Abstract
P450 aromatase (CYP19) is the terminal enzyme in the steroidogenic pathway and catalyzes the conversion of androgens to estrogens. Fundulus heteroclitus like other teleosts, express two CYP19 genes, CYP19A1 and CYP19A2. The expression of CYP19s in Fundulus was measured by in situ hybridization throughout development. In 90 dpf (day post-fertilization) fish and adult fish, CYP19A1 was expressed in the ooplasm of early stage I oocytes (primary growth stage). Expression of CYP19A1 was localized in the follicle cell layer of late stage I (previtellogenic stage) and stage II (vitellogenic stage) follicles, but by stage III (early maturational follicles) CYP19A1 expression was localized in the vitelline envelope. Overall, CYP19A1 oocyte membrane expression gradually declined from highest expression at late stage I to nondetectable levels by stage IV. Highest expression of CYP19A2 was detected in the brain including the hypothalamus from 4, 6, 8, 10, 14 dpf embryos, 90 dpf fry fish and adult fish brain. In females compared to males, there was higher CYP19A2 expression in olfactory bulb. In addition to the brain, there was strong CYP19A2 signal in adrenal/kidney cells in 6-14 dpf embryos. This work establishes the localization and constitutive expression of CYP19s in Fundulus which can then be compared with potential disruption of CYP19A1 and CYP19A2 expression and physiological consequences caused by environmental contaminants.
Keywords: Aromatase, Fundulus, CYP19, oocyte, brain
Introduction
Interference with steroid hormone biosynthesis is an important contributing mechanism that could impact proper growth, development, behavior, reproduction, and/or lead to endocrine associated cancers (Sanderson 2006). An important enzyme in regulating the balance of androgens and estrogens is the P450 enzyme aromatase or CYP19. Proper control of circulating and local estrogen concentrations are critical for diverse physiological functions including germ cell maturation in both sexes, bone, brain, and adipose physiology (reviewed by (Meyer 1999; Simpson et al., 2002)). The human CYP19 gene is a single gene for which tissue specific expression is controlled by alternate promoter splicing (Meinhardt and Mullis 2002). In contrast, in fish it is well established that there are two distinct CYP19 genes, a predominantly ovarian form (CYP19A1 or CYP19a) and a predominantly brain form (CYP19A2 or CYP19b). However, analyses at the gene sequence level suggest little functional divergence among vertebrate aromatases though transcription of these genes can be highly variable (Wilson et al., 2005). To expand our understanding of the potential physiological significance and developmental roles of CYP19A1 and CYP19A2 we have investigated the tissue specific expression of these genes in Fundulus heteroclitus (Atlantic killifish or mummichog) by in situ hybridization.
The primarily ovarian aromatase gene, CYP19A1, has been cloned from a number of fish species including those with diverse reproductive strategies including daily spawners like zebrafish and medaka, biweekly spawners (Fundulus), annual spawners (catfish, rainbow trout and goldfish) and fish capable of sex change (black porgy, wrasse, goby). Promoter analysis of fish CYP19A1s has identified cAMP-response elements, aryl hydrocarbon response elements, steroidogenic factor 1 (SF-1), TATA box, estrogen receptor recognition half sites, GATA-3, progesterone receptor response elements, Ad4BP binding motif and SRY/SOX motifs (Kazeto et al., 2001; Nocillado et al., 2007; Tanaka et al., 1995; Tchoudakova et al., 2001; Tong and Chung 2003). Using quantitative RT-PCR, unfertilized zebrafish eggs had similar levels of CYP19A1 expression as whole ovaries however these levels decreased during development until 48 hpf when CYP19A1 mRNA began to accumulate and peak at 72 hpf. In addition, the developmentally expressed CYP19A1 mRNA was not inducible by estradiol exposure (Sawyer et al., 2006). In adult ovaries of Atlantic croaker and rainbow trout, levels of CYP19A1 message were detected early in ovarian development and vitellogenesis with dramatically decreased expression later (Nakamura et al., 2005; Nunez and Applebaum 2006). The CYP19A1 mRNA expression correlated with plasma estradiol levels in both channel catfish and rainbow trout (Kumar et al., 2000; Nakamura et al., 2005). Relatively few studies have investigated CYP19A1 expression during embryonic or ovarian development by in situ hybridization or immunohistochemistry. Highest CYP19A1 mRNA expression was found in Stage III B zebrafish ovarian vitellogenic follicles (Goto-Kazeto et al., 2004; Rodriguez-Mari et al., 2005). Kobayashi and colleagues found CYP19A2 mRNA expression in goby thecal cells of previtellogenic follicles while the expression was more abundant in granulosa cells of vitellogenic follicles (Kobayashi et al., 2004). However, aromatase immunoreactivity was found in both cell types of female goby (Sunobe et al., 2005).
In contrast to CYP19A1, the neuronal CYP19A2 is inducible by estrogenic compounds and its cellular expression has been more thoroughly defined particularly in the plainfin midshipman, rainbow trout, zebrafish, goldfish, bluehead wrasse and pejerrey (Forlano et al., 2005; Forlano et al., 2001; Gelinas and Callard 1997; Goto-Kazeto et al., 2004; Marsh et al., 2006; Menuet et al., 2003; Menuet et al., 2005; Pellegrini et al., 2007; Strobl-Mazzulla et al., 2005). Aromatase expression is found in glial cells predominantly in the olfactory bulbs and hypothalamus, with expression also in the pituitary, telecephalon, and diencephalon. The unusually high capacity to synthesize estrogen in the fish brain, and the radial glial cells specifically, has been suggested as a mechanism involved in the continuous neurogenesis found in fish (Pellegrini et al., 2007). Other potential roles of neuronal aromatase include reproduction-related vocalizations in midshipman (Forlano et al., 2001) and sex determination in sea bass, medaka, pejerrey and wrasse (Blazquez and Pieferrer 2004; Marsh et al., 2006; Melo and Ramsdell 2001; Strobl-Mazzulla et al., 2005). Developmental expression of CYP19A2 has been largely investigated in zebrafish (Menuet et al., 2005; Sawyer et al., 2006; Trant et al., 2001) and more recently Fundulus (see below). In comparison to CYP19A1, in embryos CYP19A2 expression occurs sooner and reaches higher maximum levels and is estrogen inducible (Sawyer et al., 2006). Estrogen responsiveness has been associated with estrogen response elements (EREs) and ERE half-sites in the fish CYP19A2 promoter region (Kuhl et al., 2005; Tchoudakova et al., 2001).
