: 10.4049/jimmunol.170.7.3504. [DOI] [PubMed] [Google Scholar]
- 16.Pathak SS, Lich JD, Blum JS. Cutting edge: editing of recycling class II:peptide complexes by HLA-DM. J. Immunol. 2001;167:632–635. doi: 10.4049/jimmunol.167.2.632. [DOI] [PubMed] [Google Scholar]
- 17.Ramachandra L, Harding CV. Phagosomes acquire nascent and recycling class II MHC molecules but primarily use nascent molecules in phagocytic antigen processing. J. Immunol. 2000;2000;164:5103–12. doi: 10.4049/jimmunol.164.10.5103. [DOI] [PubMed] [Google Scholar]
- 18.Askew D, Chu RS, Krieg AM, Harding CV. CpG DNA induces maturation of dendritic cells with distinct effects on nascent and recycling MHC-II antigen-processing mechanisms. J. Immunol. 2000;165:6889–6895. doi: 10.4049/jimmunol.165.12.6889. [DOI] [PubMed] [Google Scholar]
- 19.Peterson M, Miller J. Invariant chain influences the immunological recognition of MHC class II molecules. Nature. 1990;345:172–174. doi: 10.1038/345172a0. [DOI] [PubMed] [Google Scholar]
- 20.Roche PA, Teletski CL, Karp DR, Pinet V, Bakke O, Long EO. Stable surface expression of invariant chain prevents peptide presentation by HLA-DR. EMBO J. 1992;11:2841–2847. doi: 10.1002/j.1460-2075.1992.tb05351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu M, Lu X, Kallinteris NL, Wang Y, Wu S, von Hofe E, Gulfo JV, Humphreys RE, Hillman GG. Immunotherapy of cancer by antisense inhibition of Ii protein, an immunoregulator of antigen selection by MHC class II molecules. Curr. Opin. Mol. Ther. 2004;6:160–165. [PubMed] [Google Scholar]
- 22.Hillman GG, Kallinteris NL, Li J, Wang Y, Lu X, Li Y, Wu S, Wright JL, Slos P, Gulfo JV, Humphreys RE, Xu M. Generating MHC Class II+/Ii− phenotype after adenoviral delivery of both an expressible gene for MHC Class II inducer and an antisense Ii-RNA construct in tumor cells. Gene. Ther. 2003;10:1512–1518. doi: 10.1038/sj.gt.3302027. [DOI] [PubMed] [Google Scholar]
- 23.Tamori Y, Tan X, Nakagawa K, Takai E, Akagi J, Kageshita T, Egami H, Ogawa M. Clinical significance of MHC class II-associated invariant chain expression in human gastric carcinoma. Oncol. Rep. 2005;14:873–877. [PubMed] [Google Scholar]
- 24.Datta MW, Shahsafaei A, Nadler LM, Freeman GJ, Dorfman DM. Expression of MHC class II-associated invariant chain (Ii;CD74) in thymic epithelial neoplasms. Appl. Immunohistochem. Mol. Morphol. 2000;8:210–215. doi: 10.1097/00129039-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Chamuleau ME, Souwer Y, Van Ham SM, Zevenbergen A, Westers TM, Berkhof J, Meijer CJ, van de Loosdrecht AA, Ossenkoppele GJ. Class II-associated invariant chain peptide expression on myeloid leukemic blasts predicts poor clinical outcome. Cancer Res. 2004;64:5546–5550. doi: 10.1158/0008-5472.CAN-04-1350. Erratum in: Cancer Res. 64(2004):7181. [DOI] [PubMed] [Google Scholar]
- 26.Koonce CH, Bikoff EK. Dissecting MHC class II export, B cell maturation, and DM stability defects in invariant chain mutant mice. J. Immunol. 2004;173:3271–3280. doi: 10.4049/jimmunol.173.5.3271. [DOI] [PubMed] [Google Scholar]
- 27.Yu M, Xu M, Savas L, Khan A. Discordant Expression of Ii and HLA-DR in Thyrocytes: A Possible Pathogenetic Factor in Hashimoto's Thyroiditis. Endocr. Pathol. 1998;9:201–208. doi: 10.1007/BF02739959. [DOI] [PubMed] [Google Scholar]
- 28.Turvy DN, Blum JS. Detection of biotinylated cell surface receptors and MHC molecules in a capture ELISA: a rapid assay to measure endocytosis. J. Immunol. Methods. 1998;212:9–18. doi: 10.1016/s0022-1759(97)00206-8. [DOI] [PubMed] [Google Scholar]
