Abstract
Chronic administration of cocaine has been shown to attenuate the functional capacity of delta opioid receptors to inhibit adenylyl cyclase activity. Abuse and withdrawal from cocaine in humans is associated with increases in anxiety and depression. Since recent research supports the role of delta opioid receptors in anxiety- and depression-like behaviors in rodents, we hypothesized that functional desensitization of delta opioid receptors contributes to anxiety- and depression-like behavioral phenotypes following short-term withdrawal from chronic administration of cocaine. To test this hypothesis, delta opioid receptor signaling and behaviors were evaluated 24 h after 14 days of binge-pattern cocaine administration (15 mg/kg three times daily at 1 h intervals) in male Sprague Dawley rats. Results showed that the inhibition of adenylyl cyclase by delta opioid receptor agonists was attenuated in the frontal cortex, nucleus accumbens and caudate putamen 24 h after cessation of cocaine administration. One day withdrawal from chronic administration of cocaine resulted in increased anxiety- and depression-like behaviors as measured by the elevated plus maze and the force swim test respectively, and no change in locomotor activity. The anxiety- and depression-like behaviors were dose-dependently reduced by acute administration of the selective delta opioid receptor agonist, SNC80. These results demonstrate that early withdrawal from cocaine resulted in increased anxiety and depression, which accompanies the desensitization of delta opioid receptor function. Furthermore, cocaine-induced anxiety- and depression-like behaviors were reversible by the delta opioid receptor agonist SNC80.
Keywords: cocaine, delta opioid receptor, rat, anxiety, depression, SNC80, adenylyl cyclase
1. Introduction
Cocaine binds to and inhibits the reuptake function of dopamine, serotonin and norepinephrine transporters (Heikkila et al., 1975; Ritz et al., 1987). Although these transporters are the direct targets of cocaine, a number of other neuropeptide and neurotransmitter systems are also affected by cocaine and play an important role in the neurochemical and behavioral effects of the drug. Dopamine and opioid systems act together to modulate locomotion, mood and motivated behaviors (Fink and Smith, 1980; Roberts and Koob, 1982). Furthermore, opioids can regulate the synthesis and release of dopamine (Moleman et al., 1984), and dopamine receptor activation, including indirect activation by cocaine, can influence the levels of endogenous opioids (Hurd et al., 1992; Olive et al., 2001; Sivam, 1989).
Chronic administration of cocaine in a binge-pattern (i.e. 15 mg/kg three times daily at 1 h intervals for 14 days) has been shown to increase levels of mu and kappa opioid receptors in discrete brain regions of the rat, but does not alter the density of delta opioid receptors (Unterwald et al., 1994). Other investigators have demonstrated similar changes in opioid receptor expression following cocaine (Azaryan et al., 1996; Hammer, 1989; Izenwasser et al., 1996). Although delta opioid receptor densities are unaltered, their function is modulated by cocaine and dopamine D1 receptor activation. Chronic, but not acute administration of cocaine or the dopamine D1 receptor agonist SKF 82958 attenuates the ability of the selective delta opioid receptor agonist D-Pen2,D-Pen5-enkephalin (DPDPE) to inhibit delta receptor-mediated adenylyl cyclase activity in the caudate putamen and nucleus accumbens of rats (Unterwald et al., 1993; Unterwald and Cuntapay, 2000).
Abuse of cocaine by humans and its subsequent withdrawal can cause psychiatric symptoms, including anxiety and depression (Basso et al., 1999; Rogerio and Takahashi, 1992; Yang et al., 1992). In rats, cocaine can induce anxiety-like behaviors (Blanchard and Blanchard, 1999), and depression-like effects have been reported in rodents exposed to cocaine prenatally (Overstreet et al., 2000; Sobrian et al., 2003) and postnatally (Magalhaes et al., 2002). The anhedonic state of rats induced by cocaine withdrawal is proportional to the amount of cocaine consumed (Markou and Koob, 1991). While these studies suggest that cocaine can produce depression- and anxiety-like states in animals, the potential involvement of the delta opioid receptor system therein has not been investigated.
The role of the delta opioid system in the regulation of anxiety and depression has been reported. Delta opioid receptor knockout mice exhibit increases in anxiety- and depression-like behaviors compared with wild-type controls (Filliol et al., 2000). Pharmacological evidence for this hypothesis has been reported by our lab and others. The selective delta opioid receptor agonist SNC80 dose-dependently reduces anxiety- (Perrine et al., 2006; Saitoh et al., 2004) and depression-like (Jutkiewicz et al., 2003) behaviors. Conversely, the selective delta opioid receptor antagonist naltrindole can be anxiogenic in rodents (Marin et al., 2003; Perrine et al., 2006; Saitoh et al., 2005). Hence, activation of delta opioid receptors by endogenous opioid peptides is critical in the physiological control of emotion-related behaviors.
Based on previous research, it is evident that cocaine can increase anxiety- and depression-like behaviors in animals. The delta opioid receptor has been implicated in depression- and anxiety-like behaviors based on pharmacological and genetic manipulation. However, the role of delta opioid receptor desensitization in cocaine-induced anxiety and depression has not been examined. In this study, the effect of early withdrawal from chronic administration of cocaine on behavior and delta opioid receptor function was investigated. Three behaviors known to be influenced by cocaine and delta opioid ligands were measured following withdrawal from cocaine, including locomotion, anxiety- and depression-like behaviors. Anxiety- and depression-like behaviors were evaluated using the elevated plus maze (Pellow et al., 1985) and the forced swim test (Lucki, 1997) respectively.
2. Methods
2.1. Animals and drug administration
Male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were housed on a 12 h light-dark cycle (7 AM-7 PM) with free access to food and water. They were housed in groups of four per cage and their weight range upon arrival was 150−200 g and upon testing was 275−375 g. Animals were allowed to acclimate for a minimum of one week during which time they were weighed and handled daily.
