Abstract
CD8+ T-lymphocytes (TCD8+) recognize minimal peptides of 8–10 residues which are the products of intracellularly processed proteins and are presented at the cell surface by major histocompatibility complex class I molecules. An important step in this process is the translocation of processed proteins from the cytosol across the endoplasmic reticulum membrane, mediated by transporter associated with antigen-processing proteins or alternatively by endoplasmic reticulum-insertion signal sequences located at the NH2-terminus of the precursor molecules. We report here that the addition of an endoplasmic reticulum-insertion signal sequence at the NH2-terminus of TCD8+ epitopes from chicken ovalbumin (amino acids 257–264) or a naturally occurring tumor antigen expressed by the murine mastocytoma P815 (P1A amino acids 35–43) significantly enhanced the priming of specific TCD8+ in vivo. The signal sequence did not enhance peptide immunogenicity by merely increasing the hydrophobicity of the peptide, since ovalbumin amino acids 257–264 peptide with the signal sequence at its COOH-terminus did not demonstrate enhanced efficacy. The signal sequence did not act as a helper epitope, since TCD8+ responses were not diminished in class II-deficient transgenic mice or in mice depleted of CD4+ T-cells in vivo. Importantly, a single immunization with the fusion peptide significantly prolonged survival of mice challenged with E.G7OVA, a thymoma transfected with the complementary DNA of chicken ovalbumin.
INTRODUCTION
TCD8+3 can play an important role in eradicating tumor cells (1, 2). Unlike antibodies which bind foreign proteins in their native form, TCD8+ recognize short fragments of intracellular antigens, 8–10 amino acids in length, complexed with MHC class I molecules (3-5). Cytosolic peptides are transported across the ER membrane with the help of the ATP-dependent transporter associated with antigen processing (6). An alternative mode of transport into the ER is via insertion signal sequences, located at the NH2-terminus of the precursor molecules (7-10). Peptides complexed with class I molecules are then transported to the cell surface for recognition by TCD8+ (4). The identification of peptide sequences recognized by TCD8+ have led to attempts to directly immunize animals with synthetic peptides (11, 12). These approaches have met with mixed success. Several strategies to improve immunization with peptides have been reported. Successful generation of TCD8+ responses in vivo has been described using peptides formulated with immunostimulating complex (13), entrapped in liposomes (14), osmotically loaded into syngeneic splenocytes (14), or coated on the surface of syngeneic splenocytes (15). Synthetic viral peptides covalently linked to a lipophilic compound were also capable of inducing a TCD8+ response (16). We hypothesized that, if the antigens (i.e., synthetic peptides) are taken up by antigen-presenting cells and gain access to the cytoplasmic compartment, then a fusion peptide with an ER signal sequence at the NH2-terminus of the minimal peptide would translocate into the ER and enhance presentation to TCD8+.
In this report, we demonstrate for the first time that fusion peptides with an ER signal sequence on the NH2-terminus of the minimal peptide are much more effective than the minimal peptides alone in generating specific TCD8+ responses. Furthermore, we found that the TCD8+ response is MHC class II independent, cannot be attributed to increased hydrophobicity of the fusion peptide, and is very effective in prolonging the survival of tumor-challenged mice.
MATERIALS AND METHODS
Cell Lines and Animals
EL-4 is a thymoma cell line of H-2b haplotype. E.G7OVA is a clone of EL-4 stably transfected with chicken ovalbumin complementary DNA (a kind gift from Dr. M. Bevan, Howard Hughes Medical Institute, Seattle, WA) (17). P815 is a mastocytoma cell line of H-2d haplotype, naturally expressing a tumor antigen called P1A (18). A minimal antigenic peptide (P1A35–43) recognized by TCD8+ has been determined and corresponds to residues 35–43 of the P1A gene product (19). CT-26 is a murine colon tumor line of H-2d haplotype (20). All of the cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, 10 mm 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer, 2 mm glutamine, 50 units/ml penicillin, 50 μg/ml streptomycin, 50 μg/ml gentamicin, and 250 ng/ml fungizone. In addition, 400 μg/ml G418 was added to the medium for the E.G7OVA line.
Female C57BL/6N and DBA/2 mice (8–10 weeks of age) were provided by Frederick Cancer Research and Development Facility, NIH (Frederick, MD), and were used 1 week after their arrival at our animal facility. Female transgenic MHC class II-deficient mice (21) were obtained from GenPharm International (Mountain View, CA).
Synthetic Peptides
The sequence of the OVA peptide (amino acids 257–264) used is SIINFEKL. The sequence of the P1A peptide (amino acids 35–43) is LPYLGWLVF. The sequence of the fusion peptide ESP1A is MRYMILGLLALAAVCSAALPYLGWLVF. The fusion peptides ESOVA (RYMILGLLALAAVCSAMSIINFEKL) and OVAES (SIINFEKLRYMILGLLALAAVCSAM) are composed of an ER signal sequence situated at the NH2-terminus or the COOH-terminus, respectively, of the OVA257–264 peptide. The signal sequence that we used originates from the E3/19 protein of adenovirus type 2 (10). All of the peptides were supplied by Peptide Technologies Corp. (Gaithersburg, MD). The identity of all peptides was determined by amino acid analysis.