- 29.Li P, Haque MA, Blum JS. Role of disulfide bonds in regulating antigen processing and epitope selection. J Immunol. 2002;169:2444–2450. doi: 10.4049/jimmunol.169.5.2444. [DOI] [PubMed] [Google Scholar]
- 30.Hiraiwa A, Yamanaka K, Kwok WW, Mickelson EM, Masewicz S, Hansen JA, Radka SF, Nepom GT. Structural requirements for recognition of the HLA-Dw14 class II epitope: a key HLA determinant associated with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 1990;87:8051. doi: 10.1073/pnas.87.20.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roche PA, Teletski CL, Stang E, Bakke O, Long EO. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc. Natl. Acad. Sci. USA. 1993;90:8581–8585. doi: 10.1073/pnas.90.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinet V, Vergelli M, Martin R, Bakke O, Long EO. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature. 1995;375:603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 33.Ma C, Whiteley PE, Cameron PM, Freed DC, Pressey A, Chen SL, Garni-Wagner B, Fang C, Zaller DM, Wicker LS, Blum JS. Role of APC in the selection of immunodominant T cell epitopes. J. Immunol. 1999;163:6413–6423. [PubMed] [Google Scholar]
- 34.Haque MA, Hawes JW, Blum JS. Cysteinylation of MHC class II ligands: peptide endocytosis and reduction within APC influences T cell recognition. J. Immunol. 2001;166:4543–4551. doi: 10.4049/jimmunol.166.7.4543. [DOI] [PubMed] [Google Scholar]
- 35.Hill CM, Liu A, Marshall KW, Mayer J, Jorgensen B, Yuan B, Cubbon RM, Nichols EA, Wicker LS, Rothbard JB. Exploration of requirements for peptide binding to HLA DRB1 0101 and DRB1 0401. J. Immunol. 1994;152:2890. [PubMed] [Google Scholar]
- 36.Haque MA, Li P, Jackson SK, Zarour HM, Hawes JW, Phan UT, Maric M, Cresswell P, Blum JS. Absence of gamma-interferon-inducible lysosomal thiol reductase in melanomas disrupts T cell recognition of select immunodominant epitopes. J. Exp. Med. 2002;195:1267–1277. doi: 10.1084/jem.20011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dodi AI, Brett S, Nordeng T, Sidhu S, Batchelor RJ, Lombardi G, Bakke O, Lechler RI. The invariant chain inhibits presentation of endogenous antigens by a human fibroblast cell line, 1994. Eur. J. Immunol. 1994;24:1632–1639. doi: 10.1002/eji.1830240727. [DOI] [PubMed] [Google Scholar]
- 38.Frauwirth K, Shastri N. Introducing endogenous antigens into the major histocompatibility complex (MHC) class II presentation pathway. Both Ii mediated inhibition and enhancement of endogenous peptide/MHC class II presentation require the same Ii domains. Immunology. 2001;102:405–415. doi: 10.1046/j.1365-2567.2001.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frauwirth K, Shastri N. Mutation of the invariant chain transmembrane region inhibits II degradation, prolongs association with MHC class II, and selectively disrupts antigen presentation. Cell. Immunol. 2001;209:97–108. doi: 10.1006/cimm.2001.1796. [DOI] [PubMed] [Google Scholar]
- 40.Stumptner-Cuvelette P, Benaroch P. Multiple roles of the invariant chain in MHC class II function. Biochim. Biophys. Acta. 2002;1542:1–13. doi: 10.1016/s0167-4889(01)00166-5. [DOI] [PubMed] [Google Scholar]
- 41.Germain RN, Hendrix LR. MHC class II structure, occupancy and surface expression determined by post-endoplasmic reticulum antigen binding. Nature. 1991;353:134. doi: 10.1038/353134a0. [DOI] [PubMed] [Google Scholar]
