Abstract
ROP small G proteins function as molecular switches in diverse signaling processes. Here, we investigated signals that activate ROP2 in guard cells. In guard cells of Vicia faba expressing Arabidopsis thaliana constitutively active (CA) ROP2 fused to red fluorescent protein (RFP-CA-ROP2), fluorescence localized exclusively at the plasma membrane, whereas a dominant negative version of RFP-ROP2 (DN-ROP2) localized in the cytoplasm. In guard cells expressing green fluorescent protein–ROP2, the relative fluorescence intensity at the plasma membrane increased upon illumination, suggesting that light activates ROP2. Unlike previously reported light-activated factors, light-activated ROP2 inhibits rather than accelerates light-induced stomatal opening; stomata bordered by guard cells transformed with CA-rop2 opened less than controls upon light irradiation. When introduced into guard cells together with CA-ROP2, At RhoGDI1, which encodes a guanine nucleotide dissociation inhibitor, inhibited plasma membrane localization of CA-ROP2 and abolished the inhibitory effect of CA-ROP2 on light-induced stomatal opening, supporting the negative effect of active ROP2 on stomatal opening. Mutant rop2 Arabidopsis guard cells showed phenotypes similar to those of transformed V. faba guard cells; CA-rop2 stomata opened more slowly and to a lesser extent, and DN-rop2 stomata opened faster than wild-type stomata in response to light. Moreover, in rop2 knockout plants, stomata opened faster and to a greater extent than wild-type stomata in response to light. Thus, ROP2 is a light-activated negative factor that attenuates the extent of light-induced changes in stomatal aperture. The inhibition of light-induced stomatal opening by light-activated ROP2 suggests the existence of feedback regulatory mechanisms through which stomatal apertures may be finely controlled.
INTRODUCTION
Guard cells play a critical role in plant growth and development by optimizing gas exchange under variable environments. For this purpose, guard cells have developed a highly elaborate signaling network. Guard cells sense a variety of environmental and internal conditions, such as light and circadian clock signals, atmospheric CO2 levels, humidity, temperature, hormones, and pathogens. Signaling pathways are coordinated and finally converge on cellular responses that cause guard cell volume changes: activation/inactivation of ion channels, reorganization of the actin cytoskeleton, endocytosis/exocytosis, as well as changes in gene expression. Signaling molecules that regulate these responses include cytosolic Ca2+, protein kinases and phosphatases, reactive oxygen species, heterotrimeric G proteins, and ROP small G proteins (Assmann and Wang, 2001; Schroeder et al., 2001).
Plants possess a distinct family of Rho GTPases, named ROP (Yang and Watson, 1993; Winge et al., 1997), but apparently lack orthologs of Cdc42, Rac, and Rho. A number of ROPs have been reported from various plant species (Yang and Watson, 1993; Bischoff et al., 1999; Gu et al., 2004). The Arabidopsis thaliana genome encodes 11 ROP genes categorized into four phylogenetic groups, which share high homology. Multiple ROPs of each group may play different or partially overlapping roles in the regulation of specific pathways, depending on their spatiotemporal expression and localization in the cell (Yang and Watson, 1993; Gu et al., 2004). Recent studies in Arabidopsis and rice (Oryza sativa) indicate that ROP participates in many different signaling pathways, leading to the suggestion that ROP acts as an important versatile molecular switch in plants in place of Cdc42, Rac, and Rho (for review, see Yang and Watson, 1993). This is consistent with findings in animal cells, in which many different small G proteins are activated in a signal-dependent manner and function as molecular switches in signaling (Bourne et al., 1990; Van Aelst and D'Souza-Schorey, 1997).
The ROP small GTPases play critical roles in growth and the determination of growth sites in developing epidermal cells and pollen tubes by regulating the dynamics of the actin cytoskeleton and/or establishing a cytosolic Ca2+ gradient (Yang and Watson, 1993; Fu and Yang, 2001, Fu et al., 2001, 2005; Gu et al., 2005). ROP1 and the closely related ROP5/AtRAC2 are localized at the growing pollen tube tip (Lin et al., 1996; Kost et al., 1999; Li et al., 1999). Another group of ROPs (ROP2, ROP4, and ROP6) positively regulate root hair initiation and tip growth as well as lobe expansion in leaf epidermal pavement cells (Fu et al., 2001, 2005; Molendijk et al., 2001; Jones et al., 2002). ROP2, localized at the expanding lobes of leaf pavement cells, regulates the assembly of growth site–localized actin filaments (Fu et al., 2001, 2005). ROP small GTPases are involved in reactive oxygen species production as well. ROP-regulated reactive oxygen species (H2O2) production seems to be essential for secondary cell wall development in cotton (Gossypium hirsutum) fibers (Potikha et al., 1999), resistance to pathogen attack in rice (Kawasaki et al., 1999), phosphatidic acid–induced leaf cell death (Park et al., 2000), and oxygen deprivation responses (Baxter-Burrell et al., 2002) in Arabidopsis. Hormonal signaling is also ROP-dependent (Lemichez et al., 2001; Li et al., 2001; Zheng et al., 2002; Tao et al., 2002). Multiple aspects of development are affected in constitutively active (CA-rop2) and dominant negative (DN-rop2) Arabidopsis plants, suggesting that many hormonal signals require ROP (Li et al., 2001). There is evidence that a subset of ROP GTPases function in auxin signaling to downstream responsive genes (Tao et al., 2002). In addition, the involvement of ROPs in abscisic acid pathways is supported by several studies. Abscisic acid inactivates ROPs, and expression of CA-rop6 (CA-AtRac1) inhibited stomatal responses to abscisic acid (Lemichez et al., 2001). A rop10 knockout mutant showed hypersensitive responses to abscisic acid during stomatal closure and inhibition of root elongation (Zheng et al., 2002). Therefore, ROP6 and ROP10 are negative regulators of abscisic acid responses.
Rho small G proteins are regulated by many other proteins. For example, in animal cells, RhoGDI binds GDP-bound forms of Rho GTPases and inhibits GDP–GTP exchange, thus inhibiting activation of these small G proteins (Hancock and Hall, 1993). In plants, At RhoGDI1 has been shown to bind wild-type and active forms of ROP4 and ROP6 but not the GDP-bound forms of the same G proteins (Bischoff et al., 2000).
Since ROP proteins are involved in diverse processes, they are expected to interact with many different partners that translate the activation status of the ROPs into specific responses. Recently, ROP-interactive CRIB motif–containing proteins (RICs) were identified as direct ROP targets, which specifically interact with GTP-bound ROP1 (Wu et al., 2001; Fu et al., 2005; Gu et al., 2005). RICs are diverse molecules that share homology only in the CRIB motif, which is necessary for ROP binding, and they are suggested to be adaptors that link ROPs to diverse effector molecules. Indeed, expression of the RIC genes in tobacco (Nicotiana tabacum) pollen tubes causes distinct phenotypes, implying distinct functions for various RICs, which may constitute the basis for the functional versatility of ROP proteins (Wu et al., 2001). Multiple RICs can interact with one ROP protein in a single cell type, as shown in the regulation of interdigitating growth of pavement cells by RIC1 and RIC4, which commonly interact with ROP2 to form complex cell shapes (Fu et al. 2005; Gu et al., 2005).
ROP2 is expressed in all vegetative tissues and belongs to the largest ROP subgroup (Winge et al., 1997; Li et al., 1998, 2001). Although ROP2 has been suggested to play a role in multiple signaling pathways (Li et al., 2001), the signals that activate this protein are not well understood. Here, we investigated which physiological signals activate ROP2 proteins in guard cells and how active ROP2 modulates stomatal movements. Our results show that light causes the translocation of ROP2 to the plasma membrane, suggesting that it is activated by a light stimulus. Moreover, we show that active ROP2, which in turn recruits RIC7, prevents excessive stomatal opening upon perception of light. The inhibition of light-induced stomatal opening by activated ROP2 suggests the existence of a regulatory mechanism by which stomatal opening is reduced, contributing to water conservation under conditions that induce excessive stomatal opening. ROP2 is a negative factor activated by light, with ROP2 activity leading to attenuation of the light response of guard cells. Therefore, this work describes an additional mode of regulation of stomatal aperture in the light.
