Abstract
Chromosome length in Drosophila is maintained by targeted transposition of three non-long terminal repeat (non-LTR) retrotransposons, HeT-A, TART and TAHRE, to the chromosome ends. The length and composition of these retrotransposon arrays can vary significantly between chromosome tips and between fly stocks, but the significance and consequences of these length differences are not understood. A dominant genetic factor, Tel, has been described that causes a several-fold elongation of the retrotransposon arrays at all telomeres. We used this strain to assess possible affects of extended telomeres on the organism. While we found no effect on life span of the adults, we could demonstrate a correlation between long telomeres and reduced fertility and fecundity in individual females, which is also reflected in abnormal oocyte development.
Introduction
DNA sequences at the very ends of linear eukaryotic chromosomes are the products of telomere elongation. In most eukaryotes, these sequences are simple repeating units that are synthesized by telomerase, but in Drosophila melanogaster they consist of tandem head-to-tail arrays of telomere-specific non-long terminal repeat (non-LTR) retrotransposons with their oligo(A) tails facing towards the centromere (Mason and Biessmann 1995).
A model for terminal elongation by retrotransposition in D. melanogaster, based on analogy with other retroelements, has recently been described in some detail (Walter and Biessmann 2004; Pardue et al. 2005). This model proposes that transcripts originating from the telomeric retrotransposon array leave the nucleus to serve as mRNA for the translation of the element-encoded polypeptide(s). The HeT-A–encoded GAG-like protein, which contains three nuclear localization signals, may bind RNA and facilitate entry of transposon nucleic acid into the nucleus where it forms distinct foci (Rashkova et al. 2002a; Rashkova et al. 2002b). After docking to a chromosome end, perhaps mediated by a protein-protein interaction between the GAG-protein and the terminal end-binding complex, a reverse transcriptase might use the free 3’ hydroxyl group at the chromosome end as primer to copy the RNA intermediate into the first DNA strand. The processes regulating transcription and transposition are still unknown.
Thus, chromosome length in Drosophila is maintained by targeted transposition of three non-LTR retrotransposons, HeT-A, TART and TAHRE, to the chromosome ends. In addition, telomeres may also accumulate retrotransposon sequences by terminal gene conversion (Kahn et al. 2000). Consequently, natural chromosome ends in Drosophila consist of long tandem arrays of these retroelements (Mason and Biessmann 1995; Pardue and DeBaryshe 2003; Abad et al. 2004). The length and composition of these HeT-A/TART/TAHRE (abbreviated HTT) arrays can vary significantly between chromosome tips and between fly stocks (Walter et al. 1995; Biessmann et al. 1997; Melnikova and Georgiev 2002; Siriaco et al. 2002; Abad et al. 2004), but the significance and consequences of these length differences are not well understood.
Two dominant genetic factors have recently been described that cause a several-fold elongation of the HTT arrays at all telomeres, Telomere elongation, Tel (Siriaco et al. 2002) and Enhancer of terminal gene conversion, E(tc) (Melnikova and Georgiev 2002). Both have been mapped genetically to the same region on the right arm of chromosome 3, which raises the possibility that they might be allelic. To date, nothing is known about the affected gene(s) that cause this “long telomere” phenotype. Other genetic conditions can also cause elongated telomeres in Drosophila; for instance, heterozygous mutations in Su(var)205, the gene encoding the HP1 capping protein (Savitsky et al. 2002; Perrini et al. 2004).
Here we investigated whether the extended HTT arrays at telomeres in the Tel strain are associated with any detectable and measurable phenotype. We found that extended HTT arrays do not cause any changes in life span of the adults, but they significantly reduce fecundity and fertility of the females. Moreover, chromosomes with longer telomeres are also transmitted less efficiently though the female germ line than those with shorter telomeres. In ovaries of Tel or heterozygous Su(var)20502 females, both having extended HTT arrays, abnormally developing egg chambers are detectable that resemble those found in females that are heterozygous for the gene proliferation disruptor (prod). The prod gene encodes a 346-amino-acid protein that localizes strongly to the centric heterochromatin of the second and third chromosomes as well as to >400 euchromatic sites (Török et al. 1997). Homozygous prod null mutations are late larval lethal and exhibit a decreased mitotic index, defects in anaphase chromatid separation and incomplete chromosome condensation. Heterozygous prod flies do not show extended HTT arrays, but HeT-A transcript levels are significantly elevated in ovaries suggesting that the PROD protein may act as a transcriptional repressor of HeT-A (Török et al, submitted). Since we have recently discovered that the PROD protein binds to HeT-A at all telomeres (Török et al, submitted), we propose that the increased copies of HeT-A elements at the telomeres of Tel and Su(var)20502 females could bind more PROD protein, thus titrating it from its centromeric location. Such re-distribution may cause the observed effects on ovarian development and consequently on female fecundity and fertility.
Materials and methods
Drosophila stocks and crosses
All Drosophila stocks were maintained at 25°C on a standard yeasted corn meal-molasses medium. The Gaiano III (GIII) stock carries the third chromosome with the Tel mutation in an Oregon R background (Siriaco et al. 2002). The prodk8810 stock is described in Török et al. (1997) and was obtained from the Bloomington stock center. Su(var)20502 is mutation in the HP1 protein (Eissenberg et al. 1990) that is required for telomere capping (Fanti et al. 1998). It also was obtained from the Bloomington stock center.
For longevity assays 80 to 100 males and females were aged together at 25°C. Every five days flies were transferred into fresh vials and dead flies were counted until less than 10 flies were alive in the majority of the vials. These experiments were repeated 6 times and the results were pooled.
Fecundity assays were performed as described previously (Clare and Luckinbill 1985). In a first experiment, virgin females and males from GIII or Oregon R were kept separately for two days and then mated in single pairs. They were transferred into fresh vials every second day for seven days and eggs were counted after each transfer under a stereomicroscope. To determine fertility, the number of pupae developing from all eggs was counted. In a second experiment, 100 virgin females of GIII were aged for two days and then mated individually with two day-old single Oregon R males. Single pairs were transferred into fresh vials every second day for five days and eggs were counted after each transfer.
From this experiment, fifteen GIII females were chosen for further analysis according to the number of eggs they had lain. DNA was extracted from these fifteen females for determination of HeT-A copy number by qPCR (see below) and their F1 offspring were collected. Six individuals (male and female) were picked at random from the F1 generation for DNA extraction and qPCR. After mating inter se, the F3 generation was sampled again by picking ten virgin females from each line and crossing them individually to Oregon R males. Fecundity and fertility were determined as above, and after five days of egg-laying, ovaries were dissected from the females for DAPI staining and microscopy, and DNA was extracted from the carcasses for qPCR.
To test for nondisjunction, Gaiano III and Oregon R females, 2–4 days old, were crossed individually to two y v/BS Y y+ males. After three days the parents were transferred to fresh food for an additional four days, then discarded. Progeny were counted several times between day 12 and day 17 for each vial. To account for the loss of progeny aneuploid for the X chromosome, the frequency of nondisjunction was calculated as 2(B females + y v males)/(total progeny + B females + y v males).
DNA extraction and quantitative Real-Time-PCR (qPCR)
DNA was extracted as described previously (Török et al., submitted) from single flies or the bodies of females from which the ovaries had been dissected. A BioRad iCycler iQ™ Real-Time PCR cycler (BioRad, Hercules, CA) was used with SYBR Green as detection reagent. Reactions were performed in 96-well microtiter plates in 20 µl volumes containing 10 µl iQ SYBR Green Supermix (BioRad, Hercules, CA), 40 ng genomic DNA and 200 nM of each primer. Reactions were done using the following program: 3 min at 95°C followed by 40 cycles with 10 sec at 95°C and 45 sec at 50°C. A melting curve analysis was done at the end of each reaction to ensure quality of the amplified product. The competitive threshold cycle method was used to quantitate the amount of each telomeric retrotransposon. In every microtiter plate, a control curve was generated with each primer pair, and each sample point was done in at least 3 replicates. The determined values were averaged and normalized with the RpS17 copy number in every DNA preparation.