Immunizations
C57BL/6N and DBA/2 mice were immunized with the synthetic peptides emulsified in IFA or PBS. Each mouse received 200 μg of peptide (50 μg in each hind footpad and 100 μg in the base of the tail). Transgenic MHC class II-deficient mice were immunized with the same synthetic peptides in PBS. Each transgenic mouse received 100 μg of peptide in the base of tail. In all immunization experiments spleen cells were isolated and restimulated in vitro 10 days after the immunization. For protection experiments, groups of five C57BL/6N mice were immunized with 200 μg of peptide OVA, ESOVA, or OVAES in IFA or PBS. Ten days later, 104 E.G7OVA cells/mouse were injected i.p. and the mice were observed to determine survival rates.
In Vitro Sensitization and Cytolytic Assay
In vivo primed spleen cells were cultured for 6 days in complete medium with 100 mm sodium pyruvate, 10 mm nonessential amino acids, 50 μm 2-mercaptoethanol, and 1 μg/ml OVA257–264 or P1A35–43 peptide. Target cells, i.e., P815 cells, CT-26 cells, CT-26 cells pulsed with P1A35–43, EL-4 cells, EL-4 cells pulsed with OVA257–264, or E.G7OVA cells, were labeled with 51Cr for 90 min and added to serially diluted effectors in 96-well microplates. Peptide-pulsed target cells were incubated with 1 μg of OVA257–264 or P1A35–43 during labeling. After a 6-h incubation at 37°C, supernatants were harvested and counted in a gamma counter. The specific lysis of target cells was determined as follows:
Antibodies and Depletion Studies
Culture supernatants of anti-CD4 monoclonal antibody GK1.5 (TIB 207; American Type Culture Collection) were purified by precipitation with ammonium sulfate and were used at a final concentration of 100 μg/ml. Ascitic fluids of hybridoma 2.43 (anti-CD8) (TIB 210; American Type Culture Collection) were harvested and diluted into Hanks' balanced salt solution prior to use in vivo. For in vivo depletion, C57BL/6N mice were given i.v. injections of 100 μg of GK1.5 or of empirically determined levels of 2.43 monoclonal antibodies 4 days before immunization with peptides and 2 and 8 days after immunization. Using fluorescein isothiocyanate-labeled anti-CD4 and anti-CD8 antibodies, FACS analyses were performed 1 day before immunization and 6 and 10 days after immunization with the peptides.
RESULTS AND DISCUSSION
To test the hypothesis that the use of a translocating signal sequence would enhance the generation of TCD8+, we utilized EL-4/E.G7OVA and P815/P1A model systems. E.G7OVA cells differ from the parent EL-4 thymoma cells in that they have been transfected with the complementary DNA of ovalbumin and thus express OVA257–264 in conjunction with the murine H-2Kb molecules (17). P815 cells naturally express a 9-amino acid peptide fragment of the tumor antigen P1A in conjunction with H-2Ld (19). We tested whether specific TCD8+ responses could be elicited by immunization with OVA minimal peptide (OVA257–264), P1A minimal peptide (P1A35–43), or fusion peptides composed of an ER signal sequence from adenovirus type 2 (10) fused to the NH2-terminus (ESOVA and ESP1A) or the COOH-terminus (OVAES) of the minimal peptide.
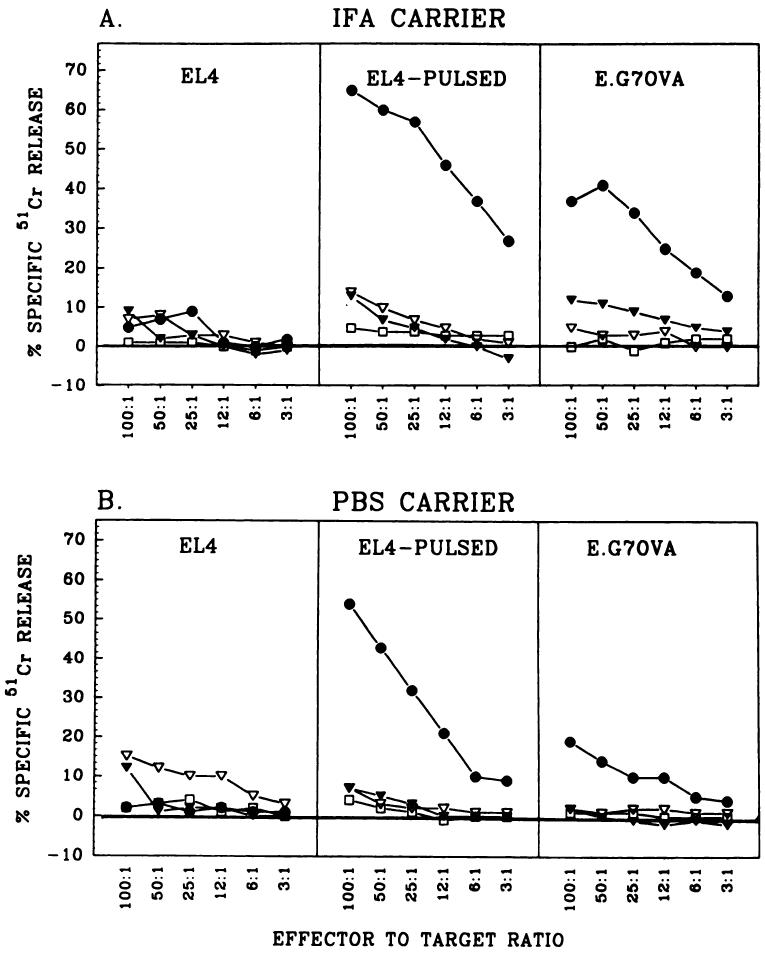
The induction of OVA-specific secondary TCD8+ responses with different synthetic peptides is shown in Fig. 1. The fusion peptide ESOVA, injected in IFA (Fig. 1A) or PBS (Fig. 1B), induced the highest level of priming for an OVA-specific TCD8+ response. In contrast, synthetic peptides OVA257–264 and OVAES emulsified in IFA or PBS failed to prime the mice for specific TCD8+ production. The induced TCD8+ lysed EL-4 cells pulsed with OVA257–264 as well as E.G7OVA cells. Nonpulsed EL-4 cells were not lysed, indicating that the TCD8+ response was antigen specific. Fig. 1 shows one representative experiment, which was repeated six times with similar results.
