Abstract
Adoptive T-cell immunotherapy combined with non-myeloablative lymphodepletion has emerged as the most effective immunotherapy treatment for patients with metastatic melanoma (objective response rates of 50%). The mechanisms underlying this major advance in the field of immunotherapy include the elimination of regulatory elements and increased access to activating cytokines. This results in the activation of low-affinity T cells, enabling them to destroy tumors. We propose that a more complete depletion of the patient’s immune system followed by transplantation with hematopoietic stem cells, which can be genetically modified, would be more effective in the treatment of metastatic cancer.
Introduction
In the past decade, our understanding of antigen-specific tumor-cell recognition and destruction has grown greatly. Much of the recent work in the field of immunotherapy has concentrated on the development of active immunization using newly discovered antigens, but the results in animal and human trials have disappointed many of the most active advocates of cancer vaccines [1]. Prophylactic vaccines for infectious diseases are highly effective, but vaccines are not currently therapeutic in the treatment of established infectious disease, such as HIV, chronic hepatitis, tuberculosis or influenza. Similarly, therapeutic vaccines for the treatment of solid tumors of non-hematopoietic cell origin have not yet been effective, although the reasons for this remain in the realm of speculation. It is plausible that the current anti-tumor vaccines do not sufficiently activate tumor-reactive T cells to a state in which they are capable of tumor destruction. Regulatory elements and poor access to activating cytokines and co-stimulatory molecules might control the activities of anti-tumor T cells in a host with established cancer. Additionally, tumor antigens are poor ‘targets’ because they are often self-antigens with low-affinity epitopes. In many cases, these epitopes are undefined [2]. Thus, tumor vaccines might fail to generate appropriate numbers of highly activated T cells that are capable of mediating a therapeutic response in patients with established metastatic tumors.
The most promising current approach for the treatment of metastatic melanoma in immunotherapy is adoptive T-cell transfer (ACT), which is given to lymphodepleted patients (see also the review by W Overwijk, in this themed issue; [3]) [1,4,5]. In ACT, autologous tumor-infiltrating lymphocytes (TILs), are extracted, expanded ex vivo and re-administered into the patient where they specifically destroy antigen-expressing tumor cells. Lymphodepletion can create an environment in the patient where the T cells against low affinity epitopes — whether of defined or unknown specificity — can be activated sufficiently to destroy tumor cells [4]. Autologous tumor-reactive T cells transfused into a lymphodepleted patient experience great exposure to activating cytokines, they are sensitized to recognize low affinity antigens and are less susceptible to suppression by regulatory elements.
This review will discuss the reasons why partial lymphodepletion in combination with ACT can be effective, and why complete ablation with hematopoietic stem cell transplant might be even more beneficial in adoptive immunotherapy.
T-cell activation in a lymphopenic host
In animal models, homeostatic expansion results in the activation of T cells. Naïve T cells transferred into a lymphopenic host undergo homeostatic proliferation and thereby acquire a memory phenotype, as manifested by the expression of memory T-cell markers (high CD44, Ly6VC and CD122) [6–9]. This proliferation is accompanied by T-cell activation, as demonstrated by enhanced in ex vivo IFN-γ release and cytolysis [6,8,9]. In vivo, T-cell activation in the lymphodepleted setting is revealed by the inhibition of tumor growth when polyclonal autologous T cells are transfused into sub-lethally irradiated mice [10]. King et al. [11] found that lymphopenia triggered autoimmunity through homeostatic proliferation of T cells in nonobese diabetic mice (NOD) and proposed that IL-21 was a homeostatic cytokine of importance in the development of activated T cells in a lymphopenic host [11]. In the lymphocytic choriomeningitis virus (LCMV) model, a significant reduction of the viral titers was achieved by transferring LCMV TCR-specific CD8+ cells into a lymphodepleted host. [12] These examples demonstrate the therapeutic potential of T cells undergoing homeostatic proliferation and concomitant activation, even in the absence of antigen-specific vaccination.
Partial ablation enhances success in immunotherapy
Recent work in our laboratory has revealed that partial lymphodepletion can significantly augment the anti-tumor efficiency of transferred T cells when combined with a tumor-antigen-specific vaccination. Mice with large B16 tumor burden were treated with transgenic T cells (called pmel-1) specific for the self and tumor antigen gp100 given in combination with IL-2 and virus expressing a modified form of gp100. This tripartite treatment resulted in significant tumor reduction [13]. Non-myeloablative lymphodepletion greatly enhanced the tumor treatment effect (L Gattinoni et al., unpublished).