Cocaine HCl and SNC80 (generously provided by NIH/NIDA) were injected in a volume of 1 ml/kg body weight. Cocaine and its saline control were injected intraperitoneally (ip), and SNC80 was administered subcutaneously (sc). SNC80 was prepared at 20 mg/ml in normal saline at pH 4.5−5 (with HCl), and then diluted with normal saline to achieve 1 and 5 mg/ml SNC80 concentrations. Saline was used as control for SNC80 because previous studies showed no difference between saline and vehicle control (Perrine et al., 2006).
Cocaine (15 mg/kg) or saline (1 ml/kg) was administered three times daily at 1 h intervals in the morning for 14 days. Twenty-four hours after the last injection, rats were either tested on one of the three behavioral measures or killed and brains dissected for analysis of delta opioid receptor function. All behavioral tests were performed between 10 AM and 2 PM. Each animal was tested only once. Potential discomfort to rats was minimized and all procedures were conducted in accordance with the National Institutes of Health's Guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and approved by Temple University's Institutional Animal Care and Use Committee.
2.2. Adenylyl Cyclase Measurement
Rats were lightly anesthetized with CO2 before undergoing guillotine decapitation. Brains were removed and the caudate putamen, nucleus accumbens, and frontal cortex were dissected immediately on ice. Crude membranes were prepared from fresh brain tissue of individual animals. Tissue homogenates (20−35 μg protein) were incubated in buffer consisting of 80 mM Tris HCl (pH 7.4), 10 mM theophylline, 1 mM MgSO4, 0.8 mM EGTA, 30 mM NaCl, 0.25 mM ATP, and 0.01 mM GTP. Samples were incubated in the absence or presence of 0.1−100 μM DPDPE or SNC80 in triplicate for 5 min at 30°C. Adenylyl cyclase activity was terminated by placing the tubes into boiling H2O for 2 min. The amounts of cAMP formed under basal conditions and in the presence of the delta opioid receptor agonists were determined by a [3H]cAMP binding protein assay and comparison to a standard curve as previously described (Brown et al., 1971). Adenylyl cyclase activity in the presence of agonist is presented as a percent of activity under basal conditions.
2.3. Elevated Plus Maze
The elevated plus maze was used to measure anxiety-like behaviors 24 h following the last injection of cocaine or saline. The plus maze (Coulbourn Instruments, Allentown, PA) consists of four equal-sized runways (45 cm long × 10 cm wide) laid-out in the shape of a plus sign and elevated off the ground by 52 cm (Handley and McBlane, 1993). Two of the arms are enclosed by a solid wall 30 cm high on the long sides of the arms (closed arms), whereas the other two arms have no sides and are thus open (open arms). A ledge (0.5 cm high) is present around the perimeter of the open arms of the maze. Test room lighting was adjusted from normal level (∼450 lux) to a dimmer setting of 150−210 lux, where open arm light levels were ∼200 lux and closed arm light levels were ∼160 lux. An entry was counted when the superior portion of the rat including the head and neck, the shoulders, the forelimbs and forepaws, and the thoracic region moved into an arm.
SNC80 (1, 5, or 20 mg/kg, sc) was administered 60 min prior to testing (23 h after the last saline or cocaine binge-pattern injection). For anxiety assessment, each rat was placed on an open arm of the maze facing the center and their behavior was recorded on videocassette for 10 min. The experimenter was not blind to the study; however, scoring was confirmed by two raters blind to the conditions. Anxiety-like behavior was determined by calculating the amount of time and number of entries each rat made in the open and closed arms and reported as a percentage of the total time or number of entries.
2.4. Forced Swim Test
The forced swim test was used to measure depression-like behaviors 24 h following chronic administration of saline or cocaine. Six hours after the last cocaine or saline injection, rats were exposed to a 15 min pre-test swim. The pretest facilitates the development of immobility during the test session and increases the sensitivity for detecting behavioral effects (Lucki, 1997). Rats were placed into a glass cylinder (46 cm high × 20 cm diameter) filled to a depth of 36 cm with room temperature (23 ± 1°C) H2O for 15 min. Animals were removed from the water, dried by the experimenter and placed back into their home cages. Twenty-three hours after the last cocaine or saline injection (17 h after the pre-test swim) animals were injected with a challenge dose of SNC80 (1 or 5 mg/kg, sc). One hour later, rats were placed in the swim chamber for 5 min and the session was recorded. Behavioral scoring utilized a sampling technique (Detke et al., 1995; Lucki, 1997), where at the end of each 5 s period during the 5 min test session, a scorer rated the rat's behavior as either immobile, swimming or climbing, for a total of 60 observations. Swimming was defined as directed movement throughout the swim chamber. Climbing activity (i.e. thrashing) consisted of upward directed movements of the forepaws against the side of the swim chamber. Immobility was assigned when no additional activity was observed other than that necessary to keep the rat's head above the water. Two raters who were blind to the treatment conditions performed the behavioral scoring and their scores were averaged.
2.5. Locomotor Activity
Automated activity monitors were used to measure ambulatory activity. Twenty-two and one-half hours after the last binge-pattern injection, rats were placed in the activity monitors. Ambulatory activity was monitored for 30 min prior to and 120 min after SNC80 (1 or 5 mg/kg, sc) or saline (1 ml/kg, sc) administration. The 120 min testing period included the 1 h before and 1 h after the time point marking 24 h after the last binge-pattern injection. Behavioral activity was measured by a computerized monitoring system (Digiscan DMicro, Accuscan Inst, Columbus, OH), which consists of a metal frame containing 16 parallel infrared photobeams and receivers into which a standard plastic rat cage (42 × 20 × 20 cm) is placed. Photobeam breaks were recorded and stored on a computer linked to the activity monitors. Ambulatory activity was determined by the number of times the animal broke consecutive light beams.