Primers
Matching primer sets with Tm of 59–62°C (synthesized by Sigma-Proligo., Boulder, CO) were designed for each retrotransposon and for the ribosomal protein gene S17 (RpS17) to give fragments from cDNA of 100 to 200 bp. Primers were checked individually by pairwise BlastN searches against all four retrotransposons and genomic DNA for specificity to their particular transposon, and functionally by PCR with genomic DNA for amplifying a single DNA fragment of the predicted size (195 bp for RpS17, 152 bp for HeT-9D4GAG-ORF, 169 bp for TAHRE-GAG-ORF, 181 bp for TART-GAG-RT-junc, and 124 bp for Jockey-GAG-RT-junc). GenBank accession numbers of Sequences used to design primers are X68130 for HeT-A element 9D4, AJ542581 for TAHRE, AY561850 for TART-A1, M22874 for jockey and M22142 for RpS17.
RpS17-F: 5’AAGCGCATCTGCGAGGAG3’
RpS17-R: 5’CCTCCTCCTGCAACTTGATG3’
HeT-9D4GAG-ORF-F: 5’TTGTCTTCTCCTCCGTCCACC3’
HeT-9D4GAG-ORF-R: 5’GAGCTGAGATTTTTCTCTATGCTACTG3’
TAHRE-GAG-ORF-F: 5’CTTCCCCTCCGCTCTCATC3’
TAHRE-GAG-ORF-R: 5’CCTAGATCTGCATTTGTATTAGTAGCTG3’
TART-GAG-RT junc-F: 5’CAAAAAATCCTTTCCGAGATCC3’
TART-GAG-RT junc-R: 5’GGGCATCAATATTTAGAATGAACAG3’
Jockey-GAG-RT-junc-F: 5’ACGACTCAATCTAGGGCTCGTG3’
Jockey-GAG-RT-junc-R: CGTCCATTCTCGTATTGATGG3’
Ovary staining and microscopy
Ovaries from 5 day-old females were dissected in Ringer solution and fixed in 4% formaldehyde in PBS for 2 hrs, washed in PBS and stained briefly with 0.1 mg/ml DAPI. Stained ovaries were observed in a Zeiss Axioskop epifluorescence microscope equipped with a 63x objective and a Photometrics Sensys cooled CCD camera.
In situ hybridization and staining of polytene chromosomes with anti-PROD antibody
Fluorescence in situ hybridization (FISH) with HeT-A DNA fragments to polytene chromosomes was done as described (Golubovsky et al. 2001). The 5.3 kb EcoRI fragment of the HeT-A element 9D4 was labeled with digoxigenin by random priming and hybridization was detected by FITC-conjugated anti-dig Fab fragments (Roche, Indianapolis, IN). Chromosomes were counterstained with propidium iodide. Staining of polytene chromosomes with anti-PROD antibody was done as described recently (Török et al., submitted). Briefly, salivary glands of 3rd instar larvae were dissected in 45% acetic acid and fixed on slides. After blocking in 2% BSA, 2% non-fat dry milk powder and 0.2% Tween in PBS for 1 hour, chromosomes were incubated form 1 hour with purified rabbit-anti-PROD antibody diluted 1:1000 in blocking solution (Török et al., 1997). Slides were washed twice for 15 minutes in 1 × PBS containing 300 mM NaCl and 0.2% Tween, rinsed in PBS and after 30 minutes blocking, incubated for 1 hour with Alexa Fluor488-conjugated Goat anti-rabbit secondary antibody, diluted 1:500 (Molecular Probes). Slides were rinsed in PBS, followed by rinsing in 100 mM Tris (p.H.7.5) and staining with DAPI.
Results
Copy number of telomeric retrotransposons in Gaiano III carrying Tel
In order to eliminate most effects due to genetic background, we used the strain Gaiano-III (GIII) (Siriaco et al. 2002), which contained all Oregon R chromosomes except for chromosome 3 from the original Gaiano strain that contained the dominant Tel mutation. The GIII stock has been maintained for more than 100 generations, which caused elongation of the terminal HTT arrays on all chromosomes. We confirmed that our GIII strain had elongated HTT arrays by determining the copy numbers of HeT-A, TAHRE and TART in the genome by quantitative PCR (qPCR) with DNA using the Oregon R wild type strain as reference. Primer sets were designed that are specific to the GAG-ORF of HeT-A and TAHRE and to the GAG-RT junctions of TART and jockey (Fig. 1A). Ct values were normalized in each case to those obtained for the ribosomal protein gene S17 (RpS17) in each DNA sample to eliminate differences due to DNA concentration. The GIII strain showed a clear elevation in copy number of the three telomeric retrotransposons (Fig. 1B), with a 6-fold increase of HeT-A, a 5-fold increase in TAHRE elements, and a 4-fold increase in TART elements over Oregon R. The non-telomeric retrotransposon jockey, which is the closest relative to HeT-A (Biessmann et al. 1992) was used as a control. jockey displayed only a very slight increase in copy number in GIII compared to Oregon R. Our values for HeT-A are consistent with those determined by different techniques by Siriaco et al. 2002, who estimated an approximately 7-fold increase of overall telomere length in GIII.
Figure 1.
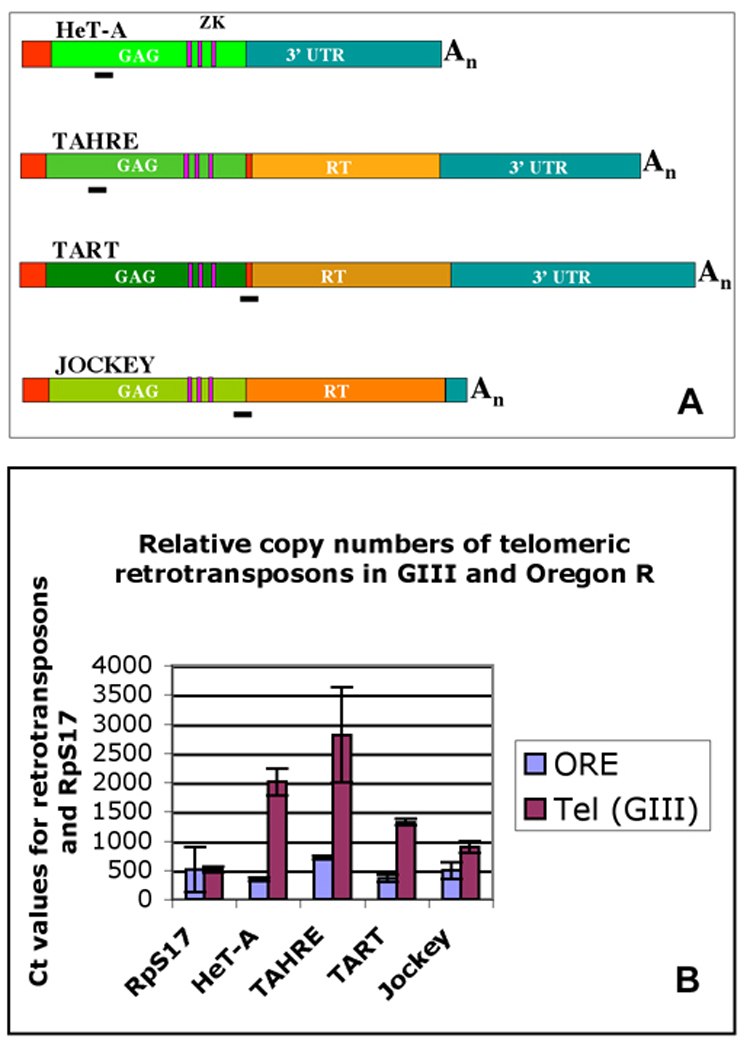
(A) Schematic map of three telomeric retrotransposons and jockey with the positions of fragments that were specifically amplified in qPCR to determine genomic copy numbers of each element. (B) Relative copy numbers (expressed as Ct values from qPCR) of telomeric retrotransposons and jockey in the GIII and Oregon R strains with respect to the copy number of the ribosomal protein gene S17 (RpS17).