RESULTS
ROP2 Is Expressed in Mature Guard Cells
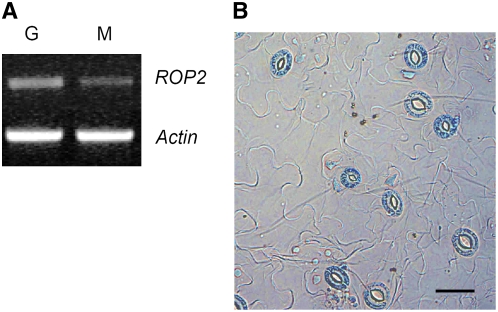
RT-PCR and microarray data using RNA extracted from Arabidopsis guard cell and mesophyll cell protoplasts identified ROP2 expression in both cell types (Figure 1A; see Supplemental Figure 1 online). The expression level was higher in guard cells than in mesophyll cells. ROP2 expression in mature guard cells was confirmed by β-glucuronidase (GUS) activity assay of transgenic Arabidopsis expressing ROP2 promoter:GUS fusions (Figure 1B).
Figure 1.
ROP2 Gene Expression in Arabidopsis Guard Cells.
(A) ROP2 PCR products were amplified from 1 μg of total RNA from guard cell (G) and mesophyll cell (M) protoplasts. PCR was repeated twice with similar results. Actin was used as a loading control.
(B) Histochemical assay of GUS activity in 4-week-old Arabidopsis seedlings expressing ROP2 promoter:GUS fusions. Bar = 50 μm.
Active ROP2 Is Localized at the Plasma Membrane of Guard Cells
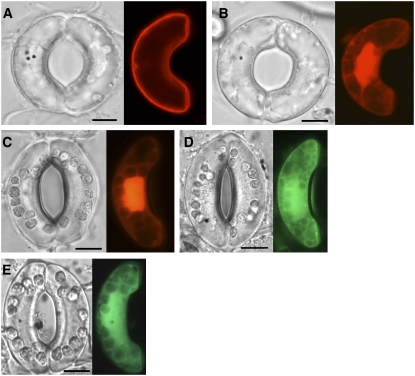
The activation of Rho proteins is often accompanied by translocation to the plasma membrane (Quinn et al., 1993; Heyworth et al., 1994), where Rho interacts with downstream effector molecules. If this is also true for ROP2, we reasoned that CA- and DN-ROP2 may have different localizations in the cell. Indeed, red fluorescent protein (RFP)–CA-ROP2 was localized exclusively at the plasma membrane of Vicia faba guard cells (Figure 2A; n = 30), whereas most of the RFP-DN-ROP2 was found in the cytoplasm in all cells examined (Figure 2B; n = 100), with a distribution similar to that of soluble RFP (Figure 2C). These results suggest that active ROP2 is localized at the plasma membrane, where it may interact with effectors and modulate various downstream signaling pathways.
Figure 2.
Localization of ROP2 in V. faba Guard Cells.
(A) An intact V. faba guard cell transformed with RFP-CA-ROP2.
(B) An intact V. faba guard cell transformed with RFP-DN-ROP2.
(C) An intact V. faba guard cell transformed with RFP alone.
(D) An intact V. faba guard cell transformed with GFP-ROP2.
(E) An intact V. faba guard cell transformed with GFP alone.
In all panels, the guard cells at left were transformed by biolistic bombardment and the cells at right were not. A fluorescence image (right) and the corresponding bright-field image (left) of the same cell are shown. The guard cells shown in (D) and (E) were kept under darkness after bombardment until observation, and those in (A) to (C) were irradiated with white light for 3 h before observation. Black dots in bright-field images are gold particles. Focus was on the mid-plane of the cells. Bars = 10 μm.
In intact V. faba guard cells transformed using particle bombardment (biolistic transformation), green fluorescent protein (GFP)–ROP2 fluorescence was detected at the plasma membrane and also in the cytoplasm (Figure 2D). By contrast, soluble GFP was dispersed throughout the cytoplasm and was not found at the plasma membrane (Figure 2E). These data suggest that only a fraction of the ROP2 small GTPase is in the active state in V. faba guard cells in the absence of a stimulus.
Light Induces the Translocation of ROP2 to the Plasma Membrane
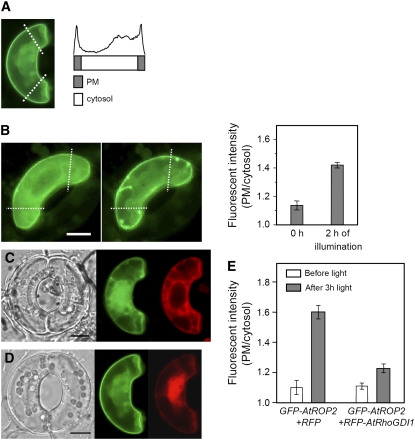
To identify physiological stimuli that activate ROP2 protein in guard cells, we subjected V. faba guard cells expressing GFP-ROP2 to light irradiation, a potent stimulus for guard cell opening, and observed the localization of GFP-ROP2. GFP fluorescence images of transformed cells were analyzed by scanning the pixel intensity along lines perpendicular to the axis of the cell. Intensity values at the boundaries (plasma membrane) of the cells were averaged and compared with the average values obtained from the cell interior (cytosol) (Figure 3A). Guard cells illuminated for 3 h with white light of 250 μmol·m−2·s−1 had higher relative fluorescence at the plasma membrane compared with dark control cells. The intensity ratio of the plasma membrane to the cell interior was 1.6 ± 0.05 (n = 32) for illuminated cells and 1.1 ± 0.05 (n = 36) for cells held in the dark, demonstrating a statistically significant (P < 0.02) movement of GFP-ROP2 to the plasma membrane upon light stimulation (Figure 3E, left). When changes in individual cells were followed (Figure 3B), the intensity ratio increased from 1.1 ± 0.03 to 1.4 ± 0.02 after 2 h of light irradiation (n = 11, P < 0.02), although the stomatal aperture change was restricted by mounting the epidermal pieces between the cover slip and the slide glass during the observations. On average, the fluorescence intensity of GFP-ROP2 in the cytosol did not change during this time, indicating that the increase in intensity ratio induced by light was attributable to the increase in fluorescence intensity of GFP-ROP2 at the plasma membrane (see Supplemental Figure 2 online). As a control, the fluorescence intensity of AHA2-GFP, a plasma membrane marker that is constitutively localized to the plasma membrane, was also assayed and found not to change in response to light (see Supplemental Figure 3 online). We also show that, in guard cells of open stomata, GFP-ROP2 was not colocalized with markers of Golgi, endoplasmic reticulum, or the prevacuolar compartment/vacuolar membrane. Additionally, GFP-ROP2 did not delineate the vacuole; rather, it colocalized with the plasma membrane marker FM4-64 (see Supplemental Figure 4 online). Together with our previous observation that active ROP2 is localized at the plasma membrane of V. faba guard cells (Figure 2), this light-induced translocation of ROP2 to the plasma membrane implies light activation of ROP2.
Figure 3.
Light-Dependent Translocation of GFP-ROP2 to the Plasma Membrane in Guard Cells.
(A) Cartoon describing how GFP-ROP2 localization to the plasma membrane (PM) and cytosol was measured. From the fluorescent images of guard cells expressing GFP-ROP2, GFP intensity was line-scanned along two lines drawn at right angles to the long axis of the cells, at ∼25% distance from both ends (white dotted lines in the left panel). The average pixel intensities of the plasma membrane (gray bar) and the cytosol (white bar) were obtained from the GFP intensity profiles as indicated.
