Abstract
Physiological functions of organic cation transporters (OCTs) in the placenta include transporting essential nutrients from the maternal to fetal circulations. OCTN2 transports carnitine with high affinity, and the transport of several drugs has also been shown to be mediated by this transporter. In this work, the role of phosphorylation and dephosphorylation mechanisms in regulating OCTN2 was investigated by observing the effects of various activators and inhibitors of kinases and phosphatases on the uptake of carnitine in BeWo cells, a human choriocarcinoma trophoblast cell line frequently used as an in vitro model of the rate-limiting barrier for maternal-fetal exchange. Preincubation with genistein resulted in significant increases in both alkaline phosphatase (ALP) activity and carnitine uptake. Levamisole, an ALP inhibitor, caused a more substantial decrease in carnitine uptake than expected from its corresponding decrease in ALP activity. It was determined that levamisole competitively inhibits carnitine uptake, with a Ki value of 1.01 ± 0.05 mM, and this effect has a greater role in decreasing carnitine uptake than any indirect effects of ALP inhibition upon OCTN2 function. Progesterone also competitively inhibited carnitine uptake (Ki = 48.6 ± 5.0 μM), but had no effect on ALP activity in BeWo cells.
Keywords: organic cation transporters, OCTN2, placenta, carnitine, phosphorylation, levamisole
1. Introduction
Understanding transport mechanisms in the placenta is essential to reduce the risks of fetal drug exposure. Transporters in the trophoblast cell layer of the human placenta serve to mediate the transfer of nutrients from mother to fetus, eliminate waste products, and limit the transplacental passage of certain substances. Physiological functions of organic cation transporters (OCTs) in the placenta include transporting essential nutrients such as carnitine from the maternal to fetal circulations, but certain drugs can also be transported by OCTs and thus interfere with nutrient exchange.
The organic cation transporter OCTN2, which is present in the placenta as well as in other tissues such as the kidney and brain, transports carnitine with high affinity in a sodium-dependent manner. Because the fetus cannot adequately supply itself with carnitine, placental transport of this nutrient is of high importance [1]. Low fetal concentrations can lead to carnitine deficiency in infants, symptoms of which include muscle weakness, cardiomyopathy, Reye's syndrome, hypoketotic hypoglycemia, and sudden infant death [2,3].
Regulation of drug transporters can serve as a way to control the extent of transporter-mediated drug influx or efflux. Knowledge of conditions that affect transporter function could forewarn pharmaceutical scientists of possible pharmacokinetic abnormalities. In theory, drugs that upregulate or downregulate specific transporters could be designed and administered to modulate the disposition of other drugs, but this concept does have some limitations [4].
The regulation of organic cation transport has been reported involving several phosphorylation pathways. The addition or subtraction of phosphate groups on proteins causes a change in local charge, which can affect the protein's conformation and biological activity. Ciarimboli et al. report that OCT1 activity decreased upon protein kinase A (PKA) activation and upon inhibition of calmodulin, calmodulin-dependent kinase II, and p56lck tyrosine kinase [5]. This same group also determined that OCT2 uptake is inhibited by phosphatidylinositol 3-kinase (PI3K) and PKA and activated by calmodulin-dependent signaling [6]. OCT3 regulation by phosphorylation/dephosphorylation mechanisms was investigated by Martel et al., who observed reduced MPP+ uptake in the presence of Ca2+/calmodulin pathway inhibitors, phosphodiesterase inhibitors, and an alkaline phosphatase (ALP) inhibitor [7]. PDZ domain-containing proteins have been shown to interact with the SLC superfamily, and shortly after Gisler et al. reported the interaction of PDZK1 with the C-terminal portion of OCTN1 [8], PDZK1 was shown to interact with the C-terminus of OCTN2 and regulate its function [9].
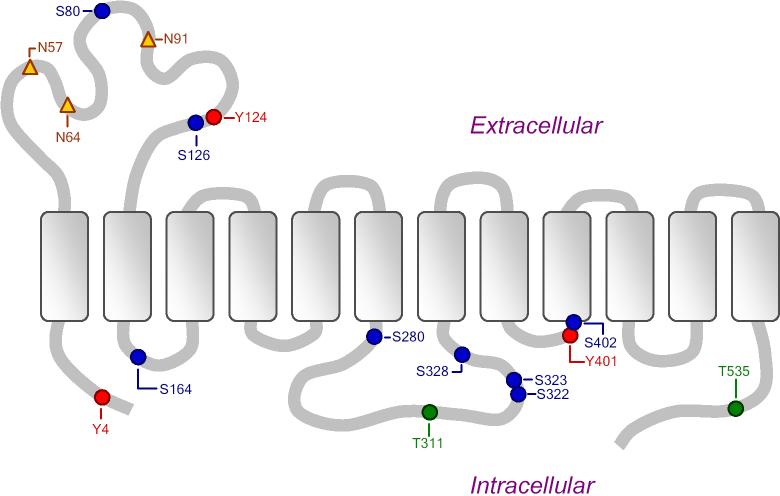
Figure 1 shows the predicted phosphorylation sites on the OCTN2 transporter protein, as predicted by NetPhos 2.0 [10] and ProSite Motif Search [11] software. Thirteen putative phosphorylation sites appear in this figure. These include predicted PKC sites at S80, S164, S280, and S322; a PKA/PKG site at S402; and a Casein kinase II phosphorylation site at T311 [12]. Three of the potential phosphorylation sites are on the extracellular loop between transmembrane domains 1 and 2.