In melanoma patients the most dramatic treatment response was achieved in an ACT therapy protocol combined with partial ablation via chemotherapy. Autologous T cells were expanded ex vivo and transfused into a lymphodepleted patient. The objective clinical response rate exceeded 50% in partially ablated patients, using strict World Health Organization (WHO) criteria in a group of heavily pretreated (surgery, chemotherapy, radiotherapy and other biological therapies) patients with stage IV melanoma. An additional 30% of patients experienced partial or mixed responses [4,5]. Thus, homeostatic proliferation expands and activates autologous polyclonal anti-tumor T cells regardless of their epitope specificity and differentiation stage.
The importance of T-cell receptors in homeostatic proliferation
The signal strength needed to trigger proliferation and activation of T cells in a lymphodepleted host is considerably reduced — even low-affinity antigens such as self-antigens become sufficient triggering signals [6,8,9,14–18]. Although controversy remains, some authors have even claimed that homeostatic proliferation might occur in the absence of MHC presentation [19,20]. Most anti-tumor T cells target self-antigens for which they have low affinities. The lymphopenic environment facilitates the activation and proliferation of these poorly reactive T cells. Nevertheless, there is evidence that homeostatic proliferation of T cells is regulated by clonal competition and is dependent on the avidity of the self-peptide–MHC complex [21–23]. Clones that can expand most rapidly dominate the replete T-cell repertoire, whereas the other clones die out [24]. Consistent with these findings, it has also been reported that naïve monoclonal T cells expand in number when transferred into a TCR transgenic host of differing clonotype, but do not expand in a host of identical clonotype [25,26].
This is of special interest for adoptive T-cell immunotherapists because the long-term survival of tumor-reactive clones is desired to protect from tumor recurrence. Whether there exists a special phenotype or certain tumor-specific epitopes that might help for long-term survival remains an interesting field of research. Adoptive transfer after lymphodepletion in patients with objective responses leads to the persistent skewing of the T-cell repertoire towards tumor-reactive T cells [27•]. An adult patient has strongly reduced thymic output and therefore the repleting T cells are mostly from transferred T cells or the few remaining, non-depleted endogenous T cells. This steers the patient to tumor immunity through tumor-reactive T cells and to autoimmunity against self or tumor antigens [4].
The role of co-stimulatory molecules and cytokines in homeostatic expansion
At least two signals — TCR stimulation and a co-stimulatory signal — are required for proliferation and activation of T cells. Homeostatic T-cell proliferation differs from antigen-driven proliferation with respect to its requirements for co-stimulatory molecules: neither the CD28–B7 interaction nor the CD40–CD40L interaction [16,28] are required for homeostatic expansion. Although 4-1BB is involved in the allogeneic T-cell response, it is not required for homeostatic proliferation [29]. The only co-stimulatory signal that was recently shown to be necessary for optimal homeostatic proliferation of CD4+ and CD8+ cells was through CD24 [30]. The findings described above suggest that signals through co-stimulatory molecules are less critical for T-cell activation in a lymphodepleted setting.
By contrast, key cytokines (especially IL-7 and IL-15) are crucial for the homeostatic proliferation of naïve and memory T cells. IL-7 is required for the homeostatic expansion of naïve and mature T cells, where IL-7 initiates T-cell proliferation [31–35], as well as for the survival of naïve T cells in a replete host [31,33]. Additionally, exogenously administered IL-7 can promote antigen-independent proliferation of T cells in a lymphoreplete host [36,37•,38]. Conversely, IL-7 receptor blocking leads to a decrease in T-cell numbers [39,40]. Thus, low levels of IL-7 mediate T-cell survival, whereas higher levels of IL-7 induce proliferation. Higher levels of IL-7 can be achieved by exogenous administration of IL-7 or, in lymphodepleted settings, by increased cytokine availability through decreased competition for endogenously produced IL-7.