Both CYP19 genes have been previously cloned in Fundulus (Greytak et al., 2005; Patel et al., 2006). Fundulus have been used as an environmentally relevant toxicology model organism to study endocrine disruption (Boudreau et al., 2005; Dube and MacLatchy 2001; Greytak and Callard 2007; Kelly and Di Giulio 2000), environmental carcinogenesis and polycyclic aromatic hydrocarbon (PAH) toxicity (Billiard et al., 2006; Vogelbein et al., 1990; Wang et al., 2006), and chemically mediated changes in gene expression (Meyer et al., 2005; Paschall et al., 2004; Powell et al., 2000). The embryonic developmental stages of Fundulus have been described (Armstrong and Child 1965). Furthermore, in both field and laboratory conditions, the follicular cycle of Fundulus is consistently reproduced at 2 week intervals year around leading to the proposal that Fundulus could be used as a general model organism for cyclic reproductive activity (Hsiao et al., 1996). The three phases of Fundulus ovarian development (recruitment, maturation and ovulation) have been well characterized with respect to timing, vitellogenesis, and steroid responsiveness (Cerda et al., 1996; Cerda et al., 1998; Petrino et al., 1990; Petrino et al., 1989a; Petrino et al., 1989b; Selman and Wallace 1983; Subhedar et al., 1997; Wallace and Selman 1985). Less work has characterized the roles of the CYP19s specifically in Fundulus. In vitro, Fundulus ovarian follicle cells were capable of 17α-hydroxy-20β-dihydroprogesterone, testosterone and estrogen production when Fundulus pituitary extract was added, however theca and surface epithelium preparations were incapable of estrogen production (Petrino et al., 1989a) suggesting that aromatase activity was present in follicle but not thecal cell layers. In laboratory control Fundulus brain CYP19A2 mRNA expression was not different between males and females although females had higher enzyme activity (Patel et al., 2006). In whole homogenized brain, CYP19A2 expression was about 100-fold higher than CYP19A1 which was consistent with other fish species. Adult females also had ∼700-times more CYP19A1 in their gonads compared to males (Patel et al., 2006). Furthermore, as measured by quantitative PCR, whole embryos also had about 5-fold higher CYP19A2 compared to CYP19A1. Greytak and coworkers have studied CYP19 expression in wild fish collected from polluted and non-polluted sites and found CYP19A2 expression 2-fold higher in polluted fish compared to non-polluted fish, but no site differences were found in CYP19A1 expression. Both CYP19A1 and CYP19A2 were lower in reproductively inactive Fundulus compared to reproductively active fish collected in June and July (Greytak et al., 2005). To add insight into when during development and in what tissue types the CYP19s are expressed, we have used in situ hybridization to measure CYP19 expression throughout Fundulus development.
Materials and methods
Fundulus eggs
A parental population of F. heteroclitus collected from an uncontaminated site at the New River inlet near Beaufort, North Carolina was raised under the University Institutional Animal Care and Use Committee (IACUC) approved conditions. Sexually mature fish were bred and kept in salt water (20-25 ppt). The fish were maintained at a 14:10 light-dark cycle in summer and 10:14 light-dark cycle in winter. Adult fish were fed twice daily with tropical flake fish food (Tetramin, Tetra Werke, Germany) and live brine shrimp. First generation offspring, from wild parents, were used for the studies described here.
Whole mount in situ hybridization
1-Phenyl-2-thiourea (0.003%) was included the embryos’ water from 5 dpf to prevent pigmentation of Fundulus larva. Fundulus embryos were fixed in 4% (w/v) paraformadehyde in phosphate saline solution (pH 7.4) overnight and stored in -20°C freezer after removal of the membrane by watchmaker’s forceps. Whole mount in situ hybridization was carried out as described elsewhere (Dong et al., 2002). Probes were generated by PCR amplification from Fundulus CYP19A1 or CYP 19A2 plasmids (originally cloned by Patel et al., 2006) AY713118 or AY494837). The CYP19A1 corresponded to nucleotides 1-1660, while the CYP19A2 was 717 bp and corresponded to nucleotides 183-1000. Probes were purified and subcloned in pGEM-TEasy Vector (Promega, Madison, USA). Antisense and sense digoxigenin probes were synthesized by in vitro transcription using T7 and SP6 polymerase on template DNA linearized with Apa I or Spe I, respectively. Following hybridization overnight at 65°C, embryos were washed with 2x SSC and 0.2x SSC twice for 30 min, respectively. After blocking with 2% blocking reagent (Roche), embryos were incubated overnight with 4000x diluted anti-DIG antibody conjugated with alkaline phosphatase (Roche) at 4°C. The color reaction was carried out by incubation with BM-purple substrate (Roche).
Paraffin sectioning and in situ hybridization
Fundulus tissues were fixed in 4% (w/v) paraformadehyde in phosphate saline solution (pH 7.4) overnight, followed by dehydration in increasing gradients of ethanol (70%, 80%, 90%, 95%, 100%I and 100%II). After cleared in Clearify™ (American Master Tech Scientific, Inc), tissues were embedded in molten paraffin (Paraplast embedding media paraplast X-tra, Sigma). Sections of 7 μm thickness were cut using a microtome (OLYMPUS CUT 4055, Olympus American Inc). The sections were cleared in gradient of ethanol (100%-70%) and hybridized with the antisense probe of Fundulus CYP19A1 as described above. Following hybridization overnight at 50 °C, sections were washed with 2x SSC and 0.2x SSC twice for 20 min, respectively. After blocking with 2% blocking reagent (Roche), embryos were incubated overnight with 4000x diluted anti-DIG antibody conjugated with alkaline phosphatase (Roche) at 4°C. The color reaction was carried out by incubation with BM-purple substrate (Roche).
Expression quantification and statistics
For measuring the intensity of CYP19A1 or CYP19A2 expression, pictures were obtained with digital camera (OPTRONICS) with a light microscope (BX40, Olympus), To quantitate, regions of interest (ROIs) were traced and mean pixel intensity was analyzed by means of KODAK 1D image Analysis Software. Results are presented as mean ± SE. Significant differences between means were determined by one-way ANOVA followed by Newman-Keuls Multiple Comparison Test (p < 0.05). For analysis of adult brain CYP19A2 expression, a two-way ANOVA was done to measure potential interaction between brain region and sex.
Results
Expression of CYP19A1 in developing Fundulus
To investigate the distribution of CYP19A1 gene, 2, 3, 4, 6, 8, 10, 14, 30 and 90 dpf Fundulus were fixed, and in situ hybridization using antisense 1660 base pair probe of CYP19A1 was carried out. No signal was detected at 2, 3, 4, 6, 8, 10, and 14 dpf in Fundulus (data not shown). As shown in Fig. 1. A, B, C, expression of CYP19A1 was first detected (blue precipitate) in the ooplasm of early stage I oocytes (primary growth stage, n=5) as strong and evenly distributed signals in the ovary of 30 and 90 dpf old Fundulus. No CYP19A1 signal was detected in brain or other tissues (data not shown) or when the sense probe was used (Fig. 1D). Hematoxylin-eosin-stain (H&E stain) was used to show histological features and to compare with CYP19A1 expression by in situ hybridization (Fig. 1. E and F).