Figure 1.
Putative phosphorylation sites (circles) and N-glycosylation sites (triangles) on transporter protein OCTN2, as predicted by NetPhos 2.0 and ProSite Motif Search software. Figure adapted from Lahjouji et al. Molecular Genetics and Metabolism 73 (2001) 287−297.
In this work, the role of phosphorylation/dephosphorylation in regulating OCTN2 was investigated by observing the effects of various activators and inhibitors of several kinases and phosphatases on the uptake of carnitine in BeWo cells, an in vitro trophoblast model of the rate-limiting barrier for maternal-fetal exchange within the placenta. OCTN2-mediated carnitine uptake was previously characterized in the BeWo cell line and shown to be saturable (Km = 9.8 ± 2.4 μM, Vmax = 800 ± 70 pmol/mg protein/30 min), with a non-saturable constant of 2.8 ± 0.3 μL/mg protein/30 min [13].
2. Materials and Methods
2.1 Cell Culture and Uptake Experiments
Protocols regarding the revival, maintenance, passage, and growth of BeWo cells for uptake studies were followed as provided in the literature [14]. Briefly, BeWo cells between passage numbers 31 and 49 were seeded in 12- or 24-well plates at a density of 12,500 cells/cm2 in Dulbecco's Modified Eagle's medium (DMEM) containing 10% fetal bovine serum, 1% penicillin-streptomycin, 1% glutamine, and 1% non-essential amino acids. The cells were incubated at 37°C under 5% CO2, 95% air, and saturated relative humidity for 4−6 days, at which point the cells were washed and equilibrated with warm HBSS. [3H]-L-carnitine (with or without inhibitors) was added to the cells after the specified preincubation times, and after the appointed duration of each uptake study, the carnitine solutions were removed by aspiration and the cells were washed three times with ice-cold HBSS and then lysed. The cell lysate was analyzed by liquid scintillation counting, and the protein content was determined using a kit from Pierce (Rockford, IL) with bovine serum albumin as the standard.
Carnitine uptake following preincubation and in the presence of various substances affecting phophatase and kinase activities was investigated in BeWo cells to assess the effects of phosphorylation in regulating OCTN2 function. Forskolin (PKA activator), phorbol 12-myristate 13-acetate (PMA, a PKC activator), 8-bromo-cGMP (PKG activator), orthovanadate (protein tyrosine phosphatase inhibitor), okadaic acid (protein serine/threonine phosphatase inhibitor), emodin (Casein kinase 2 inhibitor), genistein (protein tyrosine kinase inhibitor), 3-isobutyl-1-methylxantine (IBMX, a non-selective phosphodiesterase inhibitor), levamisole (ALP inhibitor), progesterone, L-leucine, and alkaline phophatase from bovine kidney were obtained from Sigma-Aldrich (St. Louis, MO). L-Phenylalanine was from Acros Organics (Fair Lawn, NJ). Forskolin, emodin, genistein, IBMX, and progesterone were solubilized in HBSS containing 1% ethanol (and compared to controls in 1% ethanol). PMA was solubilized in 0.5% DMSO (and compared to controls in 0.5% DMSO). Inhibitor concentrations and preincubation times closely resembled levels reported in the literature for similar work [15,16].
2.2 Alkaline Phosphatase Activity
The activity of ALP in BeWo cells and external cell membrane fragments was investigated by monitoring the conversion of 4-nitrophenylphosphate (Sigma, St. Louis) to 4-nitrophenol. The enzyme removes the phosphate group off of 4-nitrophenylphosphate, leaving the hydroxyl group. A solution of 4-nitrophenylphosphate in HBSS was added to the cells or membrane vesicles and after 60 minutes, a 450 μL sample of HBSS was diluted into 900 μL of 3 N NaOH to stop the reaction. Standards containing known concentrations of 4-nitrophenol (Sigma, St. Louis) were likewise diluted in NaOH. The concentration of 4-nitrophenol in samples and standards was quantified by UV detection at 410 nm with a Bio-mini DNA/RNA/Protein Analyzer (Shimadzu, Columbia, MD).
The external cell membranes were prepared following the protocol of Jin and Audus [17]. Briefly, cells from a 150 cm2 flask were washed three times with 10 mL warm PBS and then scraped into 10 mL PBS. The cells were spun at 450 × g for 6 minutes, and the cell pellet was resuspended in about 800 μL PBS. Sonication with a Sonic Dismembrator Model 500 (Fisher Scientific, Hampton, NH) was at 20% amplitude for 10 seconds at a time, three times, with the sample kept on ice for at least one minute in between each sonication. Following centrifugation at 15,000 × g for 20 minutes, the soluble (cytosolic) fraction was removed and the insoluble fraction was resuspended in HBSS and distributed into a 24-well plate for the same reaction of 4-nitrophenylphosphate to 4-nitrophenol to monitor ALP activity.
2.3 Statistical Analysis
Data from uptake experiments and determinations of ALP activity relative to controls were compared by single factor ANOVA tests; between 3−6 replicates were analyzed in each case, and differences were deemed statistically significant if p < 0.05. Ki values were calculated from the slopes of the Lineweaver-Burk plots, using the following equation:
where [Inhibitor] is the inhibitor concentration, mInhibitor is the slope regressed from the plot of the inverse of carnitine concentration (x-axis) versus the inverse of carnitine uptake (y-axis) in the presence of inhibitor, and mControl is the slope regressed from the same plot in the absence of inhibitor.