Similar to IL-7, IL-15 is a cytokine that signals through the common γ-chain (γC, also called CD132). Although IL-15 is not required for in vivo expansion of naïve T cells, memory CD8+ T cells clearly benefit from IL-15 signals [33,34,41,42]. In our own model of adoptively transferred T cells in the treatment of large, established tumors (pmel-1), we found that the absence of IL-15 in the lymphodepleted state significantly compromised tumor treatment [43• •]. Another common γC cytokine, IL-21, is a candidate homeostatic factor, and IL-21 receptor knockout mice have impaired CD8+ expansion and cytotoxicity [44•]. These observations suggest that ACT therapy benefits not only from the homeostatic cytokine accumulation that occurs in a lymphopenic host but also from exogenous administration of cytokines such as IL-7, IL-15 and IL-21. These findings are indeed what recent experiments in our laboratory have revealed — each of these cytokines given alone can augment ACT therapy in the pmel-1 mouse system, even more so when given in combination [44•].
The impact of lymphodepletion on CD4+CD25+ T regulatory cells
As described above, low (basal) levels of homeostatic cytokines in a lymphoreplete host prevent the activation and proliferation of self- or tumor-reactive T cells. Another mechanism that also protects the host from self-or tumor-reactive T cells is the activity of CD4+CD25+ T regulatory cells (Tregs) [45–47]. Depletion of Tregs augments tumor and autoimmunity, whereas the adoptive transfer of Tregs suppress anti-tumor T-cell responses as well as autoimmunity in a variety of models [48]. Treg cells are also likely to be important in the anti-tumor immune response in humans [49••,50••,51–53].
It seems, therefore, that the enhanced recognition of low-affinity antigens by T cells in lymphopenic hosts is triggered by higher levels of cytokines and by reduced inhibitory elements leading to the proliferation and activation of self- or tumor-specific T cells.
Complete ablation, ACT and HSC transfer: a way to fight tumors expressing unknown antigens?
Low-affinity tumor antigens are poorly recognized in a lymphoreplete host that possesses strong regulatory elements and low basal levels of homeostatic cytokines. Under these ost conditions, adoptively transferred tumor-reactive T cells can only have anti-tumor activity under the influence of a strong ‘danger’ stimulus in vivo, such as a modified epitope ligand expressed by a recombinant viral vector [2,13]. In a partially ablated host (5 Grey, total body irradiation, non-myeloablative), regulatory elements are greatly reduced and cytokine levels are greatly increased. These conditions help favor T-cell reactivity to low-affinity self- or tumor antigens, but a subset of host cells survives partial ablation, and functions as regulatory elements and ‘sinks’ for activating homeostatic cytokines (CA Klebanoff et al., unpublished; see Update). In our own pmel-1 model, vaccination is still required under the condition of partial ablation for the treatment of large established tumors (L Gattinoni et al., unpublished).
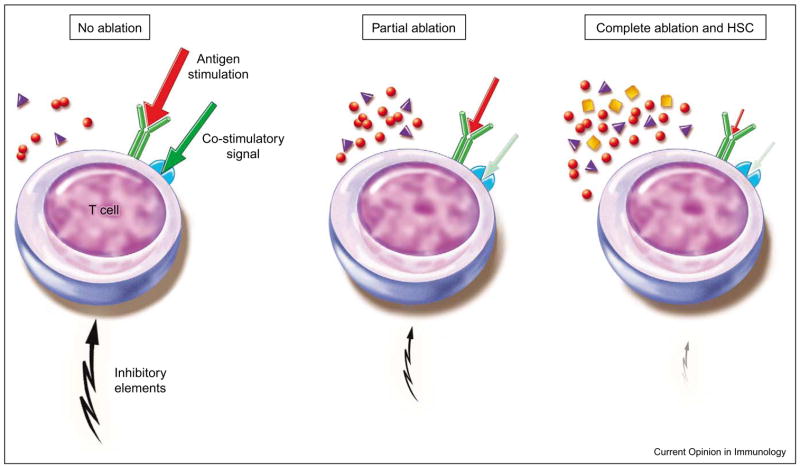
Complete ablation (9 Grey, total body irradiation, myeloablative) further eradicates elements that suppress tumor-reactive transferred T cells and is likely to further reduce host cells that consume homeostatic cytokines (Figure 1). Complete ablation also harms hematopoietic stem cells (HSCs) in the bone marrow, thus requiring HSC reconstitution [54]. These cells proliferate strongly and differentiate into the cellular elements that reconstitute the host. HSCs might also have an impact on the remaining host cell population as well as on co-administered adoptively transferred T cells.
Figure 1.