(B) Light illumination induces GFP-ROP2 translocation to the plasma membrane. The intensity of GFP-ROP2 localized to the plasma membrane was measured as described for (A) before and after 2 h of illumination from individual guard cells. The resulting pixel intensity of plasma membrane/cytosol is displayed (right panel; means ± se; n = 11, P < 0.02). Stomatal movements are inhibited by mounting the epidermal pieces between the cover slip and the slide glass during observations. Focus was on the mid-plane of the cells. Bar = 10 μm.
(C) and (D) Intact V. faba guard cells transformed with GFP-ROP2 and RFP-RhoGDI1 (C) or GFP-ROP2 and RFP alone (D) and incubated under light for 3 h. Bars = 10 μm.
(E) Relative pixel intensity at the plasma membrane of GFP-At ROP2 from guard cells transiently transformed with GFP-ROP2 together with RFP-RhoGDI1 (C) or with RFP (D) in darkness or after 3 h of illumination. Results from three independent experiments are shown (means ± se; n = 32 to 60).
To further examine the light-induced activation of ROP2, we used At RhoGDI1, which has been shown to interact with wild-type and constitutively active forms of ROP4 and ROP6 (Bischoff et al., 2000). RhoGDIs sequester Rho GTPases in the cytosol, thereby inactivating Rho GTPases. When biolistically introduced into guard cells together with GFP-ROP2, RFP-RhoGDI1 inhibited the light-induced translocation of GFP-ROP2 to the plasma membrane (Figures 3C and 3E, right; n = 60). Control guard cells that expressed free RFP together with GFP-ROP2 showed normal light-induced translocation of GFP-ROP2 to the plasma membrane (Figures 3D and 3E, left; n = 32). RFP-DN-ROP2 remained in the cytoplasm even under prolonged light irradiation (n > 100; Figure 2B), while RFP-CA-ROP2 was always found at the plasma membrane regardless of the light condition (n > 100; Figure 2A; data not shown for darkness). The activity-dependent localization of ROP2 and the RhoGDI-mediated inhibition of the light-dependent translocation indicate that light activates ROP2.
Active ROP2 Suppresses the Stomatal Opening of Vicia faba Guard Cells
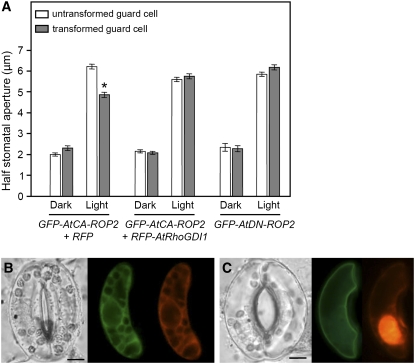
To understand the role of ROP2 at the plasma membrane, we measured apertures of stomata bordered by V. faba guard cells transiently expressing mutant ROP2 proteins and compared them with those of untransformed guard cells. Expression of GFP-CA-ROP2 suppressed light-induced stomatal opening movements of V. faba (Figure 4A, left). When illuminated for 3 h, half-stomata bordered by guard cells overexpressing GFP-CA-ROP2 opened less (4.9 ± 0.13 μm) than those bordered by untransformed guard cells (6.2 ± 0.12 μm; P < 0.02, one-tailed t test). RFP-CA-ROP2 showed a similar inhibitory effect on light-induced stomatal opening (data not shown). These results were surprising since no other light-activated factors have been reported to inhibit light-induced stomatal opening. Therefore, we further tested the inhibitory effect of active ROP2 using RhoGDI1, which inhibited light-dependent translocation of the wild-type ROP2 to the plasma membrane (Figures 3C and 3E, right). RFP-RhoGDI1 biolistically introduced into guard cells together with GFP-CA-ROP2 also inhibited the localization of GFP-CA-ROP2 to the plasma membrane (Figure 4B; n = 104), whereas free RFP introduced into guard cells did not have this effect (Figure 4C; n = 92). Furthermore, RFP-RhoGDI1 abolished the inhibitory effect of GFP-CA-ROP2 on stomatal opening (Figure 4A, center; n = 104), while GFP-DN-ROP2 did not alter light-induced stomatal opening (Figure 4A, right). These results suggest that ROP2 protein is a negative factor that attenuates the extent of light-induced changes in stomatal aperture.
Figure 4.
Effects of RhoGDI1 Expression on CA-ROP2–Induced Stomatal Movements and Localization of CA-ROP2.
(A) Light-induced opening of guard cells transformed with GFP-CA-ROP2 and RFP, GFP-CA-ROP2 and RFP-RhoGDI1, or GFP-DN-ROP2, compared with their untransformed neighbor cells. Stomatal apertures were measured after 3 h of exposure to light. Results from three independent experiments are shown (means ± se; n > 90). * P < 0.02.
(B) and (C) Intact V. faba guard cells transiently transformed with GFP-CA-ROP2 together with RFP-RhoGDI1 (B) or RFP (C). Bars = 10 μm.
Active ROP2 Negatively Regulates Light-Induced Stomatal Opening in Arabidopsis
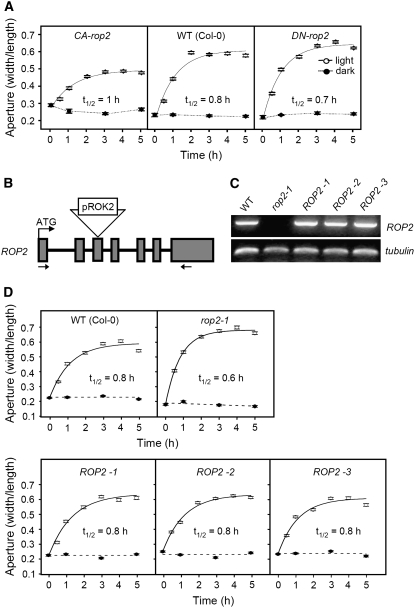
To test whether ROP2 modulates stomatal movement in Arabidopsis guard cells as shown in V. faba guard cells, stomatal responses of transgenic Arabidopsis plants that express CA- or DN-rop2 were compared with those of wild-type Arabidopsis. Fully expanded young rosette leaves of 4- to 5-week-old CA1 and DN2-3 plants (Li et al., 2001) were analyzed.
For the stomatal opening assay, the leaves were incubated overnight in stomata assay buffer in the dark, then the initial stomatal aperture was measured. CA-rop2 plants were larger in initial stomatal aperture than wild-type and DN-rop2 plants in terms of both width:length ratio (Figure 5A; P < 0.02) and absolute values (wild type, 1.8 ± 0.06 μm; CA-rop2, 2.6 ± 0.07 μm; DN-rop2, 1.7 ± 0.06 μm). This suggests that ROP2 activation suppresses stomatal closure at night. We next illuminated leaves with white light with an intensity of 250 μmol·m−2·s−1 from the beginning of the photoperiod and compared stomatal responses in the early photoperiod (0 to 5 h) (Figure 5A).
Figure 5.
Light-Induced Stomatal Opening in Wild-Type and Mutant rop2 Arabidopsis.
(A) Light-induced stomatal opening in CA-rop2, wild-type (ecotype Columbia [Col-0]), and DN-rop2 Arabidopsis.
(B) Gene structure of ROP2 and site of the T-DNA insertion in the rop2 knockout line in rop2-1. Boxes represent exons. Arrows indicate the regions of ROP2 from which the ROP2-specific primers were designed for RT-PCR. pROK2, T-DNA present in the SALK Arabidopsis mutants.
(C) RT-PCR amplification of ROP2 mRNA. ROP2 transcript was amplified from wild-type and three independent complemented lines (ROP2-1, ROP2-2, and ROP2-3) but not from rop2-1. Tubulin was amplified as a positive control.
(D) Light-induced stomatal opening in wild-type (Col-0), rop2-1, and three independent lines of ROP2-complemented rop2-1 Arabidopsis. White light with an intensity of 250 μmol·m−2·s−1 was used to irradiate the plants. Results (means ± se; n > 250) from four independent experiments are shown. In each data set, the extent of increase in stomatal aperture and the time to reach half-maximum stomatal aperture (t1/2) were obtained by fitting the data from illuminated samples to a first-order exponential function.