3. Results
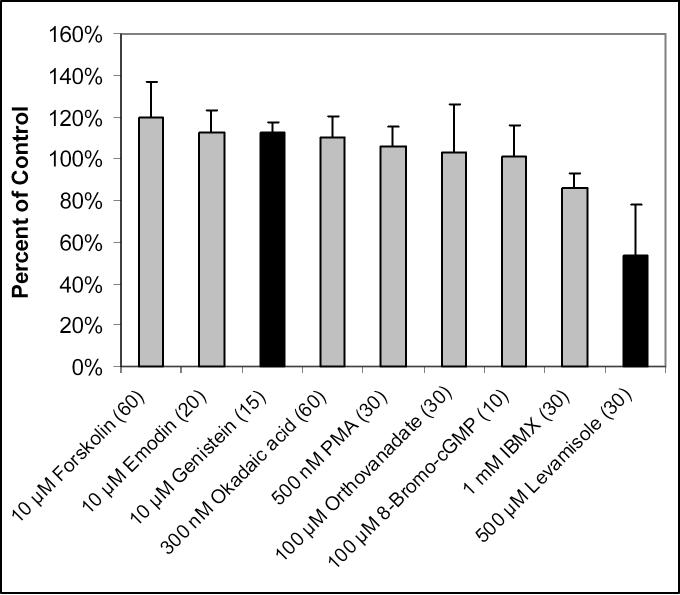
The uptake of carnitine was measured in the presence of various activators or inhibitors of kinases and phosphatases, following preincubation of the BeWo cells with these compounds. Figure 2 shows that in only two cases was there a statistically significant increase or decrease in carnitine uptake. Preincubation with 500 μM levamisole, an ALP inhibitor, significantly reduced carnitine uptake in BeWo cells (204 ± 50 fmol/mg protein) compared to control (382 ± 65 fmol/mg protein, p < 0.01). Preincubation with 10 μM genistein, a protein tyrosine kinase inhibitor, increased the uptake of carnitine from 505 ± 42 to 567 ± 28 fmol/mg protein (p < 0.05). Inhibitors or activators of protein tyrosine phosphatase, protein serine/threonine phosphatase, casein kinase II, and protein kinases PKA, PKC, and PKG had no significant effects on carnitine uptake.
Figure 2.
Effects of various compounds on the uptake of 10 nM [3H]-L-carnitine in BeWo cells. Uptake was measured for 15 or 30 minutes at 37°C in the presence of compound concentrations shown with preincubation times (in minutes) in parentheses. Data are presented as percent of control (uptake of carnitine in the absence of these compounds). Data points represent the average and standard deviation from 3−6 determinations. Bars representing statistically significant (p < 0.05) data are colored in black.
The inhibitory effect of levamisole upon ALP activity was confirmed as it reduced 4-nitrophenol formation to 80.7% of control (p < 0.05). ALP activity in BeWo cells, assessed by monitoring the enzyme's removal of the phosphate group from 4-nitrophenylphosphate, was also investigated in the external membrane fragments in order to verify that the conversion of 4-nitrophenylphosphate being monitored for ALP activity is not an intracellular reaction. In Figure 3, it is seen that when corrected for protein content, the majority of the ALP assay reaction is occurring in the external membrane fractions of the cells. It is also apparent that levamisole's inhibition of ALP is much stronger (27.0 ± 6.8% of control, compared to 80.7 ± 13.8%) when looking at just the external membranes. It was determined that the external membranes contain approximately 45% of total cellular protein.
Figure 3.
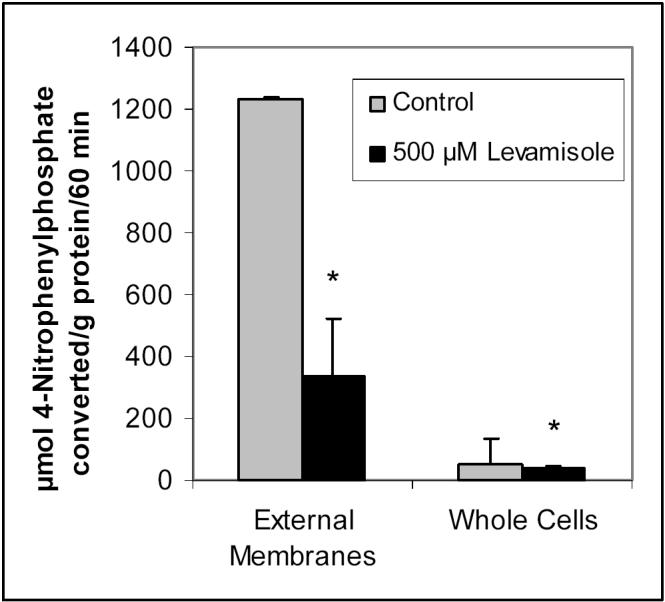
Alkaline phosphatase activity in external membrane fragments compared to whole BeWo cells, and inhibition of ALP activity by levamisole. BeWo cells or cell membrane fractions were preincubated with 500 μM levamisole followed by a 60-minute reaction of 1 mM 4-nitrophenylphosphate conversion to 4-nitrophenol. Data represent the average and standard deviation of 4 determinations, corrected for protein content. * Significantly different from control (p < 0.05).