In a lymphoreplete host, low basal cytokine levels and strong inhibitory elements are present. Therefore, strong antigen stimulation combined with a co-stimulatory signal is needed to activate T cells. Partial ablation reduces regulatory elements as well as other cytokine consuming host cells and leads to elevated cytokine levels. Low affinity antigens are sufficient to induce T-cell activation even with weaker co-stimulatory signals. In complete ablation with hematopoietic stem cell (HSC) transplants, host cells are further diminished and additional factors are released or induced by, for example, HSCs, cytokine levels are presumably even more elevated. Therefore, the need for co-stimulatory signals and T-cell receptor stimulation might be further reduced.
Collectively, complete ablation together with HSC transfer reduces regulatory elements and elevates cytokine levels to such an extent that transferred T cells gain the ability to recognize self- or tumor-reactive antigens (Figure 2), and the need for an additional antigen-over-expressing vaccine is virtually eliminated (C Wrzesinski et al., unpublished). As tumor-reactive T cells are mostly polyclonal and often of unknown epitope specificity, the elimination in vivo vaccination would be of profound importance to the clinic [1].
Figure 2.
Hematopoietic stems cells (HSCs) transferred into a completely ablated host have a high proliferative capacity. The factors required for this proliferation are provided by the ablated host or by the HSC themselves. These factors not only influence the HSCs but also impact on both the remaining host cells and adoptively transferred T cells, leading to their proliferation or survival. For tumor treatment, transferred tumor antigen-specific T cells need to be provided not only in sufficient numbers, but they must also be activated. In this lymphopenic environment, the mechanisms shown in Figure 1 could activate T cells. Alternatively, T cells transferred after in vitro activation maintain their state of activation in the lymphopenic environment.
Autologous transplant with gene-modified hematopoietic stem cells
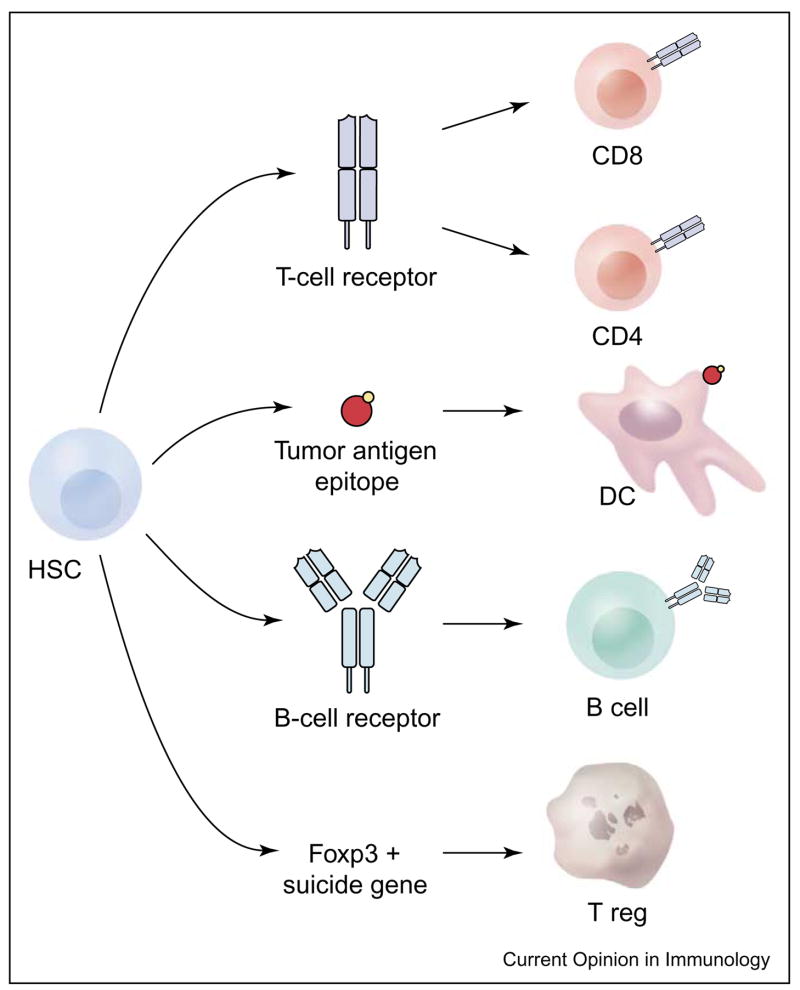
Post-transplantation, HSCs proliferate and differentiate into a variety of blood cell types, including T cells, B cells and dendritic cells, involved in the long-term immune response. Therefore, the modification of transferred HSCs could be exploited to maximize the therapeutic effect of any HSC-derived cell. By choosing an appropriate promoter, the modification could be limited to only a certain cell type (Figure 3).