Within 30 min, stomatal opening of Arabidopsis plants became apparent, and the maximum apertures were obtained by 3 h. Among the three groups of plants, CA-rop2 Arabidopsis mutants opened their stomata the slowest and the least, consistent with our results in V. faba. The maximum stomatal opening was 160% of the initial level, and the half-maximum opening was obtained by 1 h (half-life = 1 h). In wild-type Arabidopsis, the maximum stomatal opening was 260% of the initial level, and half-maximum opening was reached by 0.8 h. By contrast, DN-rop2 stomata opened faster and the extent of aperture increase was larger. By 0.7 h, DN-rop2 mutants had reached their half-maximum, and the maximum stomatal opening was 290% of the initial level. The difference in stomatal opening was statistically significant between wild-type and DN-rop2 at early time points; the stomatal apertures of DN-rop2 were significantly different from those of the wild type at 0.5 and 1 h (P < 0.02). In short, active ROP2 reduced the extent of opening and slowed stomatal opening in the light in Arabidopsis, similar to the results seen in V. faba. When light intensity was reduced to 150 μmol·m−2·s−1, the difference in final stomatal apertures became less pronounced (data not shown), but CA-rop2 stomata still opened more slowly than the wild type or DN-rop2 (half-life = 1.2 h for CA-rop2, 0.8 h for the wild type, and 0.7 h for DN-rop2), similar to bright-light conditions.
To further confirm the role of ROP2 in the modulation of stomatal movement in Arabidopsis guard cells, stomatal responses of ROP2 knockout Arabidopsis plants were compared with those of the wild type. ROP2 knockout seeds were obtained from the Salk Institute Genomic Analysis Laboratory. This knockout plant has a T-DNA insertion in the third exon of the At ROP2 gene (rop2-1; http://signal.salk.edu/cgi-bin/tdnaexpress; SALK stock number SALK_055328) (Figure 5B; see Supplemental Figure 5 online). To confirm that the homozygous rop2-1 line does not produce the ROP2 transcript, RT-PCR was performed using total RNA extracted from the plants. PCR using a pair of primers specific to ROP2, at regions in the first and last exons of the gene, did not produce any product from the homozygous knockout plants, but the transcript was present in wild-type Columbia plants (Figure 5C). Fully expanded young rosette leaves were irradiated with white light with an intensity of 250 μmol·m−2·s−1, and stomatal responses early in the photoperiod (0 to 5 h) were compared (Figure 5D). In wild-type Arabidopsis, the maximum stomatal opening was 260% of the initial level, and it took 0.8 h to reach half-maximum stomatal opening. By contrast, in rop2-1 Arabidopsis, stomata opened faster and the extent of aperture increase was larger. At 0.6 h, stomata reached half-maximum opening and their maximum aperture was 380% of the initial level. The difference in stomatal opening was statistically significant between the wild type and rop2-1 at early time points; the stomatal apertures of rop2-1 were significantly different from those of the wild type at 0.5 and 1 h (P < 0.02). Thus, the absence of ROP2 increased the extent of opening and accelerated opening in the light in Arabidopsis. To clarify that this phenotype was indeed due to the T-DNA insertion into ROP2 and to rule out the possibility that a linked mutation actually caused the observed stomatal phenotype, we complemented rop2-1 with ROP2 expressed under the control of its own promoter (Figure 5C). Three independent complemented lines expressing ROP2 showed light-induced stomatal opening similar to that of the wild type (Figure 5D); the maximum stomatal opening was 270% in ROP2-1 and was 260% of initial levels for ROP2-2 and ROP2-3. It took 0.8 h for all three lines to reach half-maximum stomatal apertures. This strongly supports the hypothesis that ROP2 protein is a negative factor that attenuates the extent of light-induced changes in stomatal aperture.
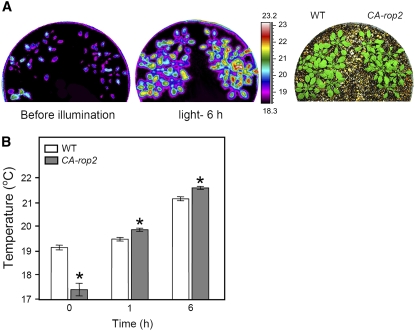
CA-rop2 Plants Transpire Less Water Than Wild-Type Plants in the Light
The inhibition of stomatal opening by light-induced ROP2 activation might contribute to water conservation during the daytime. Water loss at the leaf surface induces a drop in the leaf temperature, which can be measured using an infrared camera (Merlot et al., 2002). We used this method to compare water loss from CA-rop2 mutant and wild-type plants under bright light (250 μmol·m−2·s−1). Consistent with the larger initial stomatal aperture of CA-rop2 mutants before illumination (Figure 5A, 0 h), the temperature of CA-rop2 mutant leaves at the beginning of the photoperiod (time 0) was significantly lower than that of wild-type leaves (Figure 6). Upon illumination with a light source that heated up the air around the plants, the leaf temperature of both CA-rop2 mutant and wild-type plants increased rapidly. After 1 h of illumination, the temperature difference reversed, and the temperature of the CA-rop2 mutant was higher than that of the wild-type plants (Figure 6). The high leaf temperature of CA-rop2 leaves in the light indicates reduced transpiration, which is consistent with the slower and reduced stomatal opening under bright light in these mutant plants (Figure 5, 1 h). These data suggest that active ROP2 inhibits stomatal opening and thereby contributes to water conservation during the daytime.
Figure 6.
Leaf Temperature of White Light–Illuminated CA-rop2 Mutant and Wild-Type Arabidopsis.
(A) Infrared images of wild-type and CA-rop2 plants showing leaf temperature (left two panels) and the bright-field image (right panel).
(B) Leaf (surface) temperature of wild-type and CA-rop2 plants measured from images obtained by infrared thermography, as in (A), and analyzed by ThermaCAM reporter software. Plants were grown for 4 weeks under well-watered conditions. Experiments began at time 0 of the photoperiod, and the plants were irradiated with white light at an intensity of 250 μmol·m−2·s−1. Results (means ± se; n = 90) from three independent experiments are shown. * P < 0.02.
RIC7 Is a Downstream Effector of Active Rop2 GTPase
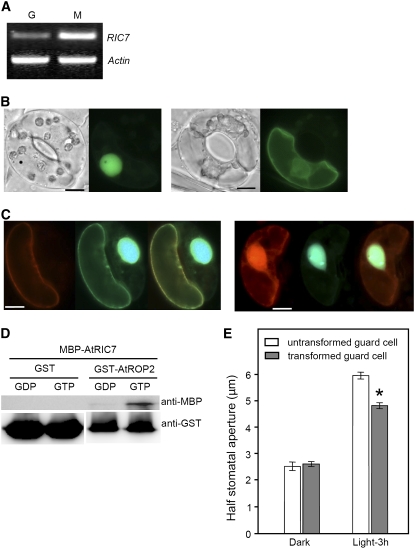
RICs are downstream effectors of many ROP GTPases, and the 11 RICs in Arabidopsis are thought to propagate ROP signals to specific cellular responses (Wu et al., 2001; Fu et al., 2005). We investigated which RIC proteins might participate in ROP2-mediated stomatal responses to light.
We expected that the RIC that functions downstream of ROP2 in guard cells would show the same cellular localization pattern and induce the same stomatal response to light as ROP2. To identify RIC interactions with ROP2, we first identified which RICs are expressed in guard cells of Arabidopsis using RT-PCR, and we found that RIC1, -3, -4, -7, -9, and -10 are expressed (Figure 7A for RIC7; data not shown for others). These RICs were then fused to GFP under the control of a 35S promoter and bombarded into V. faba guard cells, and the localization of green fluorescence was observed. Only GFP-At RIC7 was translocated to the plasma membrane in a light-dependent manner, similar to ROP2. GFP-RIC7 was localized predominantly in the nucleus before illumination (Figure 7B, left panel), but upon light irradiation for 3 h, it was found at the plasma membrane and in the cytoplasm (Figure 7B, right panel).
Figure 7.
RIC7 as a Downstream Target of Active ROP2.