Figure 1.
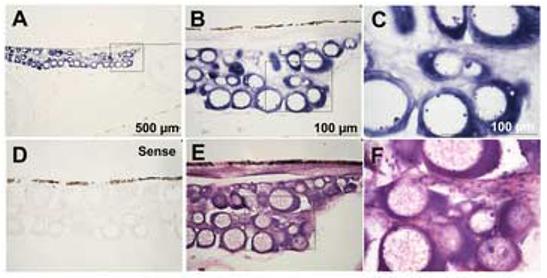
Expression of CYP19A1 in 3 month old (90 dpf) oocytes visualized by in situ hybridization using antisense (A-C) and sense (D) CYP19A1 probes and H&E staining (E and F). Expression of CYP19A1 mRNA was detected in the ooplasm of stage I oocytes (primary growth stage) as strong and evenly distributed signals in 3 month old fish. B is magnification of A; C and F are magnification of B and E, respectively. n=5.
Expression of CYP19A1 in the ovary of adult Fundulus
For analysis of CYP19A1 expression in the oocytes, developing oocytes were divided into stage I (previtellogenic up to 0.6 mm diameter), stage II (vitellogenesis 0.6 - 1.3 mm), stage III (maturation 1.3 - 1.9 mm with slight protein accumulation) and stage IV (maturation without protein accumulation) as defined by (Wallace and Selman 1985). We have further divided the stage I follicles into three size based categories: early stage I (stage Ia, 0 - 0.15 mm), mid stage I (stage Ib, 0.15 - 0.25 mm), and late stage I (stage Ic, 0.25 - 0.6 mm).
Similar to ovary of 90 dpf Fundulus, expression of CYP19A1 mRNA was detected in the ooplasm of stage Ia oocytes as strong and evenly distributed signals in the ovary of adult Fundulus (Fig. 2 E, M). In the stage Ib oocytes, the signal gradually declined at both the ooplasm and membrane of oocytes and became barely detectable (Fig. 2 F, G, N and O). From stage Ic, the CYP19A1 expression was found only in the outer region of oocytes (Fig. 2.H and P). From stage Ia to stage Ib, mean intensity of CYP19A1 expression in the ooplasm was measured and expression significantly decreased between the stage Ia and stage Ib (Fig. 3).
Figure 2.
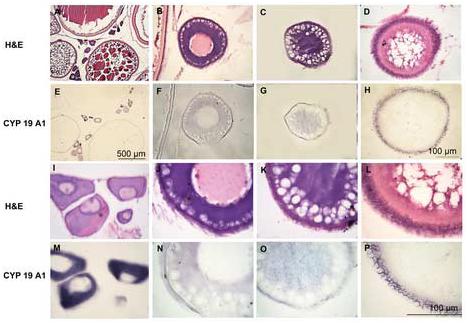
Expression of CYP19A1 in adult oocytes. Adult ovaries were sectioned and visualized by in situ hybridization using CYP19A1 antisense probe (E, F, G, H, M, N, O and P) or H&E staining (A, B, C, D, I, J, K and L). Expression of CYP19A1 mRNA was detected in the ooplasm of stage Ia oocytes (primary growth stage, E and M) as strong and evenly distributed signals in adult fish. Signal gradually decreased and became barely detectable by stage Ib oocytes (previtellogenic follicle stage, F, G, N, and O). Oocytes were characterized by the presence of yolk granules in ooplasm. In the stage Ic, expression was detected in the follicular cell layer (H and P). I, J, K and L are higher magnification of A, B, C and D; M, N, O and P are higher magnification of E, F, G and H.
Figure 3.
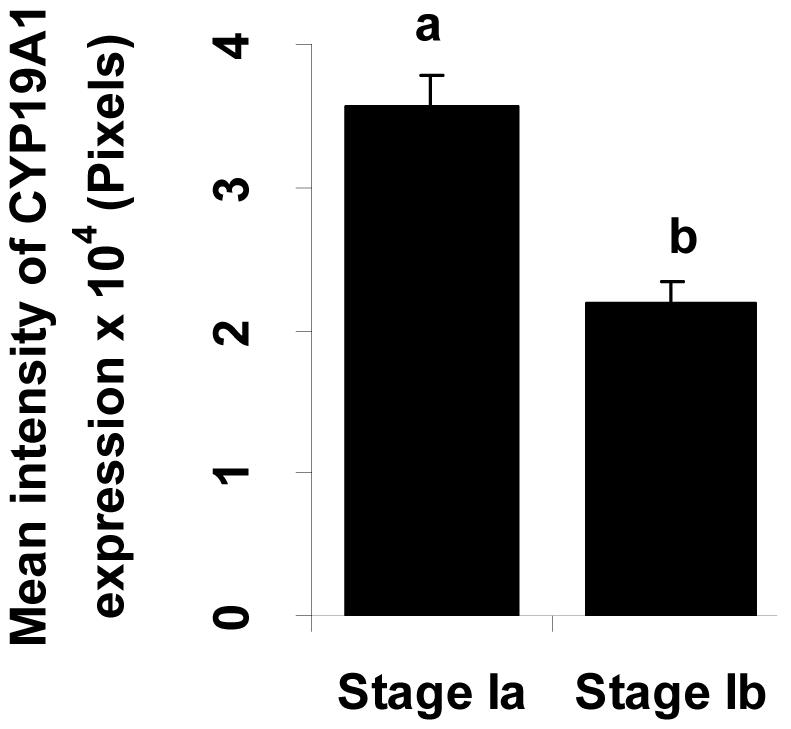
Intensity of CYP19A1 expression at early stage I (stage Ia) to mid stage I (Stage Ib) in the oocytes. Expressed regions were analyzed by means of KODAK 1D image Analysis Software (Creating regions of interest, ROIs). Bars with different letters are statistically different (p<0.05). Each bar represents the mean ± S.E. from 3-5 oocytes per developmental stage (stage Ia and Ib) from 5 fish.
Figure 4 provides further details of the follicular expression of CYP19A1 in adults. In stage Ic oocytes, the CYP19A1 expression was isolated to the follicular cell layer and expression was wavy possibly associated with outer cell membranes of individual follicle cells (for electron micrograph see (Selman and Wallace 1983)). From stage II to III expression appeared to transfer to the acellular vitelline envelope, and by stage IV there was no apparent CYP19A1 expression.
Figure 4.

Expression of CYP19A1 in the membrane of adult oocytes. Expression of CYP19A1 mRNA was detected in the membrane in the stage Ib (previtellogenic follicle stage, A); stage Ic (B); stage II (vitellogenic, C and D); stage III (early maturational follicles, E) oocytes. No signal was detected in stage IV (maturational follicles, F) oocytes. G and H are H&E stain of C and F, respectively. Arrow indicates expression in follicular cell layer and/or vitelline envelop in A-E. Bar=100 μm.