As determined previously (Siriaco et al. 2002), our GIII strain showed frequent associations of telomeres of polytene chromosomes. These telomere-telomere associations are often connected by bridges, which are labeled by HeT-A probes (Fig. 2A–C). Telomere associations also occur among diploid chromosomes, and their frequency is up to ~70-fold increased over Oregon R (Siriaco et al. 2002). Fig. 2D shows an immunohistochemical staining of a Tel polytene chromosome squash with anti-PROD antibody, documenting intense labeling of the telomeres as well as the bridge connecting the telomeres.
Figure 2.
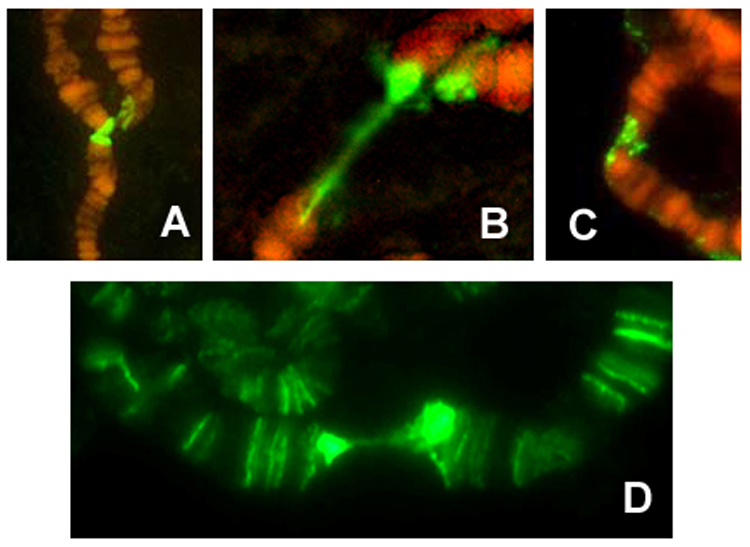
(A–C) Fluorescence in situ hybridizations (FISH) with a HeT-A probe to the bridged telomere-telomere association in polytene salivary chromosomes of GIII flies. (D) Immunofluorescence staining of a Tel polytene chromosome squash with anti-PROD antibody, showing association of the PROD protein with the telomeres and the bridges that connect the frequent telomere associations in this strain.
Effect of telomere length on life span, fecundity and fertility
To determine whether the elongated telomeres in the GIII strain had any effect on life span we tested survival of six independent sets of 100 flies for survival on yeasted corn meal-molasses fly food at 25°C. Oregon R, Canton S and y w laboratory stocks served as references. We could not detect any significant difference in survival between GIII and Oregon R flies (Fig. 3).
Figure 3.
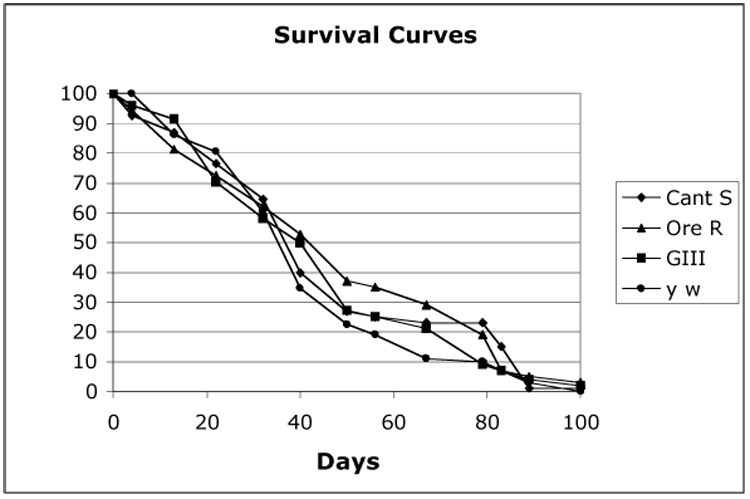
Survival curves of GIII, Oregon R, Canton S and y w flies at 25°C.
Forty single pair crosses with GIII virgin females and GIII males were used to determine whether the GIII strain exhibited a change in fecundity or fertility. The total number of eggs laid over a period of seven days and the total number of pupae and adults that developed from these eggs were counted (Fig. 4A). Oregon R pairs were used as controls (Fig. 4B). Our results showed significant variation in fecundity and fertility among GIII pairs compared to Oregon R pairs. Moreover, the fecundity of GIII was only 50% of Oregon R and fertility was 20% (Fig. 4C). These data suggested a potential negative influence of telomere length on reproduction and development in Drosophila.
Figure 4.
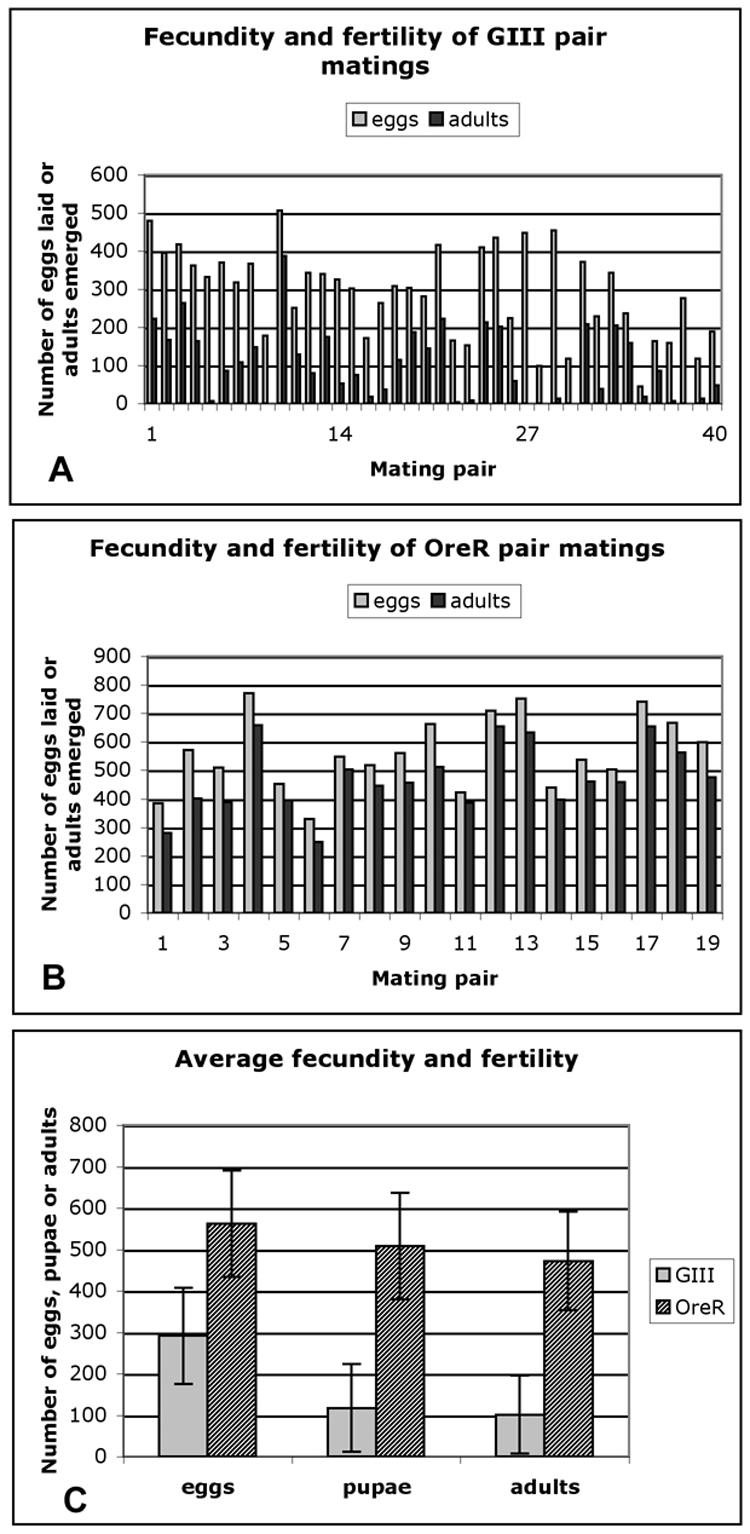
(A) Fecundity and fertility of forty GIII single pair matings; (B) Fecundity and fertility of twenty Oregon R single pair matings; (C) Average of fecundity and fertility of GIII and Oregon R pairs.