(A) RIC7 PCR products amplified from 1 μg of total RNA from guard cell (G) and mesophyll cell (M) protoplasts. PCR was repeated twice with similar results.
(B) Light-dependent translocation of GFP-RIC7 from the nucleus to the plasma membrane in guard cells. Intact V. faba guard cells were biolistically transformed by GFP-RIC7 and kept in the dark (left panel) or irradiated with white light at an intensity of 250 μmol·m−2·s−1 for 3 h (right panel). In both panels, only the cells at left were transformed; a fluorescence image (right) and the corresponding bright-field image (left) of the same cells are shown. Bars = 10 μm.
(C) CA-ROP2-dependent translocation of RIC7 from the nucleus to the plasma membrane. Intact V. faba guard cells were biolistically transformed by GFP-RIC7 mixed with RFP-CA-ROP2 (left panel) or RFP-DN-ROP2 (right panel) and kept in the dark. In each panel, the RFP, GFP, and merged images are shown from left to right. In the left panel, a pair of guard cells, one transformed with both GFP-RIC7 and RFP-CA-ROP2 (left cell) and one transformed with GFP-RIC7 alone (right cell), are shown. Note that GFP-RIC7 is localized at the plasma membrane only when coexpressed with RFP-CA-ROP2 (left guard cell) and not when expressed alone (right guard cell). By contrast, when GFP-RIC7 is expressed with RFP-DN-ROP2 (right panel), GFP-RIC7 is found only in the nucleus. Bars = 10 μm.
(D) Preferential binding of RIC7 to GTP-loaded ROP2. In vitro binding assays were performed using MBP-RIC7 and GST-ROP2 preloaded with GTP or GDP. MBP-RIC7 pulled down by GST-ROP2 was detected by an anti-MBP antibody, and GST-ROP2 was detected by an anti-GST-horseradish peroxidase conjugate (top panel). Similar amounts of GTP- and GDP-loaded GST-ROP2 were used in the binding assays, as shown in the bottom panel. GST alone was used as a negative control for GST-ROP2.
(E) Light-induced opening of stomata bordered by guard cells transformed with GFP-RIC7. GFP-RIC7 was expressed in intact V. faba guard cells, and the effect on light-induced stomatal opening was compared with that of untransformed neighbor cells. Combined results from three independent experiments are presented (means ± se; n > 70 for each). GFP-RIC7 expression suppressed the stomatal opening in response to light (* P < 0.02).
The plasma membrane localization of GFP-RIC7 was dependent on ROP2 activation (Figure 7C). In the two sister guard cells expressing GFP-RIC7 alone or together with RFP-CA-ROP2, respectively, the localization of green fluorescence was dramatically different. When coexpressed with RFP-CA-ROP2, GFP-RIC7 was localized exclusively at the plasma membrane even in the dark (Figure 7C, left panel, cell at left; n = 33), while GFP-RIC7 alone showed typical nuclear localization (Figure 7C, left panel, cell at right). In contrast with RFP-CA-ROP2, RFP-DN-ROP2 expression did not change GFP-RIC7 localization (Figure 7C, right panel; n = 50). Based on these results, we hypothesized that RIC7 is recruited by active ROP2 and functions as a downstream effector of ROP2 to control light-mediated stomatal movement.
To test our prediction that RIC7 interacts with the active form of ROP2, we used an in vitro pull-down assay (Figure 7D). Maltose binding protein (MBP)–fused RIC7 was pulled down with GTP- or GDP-loaded glutathione S-transferase (GST)–ROP2, and the amount of RIC7 bound to the two differently loaded ROP2 proteins was compared by immunoblotting using an anti-MBP antibody. As a control, GST alone preincubated with GDP or GTP was tested for MBP-RIC7 binding. Our results show that RIC7 preferentially binds to GTP-loaded active ROP2 but not to GST alone (Figure 7D).
If RIC7 is a downstream effector of active ROP2, RIC7 should affect stomatal opening in a manner similar to that of active ROP2. We tested this hypothesis by expressing GFP-RIC7 in V. faba guard cells and comparing their light-induced stomatal response with those of neighboring untransformed cells. GFP-RIC7 expression suppressed light-induced stomatal opening, similar to what was seen in GFP-CA-ROP2, in V. faba guard cells (Figures 4A and 7E). Upon 3 h of light irradiation, half-maximal stomatal aperture bordered by guard cells overexpressing GFP-RIC7 was 4.8 ± 0.11 μm, while that of untransformed controls was 6.0 ± 0.13 μm (Figure 7E; P < 0.02). These results suggest that RIC7 is recruited to the plasma membrane by active ROP2 and participates in the prevention of excessive stomatal opening.
DISCUSSION
Water conservation in plants during the daytime under high light intensity is an important aspect of the dynamic regulation of guard cell movement. However, the mechanism underlying this regulation is poorly understood. Here, we investigated the roles of ROP2 protein in mature guard cells and present experimental results that suggest a role for this G protein in water conservation in plants. We propose that ROP2 is activated by light, as it is translocated to the plasma membrane in response to light, and that this translocation/activation can be inhibited by coexpression with RhoGDI1 (Figure 3). Activated ROP2 retards stomatal opening, as indicated by the slow and reduced opening of CA-rop2 stomata and the accelerated opening of DN-rop2 and rop2-1 stomata (Figures 4 and 5). To the best of our knowledge, light activation of a negative factor that inhibits the response of guard cells to light has not been reported previously, although such responses would seem to be necessary to prevent excessive opening and consequent excessive water loss from plant leaves. Activation of both positive and negative factors by a stimulus may allow fine-tuning of the speed and extent of the organism's response to the stimulus. Our conclusion is supported by the identification of a downstream target protein recruited by active ROP2, which also inhibits light-induced stomatal opening (Figure 7). Therefore, light activation of ROP2 may reduce water loss from plants, consistent with the increased temperature of CA-rop2 leaves in the light (Figure 6).
Many lines of evidence support the correlation between ROP2 activation and the localization of ROP2 at the plasma membrane. First, CA-ROP2 was localized preferentially at the plasma membrane; by contrast, DN-ROP2 was localized mainly in the cytoplasm (Figure 2). These data indicate that ROP2 localization at the plasma membrane depends on its activity. Second, RIC7, a putative downstream effector of ROP2, which preferentially interacted with the GTP-bound active form of ROP2 in an in vitro pull-down assay (Figure 7D), colocalized with ROP2 at the plasma membrane only upon illumination. Third, RhoGDI1, a guanine nucleotide dissociation inhibitor that sequesters Rho GTPases in the cytosol, inhibited the plasma membrane localization of CA-ROP2 (Figure 4). Stimulus-dependent translocation of small G proteins has been shown in yeast and animal cells and is required to initiate responses to the stimulus. For example, RAC2 in the cytosol of human neutrophils and phagocytes translocates to the plasma membrane together with other cytosolic components, forms a large NADPH oxidase complex, and initiates the production of reactive oxygen species, which kill invading pathogens (for review, see Werner, 2004).
Light, a highly important stimulus in guard cells, induced ROP2 translocation from the cytosol to the plasma membrane (Figure 3), suggesting that light activates ROP2 in guard cells. Translocation was observed under microscopy conditions that physically reduce the light-induced stomatal aperture change (Figure 3B), which eliminates the possibility that the translocation was a visual artifact of vacuole expansion and consequent accumulation of the cytoplasm near the cell periphery. Our experiments with RhoGDI1 showed that the plasma membrane localization of active ROP2 is essential for ROP2 to function as a negative regulator of stomatal responses. RhoGDI1 interacts with wild-type and constitutively active forms of ROP4 and ROP6, close homologs of ROP2 (Bischoff et al., 2000); thus, its overexpression was expected to inhibit ROP2 activity by sequestering the protein in the cytoplasm. When expressed in guard cells together with GFP-ROP2, RhoGDI1 inhibited the light-induced translocation of GFP-ROP2 to the plasma membrane (Figure 3C). RhoGDI1 also inhibited the localization of GFP-CA-ROP2 to the plasma membrane (Figure 4B) and abolished the inhibitory effect of CA-ROP2 on stomatal opening (Figure 4A). Together, these results suggest that ROP2 is activated and recruited to the plasma membrane by light and functions as a negative factor for light-induced stomatal opening.