Fig. 1.
ESOVA but not OVAES or OVA enhances priming in vivo. Specific anti-OVA immune response was elicited by immunization with synthetic peptides in IFA (A) or PBS (B). C57BL/6N mice were immunized with 200 μg/mouse ESOVA (●), OVAES (▽), OVA (▼), or PBS (□). After 10 days, recipient spleen cells were stimulated against OVA minimal peptide in vitro for 6 days and their cytotoxic activity was determined with EL-4 cells (left), EL-4 cells pulsed with OVA257–264 for 90 min (middle), or E.G7OVA cells (right).
The exact mechanism of the successful in vivo priming using the fusion peptide ESOVA is not clear. We hypothesized that the signal sequence fused to the NH2-terminus of the minimal peptide can help in translocation through the ER membrane, thus introducing OVA257–264 into the class I presentation pathway. Indeed, ESOVA was much more effective than OVA257–264 or OVAES in generating specific anti-OVA responses in vivo. The immunizations with the minimal peptide OVA257–264 were not effective, which confirms the findings of other authors (22). OVAES was not as effective as ESOVA, probably because the signal peptidase requires NH2-terminal localization of the signal sequence to cleave off the minimal peptide. This possibility was suggested by Bacik et al. (23) in a recent paper showing that transporter associated with antigen processing-deficient T2 cells could be sensitized to lysis by TCD8+ when infected with recombinant vaccinia viruses expressing minimal peptides situated COOH-terminal, but not NH2-terminal, to the signal sequences.
There are several other possible explanations for the immunogenicity of the fusion peptides with the signal sequence at the NH2-terminus of the minimal peptide. The signal sequence at the NH2-terminus may prolong the accessibility of the minimal peptide to TCD8+ precursor cells, due to the greater hydrophobicity or greater resistance of fusion peptides to proteolytic enzymes. However, immunizations with OVAES, whose hydrophobicity was determined to be nearly identical to that of ESOVA by using the GCG software package, were not successful in priming against OVA. Another possibility is that the signal sequence helps the process of peptide translocation through the cytoplasmic membrane by mediating the attachment of the fusion peptides to the membrane. This mechanism has been suggested by Deres et al. (16) in reference to the successful priming of TCD8+ by a synthetic viral peptide covalently attached to a synthetic analogue of the active moiety of a major lipoprotein of Escherichia coli. It is also possible that the fusion peptides can associate with cells that are then phagocytosed by specialized antigen-presenting cells. These cells could then introduce the degradation products into their class I presentation system, as suggested by Bevan (24).