2.6. Data Analysis
Concentration effect curves for DPDPE and SNC80 inhibition of adenylyl cyclase activity in control versus cocaine-injected rats were evaluated by two-way analysis of variance (two-way ANOVA) followed by Bonferroni's post-hoc analysis of individual delta agonist concentrations. Scores from the elevated plus maze and forced swim test were analyzed using a t-test or one-way ANOVA followed by Bonferroni's multiple comparison post-hoc analysis. One-way repeated-measures ANOVA followed by a Bonferroni's multiple comparison post-hoc analysis was used to analyze activity time course data. Statistical significance was considered at P<0.05. An inter-rater reliability coefficient was calculated for data where the scores of two raters were averaged. Inter-rater correlation coefficients were calculated for all scores and found to be high (>0.9). All data are expressed as the mean ± standard error of the mean (SEM).
3. Results
3.1. Delta opioid receptor regulated adenylyl cyclase activity was attenuated after withdrawal from cocaine
Figure 1 shows adenylyl cyclase activity as a percent of basal cyclase activity in discrete rat brain regions obtained 24 hours after the last injection of cocaine or saline. Figures 1A and 1B show that the two selective delta opioid receptor agonists DPDPE and SNC80 inhibited adenylyl cyclase activity in a concentration-dependent manner in the caudate putamen of control saline-injected rats. Further, adenylyl cyclase activity in the caudate putamen of rats undergoing 24 h withdrawal from chronic administration of cocaine was significantly attenuated as compared to saline-injected rats for both delta receptor agonists (two-way ANOVA: figure 1A treatment F1,95=80.17 P<0.0001, DPDPE concentration F4,95=9.87 P<0.0001; figure 1B treatment F1,70=66.32 P<0.0001, SNC80 concentration F4,70=6.60 P=0.0001). Delta opioid receptor-mediated inhibition of adenylyl cyclase activity was also significantly reduced in the nucleus accumbens (two-way ANOVA: figure 1C treatment F1,70=27.54 P<0.0001, DPDPE concentration F4,70=3.45 P=0.0125; figure 1D treatment F1,60=34.88 P<0.0001, SNC80 concentration F4,60=2.82 P=0.0328) and frontal cortex (two-way ANOVA: figure 1E treatment F1,100=14.27 P=0.0003, DPDPE concentration F4,100=5.40 P=0.0006; figure 1F treatment F1,55=17.67 P<0.0001, SNC80 concentration F4,55=3.86 P=0.0078). Baseline levels (pmol) of cAMP were not significantly different between tissue obtained from cocaine- and saline-injected animals (data not shown). These data indicate that delta opioid receptor function was attenuated in the caudate putamen, nucleus accumbens, and frontal cortex following 24 h withdrawal from chronic administration of cocaine.
Figure 1. Delta opioid receptor regulated adenylyl cyclase activity is attenuated after withdrawal from cocaine.
Sprague Dawley rats were injected with cocaine or saline in a binge-pattern for 14 days. Twenty-four hours after the last injection, discrete brain regions were dissected, and the ability of the selective delta opioid receptor agonists, DPDPE or SNC80 0.1−100 μM, to inhibit adenylyl cyclase activity was measured. In all brain regions tested, namely the caudate putamen (A & B), nucleus accumbens (C & D) and frontal cortex (E & F), DPDPE and SNC80 produced a robust and dose-dependent inhibition of cAMP formation in tissue from saline-injected animals. Delta opioid receptor-meditated inhibition of cyclase activity was significantly attenuated in the caudate putamen (A & B), nucleus accumbens (C & D) and frontal cortex (E & F) in animals injected with binge-pattern cocaine compared with saline. Values are expressed as the mean ± SEM (N=6−13) and significant Bonferroni posthoc tests of individual delta agonist concentrations are indicated by *P<0.05, **P<0.01 and ***P<0.001.
3.2. Withdrawal from chronic administration of cocaine increased anxiety-like behavior, which was reversed by acute administration of a delta opioid receptor agonist
Anxiety-like behavior was assessed 24 h after the last injection of cocaine or saline using the elevated plus maze. Figures 2A and 2B illustrate the effects of chronic administration of cocaine or saline on open arm entries and time in open arms (respectively). Open arm entries (figure 2A, t-test: t16=4.29, P=0.0006) and time in open arms (figure 2B, t-test: t16=3.59, P=0.002) were significantly reduced in rats administered cocaine for 14 days compared to saline-injected controls. The data show that withdrawal from cocaine increased both measures of anxiety-like behavior.
Figure 2. Withdrawal from cocaine increased anxiety-like behavior, which was reversed by pretreatment with a delta opioid receptor agonist.
Sprague Dawley rats were injected with cocaine or saline for 14 days. Twenty-three hours after the last injection rats were challenged with saline or SNC80 (1, 5 or 20 mg/kg), and 1 h later anxiety-like behavior was tested using the elevated plus maze, where dependent measures included open arm entries (A & C) and time spent in open arms (B & D). The number of open arm entries (A) and time in open arms (B) of rats undergoing 1 day withdrawal from chronic administration of cocaine were significantly reduced compared with those injected with saline, indicating an increase in anxiety-like behaviors during cocaine withdrawal. Administration of SNC80 1 h prior to behavioral testing significantly and dose-dependently reversed the anxiogenic effects of withdrawal from cocaine as determined by open arm entries (C) and time in open arms (D). Data are expressed as the mean ± SEM (N=8−11) and *P<0.05, **P<0.01 and ***P<0.001 indicates significant t-test (A & B) or ANOVA followed by posthoc analysis (C & D).