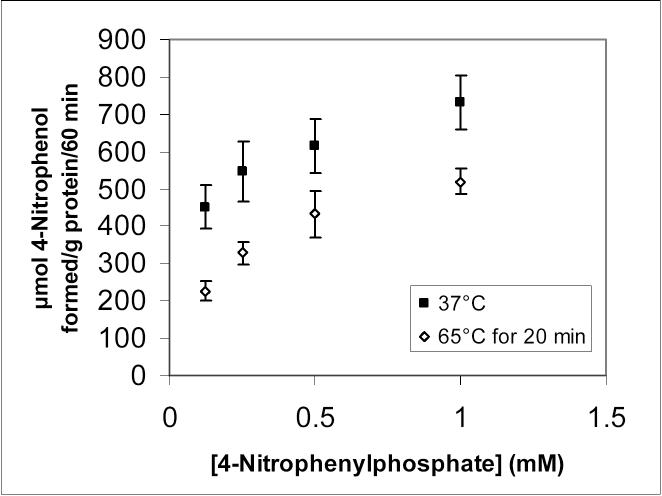
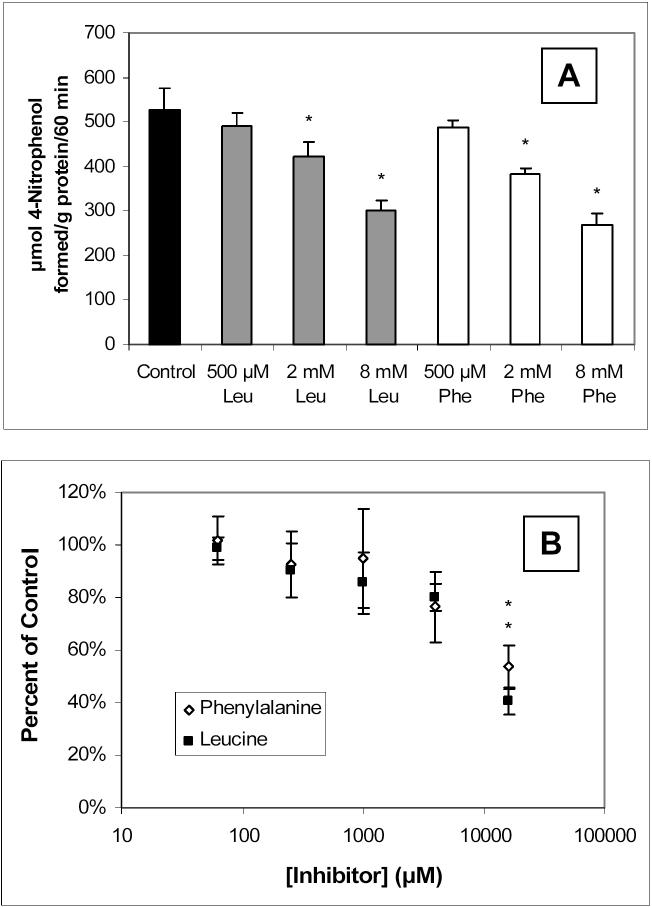
ALP activity was further characterized in the external membranes by studying the effects of heat-inactivation and other ALP inhibitors. Figure 4 shows the partial reduction of ALP activity following a 20-minute preincubation of the external membrane fragments at 65°C. Inhibition of the ALP activity in the external membranes by leucine and phenylalanine, together with the inhibition of carnitine uptake in whole BeWo cells by these two amino acids, is presented in Figure 5.
Figure 4.
Measurement of ALP activity in BeWo cell membrane fragments at 37°C following 20-minute preincubation at 37°C (control) or 65°C. Data points represent the average and standard deviation of 4 determinations. The activity at 65°C is significantly lower than the activity at 37°C at every concentration of 4-nitrophenylphosphate studied (p < 0.05).
Figure 5.
Inhibition of ALP activity by various concentrations of leucine (Leu) and phenylalanine (Phe) in external BeWo cell membranes (A) measured by the conversion of 300 μM 4-nitrophenylphosphate to 4-nitrophenol; and the inhibition of 10 nM [3H]-L-carnitine uptake in BeWo cells by leucine and phenylalanine (B). Uptake was measured at 37°C for 15 minutes. All data points represent the average and standard deviation for 3−4 determinations. * Significantly different from the control uptake of 0.443 ± 0.056 pmol/mg protein/15 min (p < 0.05).
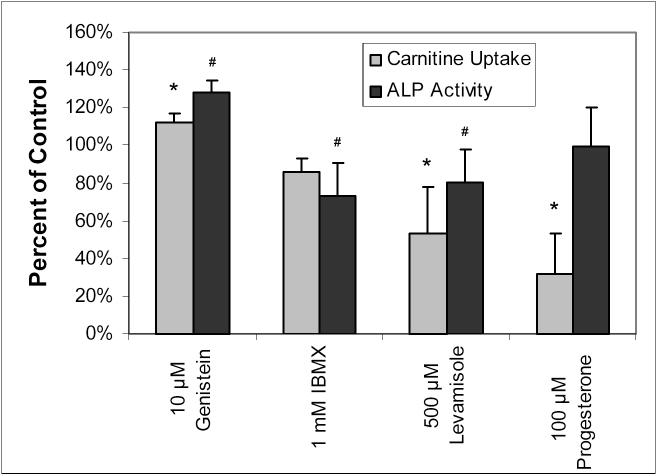
Of the three compounds having substantial effects on carnitine uptake in Figure 2, levamisole's inhibition of ALP activity has already been mentioned. The effects of genistein and IBMX on the conversion of 4-nitrophenylphosphate to 4-nitrophenol in whole BeWo cells was investigated, and the results appear in Figure 6. The influence of progesterone preincubation on carnitine uptake and ALP activity in BeWo cells was also investigated and shown to cause a significant reduction in carnitine uptake (to 32.1 ± 21.7% of control), but no change in ALP activity (99.2 ± 21.0% of control).