To further investigate a possible correlation between telomere length and fertility, we set up 100 single pair crosses between individual GIII virgin females and Oregon R males and determined fecundity and fertility as above. As before, a wide variation in fecundity was observed. From these 100 pairs (generation G0), fifteen females were selected that represented the highest and lowest levels of fecundity. After they had laid eggs for five days, the HeT-A copy number in their genomes was determined by qPCR. When relative telomere length (expressed as HeT-A copy number normalized to RpS17) was compared to fecundity and fertility (Fig. 5A), it became obvious that GIII females with shorter telomeres had laid significantly more eggs and produced more pupal progeny. Plotting the HeT-A/RpS17 ratio against the number of pupae produced by a single GIII female (Fig. 5B), we found that GIII females with short telomeres (# 28, 25, 41, 4 and 8, having ratios of HeT-A/RpS17 <2.25) produced between 150 and 220 pupae. By contrast, females with long telomeres (# 11, 22, 16, 61, 30, 17, 3, 2, 47 and 15, having ratios of HeT-A/RpS17 >3.8) produced only between 0 and 80 pupae. The discrepancy between fecundity and fertility is surprisingly high in this group with long telomeres, suggesting that many eggs did not develop into adults. The lethal phase is in the embryo as many eggs did not hatch. DAPI staining of unhatched embryos revealed that developmental arrest occurs during the early syncytial nuclear division cycles (not shown).
Figure 5.
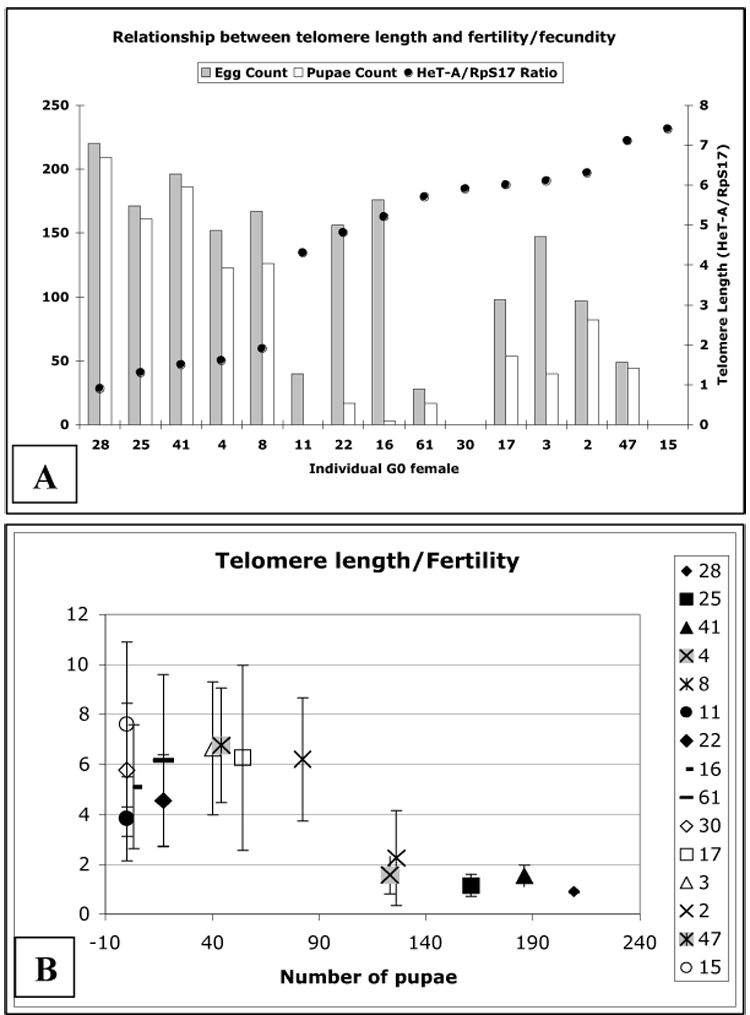
(A) Comparison of telomere length (black dots) (expressed as ratio of genomic copy number of HeT-A to that of the RpS17 gene) to fecundity and fertility in the G0 generation of 15 selected single GIII females crossed to Oregon R males. The designation of individual founder females (lines) is shown on the X-axis and the data are arranged according to increasing telomere length. (B) Correlation of telomere length with fertility in 15 different GIII females. Their numerical designations are the same as in (A) and are shown in the legend.
We then determined telomere length in the offspring of selected pairs of the above matings. Six flies were picked at random from the F1 generation of founder females #25 and 8 (short telomeres) and #2, 61 and 17 (long telomeres) and HeT-A copy number was determined by qPCR. Since the HeT-A/RpS17 ratio had been determined from each of the founder females (Fig. 5), and the average HeT-A/RpS17 ratio as determined from six individual Oregon R flies was 0.6±0.24, we can predict a HeT-A/RpS17 ratio for the F1 flies assuming an equal transmission of all maternal and paternal chromosomes. These predicted telomere lengths are compared with the determined values in Table 1. While the predicted and determined telomere lengths are consistent for the F1s from founder females with short telomeres (#25 and 8), the measured F1 telomere lengths from founder females with long telomeres (#61, 2 and 17) are significantly shorter than predicted. We interpret these results as an indication that chromosomes with long telomeres may be transmitted less efficiently through the germ line than chromosomes with short telomeres.
Table 1.
Predicted and observed telomere lengths in the F1 generation of single pair crosses between GIII founder females and Oregon R males
| G0 founder female | HeT-A/RpS17 ratio in G0 founder female | HeT-A/RpS17 ratio in F1 individuals (predicted) | HeT-A/RpS17 ratio in F1 individuals (determined) | P-value |
|---|---|---|---|---|
| 25 | 1.16±0.45 | 0.88±0.22 | 1.12±0.78 | 0.497 |
| 8 | 2.25±1.90 | 1.42±0.95 | 2.56±0.94 | 0.115 |
| 61 | 6.14±3.45 | 3.37±1.72 | 1.72±1.36 | 0.046 |
| 2 | 6.21±2.46 | 3.40±1.23 | 2.00±0.64 | 0.013 |
| 17 | 6.25±3.70 | 3.43±1.85 | 1.50±0.76 | 0.007 |
Meiosis and gametogenesis are very different in the two sexes of D. melanogaster, as no synaptonemal complex is formed and no recombination occurs during spermiogenesis. To ask whether long telomeres also affect transmission of chromosomes through the male germ line, fecundity and fertility were determined in side-by-side comparisons of fifteen single-pair matings of GIII females with Oregon R males and of GIII males with Oregon R females (Fig. 6A). Compared to Oregon R pairs, both reciprocal crosses showed about a fifty percent reduced fecundity and fertility, indicating that longer telomeres affect the female as well as the male germ line.
Figure 6.