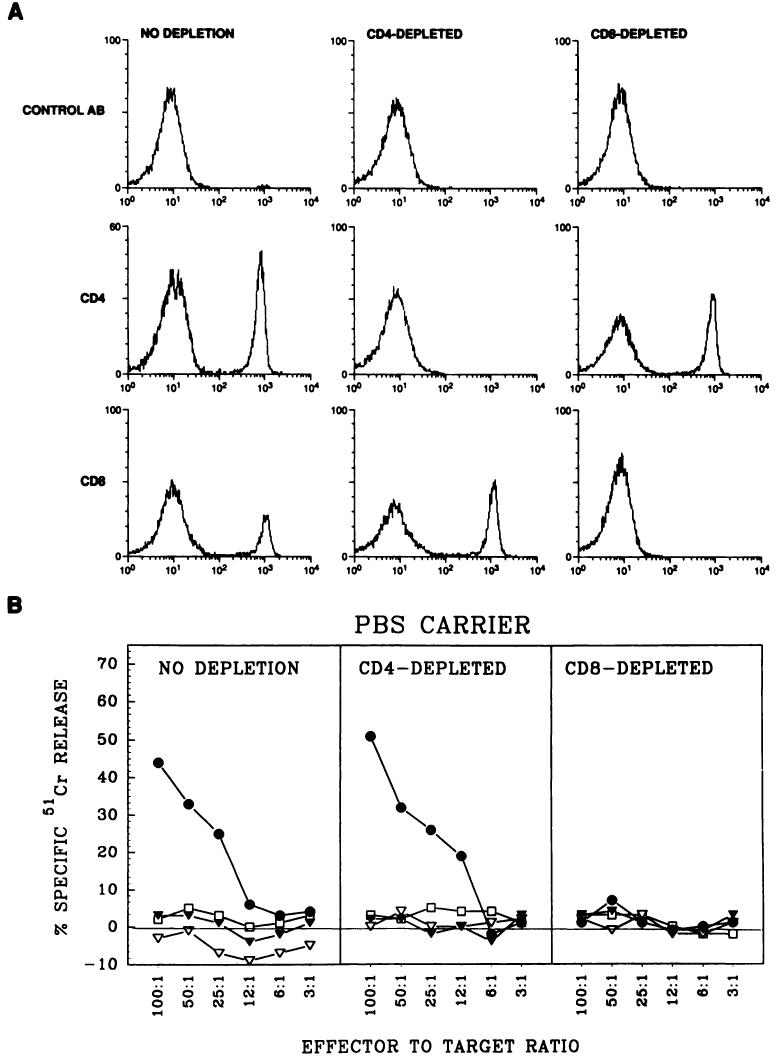
Covalent linkage of synthetic TCD4+ epitopes to TCD8+ epitopes has been found to enhance the generation of TCD8+ against specific antigens (25, 26). Thus, another possibility was that the signal sequence was acting as a TCD4+ epitope. To explore this, we depleted C57BL/6N mice of TCD4+ or TCD8+ by using monoclonal antibodies specific for these T-cell subsets. Mice received the antibodies i.v. 4 days before and 2 and 8 days after the immunization with peptides. FACS analyses shown on Fig. 2A were performed on the day of the secondary in vitro sensitization of splenocytes 2 days after the last injection with anti-CD4 and anti-CD8 antibodies. The FACS profiles show a complete depletion of TCD4+ or TCD8+ in mice treated with the relevant antibodies. We confirmed these results in two other FACS experiments performed 1 day before immunization and 6 days after immunization, to ensure that no TCD4+ or TCD8+ were available during the whole period of in vivo priming with peptides (data not shown).
Fig. 2.
ESOVA enhances priming in vivo in mice depleted of TCD4+. A, FACS analysis of splenocytes isolated from normal nondepleted (left column), TCD4+-depleted (middle column), or TCD8+-depleted (right column) mice. Mice were given i.v. injections, three times at intervals of 6 days, of antibody GK1.5 (anti-CD4) or 2.43 (anti-CD8). FACS analysis was performed on the day of secondary stimulation of splenocytes, using fluorescein isothiocyanate-labeled anti-CD4 and anti-CD8 antibodies. B, cytotoxic activity in nondepleted (left), TCD4+-depleted (middle), or TCD8+-depleted (right) mice. C57BL/6N mice were depleted of TCD4+ or TCD8+ as described in the text. The mice were immunized with ESOVA (●), OVAES (▽), OVA (▼), or PBS (□). After 10 days, recipient spleen cells were stimulated against OVA minimal peptide in vitro for 6 days and their cytotoxic activity was determined with EL-4 cells pulsed with OVA257–264 for 90 min.
The results from a representative experiment measuring TCD8+ cytotoxic activity in mice depleted of TCD4+ or TCD8+ are shown in Fig. 2B. We found that TCD4+ depletion did not affect the generation of specific TCD8+ responses after priming with ESOVA. In contrast, in vivo depletion of TCD8+ completely blocked the generation of OVA-specific TCD8+ responses. The finding that TCD4+ help was not necessary for the generation of specific TCD8+ responses is consistent with earlier reports involving some viral systems (27-30). However, in some cases TCD4+ help has been found to be important for TCD8+ generation (31-33). One model that could explain the observed TCD8+ response in ESOVA-primed mice is that two distinct CD8+ populations exist in mice depleted of TCD4+, i.e., cytotoxic CD8+ and helper CD8+ T-cells. The latter population could possibly substitute for the IL-2-producing TCD4+ in generating the observed responses (29). Mizuochi et al. (28) found that the functions of CD8+ helper T-cells are mediated at least in part by lymphokines, including IL-2. In agreement with these findings, we also observed inhibition of TCD8+ activity after inclusion of anti-IL-2 receptor antibody in the TCD4+-deficient responder cultures from mice primed with ESOVA (data not shown). Complete abrogation of the cytotoxic responses in mice depleted of CD8+ T-cells provides clear evidence that the observed responses are mediated by CD8+ T-cells.