Compared to ovary, weak CYP19A1 expression was found in the adult testis (interstitial cells, data not shown), but positive CYP19A1 expression was not detected in brain of adult Fundulus by either whole mount or section in situ hybridization (data not shown).
Expression of CYP19A2 in developing Fundulus
Temporal changes in CYP19A2 expression during development were measured by in situ hybridization after fertilization at 2, 3, 4, 6, 8, 10, and 14 dpf. CYP19A2 expression was not detected in 2 dpf embryos (data not shown) but was first observed at 3 dpf in the brain including the midbrain. In the 4 dpf embryos, the CYP19A2 signal was found in the hypothalamus and ventral telencephalon (Fig 5). Similar to 4 dpf, CYP19A2 expression also was detected at 6, 8, 10 and 14 dpf. In addition to brain expression, CYP19A2 showed very intense staining in the adrenal tissue of the head kidney at 6, 8, 10 and 14 dpf (Fig 6).
Figure 5.
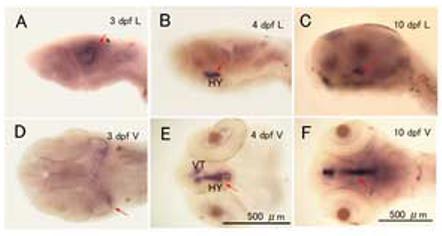
CYP19A2 expression in the brain at 3, 4 and 10 dpf. Embryos were fixed by 4% PFA, and expression of CYP19A2 mRNA in the brain was assessed by whole-mount in situ hybridization. Representative embryos are shown Laterally (A, B, and C) and Ventrally (D, E and F). Arrow indicates CYP19A2 expression in the hypothalamus (HY) and ventral telencephalon (VT), (n=10-12 embryos/age).
Figure 6.
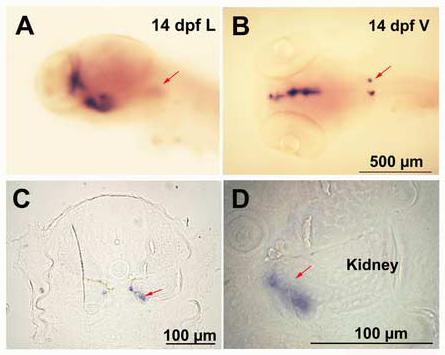
Expression of CYP 19A2 in the adrenal /kidney tissue by whole-mount in situ hybridization. The representative embryos (14 dpf) are shown in Lateral (A) Ventral (B) views and C and D are cross sections of B. D is a higher magnification of C. Arrow indicates expression in the adrenal cells in A, B, C and D (n=10-12 embryos).
In the 90 dpf Fundulus brain, CYP19A2 expression by whole mount in situ hybridization was found in the hypothalamus and ventral telencephalon, but was not found in the pituitary except where it combines with the hypothalamus (HY) or the olfactory bulb. Magnified images of the pituitary are shown in the insert photos (Fig. 7, A, B and C).
Figure 7.
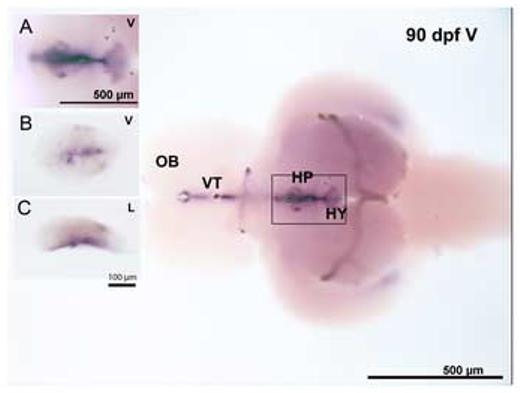
Expression of CYP19A2 in 90 dpf Fundulus brain by whole-mount in situ hybridization. The large image is a ventral view of the brain. Inset graphs show the Ventral (A and B) and Lateral (C) views of the pituitary. A is a magnification of the rectangular area, B and C are magnification of pituitary after dissection from the brain (n= 8 embryos).
Expression of CYP19A2 in the brain of adult Fundulus
To investigate the distribution of CYP19A2 expression in adult Fundulus, brains were obtained from male and female fish at similar season (February). Highest CYP19A2 mRNA expression was detected in the pituitary and hypothalamus. The ventral telencephalon and olfactory bulb had moderate and lower levels expression, respectively by whole mount in situ hybridization (Fig. 8, A and B). The distribution of CYP19A2 expression was confirmed by sectioning after whole-mount in situ hybridization (Fig. 8 C, D, E and F). The intensity of CYP19A2 within a given brain region in males and females was not significantly different except for in the olfactory bulb where female expression was higher than that in males (p<0.05, Fig. 9). In addition, very weak expression of CYP19A2 also was found in the ovary in the stage I to stage III follicles (data not shown).
Figure 8.
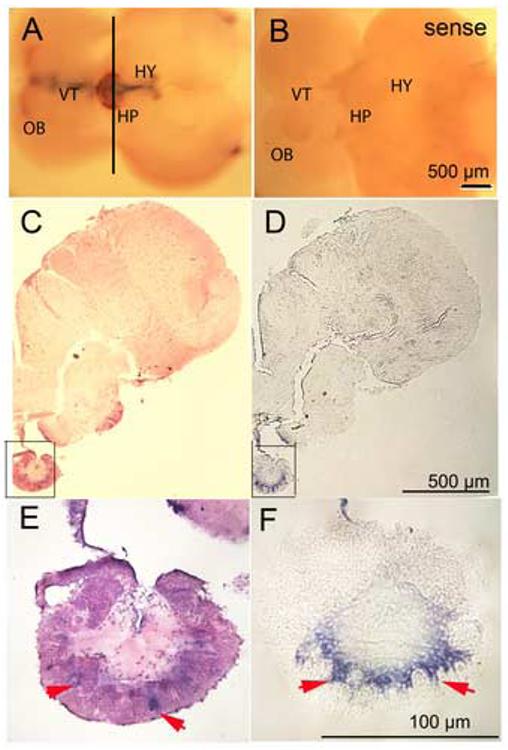
Expression of CYP19A2 in adult Fundulus brain. The CYP19A2 transcripts were found at the hypothalamus (HY), pituitary (HP), ventral telencephalon (VT), and extending to the olfactory bulb (OB). Ventral views. A, Antisense probe; B, Sense probe. C and D are sections of A at the black line. E and F are higher magnification of C and D, respectively. C and E are H&E stain. Arrows indicate CYP19A2 expression in the pituitary.
Figure 9.