Light-induced stomatal opening has been shown to be mediated by the blue light receptors PHOT1 and PHOT2, which are plasma membrane–localized protein kinases (Kinoshita et al., 2001). ROP2 activation and translocation may be triggered by the activation of phototropins, because blue light induced the translocation of ROP2 (see Supplemental Figure 6 online), although it was less effective than white light. It remains to be determined whether red light, which is absorbed by chlorophyll, can also induce the translocation of ROP2 to the plasma membrane. White light– or blue light–dependent translocation of ROP2 to the plasma membrane did not occur rapidly but took >1 h, suggesting that it may be a component of a feedback regulation located downstream in the signal transduction pathway, rather than being a primary response triggered directly by the photoreceptor. If both blue and red light activate ROP2 and thereby inhibit excessive opening of stomata, it may provide an effective feedback regulatory mechanism common to the most important signals that induce stomatal opening. Further studies will be necessary to clearly answer this question.
In guard cells, multiple ROPs may function as signal transducers of a variety of stimuli. To date, ROP6 and ROP10 have been reported to participate in abscisic acid–induced stomatal responses (Lemichez et al., 2001; Zheng et al., 2002). Here, we show that ROP2 negatively regulates light-induced stomatal opening. Since ROP family members share high homology, it is possible that other ROP proteins also participate in light-regulated stomatal regulation, as multiple ROPs do in root hair and pollen tube growth (Kost et al., 1999; Li et al., 1999; Molendijk et al., 2001; Jones et al., 2002). However, ROP2 seems to play a major role, since the ROP2 knockout phenotype was similar to or stronger than that of the DN-rop2 mutant (Figure 5). The involvement of other ROPs remains to be tested.
How does activated ROP2 inhibit excessive stomatal opening? Recent reports have shown that active ROP inhibits membrane recycling (Bloch et al., 2005) or that endocytosis and membrane recycling are activated during stomatal closing (Sutter et al., 2007). Since stomatal opening requires a large increase in the surface area of the guard cell, stomatal opening signals are also likely to activate the trafficking of proteins and lipids to the plasma membrane, as well as their recycling. Therefore, the inhibition of membrane recycling by active ROP2 at the plasma membrane of guard cells is expected to delay the light-induced swelling of guard cells and the consequent stomatal opening, which is in agreement with our result showing that CA-rop2 guard cells open less and more slowly.
We identified RIC7 as a likely downstream target protein recruited by active ROP2. RIC7 translocates from the nucleus to the plasma membrane in response to light, and this response requires active ROP2 (Figure 7C). Moreover, overexpressed RIC7 inhibits light-induced stomatal opening (Figure 7E) in a manner similar to active ROP2. Since RIC7 is expressed in many cell types, it may have many other interacting partners and carry out different physiological functions in other cell types. In pollen tubes, overexpression of RIC7 results in reduced pollen tube growth, and GFP-RIC7 is localized at the apical plasma membrane and in the cytosol. In ROP1-overexpressing pollen tubes, the apex-specific localization of GFP-RIC7 is disturbed, and it is found at the plasma membrane along the shank as well as at the apical side of the tube (Wu et al., 2001). It is interesting that RIC7 translocates in a ROP-dependent manner both in pollen tubes and in guard cells. There are at least two possible mechanisms by which RIC7 could participate in the light-mediated attenuation of stomatal opening. Upon bright-light illumination, RIC7 interaction with the active ROP2 at the plasma membrane may actively suppress the light stimulation of stomatal opening. Alternatively, when localized to the nucleus, RIC7 may have a role in the positive regulation of light-induced stomatal opening. In response to high light intensity, active ROP2 accumulates and sequesters RIC7 to the plasma membrane, inactivating the function of RIC7. Further studies are necessary to understand the downstream pathways of ROP2/RIC7 that mediate the inhibition of stomatal opening.
In summary, the results described here demonstrate the light activation of ROP2, which inhibits stomatal opening. These studies establish a role for ROP2 as a negative regulator of stomatal opening. Therefore, our work has uncovered a novel mechanism for the modulation of guard cell movement, which may be important for water conservation in nature under conditions in which water is often limiting. Our work also establishes ROP2 as a signal mediator that is translocated to the plasma membrane in a stimulus-dependent manner and recruits a downstream effector protein to the plasma membrane in guard cells. The ROP2-RIC7 system may serve as a new mechanism for signaling between the plasma membrane and the nucleus. Since ROP2 is expressed in many cell types and regulates many physiological functions, similar mechanisms of the activation of ROP2 and its interaction with downstream effector molecules may occur in other plant cell types. During the formation of the jigsaw puzzle appearance of leaf pavement cells, active ROP2 is localized to the plasma membrane region in the lobe tip and sequesters RIC1 from cortical microtubules to inactivate RIC1. Further studies of ROP2 downstream signaling pathways may reveal more mechanisms for signaling between the plasma membrane and other subcellular compartments or structures in plants.
METHODS
Plant Materials
Wild-type and CA- or DN-rop2 transgenic Arabidopsis thaliana (ecotype Columbia) plants were grown in a growth chamber at 22°C with an 8-h-light/16-h-dark photoperiod. Vicia faba plants were grown in a greenhouse at 25°C. For stomatal measurements and assays of transient expression, fully expanded young leaves from 4- to 5-week-old plants were used.
Preparation of Guard Cell Protoplasts
Arabidopsis guard cell protoplasts were isolated following a method modified from Hwang et al. (1997). Epidermal strips were collected by homogenizing rosette leaves of 4- to 5-week-old plants in a blender and filtering through a 100-μm nylon mesh. Mesophyll and epidermal cells were removed by digesting epidermal peels in an enzyme solution for 1 h at 23°C, rotating at 120 rpm. The enzyme solution was composed of 12 parts of distilled water to 8 parts of basic solution (0.5 mM CaCl2, 0.5 mM MgCl2, 10 μM KH2PO4, 5 mM K+-MES, pH 5.5, and 0.45 M d-sorbitol) containing 1% (w/v) Cellulysin cellulase (Calbiochem), 0.1% (w/v) polyvinylpyrrolidone, 0.25% (w/v) BSA, and 0.5 mM ascorbic acid. The partially digested epidermal fragments were subsequently incubated in the second enzyme solution, consisting of 8 parts of distilled water, 12 parts of basic solution, 1.5% (w/v) Onozuka RS cellulase (Yakult Honsa), 0.04% (w/v) pectolyase Y-23 (Sigma-Aldrich), 0.25% (w/v) BSA, and 0.5 mM ascorbic acid, at 23°C and 90 rpm. After 20 to 40 min, released guard cell protoplasts were filtered through four layers of nylon mesh (pore size, 10 μm) and concentrated by centrifugation at 200g for 7 min.
Isolation of the rop2-1 Knockout Mutant and Generation of Complemented Lines of rop2-1
Seeds for a rop2 T-DNA insertional knockout mutant (rop2-1) generated by SIGNAL (http://signal.salk.edu/cgi-bin/tdnaexpress; SALK stock number SALK_055328) were obtained from the ABRC. Homozygous rop2-1 plants were identified by genomic PCR using ROP2-specific primers (ROP2-forward, 5′-TTTTTGCAAATTGGATTCAGGTGTA-3′; ROP2-reverse, 5′-TTCTGCAGAAACACCAAAAGGAAAG-3′), and RT-PCR analysis suggests that rop2-1 is a null mutation. The segregation ratio for kanamycin resistance suggests that rop2-1 contains a single T-DNA insertion.