Figure 3.
Hematopoietic stem cell (HSC) transplants in completely ablated patients give rise to a variety of different immunologically important blood cells. Several modifications of HSCs that are of therapeutic interest are plausible. By choosing an adequate promoter, transgene expression can be limited to a certain cell type. For example, the tumor-antigen-specific T or B cell receptors could be expressed in T or B cells, the genes encoding tumor antigens could be inserted into dendritic cells (DCs) or regulatory T cells could be eliminated through suicide genes expressed under the control of the T regulatory cell-specific Foxp3 promoter.
Dendritic cells derived from HSCs can be modified to express tumor-associated antigens in a stable and long-lasting manner. In combination with a systemic dendritic cell activating agent (anti-CD40) and mature T cells, antigen-specific T-cell expansion and activation was achieved and resulted in tumor treatment [55].
The induction of tumor-specific T cells through insertion of the T-cell receptor into HSCs is also promising. T cells with tumor specificity are rare and must be greatly expanded to achieve sufficient numbers for successful ACT therapy of metastatic tumors. This expansion process results in terminally differentiated T cells that have lost key phenotypic and functional characteristics, resulting in reduced anti-tumor activity (L Gattinoni et al., unpublished). Furthermore, the persistence of tumor-antigen-specific T cells correlates with their anti-tumor efficacy after adoptive transfer [27•]. The transduction of tumor-antigen-specific T-cell receptors into HSCs might lead to a high precursor frequency of tumor-reactive T cells as well as a lifelong persistence of these T cells, thus solving two critical problems in current ACT-based immunotherapies. Similar procedures with B-cell receptors could result in the production of monoclonal antibodies with desired specificity, such as a complement-fixing antibody with specificity for a tumor-associated antigen. T or B lymphocytes could also be modified to produce cytokines, co-stimulatory molecules or factors that block inhibition, controlled by suicide genes, to result in the constitutive activation of immunity.
Treg cells can diminish the effectiveness of the anti-tumor immune response, especially when that response is directed against self-antigens [56,57] (PA Antony et al., unpublished; see Update). Foxp3 is a trans-activating gene product that is exclusively expressed by Treg cells [58]. A suicide gene under the control of the Foxp3 promoter could be introduced into HSCs, which could then be transferred into a completely ablated host. Activation of this suicide gene would result in the complete depletion of Tregs in the patient directly before immunotherapy.
Finally, the endothelial cells that comprise the neo-vasculature of tumors are substantially derived from HSCs. Suicide genes have been directly delivered into the tumor neo-vasculature using lentiviral modified HSCs. This reportedly resulted in tumor killing followed by slower tumor growth [55].
Conclusions
Lymphodepletion before the adoptive transfer of anti-tumor T cells is the largest single advance in tumor immunotherapy in a decade. Chemotherapy in humans and total body irradiation in mice has been used primarily to induce non-myeloablative lymphodepletion. In mice, we have found that total ablation enhances ACT therapy significantly more than partial ablation. In humans, partial ablation is associated with an objective response rate of approximately 50%. A more complete lymphodepletion with autologous HSC transplant might further augment current ACT-based therapies resulting in a higher response rate in patients with metastatic cancer.
Update
A recently published paper shows for the first time that CD4+ CD25− T cells (T helper cells) co-transferred with tumor-reactive CD8+ T cells help to break the tolerance to tumor self antigens in an IL-2-dependent mechanism, but only in the absence of CD4+ CD25+ T cells (T regulatory cells) [59• •]. Therefore, for an optimal tumor treatment with adoptive T-cell transfer the absence of inhibitory effects of T regulatory cells is required.
Acknowledgments
The work referred to in the text as (CA Klebanoff et al., unpublished) has now been published [60].