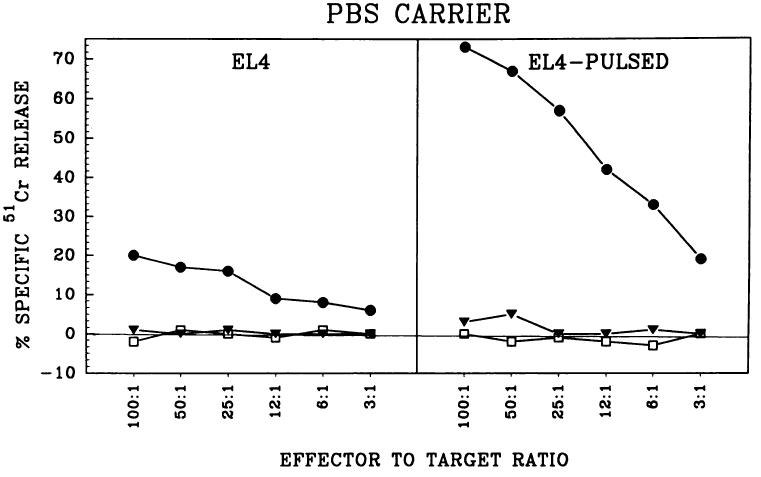
To confirm that the observed immune responses were MHC class II independent, we performed another set of experiments with MHC class II-deficient transgenic mice (21). Data presented in Fig. 3 show that immunization with ESOVA successfully induced a specific TCD8+ response in the absence of MHC class II. In fact, we observed very high specific lysis of OVA-pulsed EL-4 cells in the experiments with MHC class II-deficient mice, probably because of the higher percentage of CD8+ cells in the spleens of these mice.
Fig. 3.
ESOVA enhances priming in vivo in the absence of MHC class II. MHC class II-deficient transgenic mice were immunized with ESOVA (●), OVA (▼), or PBS (□). After 10 days, recipient spleen cells were stimulated in vitro for 6 days against OVA minimal peptide and their cytotoxic activity was determined with EL-4 cells (left) or EL-4 cells pulsed with OVA257–264 for 90 min (right).
Our experiments with mice depleted of TCD4+ and with transgenic mice suggest that TCD4+ and MHC class II expression are not required for in vivo priming of TCD8+ responses using ESOVA. However, we cannot rule out the possibility that a small population of TCD4+ cells exists in mice depleted of TCD4+. In the transgenic mice that we used, a small population of about 3% TCD4+ has been reported to exist in the periphery (21). However, if TCD4+ cells were essential for ESOVA-induced cytotoxic T-lymphocyte responses, then we would expect at least a small reduction in the cytotoxic T-lymphocyte response after treatment with GK1.5 or in the experiments with transgenic mice. Fayolle et al. (32) suggested that, to be able to induce TCD8+ responses, a synthetic peptide has to stimulate both CD4+ and CD8+ T-cell subsets, i.e., it should contain a MHC class II epitope to be able to recruit TCD4+ help for TCD8+. The possibility that ESOVA may contain an MHC class II epitope was rejected by the results of our experiments with TCD4+-depleted and transgenic mice. Palker et al. (34) were not able to induce TCD8+ activation using a hybrid human immunodeficiency virus Mr 120,000 glycoprotein synthetic peptide, even though it contained a class II epitope. Hart et al. (35) demonstrated TCD8+ activation in vitro with the same peptide coupled to a highly hydrophobic peptide representing the human immunodeficiency virus env-Mr 41,000 glycoprotein fusion domain. That study, as well as the work of Deres et al. (16), suggests that the hydrophobic nature of peptides may facilitate their association with cells and thus their entering into the class I processing pathway but also that TCD4+ help provided with class II epitopes was not sufficient for TCD8+ activation.
To determine whether immunization with ESOVA could protect mice against tumors induced by E.G7OVA cells, we performed the experiments shown in Fig. 4, which presents the combined data from three independent experiments. Fourteen of 16 mice survived more than 150 days after a single immunization with ESOVA in IFA (Fig. 4A). Six of 10 mice survived E.G7OVA tumor challenge when ESOVA was administered in PBS (Fig. 4B). None of the mice immunized with OVAES, OVA, IFA, or PBS alone survived the tumor challenge. No surviving mice could be obtained even after two immunizations with OVAES or OVA in either carrier (data not shown). The observed prolongation of survival was statistically significant in the experiments with both carriers when we compared the survival of ESOVA-primed mice to the survival of mice inoculated with the carrier alone (ESOVA in IFA, P < 0.0001; ESOVA in PBS, P < 0.0003). Most importantly, the prolongation of survival of ESOVA-primed mice, compared to OVA-primed mice, was statistically significant when we used either IFA (P < 0.0001) or PBS (P < 0.011) as carrier. These results suggest that ESOVA is efficient in generating specific immune protection even when administered in PBS. This finding correlates with the observed TCD8+ activity against E.G7OVA cells in 51Cr-release assays.
Fig. 4.

Immunoprotection of C57BL/6N mice challenged with E.G7OVA cells by a single immunization with ESOVA in IFA (A) or PBS (B). Mice were immunized with ESOVA, OVAES, OVA, or PBS 10 days before the challenge with 104 E.G7OVA cells.