To generate complemented lines of rop2-1, a 3.8-kb genomic DNA fragment containing 2.2 kb upstream of the translation start site and 209 bp downstream of the stop codon of ROP2 was obtained by PCR amplification using a pair of primers (5′-TTGTGAGTAATCGGAGAATCAACG-3′ and 5′-TCTTAGTCTGTGGACTCGAAAGGGTGACCAGC-3′) from genomic DNA and digested with SnaBI and BstEII. This fragment was then cloned into the SmaI and BstEII sites of pCAMBIA3301 vector. The cloned plasmid was introduced by electroporation into Agrobacterium tumefaciens, which was then introduced into rop2-1 plants by the floral dipping method (Clough and Bent, 1998).
ROP2 and RIC7 Expression in Arabidopsis Guard Cells
To test whether ROP2 (At1g20090) and RIC7 (At4g28556) are expressed in guard cells, RT-PCR was performed. Total RNA was extracted from Arabidopsis guard cell and mesophyll cell protoplasts prepared as described previously (Leonhardt et al., 2004). The first-strand cDNA synthesis kit (Amersham-Pharmacia Biotech) was used to synthesize cDNA. Each PCR was prepared in a 25-μL mixture (primers at 300 nM, 1× buffer, each deoxynucleotide triphosphate at 200 μM, 2.5 units of Taq polymerase [Roche], and 1 μL of guard cell or mesophyll cell cDNA). The PCR mixtures were denatured at 94°C for 3 min and followed by 27 (for actin), 30 (for ROP2), or 40 (for RIC7) cycles of amplification (94°C, 30 s; 55°C for actin and ROP2 or 48°C for RIC7, 30 s; 72°C, 1.5 min). PCR was repeated at least twice. Primers used for PCR were as follows: Actin2-forward, 5′-GGCCGATGGTGAGGATATTCAGCCACTTG-3′; Actin2-reverse, 5′-TCGATGGACCTGACTCATCGTACTCACTC-3′; ROP2-forward, 5′-CCGATCTTGCGGCAGAGATGGCGTCAAGG-3′; ROP2-reverse, 5′-CTTATCACAAGAACGCGCAACGGTTCTTATTC-3′; RIC7-forward, 5′-TACATATCTCAAGTTTTTGCAATAGAAGG-3′; RIC7-reverse, 5′-CGTCTTCAAATTGAGGCATAGATCTATCG-3′. The RT-PCR products from guard cell samples are not likely to be due to contamination from mesophyll cells because our protoplast preparation is >99% pure.
To confirm ROP2 expression in guard cells, GUS activity was assayed in mature guard cells of ROP2 promoter:GUS reporter transgenic Arabidopsis plants (Li et al., 2001). Four-week-old seedlings were stained in a 100 mM phosphate buffer, pH 7.0, containing 0.5 mM K4Fe(CN)6, 0.5 mM K3Fe(CN)6, 10 mM EDTA, 0.1% Triton X-100, and 500 μg/mL 5-bromo-4-chloro-3-indolyl-β-glucuronic acid. After 4 to 12 h of incubation at 37°C, the solution was replaced with 70% ethanol for destaining.
Transient Transformation of Guard Cells
For transient expression of Arabidopsis genes and constructs in V. faba, coding regions for At ROP2, CA1-rop2 (G15V), DN2-rop2 (D121A), RIC7, and RhoGDI1 (At3g07880) were cloned in a pUC vector fused to the C terminus of the GFP or RFP coding region under the control of the 35S promoter. V. faba guard cells were biolistically transformed following the manufacturer's protocol (Bio-Rad Laboratories). In brief, DNA-coated gold particles (diameter, 1 μm) were fired into the abaxial side of V. faba leaves at a helium pressure of 1350 p.s.i. and under a vacuum of 28 inches of mercury. Bombarded leaves were kept at 22°C in the dark. After 12 h to 3 d, epidermal fragments were peeled from the bombarded sites and fluorescence distribution was observed.
Measurement of Stomatal Apertures
Stomatal apertures were measured as described by Hwang et al. (1997). The intact rosette leaves of Arabidopsis were floated on an assay buffer containing 50 mM KCl and 10 mM K+-MES, pH 6.1. For stomatal opening in Arabidopsis, leaves were incubated in the dark overnight before starting illumination with white light at an intensity of 250 μmol·m−2·s−1. Stomatal apertures were measured (pore width/length) from abaxial epidermal strips peeled immediately before observation using an eyepiece micrometer or Interactive Measurement software (AxioVision 3.0.6; Zeiss). Stomatal apertures of V. faba were measured from the abaxial epidermal strips peeled from fully expanded young leaves. The epidermal fragments were floated on an assay buffer containing 50 mM KCl and 10 mM K+-MES, pH 6.1, and subjected to stimuli.
Assay of the Translocation of GFP-ROP2 between the Plasma Membrane and the Cytoplasm
GFP-ROP2 was introduced into V. faba guard cells by biolistic bombardment. Fluorescence images of guard cells were obtained before and after the application of a stimulus and scanned along two lines drawn at right angles to the long axis of the cell, at ∼25% distance from both ends. From the resulting intensity profiles, the average peak pixel intensity of the cell boundary (plasma membrane) and the average pixel intensity of the cell interior (cytosol) were obtained. The ratios of the two values were compared before and after exposure to a stimulus.
Infrared Thermography
Arabidopsis plants were grown under well-watered conditions (22°C, 16-h-light photoperiod) for 4 weeks. The temperature of the leaves of intact Arabidopsis plants was measured at the beginning of the photoperiod and after 1 to 6 h of illumination with white light at an intensity of 250 μmol·m−2·s−1 using a ThermaCAM S60 infrared camera (FLIR Systems). The images were analyzed using ThermaCAM Reporter software (FLIR Systems).
ROP2-RIC7 Binding Assay
The MBP-RIC7 fusion protein, GST, and GST-ROP2 protein were expressed in Escherichia coli and purified (Wu et al., 2001). GST (50 μg) or GST-ROP2 (25 μg) was preincubated with 500 μL of binding buffer (40 mM HEPES, pH 7.5, 100 mM NaCl, 5 mM MgCl2, 4% glycerol, 5 mM DTT, 1 mM phenylmethylsulfonyl fluoride, 10 mM NaF, and 20 mM glycerophosphate supplemented freshly with 2 mM Na3VO4 and 3 mM GTP or GDP) for 30 min and mixed with 2 μL of MBP-RIC7 and 100 μL of agarose-GSH beads. After 2 h of incubation at 4°C, the pellet was washed four times with binding buffer without BSA. Proteins in the pellet were separated by SDS-PAGE, and protein gel blotting was performed using affinity-purified anti-GST-horseradish peroxidase conjugate antibody (1:5000 dilution; Amersham Biosciences) or anti-MBP antibody (1:10,000 dilution; New England Biolabs). Anti-mouse antibodies conjugated to alkaline phosphatase were used to detect anti-MBP (1:5000 dilution; Promega).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database under the following accession numbers: ROP2 (At1g20090), RIC7 (At4g28556), and RhoGDI1 (At3g07880).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. ROP2 Gene Expression in Arabidopsis Guard Cells.
Supplemental Figure 2. Fluorescence Intensity Values of GFP-ROP2 at the Plasma Membrane and in the Cytosol before and after Illumination with White Light at an Intensity of 250 μmol·m−2·s−1.
Supplemental Figure 3. The Fluorescence Intensity of AHA2-GFP (H+-ATPase, a Plasma Membrane Marker [Kim et al., 2001]) Does Not Change in Guard Cells after 2 h of Irradiation with White Light at an Intensity of 250 μmol·m−2·s−1.
Supplemental Figure 4. GFP-ROP2 Is Localized to the Plasma Membrane, and Not to the Golgi, Endoplasmic Reticulum, or Vacuole, in Guard Cells of Open Stomata under Light Illumination.
Supplemental Figure 5. Molecular Characterization of the T-DNA Insertion into the Arabidopsis ROP2 Gene.
Supplemental Figure 6. Blue Light–Induced Translocation of GFP-ROP2 to the Plasma Membrane.
Supplemental Methods.