Figure 6.
Comparison of the effects of 10 μM genistein, 1 mM IBMX, 500 μM levamisole, and 100 μM progesterone on carnitine uptake and ALP activity in BeWo cells. Uptake of 10 nM [3H]-L-carnitine was measured at 37°C for 20 or 30 minutes with 30-minute preincubation of IBMX and levamisole, 15-minute preincubation of genistein, and 20-minute preincubation of progesterone. ALP activity was monitored by the 60-minute conversion of 1 mM 4-nitrophenylphosphate to 4-nitrophenol with preincubation times identical to those of the carnitine experiments. All values are expressed as percent of control (carnitine uptake or ALP activity without any preincubations or compounds present) and corrected for protein content. Data points represent averages and standard deviations of 3−4 determinations. * Significantly different from control carnitine uptake (p < 0.05). # Significantly different from control ALP activity (p < 0.05).
ALP activity and carnitine uptake were also investigated with the addition of 2 U/mL ALP to the HBSS solutions containing either carnitine or 4-nitrophenylphosphate at the commencement of the uptake or ALP activity studies, respectively (without preincubation). The additional ALP resulted in a 73-fold higher conversion of 4-nitrophenylphosphate compared to control (3300 ± 510 compared to 45.5 ± 10.8 μmol converted/g protein/60 min). However, the addition of external ALP resulted in a significantly decreased carnitine uptake (0.477 ± 0.056 pmol/mg protein/20 min) compared to control (0.589 ± 0.101 pmol/mg protein/20 min).
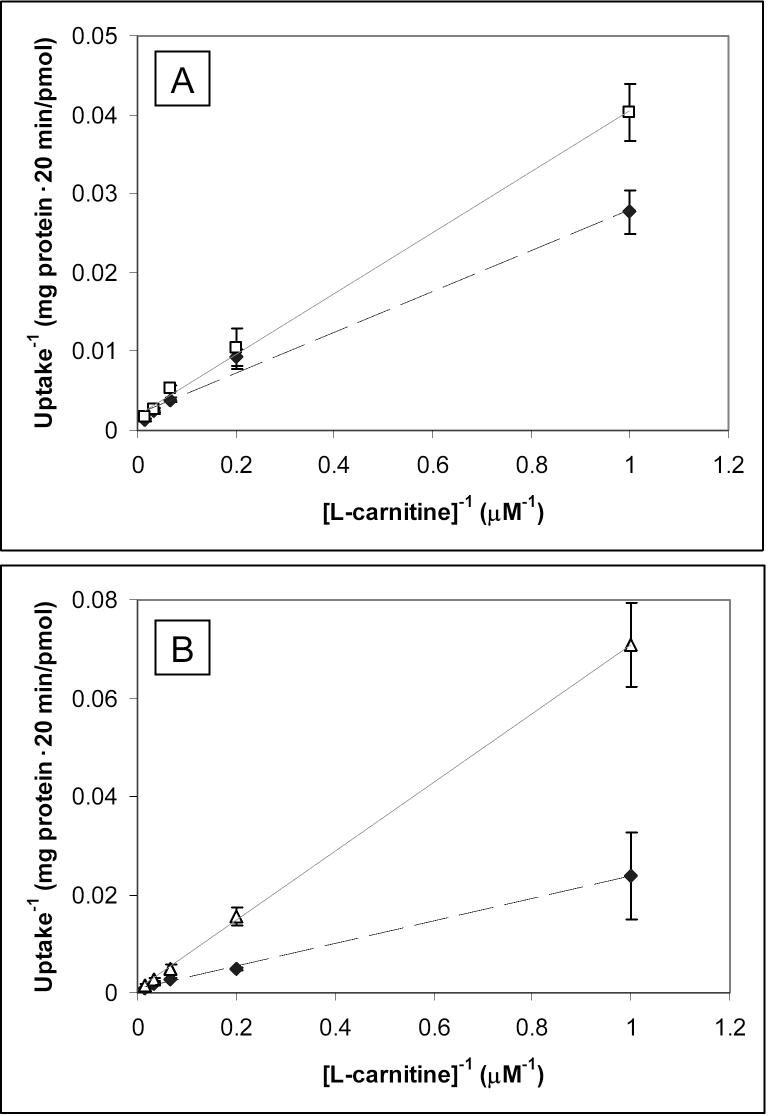
Figure 7 displays Lineweaver-Burk plots generated upon inhibition of carnitine uptake with either 500 μM levamisole (A) or 100 μM progesterone (B) with varying carnitine concentrations. The intersections of the fitted lines (all with R2 ≥ 0.99) occur at the y-axes, indicating competitive inhibition of carnitine uptake by both levamisole and progesterone. The Ki values were determined to be 1.01 ± 0.05 mM for levamisole and 48.6 ± 5.0 μM for progesterone.