Footnotes
This review comes from a themed issue on Tumour immunology
Edited by Rienk Offringa
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
• • of outstanding interest
- 1.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu Z, Theoret MR, Touloukian CE, Surman DR, Garman SC, Feigenbaum L, Baxter TK, Baker BM, Restifo NP. Poor immunogenicity of a self/tumor antigen derives from peptide-MHC-I instability and is independent of tolerance. J Clin Invest. 2004;114:551–559. doi: 10.1172/JCI21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overwijk WW. Breaking tolerance in cancer immunotherapy: time to ACT. Curr Opin Immunol. 2005;17 doi: 10.1016/j.coi.2005.01.011. in press. [DOI] [PubMed] [Google Scholar]
- 4.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14639–14645. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 7.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc Natl Acad Sci USA. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 12.Oehen S, Brduscha-Riem K. Naive cytotoxic T lymphocytes spontaneously acquire effector function in lymphocytopenic recipients: A pitfall for T cell memory studies? Eur J Immunol. 1999;29:608–614. doi: 10.1002/(SICI)1521-4141(199902)29:02<608::AID-IMMU608>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 13.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viret C, Wong FS, Janeway CA., Jr Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- 15.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 17.Bender J, Mitchell T, Kappler J, Marrack P. CD4+ T cell division in irradiated mice requires peptides distinct from those responsible for thymic selection. J Exp Med. 1999;190:367–374. doi: 10.1084/jem.190.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge Q, Rao VP, Cho BK, Eisen HN, Chen J. Dependence of lymphopenia-induced T cell proliferation on the abundance of peptide/MHC epitopes and strength of their interaction with T cell receptors. Proc Natl Acad Sci USA. 2001;98:1728–1733. doi: 10.1073/pnas.98.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke SR, Rudensky AY. Survival and homeostatic proliferation of naive peripheral CD4+ T cells in the absence of self peptide:MHC complexes. J Immunol. 2000;165:2458–2464. doi: 10.4049/jimmunol.165.5.2458. [DOI] [PubMed] [Google Scholar]
- 20.Dorfman JR, Stefanova I, Yasutomo K, Germain RN. CD4+ T cell survival is not directly linked to self-MHC-induced TCR signaling. Nat Immunol. 2000;1:329–335. doi: 10.1038/79783. [DOI] [PubMed] [Google Scholar]
- 21.Kassiotis G, Zamoyska R, Stockinger B. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J Exp Med. 2003;197:1007–1016. doi: 10.1084/jem.20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol. 2004;172:40–44. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- 23.Min B, Foucras G, Meier-Schellersheim M, Paul WE. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proc Natl Acad Sci USA. 2004;101:3874–3879. doi: 10.1073/pnas.0400606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Gruta NL, Driel IR, Gleeson PA. Peripheral T cell expansion in lymphopenic mice results in a restricted T cell repertoire. Eur J Immunol. 2000;30:3380–3386. doi: 10.1002/1521-4141(2000012)30:12<3380::AID-IMMU3380>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 25.Troy AE, Shen H. Cutting edge: homeostatic proliferation of peripheral T lymphocytes is regulated by clonal competition. J Immunol. 2003;170:672–676. doi: 10.4049/jimmunol.170.2.672. [DOI] [PubMed] [Google Scholar]
- 26.Moses CT, Thorstenson KM, Jameson SC, Khoruts A. Competition for self ligands restrains homeostatic proliferation of naive CD4 T cells. Proc Natl Acad Sci USA. 2003;100:1185–1190. doi: 10.1073/pnas.0334572100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr, Rosenberg SA. Cutting Edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. Lymphodepletion increases the persistence of adoptively transferred T cells. This paper furthermore shows a strong correlation between the rate of persistence of adoptively transferred T cells and induction of objective responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prlic M, Blazar BR, Khoruts A, Zell T, Jameson SC. Homeostatic expansion occurs independently of costimulatory signals. J Immunol. 2001;167:5664–5668. doi: 10.4049/jimmunol.167.10.5664. [DOI] [PubMed] [Google Scholar]
- 29.Blazar BR, Kwon BS, Panoskaltsis-Mortari A, Kwak KB, Peschon JJ, Taylor PA. Ligation of 4-1BB (CDw137) regulates graft-versus-host disease, graft-versus-leukemia, and graft rejection in allogeneic bone marrow transplant recipients. J Immunol. 2001;166:3174–3183. doi: 10.4049/jimmunol.166.5.3174. [DOI] [PubMed] [Google Scholar]
- 30.Li O, Zheng P, Liu Y. CD24 expression on T cells is required for optimal T cell proliferation in lymphopenic host. J Exp Med. 2004;200:1083–1089. doi: 10.1084/jem.20040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 32.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 33.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seddon B, Zamoyska R. TCR and IL-7 receptor signals can operate independently or synergize to promote lymphopenia-induced expansion of naive T cells. J Immunol. 2002;169:3752–3759. doi: 10.4049/jimmunol.169.7.3752. [DOI] [PubMed] [Google Scholar]