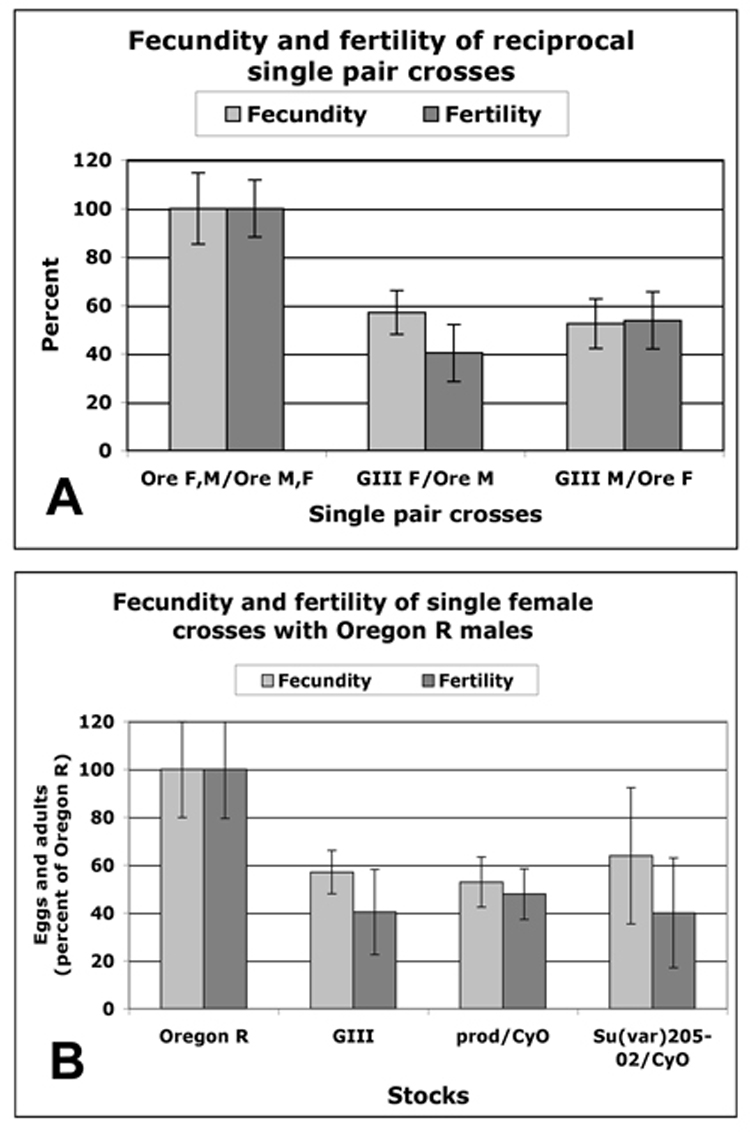
(A) Average fecundity and fertility of fifteen single pair matings between Oregon R pairs (first pair of columns), between GIII females and Oregon R males (second pair of columns) and between GIII males and Oregon R females (third pair of columns), indicating that longer telomeres affect the male as well as the female germ line. (B) Average fecundity and fertility of ten single females (as percent of Oregon R pairs) of GIII, prod/CyO and Su(var)20502/CyO single females crossed individually to Oregon R males. Error bars show STD.
To test the hypothesis that extended HTT arrays are responsible for the observed drop in fecundity and to eliminate potential interference of genetic background, we examined another strain with elongated telomeres caused by the mutation Su(var)20502. HeT-A copy number in heterozygous Su(var)20502/CyO flies was increased to about the same level as in GIII, 11-fold over Oregon R (Török et al, submitted). In a side-by-side experiment we then compared fecundity and fertility of ten females each of Oregon R, GIII, Su(var)20502/CyO and prod/CyO, which does not have extended telomeres (Török et al, submitted) in crosses with single Oregon R males. All three types of mutant females showed 53–64% fecundity and 40–47% fertility of that of Oregon R pairs (Fig. 6B).
Females with longer telomeres show defects in ovarian development
After two more generations of mass mating, the F3 generation of the GIII crosses was sampled. Ten F3 virgin females from each GIII founder line #25, 4 and 8 (short telomeres, see Fig. 5) and from founder females # 61, 2, 17 and 47 (long telomeres, see Fig. 5) were crossed individually to Oregon R males. As in G0, eggs and adult offspring were recorded for five days. From those females that produced the highest and lowest number of eggs, ovaries were dissected for microscopy and DNA was isolated from the carcasses for telomere length determination. Again, we plotted the HeT-A/RpS17 ratios of each F3 female and the number of her eggs and adult offspring (Fig. 7). As in G0, a spread of telomere lengths was observed, and in general the females with the longer telomeres exhibited a lower fecundity and fertility than those with shorter telomeres.
Figure 7.
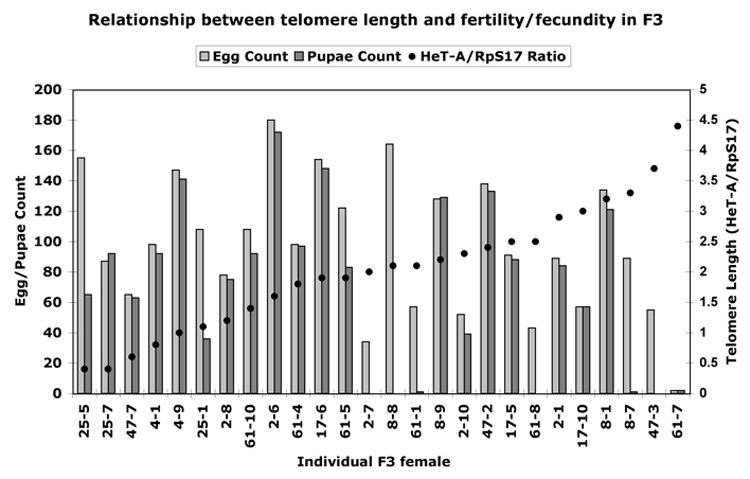
Correlation of telomere length (black dots, as ratio of genomic copy number of HeT-A to that of ribosomal protein gene S17) to fecundity and fertility in the G3 generation of selected single GIII females crossed to Oregon R males. In the numbering of individuals on the X-axis, the first number designates the original founder female (line), the second number the individual F3 female analyzed from this line. Females are arranged according to increasing telomere length.
This sampling allowed us to analyze the ovaries from individual females with known telomere length and known fertility. We concentrated on the F3 generation of the founder female 61, because it showed a range of different telomere lengths and fecundity (see Fig. 7). Representative DAPI-stained ovaries are shown in Fig. 8. F3 GIII females derived from the original 61 founder female with shorter HTT arrays (e.g. 61–4) exhibited relatively high fecundity (Fig. 7) and contained normal appearing ovaries (Fig. 8 B). By contrast, female 61-7 with long HTT arrays and very low fecundity (Fig. 7) had abnormal ovaries (Fig. 8 C), showing a degeneration of nurse cell nuclei (Fig. 8 G) with their DNA irregularly condensed in aggregates at the nuclear periphery (Fig. 8 K–M). Consequently, oocyst development mostly arrested at stage 9–10 and very few, if any, mature oocytes were produced (Fig. 8 C, G). The same phenotype was observed in other F3 females with long HTT arrays, and often these females also had a reduced number of ovarioles in one or both of their ovaries, resulting in one ovary being smaller than the other (not shown). To investigate whether the extended HTT arrays in these GIII females were responsible for the observed ovarian degeneration and the reduction in fecundity, we examined heterozygous Su(var)20502/CyO females, in which HeT-A copy number was increased 11-fold over Oregon R and fecundity and fertility were reduced by about 50% (Fig. 6). DAPI-stained ovaries of Su(var)20502/CyO females (Fig. 8 D) revealed the same developmental abnormalities as GIII females with long telomeres with a very similar degeneration phenotype of nurse cell nuclei (Fig. 8 H). A third comparison was made with prod/CyO females, which do not have extended telomeres, but exhibited an about 50% reduced fecundity and fertility (Fig. 6). In these prod/CyO females (Fig. 8 E) we observed a very similar ovarian phenotype as in GIII and Su(var)20502/CyO females including the degeneration of nurse cell nuclei and arrest at ~stage 9 (Fig. 8 I).
Figure 8.