To determine the effects of SNC80 on cocaine-induced anxiety, rats were injected with SNC80 (1, 5 or 20 mg/kg) or saline (0 mg/kg SNC80) 1 h prior to behavioral testing, which occurred 23 h after the last injection of cocaine or saline. As seen in figure 2C and 2D, SNC80 dose-dependently reduced the anxiogenic effects of withdrawal from cocaine in both measures of anxiety-like behavior. Rats treated with cocaine and injected SNC80 5 mg/kg were significantly different from those receiving cocaine and injected with saline in open arm entries (figure 2C, ANOVA: F7,64=10.4, P<0.001; coc-sal vs coc-SNC80 5 mg/kg - Bonferroni's Multiple Comparison: P<0.01) and in time in open arms (figure 2D, ANOVA: F7,65=8.3, P<0.001; coc-sal vs coc-SNC80 5 mg/kg - Bonferroni's Multiple Comparison: P<0.001). SNC80 20 mg/kg reversed the anxiogenic effects of cocaine as shown in open arm entries (figure 2C, ANOVA: F7,64=10.4, P<0.001; coc-sal vs coc-SNC80 20 mg/kg - Bonferroni's Multiple Comparison: P<0.001) and time in open arms (figure 2D, ANOVA: F7,65=8.3, P<0.001; coc-sal vs coc-SNC80 20 mg/kg - Bonferroni's Multiple Comparison: P<0.001). Rats that were injected repeatedly with saline and challenged with SNC80 (1, 5, or 20 mg/kg) showed a trend towards an anxiolytic effect following administration of SNC80; however, this difference was not statistically significant (figure 2C and 2D, ANOVA: P>0.05).
3.3. Withdrawal from cocaine increased depression-like behavior, which was reversed by a delta opioid receptor agonist
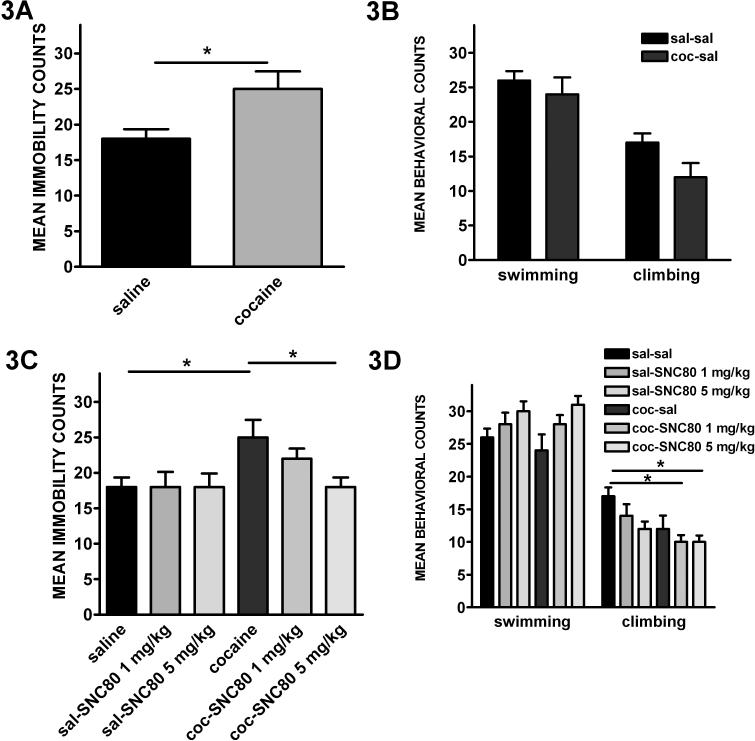
Depression-like behavior was tested using the forced swim test 24 h after the last injection of cocaine or saline. Figures 3A and 3B show the results of chronic administration of cocaine or saline on immobility (3A) and swimming and climbing behaviors (3B). Withdrawal from cocaine increased immobility, an indication of increased depression-like behavior (figure 3A, t-test: t13=2.73, P=0.017). Neither swimming or climbing measures were significantly different from saline-injected controls (figure 3B, separate t-tests: P>0.05).
Figure 3. Withdrawal from cocaine increased depression-like behavior, which was reversed by pretreatment with a delta opioid receptor agonist.
Sprague Dawley rats were injected with cocaine or saline for 14 days. Twenty-three hours after the last injection rats were challenged with saline or SNC80 (1 or 5 mg/kg), and 1 h later tested for depression-like behavior using the forced swim test, where dependent behaviors included immobility (A & C) and swimming and climbing (B & D). Immobility (A) of rats undergoing 1 day withdrawal from chronic administration of cocaine was significantly higher than those injected with saline. Administration of SNC80 1 h before testing significantly and dose-dependently reversed the depressive effects of withdrawal from cocaine as shown by a decrease in immobility scores (C). Swimming and climbing behaviors (B & D) were not significantly different between the groups. Data are expressed as the mean ± SEM (N=6−9) and *P<0.05 indicates significant t-test (A & B) or ANOVA followed by Bonferroni posthoc analysis (C & D).
To determine the effect of SNC80 on depression-like behavior produced by withdrawal from cocaine, rats were injected with SNC80 (1 or 5 mg/kg) or saline 1 h prior to behavioral testing. As seen in figure 3C, SNC80 dose-dependently reduced the immobility behavior caused by withdrawal from cocaine (ANOVA: F5,46=2.637, P=0.0371; coc-sal vs coc-SNC80 5 mg/kg - Bonferroni's Multiple Comparison: P<0.05). Swimming and climbing behaviors were not significantly different between the treatment groups (separate ANOVAs, P>0.05). Rats undergoing withdrawal from saline and injected with SNC80 (1 or 5 mg/kg) showed no significant change in swimming (figure 3D, ANOVA: F=2.413 P=0.0524); however, climbing behavior was significantly different (figure 3D, ANOVA: F=4.113, P= 0.0041; sal-sal vs coc-SNC80 1 or 5 mg/kg).