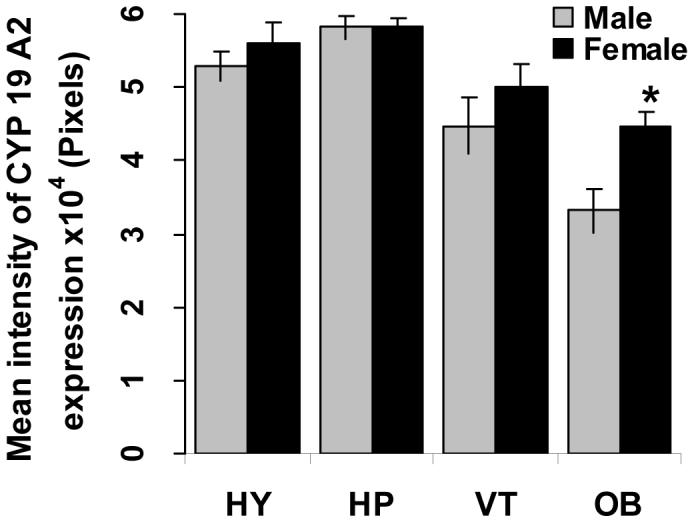
Intensity of CYP19A2 expression within a given part in the adult brain of each sex. HY: hypothalamus; VT: ventral telencephalon; OB: olfactory bulb; HP: pituitary. Asterisk indicates a significant difference between the male and female (p<0.05). Each bar represents the mean ± S.E. of 5 fish per sex.
Discussion
In this study we have used in situ hybridization to define the oocyte expression of CYP19A1 and the brain regions in which CYP19A2 is expressed during Fundulus development. Previous studies by our lab and others have measured general ovarian and brain CYP19 mRNA and aromatase activity in whole homogenized tissues (Greytak et al., 2005; Patel et al., 2006) however the cellular localization (without amplification) has not been previously reported in Fundulus. For example, while both CYP19A1 and CYP19A2 mRNA expression was detectable in Fundulus and zebrafish embryos by sensitive qRT-RT PCR methods (Kishida and Callard 2002; Patel et al., 2006), expression of CYP19A1 and CYP19A2 expression was not detected until 30 and 3 dpf, respectively, by in situ hybridization. Disruptions of CYP19A2 expression, therefore, may be more significant early in Fundulus development while variant CYP19A1 expression may impact reproductive potential of adults.
As described in the introduction Fundulus reproduction and oocyte maturation has been extensively studied particularly by the Selman and Wallace laboratories. Fundulus are asynchronous spawners with semilunar spawning. Ovaries from reproductively immature female fish (30 and 90 dpf in this study) contained many small previtellogenic oocytes that stained strongly for CYP19A1 mRNA. Similarly in adult ovaries the small previtellogenic follicles stained strongly. However, in later stage I and stage II (vitellogenic) follicles, CYP19A1 expression was isolated to the follicular cell layer and was wispy in appearance. By stage III (early maturation) there was a more solid CYP19A1 expression in the vitelline envelop which was not seen in mature stage IV follicles (Fig 4). These results are consistent with work done by Petrino and coworkers where Fundulus follicle cell preparations but not denuded oocytes or theca/surface epithelium preparations were capable of estrogen production when stimulated by pituitary extracts (Petrino et al., 1989a). CYP19A1 expression has also been recently described in the follicular layer of zebrafish vitellogenic oocytes (Goto-Kazeto et al., 2004). These results suggest that aromatase and local estrogen synthesis may be physiologically important in both early oocyte growth and during vitellogenesis.
We found very weak CYP19A1 staining in the interstial cells of the adult testis. Our previous PCR results suggested that there was approximately 700 times less CYP19A1 expression in male gonads compared to females, and there was actually more CYP19A2 in testis than CYP19A1 (Patel et al., 2006). CYP19A1 expression was also reported in goby testis but the regulatory mechanisms or the physiological significance of aromatase in the male gonad is currently unknown (Kobayashi et al., 2004).
To our knowledge, a unique finding of this study was the CYP19A2 expression detected in what we believe are adrenal like cells in the head kidney in Fundulus embryos from 6 - 14 dpf (Fig. 6). In sections of 90 dpf and adults, we did not see similar expression, however, it could have been weak and missed in the whole sections of the fish torso. Male Fundulus adrenocortical tissue possesses enzyme activities associated with steroid synthesis, specifically 3β-, 3α-, 11β-, and 17β-hydroxysteroid dehydrogenase activities (Bara 1968). Similarly, male African catfish adrenal (interrenal) tissue was capable of synthesis of at least 15 different steroids including predominantly cortisol and androstanediones (Vermeulen et al., 1995). Head kidneys from adult European sea bass have very low but detectable aromatase activity (Gonzáles and Piferrer 2003). CYP19A2 RNA expression has been detected by PCR in adult fish kidneys of several species (see review by Piferrer and Blazquez, 2005) including pejerrey (Strobl-Mazzulla et al., 2005), Nile tilapia (Kwon et al, 2001) and by Northern blot in orange spotted grouper (Zhang et al, 2004). In their study of tissue specific expression of CYP19s in Fundulus by PCR, Greytak and coworkers did not test kidney for CYP19 RNA expression (Greytak et al., 2005). However, enzymatically active aromatase has been reported in newborn pig adrenal glands whereas it is relatively absent in mature pigs (Conley et al., 1996). These data suggest that adrenal estrogen synthesis may be important in Fundulus development and further study is warranted.
The earliest expression of CYP19A2 was detected at 3 dpf in the midbrain, and by 4 dpf expression was clearly localized in the hypothalamus and telencephalon. Compared to adult CYP19A2 expression, relatively less is known about the significance of early developmental expression of brain aromatase. In control zebrafish CYP19A2 was not detected by in situ hybridization at 24, 36, and 48 hpf (Menuet et al., 2005), however 10-8 M estradiol induced CYP19A2 by 36 hpf in the preoptic area and hypothalamus and by 48 hpf in the telecephalon also. For comparison, a 29 hpf zebrafish is developmentally similar to a Fundulus at 3 dpf with respect to mid and hind brain development. Therefore, Fundulus may have earlier and higher CYP19A2 expression compared to zebrafish, or there were experimental differences that increased the sensitivity of the Fundulus in situ hybridization.
By 90 dpf of Fundulus development, the CYP19A2 expression was still predominantly in hypothalamus and telencephalon, but in adults expression was also found in the pituitary and olfactory bulb. The regions of expression were similar to those reported by in situ hybridization and immunohistochemistry in adult zebrafish (Goto-Kazeto et al., 2004; Menuet et al., 2005) with the exception that there were no sex differences in CYP19A2 RNA expression in zebrafish. In our study, we found that adult female Fundulus had significantly higher CYP19A2 expression in the olfactory bulb region. In contrast to Fundulus, adult female sea bass had significantly lower brain aromatase activities in the telencephalon, hypothalamus, pituitary and optic bulb, but not in the olfactory bulb (Gonzáles and Piferrer 2003). Sexual dimorphism in brain aromatase activity has also been reported in sections along the longitudinal axis of the Japanese medaka brain. Males had higher aromatase activity in sections containing the preoptic hypothalamic nuclei and the suprachiasmatic nucleus whereas highest activities in females were more caudal containing the periventricular dorsalis, ventralis, caudalis, tuberi anteriores, and posteriores nuclei (Melo and Ramsdell 2001).