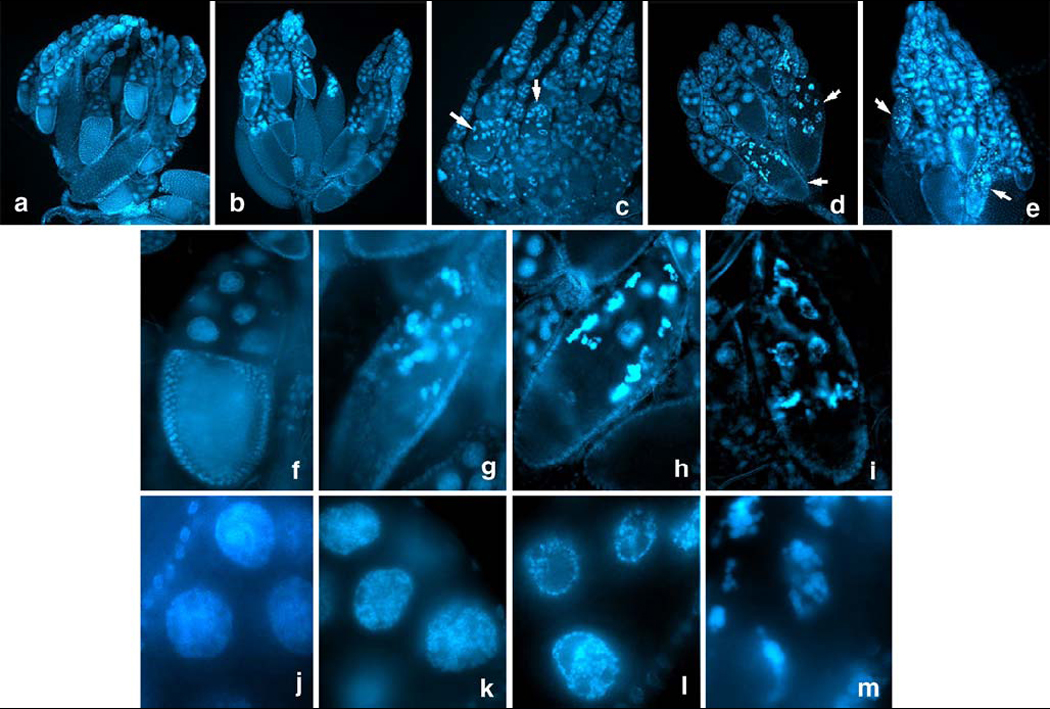
DAPI-stained ovaries dissected from Oregon R as control (A), and from selected females in generation 3 of the original GIII founder female 61 (B, C). Female 61-4 with short HTT arrays and high fecundity and fertility (see Fig. 7) contained normal appearing ovaries (B), while female 61-7 with long HTT arrays and very low fecundity and fertility (Fig. 7) had abnormal ovaries (C) characterized by degeneration of nurse cell nuclei with their DNA irregularly condensed. Ovaries of Su(var)20502/Cy females with long telomeres (D) and prod/Cy females (E), which do not have extended telomeres, revealed similar developmental abnormalities as GIII females with long telomeres (C). Arrows in C–E point to abnormal oocysts, which mostly arrested in stage 9–10. DAPI-stained normal oocysts of Oregon R, stage 9–10, are shown in (F). Abnormal oocysts of the GIII female 61-7 with long HTT arrays (G), of Su(var)20502/Cy females with long telomeres (H) and of prod/Cy females (I) reveal a very similar degeneration phenotype of nurse cell nuclei. Nurse cell nuclei of Oregon R females (J) are contrasted with nurse cell nuclei of GIII females at progressive stages of DNA condensation and aggregation at the nuclear periphery (K–M).
After these ovarian phenotypes had been established, we used them to investigate if they could be linked to the Tel locus, which had previously been mapped by meiotic recombination to region 69 on chromosome 3 between the markers sr and e (Siriaco et al. 2002). Ovaries from ten females each of the 20 different recombinant lines were used to investigate their ovarian phenotypes. Ovaries were stained with DAPI and scored for the abnormalities described above. Results showed a perfect correlation in each recombinant of the ovarian phenotype with the telomere elongation phenotype observed after 50 generations (Siriaco et al. 2002), indicating the ovarian phenotype can be mapped to the same genetic region as the Tel locus.
The reduction in female fertility could be the result of chromosome malsegregation at meiosis; we therefore measured the frequency of X chromosome nondisjunction in Gaiano III females. As both Gaiano III and Oregon R females have normal sequence unmarked X chromosomes, we crossed females to y v/BS Y y+ males. Normal chromosome segregation produced + females and BS males. Meiotic nondisjunction of the X chromosomes in these females produced BS females and y v males. Chromosome loss may also contribute to the recovery of y v males. There was no increase in X chromosome nondisjunction frequency in Gaiano III females compared with the control (Table 2).
Table 2.
X chromosome nondisjunction frequencies in Gaiano III and Oregon R females crossed to y v/BS Y y+ males.
| Progeny |
|||||
|---|---|---|---|---|---|
| Mother | + females | B males | B females | y v males | % Nondisjunctiona |
| Gaiano III | 4209 | 3713 | 2 | 0 | 0.05 |
| Oregon R | 3585 | 3399 | 2 | 2 | 0.10 |
The percent nondisjunction is calculated as 2(Bar females + y v males)/(total + Bar females + y v males). This allows for the fact that half of the exceptional progeny die because of aneuploidy for the X chromosomes.
Discussion
Telomere length and life span
Abnormal length of telomeric repeats has profound influences on cell proliferation of vertebrate cells in culture and in vivo. Many studies have demonstrated a correlation between shortened or otherwise disturbed telomeres to cellular senescence (Harley 2002). In vertebrates and yeast cells, critically short telomeres become unprotected and activate cell cycle checkpoints causing cell cycle arrest and “crisis”. Thus, defects in telomerase RNA knockout mice are first detectable in highly proliferating tissue such as the germ line (Lee et al. 1998). Studies addressing whether shortened or extended telomeres cause any advantage or disadvantage for the organism as a whole have shown that older, telomerase deficient mice with critically short telomeres exhibit decreased viability associated with diminished proliferative capacity of B and T cells (Herrera et al. 1999). However, while these telomerase-null mice had a shortened life span and a reduced ability to respond to stress, they did not show the typical symptoms of aging (Rudolph et al. 1999). Thus, telomere length is a predictor of the number of cell divisions remaining in differentiated tissues that lack telomerase, but in non-mutant organisms there is little evidence that telomere length correlates with life span, considering also that mouse telomeres are significantly longer that human telomeres (Greider 1996; Wright and Shay 2000). Other factors, such as oxidative stress, may play a more significant role in aging and reducing organismal life span than telomere erosion (Aviv et al. 2003; Kenyon 2005).
In organisms that use telomerase the length of the telomeric repeats is kept within certain limits and is controlled by many factors that regulate telomere structure and accessibility of the chromosome end to telomerase (Chan and Blackburn 2002; Vega et al. 2003; Wei and Price 2003). Disturbing this balance by mutating or over-expressing a telomere protein may lead to either elongation or shrinking of the telomere repeat array in yeast and human cells (Kyrion et al. 1992; Greenwell et al. 1995; Krauskopf and Blackburn 1996; Cooper et al. 1997; Smogorzewska et al. 2000). While shortened telomeres cause genome instability and cell cycle arrest, very few studies have investigated the effects of elongated telomeres. Telomere length can be increased in the macronucleus of Tetrahymena thermophila by growth of log phase cultures at 30°C (Larson et al. 1987). These cells grow slowly and are eventually overgrown by individuals carrying a mutation in the telomerase RNA gene resulting in shorter telomeres (Ahmed et al. 1998), suggesting that elongated telomeres have a negative effect on cell division rates.
The proposal that telomere length is associated with life span is debatable. Increasing telomere length by over-expression of the single-stranded telomeric DNA binding protein HRP-1 in Caenorhabditis elegans is said to increase life span by about 20% (Joeng et al. 2004), but another study demonstrated that life span in C. elegans is independent of telomere length (Raices et al. 2005).