3.4. Withdrawal from cocaine had no effect on spontaneous or SNC80-induced ambulatory activity
Figure 4 shows the time-course of ambulatory activity following saline or SNC80 (1 or 5 mg/kg) measured 24 h following the last injection of cocaine or saline. Repeated measures ANOVA showed a significant difference between groups (repeated measures ANOVA: F5,60=34.34, P<0.0001). No significant difference was seen between groups previously exposed to cocaine or saline (sal-sal vs coc-sal: Bonferroni's Multiple Comparison test: P>0.05). A significant effect was found between challenge doses of SNC80. Groups challenged with 1 mg/kg (P<0.01) or 5 mg/kg (P<0.001) SNC80 had significantly higher activity counts than those challenged with saline regardless of whether they previously received cocaine or saline. There were no differences in SNC80-induced activity between cocaine and saline pre-exposed groups (sal-SNC80 vs coc-SNC80: Bonferroni's Multiple Comparison test: P>0.05).
Figure 4. Withdrawal from cocaine has no effect on SNC80-stimulated ambulatory activity.
Rats were injected with cocaine or saline for 14 days. Twenty-three hours after the last injection rats were challenged with saline or SNC80 (1 or 5 mg/kg) and locomotor activity measured for 120 min. Ambulatory activity was time- and dose-dependently increased following SNC80 as determined by repeated-measures ANOVA. Bonferroni's posthoc analysis showed that 1 mg/kg (P<0.01) and 5 mg/kg (P<0.001) SNC80 induced hyperactivity when compared to saline-injected rats. Ambulatory activity was not significantly different between groups receiving saline versus cocaine at any dose of SNC80. Values are expressed as the mean ± SEM (N=7−8).
4. Discussion
The aim of the research described here was to determine the effects of early withdrawal from chronic administration of cocaine (i.e. one day after 14 day binge-pattern administration of cocaine) on neurochemistry and behavior in relation to the delta opioid receptor system. It was hypothesized that the functional desensitization of delta opioid receptors resulting from chronic exposure to cocaine contributes to anxiety- and depression-like behavioral phenotypes. The results of these studies show that early withdrawal from chronic administration of cocaine attenuated delta opioid receptor regulation of adenylyl cyclase activity and increased anxiety- and depression-like behaviors in the rat. Furthermore, delta opioid receptor activation by SNC80 reversed the cocaine-induced anxiety- and depression-like behaviors. No effect on locomotor activity was seen after chronic administration of cocaine, and SNC80-induced locomotor activity was not affected by prior cocaine administration. These data suggest that delta opioid receptor desensitization may contribute selectively to cocaine-induced anxiety and depression.
Previous data indicate that dopamine D1 receptors are involved in cocaine-induced delta opioid receptor desensitization. Direct activation of D1 receptors results in heterologous cross-desensitization of delta opioid receptor function (Unterwald and Cuntapay, 2000). Further, anatomical evidence supports the co-localization of opioid and dopamine receptors within the nucleus accumbens and caudate putamen. The opioid and dopamine systems in the nucleus accumbens and caudate putamen have been shown to be within close proximity of each other using ultrastructural microscopy (Pickel et al., 1992; Svingos et al., 1999). Dendritic spines labeled for delta opioid receptors are in direct contact with dopamine transporter-immunoreactive terminals (Svingos et al., 1999), and delta opioid receptors are expressed in the nucleus accumbens shell, a site rich in dopamine receptors (Svingos et al., 1998). Importantly, a recent study using electron microscopy immunohistochemistry directly demonstrates that delta opioid receptors and dopamine D1 receptor are co-localized in individual neurons of the striatum of rats (Ambrose et al., 2006). This opens the possibility for an intracellular mechanism by which dopamine D1 receptor activation could modulate delta opioid receptor function.
Co-morbidity of depression and cocaine dependence has been observed clinically and classical antidepressants often are ineffective treatments (Schmitz et al., 2001), suggesting a potential novel etiology. Roques and colleagues (Baamonde et al., 1992) investigated the interaction between delta opioid receptors and dopamine D1 receptors on depression-like behaviors and found that the antidepressant-like effects of enkephalins are mediated by delta opioid and dopamine D1 receptor stimulation. In their study, the mixed enkephalinase inhibitor, RB101, decreased depression scores in a rat forced swim test. This effect was attenuated by pretreatment with the delta opioid receptor antagonist naltrindole or the dopamine D1 receptor antagonist, SCH23390 (Baamonde et al., 1992). These data imply a functional interaction between delta opioid and dopamine D1 receptors in the regulation of behavioral depression. In addition to the delta opioid and dopaminergic systems, others could be involved in cocaine-mediated depression. Studies from Rothman and colleagues suggest that depression caused by withdrawal from cocaine is mediated in part by dysregulation in the adrenergic (Baumann et al., 2004) and serotonergic (Baumann and Rothman, 1998) systems. Their findings together with the results of the present study suggest that the dysregulation caused by cocaine, both directly on dopaminergic, serotonergic, and adrenergic systems and indirectly on the opioidergic system, contribute to the depression-like behaviors seen after cocaine use and withdrawal.
Clinical observations have shown that cocaine use and withdrawal can produce anxiety (Mackler and O'Brien, 1992; O'Brien, 1996), and it has been reported that users self-administer opiates to reduce cocaine-induced anxiety and irritability (Gawin, 1991; Kosten et al., 1986). Preclinical evidence supports the observation that withdrawal from cocaine causes anxiety-like behavior. In rats, cocaine can induce anxiety-like behaviors as measured in a number of pharmacological-animal models (Basso et al., 1999; Paine et al., 2002; Rogerio and Takahashi, 1992; Yang et al., 1992). The benzodiazepine, diazepam, can be effective in reducing cocaine-induced anxiety-like behavior (Ettenberg and Geist, 1991; Paine et al., 2002; Wood and Lal, 1987), however, the serotonergic agents, buspirone and SB 224289, are not (Hoplight et al., 2005; Paine et al., 2002).