Figure 7.
Lineweaver-Burk plot for inhibition of various concentrations of [3H]-L-carnitine uptake in BeWo cells at 37°C for 20 minutes following a 30-minute preincubation with 500 μM levamisole (A, open squares) or a 20-minute preincubation with 100 μM progesterone (B, open triangles). Data for the control situations (no preincubation or inhibitor present) are also presented (closed diamonds, dotted lines). Each point and error bar represents the average and standard deviation for 4−6 determinations of uptake corrected for protein content.
4. Discussion
Experiments have been carried out to investigate the possible regulation of OCTN2 in BeWo cells by phosphorylation. Although OCTN2 is predicted to have several PKC phosphorylation sites, a PKA/PKG site, and a possible casein kinase II site, preincubation with compounds that activate PKC and PKG, and an inhibitor of casein kinase II led to no significant effects on OCTN2-mediated carnitine uptake. Forskolin, an activator of PKA, did increase the carnitine uptake to 119 ± 17% of control, but the increase was not statistically significant (p > 0.05).
Regulation of OCTN2 by phosphorylation/dephosphorylation mechanisms was expected due to previous reports regarding such effects on OCT1, OCT2, and OCT3 [5-7] and the presence of putative phosphorylation sites on the OCTN2 protein (see Figure 1). Nevertheless, the results gathered while testing this hypothesis suggest that the contributions of phosphorylation in the regulation of OCTN2 are minimal in this model of the placental barrier.
Among the compounds in Figure 2, levamisole had the greatest effect on carnitine uptake, reducing it to 53.4 ± 24.3% of control. Levamisole is an inhibitor of ALP, so this inhibition of carnitine uptake suggested a link between ALP and OCTN2 transport function, and experiments were performed to further characterize the activity of ALP in BeWo cells.
There are four types of ALP: tissue-unspecific ALP (also called liver/bone/kidney ALP), intestinal ALP, placental ALP, and placental-like ALP (also called germ-cell ALP); these isoforms can be distinguished based on their thermal stabilities and sensitivities to various inhibitors [18]. Placental ALP and placental-like ALP are more stable at high temperatures than the tissue-unspecific and intestinal ALPs. The IC50 values of inhibitors such as phenylalanine, leucine, and levamisole have been tabulated for the four types of ALP [18].
Placental ALP exhibits higher density in syncytiotrophoblast than in cytotrophoblast cells, and is located in both the apical and basal membranes of trophoblast cells [19]. The characterization of ALP by thermostability and inhibition profiles was therefore carried out in this work by separating the external membranes of the BeWo cells from the cytosolic fractions. Figure 3 shows the ALP activity corrected for protein content in the external membranes compared to the whole cell, and the predominant portion of ALP activity is in the external membranes. The strong inhibition of levamisole on external membrane ALP activity, the heat stability of the ALP activity shown in Figure 4, and the similar inhibition profiles of leucine and phenylalanine on ALP activity (in Figure 5), compared to the tabulated values for the thermostability and inhibition of the different ALP isoforms [18], together suggest the involvement of both tissue-unspecific and placental-like ALP in BeWo cells. This is in agreement with literature reports of the presence of these two isoforms in BeWo cells [20,21].
Genistein is listed in the literature as an inhibitor of protein tyrosine kinase [15], and IBMX as a non-selective phosphodiesterase inhibitor [7], However, these two compounds have also been reported to affect ALP activity [22,23]. Following this thought, the effects of both genistein and IBMX on the present assay of ALP activity were investigated. Figure 6 shows that the degree of ALP stimulation (by genistein) or inhibition (by IBMX) matched moderately well with the respective increased and decreased carnitine uptake upon incubation with these two agents. This suggests that the increased OCTN2 function observed after preincubation with genistein may not be due to protein tyrosine kinase after all, but simply due to an increase in ALP activity brought about by genistein. These observations were also in agreement with the decreased inhibition of carnitine uptake upon inhibition of ALP by levamisole, thereby providing initial indications of a correlation between ALP activity and OCTN2-mediated uptake that would suggest greater transporter function in a dephosphorylated state.
Martel et al. observed reduced MPP+ uptake in the presence of an ALP inhibitor, and increased uptake of MPP+ after the addition of 2 U/mL ALP [7]. Although ALP inhibition in this work seemed to match decreases in carnitine uptake, the external addition of 2 U/mL ALP to BeWo cells did not result in increased carnitine uptake, but rather, a significant decrease, even though the measured ALP activity was very much increased. Although the decreased uptake may be due to protein binding effects, this experiment suggests that there may be other factors responsible for the observed changes in carnitine transport in the presence of the ALP inhibitor levamisole. In addition, very high concentrations of leucine and phenylalanine are required to inhibit ALP activity and carnitine uptake, which makes it difficult to attribute the effects observed in Figure 5 to a definite link between ALP and carnitine uptake by OCTN2.