We hypothesize that the signal sequence we used promotes the entrance of the antigen into the MHC class I presentation pathway by helping the translocation of the peptide through the ER membrane. While translocation usually occurs during translation, protein precursors have also been shown to be imported into the ER after their synthesis has been completed (36). The signal sequence may also help the cell membrane translocation because of its hydrophobic nature, as suggested earlier by other authors using synthetic peptides attached to hydrophobic groups (16, 35).
The possibility that ESOVA activates TCD8+ indirectly through an antigen-presenting cell population cannot be excluded. Debrick et al. (37) found that macrophages are essential for TCD8+ activation in mice infected with influenza virus. This possibility was also suggested by Bevan (24) and could be relevant to our study. Dendritic cells were found to be very effective in generating specific TCD8+ in Sendai virus infection (38), probably because of their morphology and high expression of MHC class I molecules (39). The possible role of macrophages or dendritic cells in the generation by ESOVA of the specific TCD8+ responses remains to be examined. Natural killer activity is probably not a likely explanation for our results, because E.G7OVA cells were found to be insensitive to natural killer cell-mediated lysis (40) and because of the specificity of the observed responses. Antibody-mediated tumoricidal activity can also be excluded, because Zhou et al. (14) proved with complement-mediated cytolysis assays that anti-OVA sera cannot recognize E.G7OVA cells. This result is not surprising, considering that the OVA protein synthesized by E.G7OVA cells was engineered genetically as a secretory protein (17) and should not be present on the cell surface in its native form.
To test whether our finding is applicable for other TCD8+ epitopes, we studied the tumor-associated antigen P1A, which is naturally expressed by P815 cells (18). Boon and coworkers (19) have shown that a minimal antigenic peptide recognized by TCD8+ corresponds to residues 35–43 of the P1A gene product. The induction of P1A-specific secondary TCD8+ responses is shown in Fig. 5. The fusion peptide ESP1A, injected in IFA (Fig. 5A) or PBS (Fig. 5B), induced the highest level of priming for a P1A-specific TCD8+ response, in contrast to the synthetic peptide P1A35–43, which failed to prime the mice for specific TCD8+ production. The induced TCD8+ lysed CT-26 cells pulsed with P1A35–43 as well as P815 cells, which naturally express the antigen. Nonpulsed CT-26 cells were not lysed, which confirmed that the TCD8+ response elicited by the fusion peptide was antigen specific also in the P815/P1A model system. Fig. 5 shows one representative experiment, which was repeated three times with similar results.
Fig. 5.
ESP1A but not P1A enhances priming in vivo. Specific anti-P1A immune response was elicited by immunization with synthetic peptides in IFA (A) or PBS (B). DBA/2 mice were immunized with 200 μg/mouse ESP1A (●), P1A (▼), or PBS (▽). After 10 days, recipient spleen cells were stimulated against P1A minimal peptide in vitro for 6 days and their cytotoxic activity was determined with P815 cells (left), CT-26 cells (middle), or CT-26 cells pulsed with P1A35–43 for 90 min (right).
Godelaine et al. (41) studied another Ld-binding antigen, P91A, derived from a protein located in the cytosol of the cell clone P91 from P815 cells, and they suggested that the peptide fragments that bind to MHC class I molecules can be generated in the ER (42). This finding is consistent with the hypothesis that the ESP1A peptide is being cleaved in the ER, possibly by signal peptidase.
Thus, we conclude that adding protein signal sequences to MHC class I-restricted minimal peptides may be very helpful for the development of effective synthetic vaccines against neoplastic and viral diseases. The general problem limiting the use of peptide vaccines in outbred populations is that T-cells from individuals expressing different MHC molecules recognize different peptides from tumor or viral antigens in the context of their respective self MHC. However, the use of synthetic peptides representing tumor-associated antigens which are presented by commonly expressed MHC molecules makes the use of synthetic peptide vaccines feasible. Recent progress in cloning techniques has resulted in the cloning of several antigens expressed by human melanoma cells (43-45). These important findings are leading to the development of synthetic peptide vaccines for clinical trials in humans with metastasic cancer.
ACKNOWLEDGMENTS
We thank Drs. J. Wunderlich, H. Morse III, K. Irvine, and P. Cohen for very helpful discussions; Drs. R. Feldman and P. Fitzgerald for help in assessment of the hydrophobicity of the synthetic peptides; E. Fitzgerald and A. Mixon for assistance in FACS experiments; and D. Jones for assistance in the animal experiments.
Footnotes
The abbreviations used are: TCD8+, CD8+ T-lymphocytes; TCD4+, CD4+ T-lymphocytes; ER, endoplasmic reticulum; MHC, major histocompatibility complex; IFA, incomplete Freund's adjuvant; PBS, phosphate-buffered saline; FACS, fluorescence-activated cell-sorting; IL-2, interleukin 2; OVA, ovalbumin; ESP1A, endoplasmic reticulum-insertion signal sequence-P1A; ESOVA, endoplasmic reticulum-insertion signal sequence-ovalbumin; OVAES, ovalbumin-endoplasmic reticulum-insertion signal sequence; P1A35–43, P1A amino acids 35–43; OVA257–264, ovalbumin amino acids 257–264.