Our results demonstrate that extended telomeres in the Drosophila GIII strain apparently have no effect on life span. It has been shown before that the wild type strain Canton S has lower survival rates than Oregon R, at least at 29°C (Tower 1996). However, since the GIII stock contains all chromosomes from Oregon R and only chromosome 3, carrying the Tel mutation, from the original Gaiano strain (Siriaco et al. 2002), background effects may have been mostly eliminated.
Telomere length and fecundity/fertility
We observed significant effects on female fecundity and fertility correlated with telomere length. Females with longer HTT arrays laid fewer eggs than their sisters with shorter HTT arrays. The average telomere length in Oregon R wild type flies, as determined by retrotransposon copy number of HeT-A, TART and TAHRE, is roughly 7 times shorter than in GIII flies and does not differ much between individuals. By contrast, telomere lengths are highly variable among GIII individuals, ranging from sizes that are about the same as in Oregon R and up to 8-fold longer. Because of the great variation, we did not determine the overall length distribution in the GIII strain, rather we selected 15 individuals with high or low fecundity from the original 100 single-pair matings for telomere length determinations. Our data show that these two groups exhibited corresponding telomere lengths that fell in two distinct clusters. There may be females with intermediate telomere lengths in the GIII stock associated with intermediate fecundity, representing a continuum, but these were not analyzed here.
Fertility of GIII females was also reduced because a significant number of eggs from females with longer telomeres did not develop, suggesting that elongated HTT arrays not only affect the germ line but also embryonic development.
As in G0, F3 females with the longer telomeres exhibited a lower fecundity and fertility than those with shorter telomeres. However, the telomere lengths of the F3 progeny of founder females exhibit a high range of variability and the correlation with fecundity is not as good as in the G0 crosses. The reason lies probably in the fact that the flies were propagated by crossing inter se, which results re-distribution in the population of chromosomes with long (from GIII) and others with short telomeres (from Oregon R). Due to the selection against long telomeres, the F3 generation does not provide an as good representation for flies with long telomeres as in G0. The correlation with fecundity is further compounded by the fact that determining HeT-A copy number by qPCR can only provide an average telomere length in a given fly, but is unable to distinguish between chromosomes with long and chromosomes with short telomeres. Thus, the presence of one chromosome with very long telomeres in a background of chromosomes with short telomeres may not affect overall HeT-A copy number, but may have a significant effect on fecundity.
Reciprocal crosses showed the same reduction in fecundity in GIII males and females, indicating that longer telomeres also affect spermiogenesis. Since meiosis and gametogenesis are very different in the two sexes, no synaptonemal complex is formed and no recombination occurs during spermiogenesis, this result suggests that the extended telomeres may affect the premeiotic divisions of the germ cells.
The observed correlation between telomere length and fecundity in Drosophila is opposite of that observed in mice, where reduced fecundity is caused by critically short telomeres that might engage in telomere fusions and trigger cell cycle arrest and apoptosis (Lee et al. 1998). To understand this difference, one needs to recall the specific features of the Drosophila telomere (Biessmann et al. 2005). Importantly, protection of the chromosome end from terminal fusions in Drosophila is a sequence-independent function that is mediated by specific proteins (Cenci et al. 2005), and even terminally broken chromosomes that lack any terminal retrotransposons may be protected by this capping complex (Fanti et al. 1998). Thus, in contrast to telomerase-synthesized telomeres in other eukaryotes, which depend on a minimal length and the proper association with special telomere-repeat binding proteins, some of which are needed to form a t-loop structure that ensures end protection (Griffith et al. 1999), Drosophila telomeres probably do not depend on secondary structure for end protection (Biessmann et al. 2005), although the possibility of telomeric loops involving the HTT array have been discussed (Cenci et al. 2005; Melnikova and Georgiev 2005). We, therefore, conclude that extended HTT arrays in Drosophila are properly capped and that the observed lower fecundity and fertility levels are not due to capping defects.
To further support the idea that elongated telomeres and not genetic background are responsible for the reduced fecundity and fertility, we examined Su(var)20502/CyO flies, in which HeT-A copy number was increased to about the same level as in GIII (11-fold over Oregon R), and found an about 50% reduction in fecundity and fertility, suggesting that the elevated HeT-A copy number in both strains is likely to be the cause.
How then could longer telomeres in Drosophila reduce fecundity and fertility? One possibility is that the high rates of telomere associations observed in the GIII strain (Siriaco et al. 2002) might somehow interfere with pre-meiotic or meiotic cell divisions in the germ line, thus preventing proper development of the oocysts. We find this possibility unlikely, because the telomere-telomere associations in GIII are dissolved during mitosis and do not form anaphase bridges (Siriaco et al. 2002). Another hypothesis, however, is quite attractive. We have recently reported that the protein product of the prod locus binds to HeT-A elements at telomeres (Török et al, submitted). Prod null mutations exhibit proliferation disorders in imaginal discs and cause late larval lethality (Török et al. 1997). While these phenotypes can be attributed to PROD protein localization at the centric heterochromatin, its role at the euchromatic sites and at the telomeres is unknown. We show here that prod is not entirely recessive, as heterozygous prod/CyO females exhibit significantly reduced fecundity and fertility, suggesting that reducing overall levels of PROD affects egg development. The ovarian defects of heterozygous prod/CyO females are similar to those observed in GIII and Su(var)20502/CyO females with long telomeres. It is, therefore tempting to speculate that the increased copies of HeT-A at the telomeres of GIII and Su(var)20502 females could bind more PROD protein, thus titrating it from its centromeric location. Such re-distribution is apparently not lethal but may cause the observed effects on ovarian development and consequently on female fecundity and fertility described here.
Acknowledgements
This work was partially supported by the U.S. Public Health Service grant GM-56729 to H.B., by the Hungarian National Science Foundation OTKA no. T 037422 to T.T. and in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. We thank Chris LePhong and Diana Le for excellent lab work and Matt Roper for valuable suggestions. C.B. was a participant in the AGEP Summer Research Program at UCI.