- 36.Komschlies KL, Gregorio TA, Gruys ME, Back TC, Faltynek CR, Wiltrout RH. Administration of recombinant human IL-7 to mice alters the composition of B-lineage cells and T cell subsets, enhances T cell function, and induces regression of established metastases. J Immunol. 1994;152:5776–5784. [PubMed] [Google Scholar]
- 37•.Moniuszko M, Fry T, Tsai WP, Morre M, Assouline B, Cortez P, Lewis MG, Cairns S, Mackall C, Franchini G. Recombinant interleukin-7 induces proliferation of naive macaque CD4+ and CD8+ T cells in vivo. J Virol. 2004;78:9740–9749. doi: 10.1128/JVI.78.18.9740-9749.2004. This suggests that the application of IL-7 together with adoptively transferred tumor-reactive T cells in a lymphopenic host can augment tumor treatment potential. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geiselhart LA, Humphries CA, Gregorio TA, Mou S, Subleski J, Komschlies KL. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J Immunol. 2001;166:3019–3027. doi: 10.4049/jimmunol.166.5.3019. [DOI] [PubMed] [Google Scholar]
- 39.Vivien L, Benoist C, Mathis D. T lymphocytes need IL-7 but not IL-4 or IL-6 to survive in vivo. Int Immunol. 2001;13:763–768. doi: 10.1093/intimm/13.6.763. [DOI] [PubMed] [Google Scholar]
- 40.Boursalian TE, Bottomly K. Survival of naive CD4 T cells: roles of restricting versus selecting MHC class II and cytokine milieu. J Immunol. 1999;162:3795–3801. [PubMed] [Google Scholar]
- 41.Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, Homann D. IL-7 regulates basal homeostatic proliferation of antiviral CD4+T cell memory. Proc Natl Acad Sci USA. 2004;101:9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. The application of IL-15 as supportive cytokine in ACT could circumvent negative effects of the IL-2, which has been used to date, in a similar way to the induction of regulatory T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Zeng R, Spolski R, Finkelstein SE, Oh D, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T-cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. The recently discovered cytokine IL-21 is thought to be a homeostatic cytokine. In this paper, authors show that IL-21 in combination with IL-15 promotes proliferation of naïve and memory CD8+ cells, and can augment ACT when administered in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32:3267–3275. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 48.Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diaz de Cerio A, Melero I, Prieto J, Borras-Cuesta F, Lasarte JJ. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171:5931–5939. doi: 10.4049/jimmunol.171.11.5931. [DOI] [PubMed] [Google Scholar]
- 49••.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. In this paper the authors showed for the first time that CD4+ CD25+ T cells can be generated from tumor-infiltrating lymphocytes (TILs) in cancer patients. TILs showed suppressor activity and therefore might substantially inhibit T-cell anti-tumor action at the tumor site. [DOI] [PubMed] [Google Scholar]
- 50••.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. Human tumor CD4+ CD25+Foxp3+ Treg cells from ovarian cancer patients suppress tumor-specific T-cell immunity, influence tumor growth and are associated with reduced survival. [DOI] [PubMed] [Google Scholar]
- 51.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 52.Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 53.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 54.Lorenz E, Uphoff D, Reid TR, Shelton E. Modification of irradiation injury in mice and guinea pigs by bone marrow injections. J Natl Cancer Inst. 1951;12:197–201. [PubMed] [Google Scholar]
- 55.Cui Y, Kelleher E, Straley E, Fuchs E, Gorski K, Levitsky H, Borrello I, Civin CI, Schoenberger SP, Cheng L, et al. Immunotherapy of established tumors using bone marrow transplantation with antigen gene–modified hematopoietic stem cells. Nat Med. 2003;9:952–958. doi: 10.1038/nm882. [DOI] [PubMed] [Google Scholar]
- 56.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 58.Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889–899. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 59••.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005 doi: 10.4049/jimmunol.174.5.2591. in press. This paper shows for the first time that CD4+ CD25− T cells (T helper cells) co-transferred with tumor reactive CD8+ T cells help to break the tolerance to tumor self antigens in an IL-2-dependent mechanism, but only in the absence of CD4+CD25+ T cells (T regulatory cells). Therefore, for an optimal tumor treatment with adoptive T cell transfer the absence of inhibitory effects of T regulatory cells is required. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]