REFERENCES
- 1.Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv. Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science (Washington DC) 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 3.Townsend A, Bodmer H. Antigen recognition of class I restricted T lymphocytes. Annu. Rev. Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- 4.Yewdell JW, Bennink JR. Cell biology of antigen processing and presentation to MHC class I molecule-restricted T lymphocytes. Annu. Rev. Immunol. 1992;52:1–123. doi: 10.1016/s0065-2776(08)60875-5. [DOI] [PubMed] [Google Scholar]
- 5.Rammensee HG. MHC class I structure and function. Semin. Immunol. 1993;5:73–145. [Google Scholar]
- 6.Spies T, Cerundolo V, Colonna M, Cresswell P, Townsend A, DeMars R. Presentation of viral antigen by MHC class I molecules is dependent on a putative peptide transporter heterodimer. Nature (Lond.) 1992;355:644–646. doi: 10.1038/355644a0. [DOI] [PubMed] [Google Scholar]
- 7.Rapoport TA. Transport of proteins across the endoplasmic reticulum membrane. Science (Washington DC) 1992;258:931–936. doi: 10.1126/science.1332192. [DOI] [PubMed] [Google Scholar]
- 8.Gilmore R. Protein translocation across the endoplasmic reticulum: a tunnel with toll booths at entry and exit. Cell. 1993;75:589–592. doi: 10.1016/0092-8674(93)90476-7. [DOI] [PubMed] [Google Scholar]
- 9.Hunt DF, Henderson RA, Shabanowitz J, Sakaguchi K, Michel H, Sevilir N, Cox AL, Appella E, Engelhard VH. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science (Washington DC) 1992;255:1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- 10.Anderson K, Cresswell P, Gammon M, Hermes J, Williamson A, Zweerink H. Endogenously synthesized peptide with an endoplasmic reticulum signal sequence sensitizes antigen processing mutant cells to class I-restricted cell-mediated lysis. J. Exp. Med. 1991;174:489–492. doi: 10.1084/jem.174.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aichele P, Hengartner H, Zinkernagel RM, Schulz M. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J. Exp. Med. 1990;171:1815–1820. doi: 10.1084/jem.171.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz M, Zinkernagel RM, Hengartner H. Peptide-induced antiviral protection by cytotoxic T cells. Proc. Natl. Acad. Sci. USA. 1991;88:991–993. doi: 10.1073/pnas.88.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipford GB, Hoffman M, Wagner H, Heeg K. Primary in vivo responses to ovalbumin. Probing the predictive value of the Kb binding motif. J. Immunol. 1993;150:1212–1222. [PubMed] [Google Scholar]
- 14.Zhou F, Rouse BT, Huang L. Prolonged survival of thymoma-bearing mice after vaccination with a soluble protein antigen entrapped in liposomes: a model study. Cancer Res. 1992;52:6287–6291. [PubMed] [Google Scholar]
- 15.Harty JT, Bevan MJ. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J. Exp. Med. 1992;175:1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deres K, Schild H, Wiesmüller K-H, Jung G, Rammensee H-G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature (Lond.) 1989;342:561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- 17.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 18.Van den Eynde B, Lethé B, Van Pel A, De Plaen E, Boon T. The gene coding for a major tumor rejection antigen of tumor P815 is identical to the normal gene of syngeneic DBA/2 mice. J. Exp. Med. 1991;173:1373–1384. doi: 10.1084/jem.173.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lethe B, Van den Eynde A, Van Pel G, Corradin A, Boon T. Mouse tumor rejection antigens P815A and P815B: two epitopes carried by a single peptide. Eur. J. Immunol. 1992;22:2283–2288. doi: 10.1002/eji.1830220916. [DOI] [PubMed] [Google Scholar]
- 20.Brattain MG, Strobel-Stevens J, Find D, Webb M, Sarrif AM. Establishment of mouse colonic carcinoma with different metastatic properties. Cancer Res. 1980;40:2142–2146. [PubMed] [Google Scholar]
- 21.Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science (Washington DC) 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 22.Rock KL, Fleischacker C, Gamble S. Peptide-priming of cytolytic T cell immunity in vivo using β2-microglobulin as an adjuvant. J. Immunol. 1993;150:1244–1252. [PubMed] [Google Scholar]
- 23.Bacik I, Cox JH, Anderson R, Yewdell JW, Bennink JR. TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptides is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide. J. Immunol. 1994;152:381–387. [PubMed] [Google Scholar]
- 24.Bevan MJ. Antigen recognition: class discrimination in the world of immunology. Nature (Lond.) 1987;325:192–194. doi: 10.1038/325192b0. [DOI] [PubMed] [Google Scholar]