Supplementary Material
Acknowledgments
This work was supported by a grant to Y.L. from the Crop Functional Genomics Center of the 21st Century Frontier Research Program (Grant CG1513) and the Global Research Laboratory program (Grant 2.0005412.01) funded by Ministry of Science and Technology of Korea, by a grant to Z.Y. from the U.S. Department of Energy (Grant DE-FG02-04ER15555), and by a grant to J.M.K. from the National Research Initiative of the USDA Cooperative State Research Education and Extension Service (Grant 2004-35100-14909). D.C. was partly supported by the Postdoctoral Fellowship Program of the Korea Science and Engineering Foundation.
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) are: Zhenbiao Yang (zhenbiao.yang@ucr.edu) and Youngsook Lee (ylee@postech.ac.kr).
Online version contains Web-only data.
References
- Assmann, S.M., and Wang, X.Q. (2001). From milliseconds to millions of years: guard cells and environmental responses. Curr. Opin. Plant Biol. 4 421–428. [DOI] [PubMed] [Google Scholar]
- Baxter-Burrell, A., Yang, Z., Springer, P.S., and Bailey-Serres, J. (2002). RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296 2026–2028. [DOI] [PubMed] [Google Scholar]
- Bischoff, F., Molendijk, A.J., Rajendrakumar, C.S.V., and Palme, K. (1999). GTP-binding proteins in plants. Cell. Mol. Life Sci. 55 233–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff, F., Vahlkamp, L., Molendijk, A.J., and Palme, K. (2000). Localization of AtROP4 and AtROP6 and interaction with the guanine nucleotide dissociation inhibitor AtRhoGDI1 from Arabidopsis. Plant Mol. Biol. 42 515–530. [DOI] [PubMed] [Google Scholar]
- Bloch, D., Lavy, M., Efrat, Y., Efroni, I., Bracha-Drori, K., Abu-Abied, M., Sadot, E., and Yalovsky, S. (2005). Ectopic expression of an activated RAC in Arabidopsis disrupts membrane cycling. Mol. Biol. Cell 16 1913–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne, H.R., Sanders, D.A., and McCormick, F. (1990). The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348 125–132. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Fu, Y., Gu, Y., Zheng, Z., Wasteneys, G., and Yang, Z. (2005). Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120 687–700. [DOI] [PubMed] [Google Scholar]
- Fu, Y., Wu, G., and Yang, Z. (2001). Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J. Cell Biol. 152 1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y., and Yang, Z. (2001). Rop GTPase: A master switch of cell polarity development in plants. Trends Plant Sci. 6 545–547. [DOI] [PubMed] [Google Scholar]
- Gu, Y., Fu, Y., Dowd, P., Li, S., Vernoud, V., Gilroy, S., and Yang, Z. (2005). A Rho-family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J. Cell Biol. 169 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, Y., Wang, Z., and Yang, Z. (2004). ROP/RAC GTPase: An old new master regulator for plant signaling. Curr. Opin. Plant Biol. 7 527–536. [DOI] [PubMed] [Google Scholar]
- Hancock, J.F., and Hall, A. (1993). A novel role for RhoGDI as an inhibitor of GAP proteins. EMBO J. 12 1915–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth, P.G., Bohl, B.P., Bokoch, G.M., and Curnutte, J.T. (1994). Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox. Evidence for its interaction with flavocytochrome b558. J. Biol. Chem. 269 30749–30752. [PubMed] [Google Scholar]
- Hwang, J.U., Suh, S., Yi, H., Kim, J., and Lee, Y. (1997). Actin filaments modulate both stomatal opening and inward K+-channel activities in guard cells of Vicia faba L. Plant Physiol. 115 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M.A., Shen, J.-J., Fu, Y., Li, H., Yang, Z., and Grierson, C.S. (2002). The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell 14 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki, T., Henmi, K., Ono, E., Hatakeyama, S., Iwano, M., Satoh, H., and Shimamoto, K. (1999). The small GTP-binding protein Rac is a regulator of cell death in plants. Proc. Natl. Acad. Sci. USA 96 10922–10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, T., Doi, M., Suetsugu, N., Kagawa, T., Wada, M., and Shimazaki, K. (2001). Phot1 and Phot2 mediate blue light regulation of stomatal opening. Nature 414 656–660. [DOI] [PubMed] [Google Scholar]
- Kost, B., Lemichez, E., Spielhofer, P., Hong, Y., Tolias, K., Carpenter, C., and Chua, N.-H. (1999). Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol. 145 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemichez, E., Wu, Y., Sanchez, J.-P., Mettouchi, A., Mathur, J., and Chua, N.-H. (2001). Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev. 15 1808–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt, N., Kwak, J.M., Robert, N., Waner, D., Leonhardt, G., and Schroeder, J.I. (2004). Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 16 596–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Lin, Y., Heath, R.M., Zhu, M.X., and Yang, Z. (1999). Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 11 1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Shen, J.-J., Zheng, Z., Lin, Y., and Yang, Z. (2001). The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol. 126 670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Wu, G., Ware, D., Davis, K.R., and Yang, Z. (1998). Arabidopsis Rho-related GTPases: Differential gene expression in pollen and polar localization in fission yeast. Plant Physiol. 118 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y., Wang, Y., Zhu, J.-K., and Yang, Z. (1996). Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell 8 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot, S., Mustilli, A.C., Genty, B., North, H., Lefebvre, V., Sotta, B., Vavasseur, A., and Giraudat, J. (2002). Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 30 601–609. [DOI] [PubMed] [Google Scholar]
- Molendijk, A.J., Bischoff, F., Rajendrakumar, C.S.V., Friml, J., Braun, M., Gilroy, S., and Palme, K. (2001). Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 20 2779–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J., Choi, H.J., Lee, S., Lee, T., Yang, Z., and Lee, Y. (2000). Rac-related GTP-binding protein in elicitor-induced reactive oxygen generation by suspension-cultured soybean cells. Plant Physiol. 124 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potikha, T.S., Collins, C.C., Johnson, D.I., Delmer, D.P., and Levine, A. (1999). The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 119 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn, M.T., Evans, T., Loetterle, L.R., Jesaitis, A.J., and Bokoch, G.M. (1993). Translocation of Rac correlates with NADPH oxidase activation. Evidence for equimolar translocation of oxidase components. J. Biol. Chem. 268 20983–20987. [PubMed] [Google Scholar]
- Schroeder, J.I., Allen, G.J., Hugouvieux, V., Kwak, J.M., and Waner, D. (2001). Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52 627–658. [DOI] [PubMed] [Google Scholar]
- Sutter, J.U., Sieben, C., Hartel, A., Eisenach, C., Thiel, G., and Blatt, M.R. (2007). Abscisic acid triggers the endocytosis of the Arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr. Biol. 17 1396–1402. [DOI] [PubMed] [Google Scholar]
- Tao, L.Z., Cheung, A.Y., and Wu, H.M. (2002). Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. Plant Cell 14 2745–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst, L., and D'Souza-Schorey, C. (1997). Rho GTPases and signaling networks. Genes Dev. 11 2295–2322. [DOI] [PubMed] [Google Scholar]
- Werner, E. (2004). GTPases and reactive oxygen species: Switches for killing and signaling. J. Cell Sci. 117 143–153. [DOI] [PubMed] [Google Scholar]
- Winge, P., Brembu, T., and Bones, A.M. (1997). Cloning and characterization of rac-like cDNAs from Arabidopsis thaliana. Plant Mol. Biol. 35 483–495. [DOI] [PubMed] [Google Scholar]
- Wu, G., Gu, Y., Li, S., and Yang, Z. (2001). A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets. Plant Cell 13 2841–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z., and Watson, J.C. (1993). Molecular cloning and characterization of rho, a ras-related small GTP-binding protein from the garden pea. Proc. Natl. Acad. Sci. USA 90 8732–8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Z.L., Nafisi, M., Tam, A., Li, H., Crowell, D.N., Chary, S.N., Schroeder, J.I., Shen, J., and Yang, Z. (2002). Plasma membrane-associated ROP10 small GTPase is a specific negative regulator of abscisic acid responses in Arabidopsis. Plant Cell 14 2787–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.