The exact physiological significance of brain CYP19A2 expression has not been determined, but recent work has suggested its role in estrogen dependent neurogenesis (which continuous through adulthood in fish) (Forlano et al., 2001; Pellegrini et al., 2005, 2007), sex change and behavior (Marsh et al., 2006), and regulation of the gonadal-pituitary-hypothalamic feedback loop (Melo and Ramsdell 2001; Villeneuve et al., 2007). Additional studies with estrogenic inducers and aromatase inhibitors together with measurement of CYP19A2 expression and phenotypic effects (for example (Greytak et al., 2005; Kishida et al., 2002)) will further refine the physiological significance of aromatase in fish.
In summary, both CYP19A1 and CYP19A2 expression were cell type and developmental stage dependent. CYP19A1 was diffusely expressed in early follicles and in larger follicles expression was localized to the follicular cell layer then the vitelline envelop. CYP19A2 expression was detected in adrenal/kidney cells during early Fundulus development and in brain by 3 dpf. Regional expression of CYP19A2 in the brain differed by age and expression was higher in female adult olfactory bulb compared to males. This work establishes the constitutive expression of CYP19s which can then be compared with potential disruption of CYP19A1 and CYP19A2 expression by environmental contaminants such as benzo(a)pyrene and ethinylestradiol.
Acknowledgements
This work was supported by NIEHS grant R01ES012710 and instruments provided by NCRR grant RR016476 from the MFGN INBRE Program. We thank Annette Ford, Lu Wang and Kate Argote for technical assistance and fish care. Parental Fundulus were collected and provided by Dr. Patricia McClellan-Green, Duke University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong PB, Child JS. Stages in the normal development of Fundulus heteroclitus. Biol. Bull. 1965;128:143–169. [Google Scholar]
- Bara G. Histochemical study of 3-beta-, 3-alpha-, 11-beta-, and 17-beta-hydroxysteroid dehydrogenases in the adrenocortical tissue and the corpuscles of Stannius of Fundulus heteroclitus. Gen. Comp Endocrinol. 1968;10:126–137. doi: 10.1016/0016-6480(68)90018-x. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, DiGiulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons in zebrafish. Toxicol. Sci. 2006;92:526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- Blazquez M, Piferrer F. Cloning, sequence analysis, tissue distribution, and sex-specific expression of the neural form of P450 aromatase in juvenile sea bass (Dicentrarchus labrax) Mol. Cell. Endocrinol. 2004;219:83–94. doi: 10.1016/j.mce.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Boudreau M, Courtenay SC, MacLatchy DL, Berube CH, Hewitt LM, Van Der Kraak GJ. Morphological abnormalities during early-life development of the estuarine mummichog, Fundulus heteroclitus, as an indicator of androgenic and anti-androgenic endocrine disruption. Aquat. Toxicol. 2005;71:357–369. doi: 10.1016/j.aquatox.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Cerda J, Calman BG, LaFleur GJ, Jr., Limesand S. Pattern of vitellogenesis and follicle maturational competence during the ovarian follicular cycle of Fundulus heteroclitus. Gen. Comp Endocrinol. 1996;103:24–35. doi: 10.1006/gcen.1996.0090. [DOI] [PubMed] [Google Scholar]
- Cerda J, Subhedar N, Reich G, Wallace RA, Selman K. Oocyte sensitivity to serotonergic regulation during the follicular cycle of the teleost Fundulus heteroclitus. Biol. Reproduct. 1998;59:53–61. doi: 10.1095/biolreprod59.1.53. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Corbin CJ, Hinshelwood MM, Liu Z, Simpson ER, Ford JJ, Harada N. Functional aromatase expression in porcine adrenal gland and testis. Biol. Reprod. 1996;54:497–505. doi: 10.1095/biolreprod54.2.497. [DOI] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Yamazaki K, Tsukiyama S, Imani S, Imagawa T, Stegeman JJ, Peterson RE, Hiraga T. 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: local circulation failure in the dorsal midbrain is associated with increased apoptosis. Toxicol. Sci. 2002;69:191–201. doi: 10.1093/toxsci/69.1.191. [DOI] [PubMed] [Google Scholar]
- Dube MG, MacLatchy DL. Identification and treatment of a waste stream at a bleached-kraft pulp mill that depresses a sex steroid in the mummichog (Fundulus heteroclitus) Environ. Toxicol. Chem. 2001;20:985–995. doi: 10.1897/1551-5028(2001)020<0985:iatoaw>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J. Comp. Neuro. 2005;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: Aromatase enzyme and mRNA expression identify glia as source. J. Neurosci. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas D, Callard GV. Immunolocalization of aromatase- and androgen receptor-positive neurons in the goldfish brain. Gen. Comp. Endocrinol. 1997;106:155–168. doi: 10.1006/gcen.1997.6891. [DOI] [PubMed] [Google Scholar]
- Gonzáles A, Piferrer F. Aromatase activity in the European sea bass (Dicentrarchus labraz L.) brain. Distribution and changes in relation to age, sex, and the annual reproductive cycle. Gen. Comp. Endocrinol. 2003;132:223–230. doi: 10.1016/s0016-6480(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Goto-Kazeto R, Kight KE, Zohar Y, Place AR, Trant JM. Localization and expression of aromatase mRNA in adult zebrafish. Gen. Comp. Endocrinol. 2004;139:72–84. doi: 10.1016/j.ygcen.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Greytak SR, Callard GV. Cloning of three estrogen receptors (ER) from killifish (Fundulus heteroclitus): Differences in populations from polluted and reference environments. Gen. Comp Endocrinol. 2007;150:174–188. doi: 10.1016/j.ygcen.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Greytak SR, Champlin D, Callard GV. Isolation and characterization of two cytochrome P450 aromatase forms in killifish (Fundulus heteroclitus): differential expression in fish from polluted and unpolluted environments. Aquatic. Toxicol. 2005;71:371–389. doi: 10.1016/j.aquatox.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Hsiao S-M, Limesand SW, Wallace RA. Semilunar follicular cycle of an intertidal fish: the Fundulus model. Biol. Reproduct. 1996;54:809–818. doi: 10.1095/biolreprod54.4.809. [DOI] [PubMed] [Google Scholar]