- 42.Phan UT, Arunachalam B, Cresswell P. Gamma-interferon-inducible lysosomal thiol reductase (GILT). Maturation, activity, and mechanism of action. J. Biol. Chem. 2000;275:25907–25914. doi: 10.1074/jbc.M003459200. [DOI] [PubMed] [Google Scholar]
- 43.Maric M, Arunachalam B, Phan UT, Dong C, Garrett WS, Cannon KS, Alfonso C, Karlsson L, Flavell RA, Cresswell P. Defective antigen processing in GILT-free mice. Science. 2001;294:1361–1365. doi: 10.1126/science.1065500. [DOI] [PubMed] [Google Scholar]
- 44.Kukol A, Torres J, Arkin IT. A structure for the trimeric MHC class II-associated invariant chain transmembrane domain. J. Mol. Biol. 2002;320:1109–1117. doi: 10.1016/s0022-2836(02)00563-6. [DOI] [PubMed] [Google Scholar]
- 45.Karlsson L. DM and DO shape the repertoire of peptide-MHC-class-II complexes. Curr. Opin. Immunol. 2005;17:65–70. doi: 10.1016/j.coi.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Monji T, McCormack AL, Yates E, Pious D. Invariant-cognate peptide exchange restores class II dimer stability in HLA-DM mutants 1994. J. Immunol. 1994;153:4468–4477. [PubMed] [Google Scholar]
- 47.Karlsson L, Peleraux A, Lindstedt R, Liljedahl M, Peterson PA. Reconstitution of an operational MHC class II compartment in nonantigen-presenting cells. Science. 1994;266:1569–1573. doi: 10.1126/science.7985028. [DOI] [PubMed] [Google Scholar]
- 48.Ramachandra L, Kovats S, Eastman S, Rudensky AY. Variation in HLA-DM expression influences conversion of MHC class II alpha beta:class II-associated invariant chain peptide complexes to mature peptide-bound class II alpha beta dimers in a normal B cell line. J. Immunol. 1996;156:2196–2204. [PubMed] [Google Scholar]
- 49.Lorenz RG, Blum JS, Allen PM. Constitutive competition by self proteins for antigen presentation can be overcome by receptor-enhanced uptake. J. Immunol. 1990;144:1600–1606. [PubMed] [Google Scholar]
- 50.Dong X, An B, Kierstead LS, Storkus WJ, Amoscato AA, Salter RD. Modification of the amino terminus of a class II epitope confers resistance to degradation by CD13 on dendritic cells and enhances presentation to T cells. J. Immunol. 2000;164:129–135. doi: 10.4049/jimmunol.164.1.129. [DOI] [PubMed] [Google Scholar]
- 51.Ilkovitch D, Ostrand-Rosenberg S. MHC class II and CD80 tumor cell-based vaccines are potent activators of type 1 CD4+ T lymphocytes provided they do not coexpress invariant chain. Cancer Immunol. Immunother. 2004;53:525–532. doi: 10.1007/s00262-003-0486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qi L, Rojas JM, Ostrand-Rosenberg S. Tumor cells present MHC class II-restricted nuclear and mitochondrial antigens and are the predominant antigen presenting cells in vivo. J Immunol. 2000;165:5451–5461. doi: 10.4049/jimmunol.165.10.5451. [DOI] [PubMed] [Google Scholar]
- 53.Thompson JA, Srivastava MK, Bosch JJ, Clements VK, Ksander BR, Ostrand-Rosenberg S. The absence of invariant chain in MHC II cancer vaccines enhances the activation of tumor-reactive type 1 CD4(+) T lymphocytes. Cancer Immunol. Immunother. 2007 Aug 28; doi: 10.1007/s00262-007-0381-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson CA, Petzold SJ, Unanue ER. Identification of two distinct properties of class II major histocompatibility complex-associated peptides. Proc. Natl. Acad. Sci. USA. 1993;90:1227–1231. doi: 10.1073/pnas.90.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]