In Figure 6, the observed inhibition of carnitine uptake by levamisole (to 53.4% of control) is quite a bit lower than the observed inhibition of ALP activity in whole cells (80.7%). The pKa of levamisole is 8.0 [24], which means that at a pH of 7.4, roughly 75% of the levamisole molecules would have a positively charged nitrogen atom and therefore it is possible that the inhibition of carnitine uptake by levamisole is due to a combination of ALP inhibition and competition of levamisole for the cationic binding site for carnitine on OCTN2. This was confirmed in the Lineweaver-Burk plot (Figure 7A), which shows that levamisole indeed competitively inhibits carnitine uptake, i.e., levamisole and carnitine compete for the same binding site on the OCTN2 protein. Although the Ki value (1.01 ± 0.05 mM) is not remarkably low, the observation of levamisole's competitive inhibition of OCTN2 can explain the difference between the magnitude of levamisole's inhibition of carnitine uptake and its inhibition of ALP activity in BeWo cells.
Figure 6 also shows that progesterone significantly inhibits carnitine uptake in BeWo cells, but it has no effect at all on ALP activity, as quantified here using 1 mM 4-nitrophenylphosphate hydrolysis. Progesterone has been reported to increase ALP activity [15,25], which is why it was investigated in this work to correlate ALP and OCTN2 function. However, the strong inhibition of carnitine uptake by progesterone without the accompanying impact on ALP activity suggests other effects of this hormone on OCTN2-mediated transport function. Progesterone is an essential steroid for maintenance of pregnancy, and it is involved in the regulation of the glucose transporter GLUT1, the peptide transporter PEPT1, and other enzymes [26-28]. Since the inhibition of carnitine uptake by progesterone was seen after only 20 minutes of preincubation, it is not likely that hormonal regulation of OCTN2 is responsible for these observations. Additional studies are necessary to further investigate the interactions of progesterone and other pregnancy hormones with OCTs expressed in the placenta.
In summary, these investigations were carried out to understand the possible roles of phosphorylation/dephosphorylation mechanisms affecting OCTN2-mediated carnitine uptake in BeWo cells. Experiments confirmed the expression of placental-like and tissue-unspecific ALP in BeWo cells. Although preincubation with ALP inhibitors resulted in reductions in OCTN2-mediated carnitine uptake similar to the observed reductions in ALP activity, it appears that competitive inhibition of the ALP inhibitor levamisole on the OCTN2 protein plays a more substantial role in decreasing carnitine uptake in BeWo cells than the indirect effects of ALP inhibition upon the function of this transporter. Furthermore, progesterone was also shown to be a competitive inhibitor of carnitine uptake in this model of placental trophoblasts. Future investigations should provide additional insight into the interactions of progesterone with placental OCTs, since they play an important role in transporting cationic drugs and nutrients between the maternal and fetal circulations.
Acknowledgements
The authors would like to express their appreciation to Amber Young and Hong Jin for technical assistance. Financial support was provided by the Madison & Lila Self Graduate Fellowship, the University of Kansas Department of Pharmaceutical Chemistry, and grant HD039878.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu X, Huang W, Prasad PD, Seth P, Rajan DP, Leibach FH, Chen JW, Conway SJ, Ganapathy V. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. J Pharmacol Exp Ther. 1999;290:1482–1492. [PubMed] [Google Scholar]
- 2.Wang YH, Meadows TA, Longo N. Abnormal sodium stimulation of carnitine transport in primary carnitine deficiency. J Biol Chem. 2000;275:20782–20786. doi: 10.1074/jbc.M000194200. [DOI] [PubMed] [Google Scholar]
- 3.Lahjouji K, Mitchell GA, Qureshi IA. Carnitine transport by organic cation transporters and systemic carnitine deficiency. Mol Gen Metab. 2001;73:287–297. doi: 10.1006/mgme.2001.3207. [DOI] [PubMed] [Google Scholar]
- 4.Ciarimboli G, Schlatter E. Regulation of organic cation transport. Pflugers Arch. 2005;449:423–441. doi: 10.1007/s00424-004-1355-5. [DOI] [PubMed] [Google Scholar]
- 5.Ciarimboli G, Struwe K, Arndt P, Gorboulev V, Koepsell H, Schlatter E, Hirsch JR. Regulation of the human organic cation transporter hOCT1. J Cell Physiol. 2004;201:420–428. doi: 10.1002/jcp.20081. [DOI] [PubMed] [Google Scholar]
- 6.Cetinkaya I, Ciarimboli G, Yalcinkaya G, Mehrens T, Velic A, Hirsch JR, Gorboulev V, Koepsell H, Schlatter E. Regulation of human organic cation transporter hOCT2 by PKA, PI3K, and calmodulin-dependent kinases. Am J Physiol Renal Physiol. 2003;284:F293–F302. doi: 10.1152/ajprenal.00251.2002. [DOI] [PubMed] [Google Scholar]
- 7.Martel F, Keating E, Calhau C, Grundemann D, Schomig E, Azevedo I. Regulation of human extraneuronal monoamine transporter (hEMT) expressed in HEK293 cells by intracellular second messenger systems. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:487–495. doi: 10.1007/s002100100476. [DOI] [PubMed] [Google Scholar]