- Kazeto Y, Ijiri S, Place AR, Zohar Y, Trant JM. The 5′-flanking regions of CYP19A1 and CYP19A2 in zebrafish. Biochem. Biophys. Res. Commun. 2001;288:503–508. doi: 10.1006/bbrc.2001.5796. [DOI] [PubMed] [Google Scholar]
- Kelly SA, Di Giulio RT. Developmental toxicity of estrogenic alkylphenols in killifish (Fundulus heteroclitus) Environ, Toxicol. Chem. 2000;19:2564–2570. [Google Scholar]
- Kishida M, Callard GV. Distinct cytochrome P450 aromatase isoforms in zebrafish (Danio rerio) brain and ovary are differentially programmed and estrogen regulated during early development. Endocrinology. 2002a;142:740–750. doi: 10.1210/endo.142.2.7928. [DOI] [PubMed] [Google Scholar]
- Kishida M, McLellan M, Miranda JA, Callard GV. Estrogen and xenoestrogens upregulate the brain aromatase isoform (P450aromB) and perturb the markers of early development in zebrafish (Danio rerio) Comp. Biochem. Physiol. 2002b;129:261–268. doi: 10.1016/s1096-4959(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kobayashi T, Nakamura M, Sunobe T, Morrey CE, Suzuki N, Nagahama Y. Characterization of two types of cytochrome P450 aromatase in the serial-sex changing gobiid fish, Trimma okinawae. Zoo. Sci. 2004;21:417–425. doi: 10.2108/zsj.21.417. [DOI] [PubMed] [Google Scholar]
- Kuhl AJ, Manning S, Brouwer M. Brain aromatase in Japanese medaka (Oryzias latipes): Molecular characterization and role in xenoestrogen-induced sex reversal. J. Steroid Biochem. Mol. Biol. 2005;96:67–77. doi: 10.1016/j.jsbmb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Kumar RS, Ijiri S, Trant JM. Changes in the expression of genes encoding steroidogenic enzymes in the channel catfish (Ictalurus punctatus) ovary throughout a reproductive cycle. Biol. Reproduct. 2000;63:1676–1682. doi: 10.1095/biolreprod63.6.1676. [DOI] [PubMed] [Google Scholar]
- Kwon JY, McAndrew BJ, Penman DJ. Cloning of brain aromatase gene and expression of brain and ovarian aromatase genes during sexual differentiation in genetic male and female Nile tilapia Oreochromis nitoticus. Mol. Reproduct. Develop. 2001;59:359–370. doi: 10.1002/mrd.1042. [DOI] [PubMed] [Google Scholar]
- Marsh KE, Creutz LM, Hawkins MB, Godwin J. Aromatase immunoreactivity in the bluehead wrasse brain, Thalassoma bifasciatum: Immunolocalization and co-regionalization with arginine vasotocin and tyrosine hydroxylase. Brain Res. 2006;1126:91–101. doi: 10.1016/j.brainres.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt U, Mullis PE. The aromatase cytochrome P-450 and its clinical impact. Hormone Res. 2002;57:145–152. doi: 10.1159/000058374. [DOI] [PubMed] [Google Scholar]
- Melo AC, Ramsdell JS. Sexual dimorphism of brain aromatase activity in medaka: Induction of a female phenotype by estradiol. Environ. Health Perspect. 2001;109:257–264. doi: 10.1289/ehp.01109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuet A, Anglade I, Le GR, Pellegrini E, Pakdel F, Kah O. Distribution of aromatase mRNA and protein in the brain and pituitary of female rainbow trout: Comparison with estrogen receptor alpha. J. Comp Neurol. 2003;462:180–193. doi: 10.1002/cne.10726. [DOI] [PubMed] [Google Scholar]
- Menuet A, Pellegrini E, Brion F, Gueguen MM, Anglade I, Pakdel F, Kah O. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J. Comp Neurol. 2005;485:304–320. doi: 10.1002/cne.20497. [DOI] [PubMed] [Google Scholar]
- Meyer HHD. Handbook of Experimental Pharmacology. 1999. Comparative aspects of estrogen biosynthesis and metabolism and the endocrinological consequences in different animal species; pp. 575–602. [Google Scholar]
- Meyer JN, Volz DC, Freedman JH, Di Giulio RT. Differential display of hepatic mRNA from killifish (Fundulus heteroclitus) inhabiting a Superfund estuary. Aquat. Toxicol. 2005;73:327–341. doi: 10.1016/j.aquatox.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Nakamura I, Evans JC, Kusakabe M, Nagahama Y, Young G. Changes in steroidogenic enzyme and steroidogenic acute regulatory protein messenger RNAs in ovarian follicles during ovarian development of rainbow trout (Oncorhynchus mykiss) Gen. Comp Endocrinol. 2005;144:224–231. doi: 10.1016/j.ygcen.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Nocillado JN, Elizur A, Avitan A, Carrick F, Levavi-Sivan B. Cytochrome P450 aromatase in grey mullet: cDNA and promoter isolation; brain, pituitary and ovarian expression during puberty. Mol. Cell Endocrinol. 2007;263:65–78. doi: 10.1016/j.mce.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Nunez BS, Applebaum SL. Tissue- and sex-specific regulation of CYP19A1 expression in the Atlantic croaker (Micropogonias undulatus) Gen. Comp Endocrinol. 2006;149:205–216. doi: 10.1016/j.ygcen.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Paschall JE, Oleksiak MF, VanWye JD, Roach JL, Whitehead JA, Wyckoff GJ, Kolell KJ, Crawford DL. FunnyBase: a systems level functional annotation of Fundulus ESTs for the analysis of gene expression. BMC Genomics. 2004;5:96. doi: 10.1186/1471-2164-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MR, Scheffler BE, Wang L, Willett KL. Effects of benzo(a)pyrene exposure on killifish (Fundulus heteroclitus) aromatase activities and mRNA. Aquat. Toxicol. 2006;77:267–278. doi: 10.1016/j.aquatox.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini E, Menuet A, Lethimonier C, Adrio F, Gueguen M-M, Tascon C, Anglade I, Pakdel F, Kah O. Relationships between aromatase and estrogen receptors in the brain of teleost fish. Gen. Comp. Endocrinol. 2005;142:60–66. doi: 10.1016/j.ygcen.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Pellegrini E, Mouriec K, Anglade I, Menuet A, Le PY, Gueguen MM, Marmignon MH, Brion F, Pakdel F, Kah O. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J. Comp Neurol. 2007;501:150–167. doi: 10.1002/cne.21222. [DOI] [PubMed] [Google Scholar]