Several studies show that plasma corticosterone levels in rats are elevated in response to acute and chronic administration of cocaine (Mantsch et al., 2000; Yang et al., 1992; Zhou et al., 2003). Furthermore, a corticotropin-releasing hormone antagonist can reverse cocaine-induced anxiety-like behaviors in rats (Basso et al., 1999). These studies illustrate that general activation of the hypothalamic-pituitary-adrenal (HPA) axis is triggered by cocaine. The effects of prolonged HPA axis activation, like that experienced during chronic exposure to cocaine, likely results in dysregulation of the system and induction of compensatory mechanisms to maintain homeostasis. In rats, endogenous opioids have been shown to inhibit the HPA axis and stress response (Kreek et al., 2002) and in mice, delta opioid receptor activation by endogenous enkephalins acts to maintain homeostasis of motivated and emotional behaviors (Nieto et al., 2005). Furthermore, corticosterone levels are increased following antagonism of delta opioid receptors in rats (Saitoh et al., 2005) suggesting a role for delta opioid receptors in maintaining homeostatic control of stress hormones and anxiety. Although the specific mechanism of action of the delta opioid receptor system in regulating cocaine-induced mood-related behaviors remains to be clarified, the present study adds to accumulating evidence suggesting a significant role of the delta opioid system in cocaine-induced anxiety and depression.
The data presented here indicate that the delta opioid receptor agonist SNC80 effectively reversed cocaine-induced anxiety and depression despite reduced delta opioid receptor function during short-term withdrawal from chronic administration of cocaine. There are several possible reasons for this apparent paradox. One possibility is that administration of the delta receptor agonist was able to sufficiently activate the remaining functional delta opioid receptors to produce changes in behavior. By elevating the concentration of agonist the diminished signaling of delta opioid receptors through adenylyl cyclase might have been overcome. Another possibility is that there may exist a substantial pool of spare receptors which are activated upon administration of SNC80. Studies in cell lines have shown that prolonged agonist exposure cause a loss of opioid signaling with minimal affect in binding affinity, suggesting that there is a receptor reserve (Law et al., 1982; Pak et al., 1996). Alternatively, the mood-altering effects of delta opioid agonists may be mediated by other brain regions that have an intact delta opioid receptor system. Here we assessed delta opioid receptor function only in the nucleus accumbens, caudate putamen, and frontal cortex. It is also possible that while delta opioid receptor regulation of adenylyl cyclase is attenuated following chronic administration of cocaine, signaling through another second messenger might still be intact. Further studies are necessary to resolve this issue.
Previous studies have shown that SNC80 produces anxiolytic effects in naive rats (Perrine et al., 2006; Saitoh et al., 2004). In the present study, SNC80 did not show a significant anxiolytic response in control animals that were injected with saline for 14 days. Repeated handling and injection of the animals over the 14 day injection period led to overall reduced anxiety tone in the control animals as indicated by higher baseline scores of open arm time and entries. This low level of baseline anxiety in the control animals makes it difficult to detect an anxiolytic effect on this test in the control animals, however cocaine-induced anxiety was readily reversed by SNC80. SNC80 also has been shown to decrease immobility on the rat forced swim test in naive animals at a dose of 32 mg/kg (Broom et al., 2002). The lower doses of SNC80 used in the present study (1 and 5 mg/kg) did not alter immobility scores in the control animals, but did reduce the heightened levels of immobility seen after cocaine exposure.
Clinical studies have shown that mood altering drugs exert their effects in part on serotonergic and dopaminergic neurotransmission (Holtzheimer and Nemeroff, 2006; Nutt, 2005, 2006). Preclinical research confirms that these drugs decrease anxiety- and depression-like behavior by influencing monoamines (Hindmarch, 2002). In the rat, there is evidence to suggest that the frontal cortex, nucleus accumbens, and striatum are sites of dopaminergic modulation of these behaviors. Sarkisova and colleagues demonstrated that “behavioral despair” precipitated by forced swimming causes an increase in neuronal activity as measured by c-fos expression in the frontal cortex, nucleus accumbens, and striatum (Sarkisova et al., 2003) which are the terminal projection sites of the mesocortical, mesolimbic and nigrostriatal dopaminergic pathways. Additional studies indicate that mesocortical and mesolimbic dopamine pathways are active in environment- and stress-induced behaviors (Willner et al., 1992; Zacharko and Anisman, 1991). These studies demonstrate that anxiety- and depression-like behaviors are mediated in part by dopaminergic receptors in the frontal cortex, nucleus accumbens, and caudate putamen. The interaction of the delta opioid and dopamine D1 receptors in regulating these behaviors is not completely understood. However, since our previous studies demonstrate that delta opioid receptor desensitization occurs following exposure to a dopamine D1 receptor agonist (Unterwald and Cuntapay, 2000) and that delta opioid receptor antagonists increase anxiety-like behaviors (Perrine et al., 2005), we hypothesize that D1 receptor activation secondary to cocaine administration leads to a heterologous desensitization of delta opioid receptor function which is associated with increases in anxiety-like behaviors.
In summary, the data presented here and the literature reviewed support the hypothesis that dopaminergic and opioidergic systems interact to modulate neuronal function and emotional behavior. Despite the noted interaction of these two systems at a functional and anatomical level, the molecular basis of their interaction as related to cocaine abuse and withdrawal is an area worthy of further investigation. Future research will focus on understanding the molecular basis of cocaine-induced delta opioid receptor heterologous desensitization. Meanwhile, the data presented herein demonstrate that early withdrawal from chronic administration of cocaine results in a behavioral phenotype consistent with heightened levels of anxiety and depression which may be mediated in part by loss of delta opioid receptor function. Since administration of a delta opioid receptor agonist can reverse this behavioral phenotype, therapeutics targeted at the delta opioid receptor system may prove to be useful during early abstinence from cocaine.