- 8.Gisler SM, Pribanic S, Bacic D, Forrer P, Gantenbein A, Sabourin LA, Tsuji A, Zhao ZS, Manser E, Biber J, Murer H. PDZK1: I. a major scaffolder in brush borders of proximal tubular cells. Kidney Int. 2003;64:1733–1745. doi: 10.1046/j.1523-1755.2003.00266.x. [DOI] [PubMed] [Google Scholar]
- 9.Kato Y, Sai Y, Yoshida K, Watanabe C, Hirata T, Tsuji A. PDZK1 directly regulates the function of organic cation/carnitine transporter OCTN2. Mol Pharmacol. 2005;67:734–743. doi: 10.1124/mol.104.002212. [DOI] [PubMed] [Google Scholar]
- 10.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 11.Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status in 1997. Nucleic Acids Res. 1997;25:217–221. doi: 10.1093/nar/25.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burckhardt G, Wolff NA. Structure of renal organic anion and cation transporters. Am J Physiol Renal Physiol. 2000;278:F853–F866. doi: 10.1152/ajprenal.2000.278.6.F853. [DOI] [PubMed] [Google Scholar]
- 13.Rytting E, Audus KL. Novel organic cation transporter 2-mediated carnitine uptake in placental choriocarcinoma (BeWo) cells. J Pharmacol Exp Ther. 2005;312:192–198. doi: 10.1124/jpet.104.072363. [DOI] [PubMed] [Google Scholar]
- 14.Bode CJ, Jin H, Rytting E, Silverstein PS, Young AM, Audus KL. In vitro Models for Studying Trophoblast Transcellular Transport. Methods Mol Med. 2006;122:225–239. doi: 10.1385/1-59259-989-3:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calhau C, Martel F, Soares-da-Silva P, Hipolito-Reis C, Azevedo I. Regulation of [H-3]MPP+ transport by phosphorylation/dephosphorylation pathways in RBE4 cells: role of ecto-alkaline phosphatase. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:349–356. doi: 10.1007/s00210-002-0542-1. [DOI] [PubMed] [Google Scholar]
- 16.Kim KY, Shin HK, Lee JH, Kim CD, Lee WS, Rhim BY, Shin YW, Hong KW. Cilostazol enhances casein kinase 2 phosphorylation and suppresses tumor necrosis factor-alpha-induced increased phosphatase and tensin homolog deleted from chromosome 10 phosphorylation and apoptotic cell death in SK-N-SH cells. J Pharmacol Exp Ther. 2004;308:97–104. doi: 10.1124/jpet.103.058768. [DOI] [PubMed] [Google Scholar]
- 17.Jin H, Audus KL. Effect of bisphenol A on drug efflux in BeWo, a human trophoblast-like cell line. Placenta. 2005;26(Suppl A):S96–S103. doi: 10.1016/j.placenta.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Harris H. The human alkaline phosphatases: what we know and what we don't know. Clin Chim Acta. 1990;186:133–150. doi: 10.1016/0009-8981(90)90031-m. [DOI] [PubMed] [Google Scholar]
- 19.Leitner K, Szlauer R, Ellinger I, Ellinger A, Zimmer KP, Fuchs R. Placental alkaline phosphatase expression at the apical and basal plasma membrane in term villous trophoblasts. J Histochem Cytochem. 2001;49:1155–1164. doi: 10.1177/002215540104900909. [DOI] [PubMed] [Google Scholar]
- 20.Lowe ME, Strauss AW. Expression of a Nagao-type, phosphatidylinositolglycan anchored alkaline phosphatase in human choriocarcinomas. Cancer Res. 1990;50:3956–3962. [PubMed] [Google Scholar]
- 21.Hamilton TA, Tin AW, Sussman HH. Regulation of alkaline phosphatase expression in human choriocarcinoma cell lines. Proc Natl Acad Sci U S A. 1979;76:323–327. doi: 10.1073/pnas.76.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calhau C, Martel F, Hipolito-Reis C, Azevedo I. Modulation of uptake of organic cationic drugs in cultured human colon adenocarcinoma Caco-2 cells by an ecto-alkaline phosphatase activity. J Cell Biochem. 2002;87:408–416. doi: 10.1002/jcb.10306. [DOI] [PubMed] [Google Scholar]
- 23.Wober J, Weisswange I, Vollmer G. Stimulation of alkaline phosphatase activity in Ishikawa cells induced by various phytoestrogens and synthetic estrogens. J Steroid Biochem Mol Biol. 2002;83:227–233. doi: 10.1016/s0960-0760(02)00252-2. [DOI] [PubMed] [Google Scholar]
- 24.Abdi YA, Gustafsson LL, Ericsson O, Hellgren U. Handbook of Drugs for Tropical Parasitic Infections. Taylor & Francis; Bristol, PA: 1995. [Google Scholar]
- 25.Young AM. Characterization of Efflux Transporters of the Human Trophoblast Using BeWo as a Model. University of Kansas Dissertation. 2005 [Google Scholar]
- 26.Medina RA, Meneses AM, Vera JC, Guzman C, Nualart F, Rodriguez F, los Angeles GM, Kato S, Espinoza N, Monso C, Carvajal A, Pinto M, Owen GI. Differential regulation of glucose transporter expression by estrogen and progesterone in Ishikawa endometrial cancer cells. J Endocrinol. 2004;182:467–478. doi: 10.1677/joe.0.1820467. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe K, Jinriki T, Sato J. Effects of progesterone and norethisterone on cephalexin uptake in the human intestinal cell line Caco-2. Biol Pharm Bull. 2004;27:559–563. doi: 10.1248/bpb.27.559. [DOI] [PubMed] [Google Scholar]
- 28.Maccarrone M, Bari M, Di Rienzo M, Finazzi-Agro A, Rossi A. Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros. Evidence for a synergistic effect of leptin. J Biol Chem. 2003;278:32726–32732. doi: 10.1074/jbc.M302123200. [DOI] [PubMed] [Google Scholar]