References
- Abad JP, de Pablos B, Osoegawa K, de Jong PJ, Martin-Gallardo A, Villasante A. Genomic analysis of Drosophila melanogaster telomeres: full-length copies of HeT-A and TART elements at the telomeres. Mol. Biol. Evol. 2004;21:1613–1619. doi: 10.1093/molbev/msh174. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Sheng H, Niu LM, Henderson E. Tetrahymena mutants with short telomeres. Genetics. 1998;150:643–650. doi: 10.1093/genetics/150.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Levy D, Mangel M. Growth, telomere dynamics and successful and unsuccessful human aging. Mech. Ageing. Dev. 2003;124:829–837. doi: 10.1016/s0047-6374(03)00143-x. [DOI] [PubMed] [Google Scholar]
- Biessmann H, Champion LE, O'Hair M, Ikenaga K, Kasravi B, Mason JM. Frequent transpositions of Drosophila melanogaster HeT-A transposable elements to receding chromosome ends. EMBO J. 1992;11:4459–4469. doi: 10.1002/j.1460-2075.1992.tb05547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessmann H, Prasad S, Walter MF, Mason JM. Euchromatic and heterochromatic domains at Drosophila telomeres. Biochem. Cell. Biol. 2005;83:477–485. doi: 10.1139/o05-053. [DOI] [PubMed] [Google Scholar]
- Biessmann H, Walter MF, Mason JM. Drosophila telomere elongation. Ciba Found Symp. 1997;211:53–67. doi: 10.1002/9780470515433.ch5. [DOI] [PubMed] [Google Scholar]
- Cenci G, Ciapponi L, Gatti M. The mechanism of telomere protection: a comparison between Drosophila and humans. Chromosoma. 2005;114:135–145. doi: 10.1007/s00412-005-0005-9. [DOI] [PubMed] [Google Scholar]
- Chan SW, Blackburn EH. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene. 2002;21:553–563. doi: 10.1038/sj.onc.1205082. [DOI] [PubMed] [Google Scholar]
- Clare ML, Luckinbill LS. The effects of gene-environment interaction on the expression of longevity. Heredity. 1985;55:19–26. doi: 10.1038/hdy.1985.67. [DOI] [PubMed] [Google Scholar]
- Cooper JP, Nimmo ER, Allshire RC, Cech TR. Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature. 1997;385:744–747. doi: 10.1038/385744a0. [DOI] [PubMed] [Google Scholar]
- Eissenberg JC, James TC, Foster-Hartnett DM, Hartnett T, Ngan V, Elgin SC. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 1990;87:9923–9927. doi: 10.1073/pnas.87.24.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti L, Giovinazzo G, Berloco M, Pimpinelli S. The heterochromatin protein 1 prevents telomere fusions in Drosophila. Mol. Cell. 1998;2:527–538. doi: 10.1016/s1097-2765(00)80152-5. [DOI] [PubMed] [Google Scholar]
- Golubovsky MD, Konev AY, Walter MF, Biessmann H, Mason JM. Terminal retrotransposons activate a subtelomeric white transgene at the 2L telomere in Drosophila. Genetics. 2001;158:1111–1123. doi: 10.1093/genetics/158.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell PW, Kronmal SL, Porter SE, Gassenhuber J, Obermaier B, Petes TD. TEL1, a gene involved in controlling telomere length in S. cerevisiae, is homologous to the human ataxia telangiectasia gene. Cell. 1995;82:823–829. doi: 10.1016/0092-8674(95)90479-4. [DOI] [PubMed] [Google Scholar]
- Greider CW. Telomere length regulation. Annu. Rev. Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- Harley CB. Telomerase is not an oncogene. Oncogene. 2002;21:494–502. doi: 10.1038/sj.onc.1205076. [DOI] [PubMed] [Google Scholar]
- Herrera E, Samper E, Martin-Caballero J, Flores JM, Lee HW, Blasco MA. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 1999;18:2950–2960. doi: 10.1093/emboj/18.11.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joeng KS, Song EJ, Lee KJ, Lee J. Long lifespan in worms with long telomeric DNA. Nat. Genet. 2004;36:607–611. doi: 10.1038/ng1356. [DOI] [PubMed] [Google Scholar]
- Kahn T, Savitsky M, Georgiev P. Attachment of HeT-A sequences to chromosomal termini in Drosophila melanogaster may occur by different mechanisms. Mol. Cell. Biol. 2000;20:7634–7642. doi: 10.1128/mcb.20.20.7634-7642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Krauskopf A, Blackburn EH. Control of telomere growth by interactions of RAP1 with the most distal telomeric repeats. Nature. 1996;383:354–357. doi: 10.1038/383354a0. [DOI] [PubMed] [Google Scholar]
- Kyrion G, Boakye KA, Lustig AJ. C-Terminal truncation of RAP1 results in the deregulation of telomere size, stability, and function in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992;12:5159–5173. doi: 10.1128/mcb.12.11.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson DD, Spangler EA, Blackburn EH. Dynamics of telomere length variation in Tetrahymena thermophila. Cell. 1987;50:477–483. doi: 10.1016/0092-8674(87)90501-0. [DOI] [PubMed] [Google Scholar]
- Lee HW, Blasco MA, Gottlieb GJ, Horner JW, Greider CW, Depinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- Mason JM, Biessmann H. The unusual telomeres of Drosophila. Trends Genet. 1995;11:58–62. doi: 10.1016/s0168-9525(00)88998-2. [DOI] [PubMed] [Google Scholar]
- Melnikova L, Georgiev P. Enhancer of terminal gene conversion, a new mutation in Drosophila melanogaster that induces telomere elongation by gene conversion. Genetics. 2002;162:1301–1312. doi: 10.1093/genetics/162.3.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikova L, Georgiev P. Drosophila telomeres: the non-telomerase alternative. Chromosome Res. 2005;13:431–441. doi: 10.1007/s10577-005-0992-7. [DOI] [PubMed] [Google Scholar]
- Pardue ML, DeBaryshe PG. Retrotransposons provide an evolutionarily robust non-telomerase mechanism to maintain telomeres. Annual Review of Genetics. 2003;37:485–511. doi: 10.1146/annurev.genet.38.072902.093115. [DOI] [PubMed] [Google Scholar]
- Pardue ML, Rashkova S, Casacuberta E, DeBaryshe PG, George JA, Traverse KL. Two retrotransposons maintain telomeres in Drosophila. Chromosome Res. 2005;13:443–453. doi: 10.1007/s10577-005-0993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrini B, Piacentini L, Fanti L, Altieri F, Chichiarelli S, Berloco M, Turano C, Ferraro A, Pimpinelli S. HP1 controls telomere capping, telomere elongation, and telomere silencing by two different mechanisms in Drosophila. Mol. Cell. 2004;15:467–476. doi: 10.1016/j.molcel.2004.06.036. [DOI] [PubMed] [Google Scholar]
- Raices M, Maruyama H, Dillin A, Karlseder J. Uncoupling of longevity and telomere length in C. elegans. PLoS Genet. 2005;1:e30. doi: 10.1371/journal.pgen.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashkova S, Karam SE, Kellum R, Pardue ML. Gag proteins of the two Drosophila telomeric retrotransposons are targeted to chromosome ends. Journal of Cell Biology. 2002a;159:397–402. doi: 10.1083/jcb.200205039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashkova S, Karam SE, Pardue ML. Element-specific localization of Drosophila retrotransposon Gag proteins occurs in both nucleus and cytoplasm. Proc. Natl. Acad. Sci. USA. 2002b;99:3621–3626. doi: 10.1073/pnas.032071999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, Depinho RA. Longevity, stress response, and cancer in aging telomerase- deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Savitsky M, Kravchuk O, Melnikova L, Georgiev P. Heterochromatin protein 1 is involved in control of telomere elongation in Drosophila melanogaster. Mol. Cell. Biol. 2002;22:3204–3218. doi: 10.1128/MCB.22.9.3204-3218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriaco GM, Cenci G, Haoudi A, Champion LE, Zhou C, Gatti M, Mason JM. Telomere elongation (Tel), a new mutation in Drosophila melanogaster that produces long telomeres. Genetics. 2002;160:235–245. doi: 10.1093/genetics/160.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, de Lange T. Control of human telomere length by TRF1 and TRF2. Mol. Cell. Biol. 2000;20:1659–1668. doi: 10.1128/mcb.20.5.1659-1668.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török T, Harvie PD, Buratovich M, Bryant PJ. The product of proliferation disrupter is concentrated at centromeres and required for mitotic chromosome condensation and cell proliferation in Drosophila. Gene Dev. 1997;11:213–225. doi: 10.1101/gad.11.2.213. [DOI] [PubMed] [Google Scholar]
- Tower J. Aging mechanims in fruit flies. BioEssays. 1996;18:799–807. doi: 10.1002/bies.950181006. [DOI] [PubMed] [Google Scholar]
- Vega LR, Mateyak MK, Zakian VA. Getting to the end: Telomerase access in yeast and humans. Nature Reviews Molecular Cell Biology. 2003;4:948–959. doi: 10.1038/nrm1256. [DOI] [PubMed] [Google Scholar]
- Walter MF, Biessmann H. Expression of telomere retrotransposons, HeT-A and TART, in Drosophila melanogaster is correlated with cell proliferation. Dev. Genes Evol. 2004;214:211–219. doi: 10.1007/s00427-004-0400-x. [DOI] [PubMed] [Google Scholar]
- Walter MF, Jang C, Kasravi B, Donath J, Mechler BM, Mason JM, Biessmann H. DNA organization and polymorphism of a wild-type Drosophila telomere region. Chromosoma. 1995;104:229–241. doi: 10.1007/BF00352254. [DOI] [PubMed] [Google Scholar]
- Wei C, Price M. Protecting the terminus: t-loops and telomere end-binding proteins. Cellular and Molecular Life Sciences. 2003;60:2283–2294. doi: 10.1007/s00018-003-3244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WE, Shay JW. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nat. Med. 2000;6:849–851. doi: 10.1038/78592. [DOI] [PubMed] [Google Scholar]