Acknowledgements
The authors would like to thank Kris Guardiario and Mary McCafferty for their technical assistance and Drs. Alan Cowan, Tom Gould and Michelle Page for their advice and assistance with the behavioral testing. This work was supported by NIH/NIDA grants R01DA018326 (EMU) and T32DA007237 (EMU/SAP). There are no conflicts of interest to disclose for any of the authors relating to this submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrose LM, Perrine SA, Van Bockstaele EJ, Unterwald EM. Chronic cocaine administration attenuates delta-opioid receptor function in female rats. Society for Neuroscience; Atlanta, GA: 2006. [Google Scholar]
- Azaryan AV, Coughlin LJ, Buzas B, Clock BJ, Cox BM. Effect of chronic cocaine treatment on mu- and delta-opioid receptor mRNA levels in dopaminergically innervated brain regions. J Neurochem. 1996;66:443–448. doi: 10.1046/j.1471-4159.1996.66020443.x. [DOI] [PubMed] [Google Scholar]
- Baamonde A, Dauge V, Ruiz-Gayo M, Fulga IG, Turcaud S, Fournie-Zaluski MC, Roques BP. Antidepressant-type effects of endogenous enkephalins protected by systemic RB 101 are mediated by opioid delta and dopamine D1 receptor stimulation. Eur J Pharmacol. 1992;216:157–166. doi: 10.1016/0014-2999(92)90356-9. [DOI] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Milchanowski AB, Rothman RB. Evidence for alterations in alpha2-adrenergic receptor sensitivity in rats exposed to repeated cocaine administration. Neuroscience. 2004;125:683–690. doi: 10.1016/j.neuroscience.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Rothman RB. Alterations in serotonergic responsiveness during cocaine withdrawal in rats: similarities to major depression in humans. Biol Psychiatry. 1998;44:578–591. doi: 10.1016/s0006-3223(98)00123-1. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Cocaine potentiates defensive behaviors related to fear and anxiety. Neurosci Biobehav Rev. 1999;23:981–991. doi: 10.1016/s0149-7634(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Brown BL, Albano JD, Ekins RP, Sgherzi AM. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971;121:561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology (Berl) 1991;103:455–461. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Fink JS, Smith GP. Mesolimbicocortical dopamine terminal fields are necessary for normal locomotor and investigatory exploration in rats. Brain Res. 1980;199:359–384. doi: 10.1016/0006-8993(80)90695-2. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Hammer RP,, Jr. Cocaine alters opiate receptor binding in critical brain reward regions. Synapse. 1989;3:55–60. doi: 10.1002/syn.890030108. [DOI] [PubMed] [Google Scholar]
- Handley SL, McBlane JW. An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J Pharmacol Toxicol Methods. 1993;29:129–138. doi: 10.1016/1056-8719(93)90063-k. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Orlansky H, Cohen G. Studies on the distinction between uptake inhibition and release of (3H)dopamine in rat brain tissue slices. Biochem Pharmacol. 1975;24:847–852. doi: 10.1016/0006-2952(75)90152-5. [DOI] [PubMed] [Google Scholar]
- Hindmarch I. Beyond the monoamine hypothesis: mechanisms, molecules and methods. Eur Psychiatry. 2002;17(Suppl 3):294–299. doi: 10.1016/s0924-9338(02)00653-3. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE,, 3rd, Nemeroff CB. Advances in the treatment of depression. NeuroRx. 2006;3:42–56. doi: 10.1016/j.nurx.2005.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoplight BJ, Vincow ES, Neumaier JF. The effects of SB 224289 on anxiety and cocaine-related behaviors in a novel object task. Physiol Behav. 2005;84:707–714. doi: 10.1016/j.physbeh.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Brown EE, Finlay JM, Fibiger HC, Gerfen CR. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Brain Res Mol Brain Res. 1992;13:165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Heller B, Cox BM. Continuous cocaine administration enhances mu- but not delta-opioid receptor-mediated inhibition of adenylyl cyclase activity in nucleus accumbens. Eur J Pharmacol. 1996;297:187–191. doi: 10.1016/0014-2999(95)00828-4. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Rice KC, Woods JH, Winsauer PJ. Effects of the delta-opioid receptor agonist SNC80 on learning relative to its antidepressant-like effects in rats. Behav Pharmacol. 2003;14:509–516. doi: 10.1097/00008877-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Gawin FH, Rounsaville BJ, Kleber HD. Cocaine abuse among opioid addicts: demographic and diagnostic factors in treatment. Am J Drug Alcohol Abuse. 1986;12:1–16. doi: 10.3109/00952998609083739. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Bor GL, Zhou Y, Schluger J. Relationship between endocrine functions and substance abuse syndromes: Heroin and related short-acting opiates in addiction contrasted with methadone and other long-acting opioid agonists used in pharmacotherapy of addiction. In: Pfaff D, editor. Hormones, Brain and Behavior. Academic Press; San Diego: 2002. pp. 781–830. [Google Scholar]
- Law PY, Hom DS, Loh HH. Loss of opiate receptor activity in neuroblastoma X glioma NG108−15 hybrid cells after chronic opiate treatment. A multiple-step process. Mol Pharmacol. 1982;22:1–4. [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Mackler SA, O'Brien CP. Cocaine abuse. Adv Intern Med. 1992;37:21–35. [PubMed] [Google Scholar]
- Magalhaes A, Tavares MA, de Sousa L. Postnatal cocaine exposure: effects on behavior of rats in forced swim test. Ann N Y Acad Sci. 2002;965:529–534. doi: 10.1111/j.1749-6632.2002.tb04194.x. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Effects of cocaine self-administration on plasma corticosterone and prolactin in rats. J Pharmacol Exp Ther. 2000;294:239–247. [PubMed] [Google Scholar]