- 25.Lasarte JJ, Sarobe P, Gullon A, Prieto J, Borras-Cuesta F. Induction of cytotoxic T lymphocytes in mice against the principal neutralizing domain of HIV-1 by immunization with an engineered T-cytotoxic-T-helper synthetic peptide construct. Cell. Immunol. 1992;141:211–218. doi: 10.1016/0008-8749(92)90140-k. [DOI] [PubMed] [Google Scholar]
- 26.Shirai M, Pendleton CD, Ahlers J, Takeshita T, Newman M, Berzofsky JA. Helper-cytotoxic T lymphocyte (CTL) determinant linkage required for priming of anti-HIV CD8+ CTL in vivo with peptide vaccine constructs. J. Immunol. 1994;152:549–556. [PubMed] [Google Scholar]
- 27.Vasilakos JP, Michael JG. Herpes simplex virus class I restricted peptide induces cytotoxic T lymphocytes in vivo independent of CD4+ cells. J. Immunol. 1993;150:2346–2355. [PubMed] [Google Scholar]
- 28.Mizuochi T, Hugin AW, Morse HC, III, Singer A, Buller RML. Role of lymphokine-secreting CD8+ T cells in cytotoxic T lymphocyte responses against vaccinia virus. J. Immunol. 1989;142:270–273. [PubMed] [Google Scholar]
- 29.Buller RML, Holmes KL, Hugin A, Frederickson TN, Morse HC., III Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature (Lond.) 1987;328:77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed R, Butler LD, Bhati L. T4+ T helper cell function in vivo: differential requirement for induction of antiviral cytotoxic T-cell and antibody responses. J. Virol. 1988;62:2102–2106. doi: 10.1128/jvi.62.6.2102-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao XM, Zheng B, Liew FY, Brett S, Tite J. Priming of influenza virus-specific cytotoxic T lymphocytes in vivo by short synthetic peptides. J. Immunol. 1991;147:3268–3273. [PubMed] [Google Scholar]
- 32.Fayolle C, Deriaud E, Leclerc C. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J. Immunol. 1991;147:4069–4073. [PubMed] [Google Scholar]
- 33.Hussman LA, Bevan MJ. Cooperation between helper T cells and cytotoxic T lymphocyte precursors. Ann. NY Acad. Sci. 1988;532:158–169. doi: 10.1111/j.1749-6632.1988.tb36335.x. [DOI] [PubMed] [Google Scholar]
- 34.Palker TJ, Mattheus TJ, Langlois A, Tanner ME, Martin ME, Scearce RM, Kim JE, Berzofsky JA, Bolognezi DP, Haynes BF. Polyvalent human immunodeficincy virus synthetic immunogen comprised of envelope gp120 T helper cell sites and B cell neutralization epitopes. J. Immunol. 1989;142:3612–3619. [PubMed] [Google Scholar]
- 35.Hart MK, Weinhold KJ, Scearce RM, Washburn EM, Clark CA, Palker CE, Haynes BF. Priming of anti-human immunodeficiency virus (HIV) CD8+ cytotoxic T cells in vivo by carrier-free synthetic peptides. Proc. Natl. Acad. Sci. USA. 1991;88:9448–9452. doi: 10.1073/pnas.88.21.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perara E, Rothman RE, Lingappa VR. Uncoupling translocation from translation: implications for transport of proteins across membranes. Science (Washington DC) 1986;232:348–352. doi: 10.1126/science.3961485. [DOI] [PubMed] [Google Scholar]
- 37.Debrick JE, Campbell PA, Staerz UD. Macrophages as accessory cells for class I MHC-restricted immune responses. J. Immunol. 1991;147:2846–2851. [PubMed] [Google Scholar]
- 38.Kast WM, Boog CJ, Roep BO, Voordouw AC, Melief CJM. Failure or success in the restoration of virus-specific cytotoxic T lymphocyte response defects by dendritic cells. J. Immunol. 1988;140:3186–3193. [PubMed] [Google Scholar]
- 39.Boog CJP, Boes J, Melief JM. Role of dendritic cells in the regulation of class I restricted cytotoxic T lymphocyte responses. J. Immunol. 1988;140:3331–3337. [PubMed] [Google Scholar]
- 40.Storkus WJ, Alexander J, Paine JA, Downson JR, Cresswell P. Reversal of natural killing susceptibility in target cells expressing transfected class I HLA genes. Proc. Natl. Acad. Sci. USA. 1989;86:2361–2364. doi: 10.1073/pnas.86.7.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lurquin C, Van Pel A, Mariame B, De Plaen E, Szikora J, Jannsens C, Reddehase MJ, Lejeune J, Boon T. Structure of the gene of tum– transplantation antigen P91A: the mutated exon encodes a peptide recognized with Ld by cytolytic T cells. Cell. 1989;58:293–303. doi: 10.1016/0092-8674(89)90844-1. [DOI] [PubMed] [Google Scholar]
- 42.Godelaine D, Van Pel A, Van Malderen M, Beaufay H. Presentation of mouse tum– P91A antigen from chimeric proteins with different subcellular localizations by class I molecules of the major histocompatibility complex. Eur. J. Immunol. 1993;23:1731–1734. doi: 10.1002/eji.1830230752. [DOI] [PubMed] [Google Scholar]
- 43.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science (Washington DC) 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 44.Brichard V, Van Pel A, Wölfel T, Wölfel C, De Plaen E, Lethé B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J. Exp. Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltinin L, Topalian SL, Miki T, Rosenberg SA. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc. Natl. Acad. Sci. (USA) 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]