- Petrino TR, Greeley MS, Selman K, Lin Y-W, Wallace RA. Steroidogenesis in Fundulus heteroclitus II. Production of 17α-hydroxy-20β-dihydroprogesterone, testosterone, and 17β-estradiol by various components of the ovarian follicle. Gen. Comp. Endocrinol. 1989a;76:230–240. doi: 10.1016/0016-6480(89)90154-8. [DOI] [PubMed] [Google Scholar]
- Petrino TR, Hoch KL, Lin Y-W, Wallace RA. Steroidogenesis in Fundulus heteroclitus: III. Evidence for involvement of cAMP and protein synthesis in the gonadotropic modulation of ovarian steroid production and aromatase activity. J. Exper. Zool. 1990;253:177–185. [Google Scholar]
- Petrino TR, Lin Y-W, Wallace RA. Steroidogenesis in Fundulus heteroclitus I. Production of 17α-hydroxy, 20β-dihydroprogesterone, testosterone, and 17β-estradiol by prematurational follicles in vitro. Gen. Comp. Endocrinol. 1989b;73:147–156. doi: 10.1016/0016-6480(89)90065-8. [DOI] [PubMed] [Google Scholar]
- Piferrer F, Blazquez M. Aromatase distribution and regulation in fish. Fish Physiol. Biochem. 2005;31:215–226. doi: 10.1007/s10695-006-0027-0. [DOI] [PubMed] [Google Scholar]
- Powell WH, Bright R, Bello SM, Hahn ME. Developmental and tissue-specific expression of AHR1, AHR2, and ARNT2 in dioxin-sensitive and -resistant populations of the marine fish, Fundulus heteroclitus. Toxicol. Sci. 2000;57:229–239. doi: 10.1093/toxsci/57.2.229. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mari A, Yan YL, Bremiller RA, Wilson C, Canestro C, Postlethwait JH. Characterization and expression pattern of zebrafish Anti-Mullerian hormone (Amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expr. Patterns. 2005;5:655–667. doi: 10.1016/j.modgep.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Sanderson JT. The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol. Sci. 2006;94:3–21. doi: 10.1093/toxsci/kfl051. [DOI] [PubMed] [Google Scholar]
- Sawyer SJ, Gerstner KA, Callard GV. Real-time PCR analysis of cytochrome P450 aromatase expression in zebrafish: gene specific tissue distribution, sex differences, developmental programming, and estrogen regulation. Gen. Comp Endocrinol. 2006;147:108–117. doi: 10.1016/j.ygcen.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Selman K, Wallace RA. Oogenesis in Fundulus heteroclitus. III. Vitellogenesis. J. Exp. Zool. 1983;226:441–457. doi: 10.1002/jez.1402260315. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. Aromatase - A brief overview. Annu. Rev. Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- Strobl-Mazzulla PH, Moncaut NP, Lopez GC, Miranda LA, Canario AV, Somoza GM. Brain aromatase from pejerrey fish (Odontesthes bonariensis): cDNA cloning, tissue expression, and immunohistochemical localization. Gen. Comp Endocrinol. 2005;143:21–32. doi: 10.1016/j.ygcen.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Subhedar N, Cerda J, Calman BG, Wallace RA. Changes in forebrain and pituitary dopamine and serotonin contents of female Fundulus during its biweekly reproductive cycle. Comp. Biochem. Physiol. 1997;118A:577–584. [Google Scholar]
- Sunobe T, Nakamura M, Kobayashi Y, Kobayashi T, Nagahama Y. Aromatase immunoreactivity and the role of enzymes in steroid pathways for inducing sex change in the hermaphrodite gobiid fish Trimma okinawae. Comp Biochem. Physiol A Mol. Integr. Physiol. 2005;141:54–59. doi: 10.1016/j.cbpb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fukada S, Matsuyama M, Nagahama Y. Structure and promoter analysis of the cytochrome P-450 aromatase gene of the teleost fish, medaka (Oryzias latipes) J. Biochem. 1995;117:719–725. doi: 10.1093/oxfordjournals.jbchem.a124768. [DOI] [PubMed] [Google Scholar]
- Tchoudakova AV, Kishida M, Wood E, Callard GV. Promoter characteristics of two cyp19 genes differentially expressed in the brain and ovary of teleost fish. J. Steroid Biochem. Molec. Biol. 2001;78:427–439. doi: 10.1016/s0960-0760(01)00120-0. [DOI] [PubMed] [Google Scholar]
- Tong S-K, Chung B-C. Analysis of zebrafish cyp19 promoters. J. Steroid Biochem. Mol. Biol. 2003;86:381–386. doi: 10.1016/s0960-0760(03)00347-9. [DOI] [PubMed] [Google Scholar]
- Trant JM, Gavasso S, Ackers J, Chung B, Place AR. Developmental expression of cytochrome P450 aromatase genes (CYP19a and CYP19b) in zebrafish fry (Danio rerio) J. Exper. Zool. 2001;290:475–483. doi: 10.1002/jez.1090. [DOI] [PubMed] [Google Scholar]
- Vermeulen GJ, Lambert JGD, Teitsma CA, Zandbergen MA, Goos HJT. Adrenal tissue in the male African catfish, Clarias gariepinus: localization and steroid hormone secretion. Cell Tissue Res. 1995;280:653–657. [Google Scholar]
- Villeneuve DL, Larkin P, Knoebl I, Miracle AL, Kahl MD, Jensen KM, Makynen EA, Durhan EJ, Carter BJ, Denslow ND, Ankley GT. A graphical systems model to facilitate hypothesis-driven ecotoxicogenomics research on the teleost brain-pituitary-gonadal axis. Environ. Sci. Technol. 2007;41:321–330. doi: 10.1021/es061739x. [DOI] [PubMed] [Google Scholar]
- Vogelbein WK, Fournie JW, Van Veld PA, Huggett RJ. Hepatic neoplasms in the mummichog Fundulus heteroclitus from a creosote-contaminated site. Cancer Res. 1990;50:5978–5986. [PubMed] [Google Scholar]
- Wallace RA, Selman K. Major protein changes during vitellogenesis and maturation of Fundulus oocytes. Dev. Biol. 1985;110:492–498. doi: 10.1016/0012-1606(85)90106-x. [DOI] [PubMed] [Google Scholar]
- Wang L, Scheffler BE, Willett KL. CYP1C1 messenger RNA expression is inducible by benzo[a]pyrene in Fundulus heteroclitus embryos and adults. Toxicol. Sci. 2006;93:331–340. doi: 10.1093/toxsci/kfl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JY, McArthur AG, Stegeman JJ. Characterization of a cetacean aromatase (CYP19) and the phylogeny and functional conservation of vertebrate aromatase. Gen. Comp. Endocrinol. 2005;140:74–83. doi: 10.1016/j.ygcen.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang W, Zhang L, Zhu T, Tian J, Li X, Lin H. Two distinct cytochrome P450 aromatases in the orange-spotted grouper (Epinephelus coioides): cDNA cloning and differential mRNA expression. 2004;92:39–50. doi: 10.1016/j.jsbmb.2004.05.010. [DOI] [PubMed] [Google Scholar]