- Marin S, Marco E, Biscaia M, Fernandez B, Rubio M, Guaza C, Schmidhammer H, Viveros MP. Involvement of the kappa-opioid receptor in the anxiogenic-like effect of CP 55,940 in male rats. Pharmacol Biochem Behav. 2003;74:649–656. doi: 10.1016/s0091-3057(02)01041-9. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Moleman P, van Valkenburg CF, vd Krogt JA. Effects of morphine on dopamine metabolism in rat striatum and limbic structures in relation to the activity of dopaminergic neurones. Naunyn Schmiedebergs Arch Pharmacol. 1984;327:208–213. doi: 10.1007/BF00502451. [DOI] [PubMed] [Google Scholar]
- Nieto MM, Guen SL, Kieffer BL, Roques BP, Noble F. Physiological control of emotion-related behaviors by endogenous enkephalins involves essentially the delta opioid receptors. Neuroscience. 2005;135:305–313. doi: 10.1016/j.neuroscience.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectr. 2005;10:49–56. doi: 10.1017/s1092852900009901. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry. 2006;67(Suppl 6):3–8. [PubMed] [Google Scholar]
- O'Brien CP. Recent developments in the pharmacotherapy of substance abuse. J Consult Clin Psychol. 1996;64:677–686. doi: 10.1037//0022-006x.64.4.677. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci. 2001;21:RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Moy SS, Lubin DA, Gause LR, Lieberman JA, Johns JM. Enduring effects of prenatal cocaine administration on emotional behavior in rats. Physiol Behav. 2000;70:149–156. doi: 10.1016/s0031-9384(00)00245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, ackman SL, Olmstead MC. Cocaine-induced anxiety: alleviation by diazepam, but not buspirone, dimenhydrinate or diphenhydramine. Behav Pharmacol. 2002;13:511–523. doi: 10.1097/00008877-200211000-00001. [DOI] [PubMed] [Google Scholar]
- Pak Y, Kouvelas A, Scheideler MA, Rasmussen J, O'Dowd BF, George SR. Agonist-induced functional desensitization of the mu-opioid receptor is mediated by loss of membrane receptors rather than uncoupling from G protein. Mol Pharmacol. 1996;50:1214–1222. [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Hoshaw BA, Unterwald EM. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol. 2006;147:864–872. doi: 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Schroeder JA, Unterwald EM. Behavioral sensitization to binge-pattern cocaine administration is not associated with changes in protein levels of four major G-proteins. Brain Res Mol Brain Res. 2005;133:224–232. doi: 10.1016/j.molbrainres.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Sesack SR. Cellular basis for interactions between catecholaminergic afferents and neurons containing Leu-enkephalin-like immunoreactivity in rat caudate-putamen nuclei. J Neurosci Res. 1992;31:212–230. doi: 10.1002/jnr.490310203. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav. 1982;17:901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacol Biochem Behav. 1992;43:631–633. doi: 10.1016/0091-3057(92)90203-r. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Yoshikawa Y, Onodera K, Kamei J. Role of delta-opioid receptor subtypes in anxiety-related behaviors in the elevated plus-maze in rats. Psychopharmacology (Berl) 2005;182:327–334. doi: 10.1007/s00213-005-0112-6. [DOI] [PubMed] [Google Scholar]
- Sarkisova KY, Midzianovskaia IS, Kulikov MA. Depressive-like behavioral alterations and c-fos expression in the dopaminergic brain regions in WAG/Rij rats with genetic absence epilepsy. Behav Brain Res. 2003;144:211–226. doi: 10.1016/s0166-4328(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Averill P, Stotts AL, Moeller FG, Rhoades HM, Grabowski J. Fluoxetine treatment of cocaine-dependent patients with major depressive disorder. Drug Alcohol Depend. 2001;63:207–214. doi: 10.1016/s0376-8716(00)00208-8. [DOI] [PubMed] [Google Scholar]
- Sivam SP. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J Pharmacol Exp Ther. 1989;250:818–824. [PubMed] [Google Scholar]
- Sobrian SK, Marr L, Ressman K. Prenatal cocaine and/or nicotine exposure produces depression and anxiety in aging rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:501–518. doi: 10.1016/S0278-5846(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Clarke CL, Pickel VM. Cellular sites for activation of delta-opioid receptors in the rat nucleus accumbens shell: relationship with Met5-enkephalin. J Neurosci. 1998;18:1923–1933. doi: 10.1523/JNEUROSCI.18-05-01923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Clarke CL, Pickel VM. Localization of the delta-opioid receptor and dopamine transporter in the nucleus accumbens shell: implications for opiate and psychostimulant cross-sensitization. Synapse. 1999;34:1–10. doi: 10.1002/(SICI)1098-2396(199910)34:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Cox BM, Kreek MJ, Cote TE, Izenwasser S. Chronic repeated cocaine administration alters basal and opioid-regulated adenylyl cyclase activity. Synapse. 1993;15:33–38. doi: 10.1002/syn.890150104. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Cuntapay M. Dopamine-opioid interactions in the rat striatum: a modulatory role for dopamine D1 receptors in delta opioid receptor-mediated signal transduction. Neuropharmacology. 2000;39:372–381. doi: 10.1016/s0028-3908(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport. 1994;5:1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Wood DM, Lal H. Anxiogenic properties of cocaine withdrawal. Life Sci. 1987;41:1431–1436. doi: 10.1016/0024-3205(87)90619-9. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–650. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]
- Zacharko RM, Anisman H. Stressor-induced anhedonia in the mesocorticolimbic system. Neurosci Biobehav Rev. 1991;15:391–405. doi: 10.1016/s0149-7634(05)80032-6. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ. Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of proopiomelanocortin and corticotropin-releasing hormone-receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic ‘binge’ cocaine and withdrawal. Brain Res. 2003;964:187–199. doi: 10.1016/s0006-8993(02)03929-x. [DOI] [PubMed] [Google Scholar]