Abstract
Dehydroepiandrosterone (DHEA) and its sulfate derivative (DHEAS) are the most abundant steroids produced by the human adrenal, but no receptors have been identified for these steroids, and no function for them has been established, other than as precursors for sex steroid synthesis. DHEA and DHEAS are found in brains from many species, and we have shown that enzymes crucial for their synthesis, especially P450c17 (17α-hydroxylase/c17,20 lyase), are expressed in a developmentally regulated, region-specific fashion in the developing rodent brain. One region of embryonic expression of P450c17, the neocortical subplate, has been postulated to play a role in guiding cortical projections to their appropriate targets. We therefore determined if products of P450c17 activity, DHEA and DHEAS, regulated the motility and/or growth of neocortical neurons. In primary cultures of mouse embryonic neocortical neurons, DHEA increased the length of neurites containing the axonal marker Tau-1, and the incidence of varicosities and basket-like process formations in a dose-dependent fashion. These effects could be seen at concentrations normally found in the brain. By contrast, DHEAS had no effect on Tau-1 axonal neurites but increased the length of neurites containing the dendritic marker microtubule-associated protein-2. DHEA rapidly increased free intracellular calcium via activation of N-methyl-d-aspartate (NMDA) receptors. These studies provide evidence of mechanisms by which DHEA and DHEAS exert biological actions, show that they have specific functions other than as sex steroid precursors, mediate their effects via non-classic steroid hormone receptors, and suggest that their developmentally regulated synthesis in vivo may play crucial and different roles in organizing the neocortex.
Neurosteroids including pregnenolone, allopregnanolone, dehydroepiandrosterone (DHEA), and their sulfated and lipoidal fatty acid ester derivatives are synthesized in the nervous system from cholesterol (1, 2). The same steroidogenic enzymes are found in the nervous system and in the classical steroidogenic tissues (3–9) although their extremely low level of expression in the brain and peripheral nervous system delayed their identification. DHEA and its sulfated derivative (DHEAS) and lipoidal fatty acid ester derivatives are abundant (10−8 to 10−7 M) in rodent, rabbit, monkey, dog, and human brains (10–14) but expression of P450c17, the sole enzyme having the 17α hydroxylase and c17,20 lyase activities needed for DHEA synthesis (15–17) only recently was identified in the fetal but not adult rodent brain (7). Some neurosteroids, such as allopregnanolone and pregnenolone, act through γ-aminobutyric acid (GABA)A (18–20) receptors while the neurosteroid pregnenolone sulfate may act through NMDA receptors (21, 22) rather than through classical nuclear steroid hormone receptors. Using these neurotransmitter receptors, allopregnanolone elicits anxiolytic, anticonvulsant activity (23, 24). Pregnenolone sulfate, DHEA and DHEAS enhance memory in mice and rats (25–30). There are many reports on the effects of DHEA on neural, endocrine, immune, and metabolic systems (reviewed in ref. 30). However, the molecular/genetic mechanisms of the effects have not been established in these systems. Furthermore, it is unknown if all the effects attributed to DHEA are mediated by DHEA or by its metabolites. No nuclear or any other type of receptor system has been described for DHEA or DHEAS, which have been thought to function solely as precursors for sex steroid synthesis.
P450c17 is expressed only in discrete areas of the developing brain, including neurons in the cortical subplate (7), a region involved in guiding thalamic fibers to their cortical targets and in establishing appropriate thalamo-cortical connections (31–34). Detecting P450c17 in neurons of the neocortical subplate suggested a possible role for its neurosteroid products, DHEA and the sulfate derivative of DHEA (DHEAS), in regulating motility and/or growth of cortico-thalamic projections. We therefore examined the roles of DHEA and DHEAS on the growth of neocortical neurites and determined the mechanisms by which they mediate their effects.
METHODS
Embryonic Neuronal Cultures.
Fetuses were removed on embryonic day 16.5 (E16.5) (the morning after evening mating is E0.5). Cortical hemispheres were separated from midbrain and hindbrain, and basal ganglia were removed, leaving the neocortex. After removal of the meninges, cortical tissue was placed into PBS containing 0.03% collagenase and 1 μg/ml DNase I for 30 min at 37°C. After mechanical trituration with a sterile Pasteur pipette, cells were filtered through a 40 μm nylon mesh and plated (50,000 cells per cm2) on glass coverslips coated with poly-D lysine (5 μg/cm2, Boehringer Mannheim). The culture medium was a modification of N2 serum-free medium used for culturing neuroblastoma cell lines (35) The medium was DMEM-Ham F12 1:1 (2.24 g/liter bicarbonate, no phenol red) without serum, containing glucose (3.15 g/l), l-glutamine (2 mM), insulin (5 μg/ml, Boehringer Mannheim), transferrin (5 μg/ml, Boehringer Mannheim), selenium (3 × 10−8 M, Boehringer Mannheim), progesterone (2 × 10−8 M, Sigma), putrescine (10−4 M, Sigma), and lipids (0.5 μl/ml, GIBCO/BRL). Cells were allowed to settle and attach to the coverslip for 2 h before the coverslip was inverted, as described (36). This procedure yields a pure neuronal culture (<1% contamination by nonneuronal cells) as assessed by a negative glial fibrillary acidic protein immunostaining and positive neuron-specific enolase immunostaining. Sandwiched cells were cultured for 3 days in 5% CO2 at 37°C and then treated according to the experimental needs.
Immunocytochemistry.
Cells were fixed for 20 min in 4% paraformaldehyde in 0.1 M PBS, pH 7.4. Fixed cells were preincubated for 1 h with normal goat serum (3%) and BSA (2%) in PBS containing 0.03% Triton X-100 to block background immunostaining, and then were incubated overnight with the monoclonal primary antibody directed against either bovine brain Tau-1 or against bovine brain microtubule-associated protein-2 (MAP-2) (Boehringer Mannheim), which were diluted 1:500 in PBS. Immunostaining was performed by using indirect immunofluorescence (6, 7). Cells were then washed one time (5 min) in PBS containing 0.03% Triton X-100 and three times (5 min each) in PBS without Triton X-100. Cells were then incubated with an anti-mouse fluorescein isothiocyanate conjugate at room temperature for 1 h, washed in PBS as described above, mounted, and observed under an epifluorescence microscope.
Morphometric Analysis.
For each experiment, a minimum of 35 neurons per well, three wells per treatment, were randomly counted. Experiments were performed at least three times for each treatment. To avoid counting bias and to ensure cells were not counted twice, we used the following parameters described (37): neurons whose somata abutted other somata were considered crowded and hence might not extend neurites optimally, and were not counted; cells that remained round and did not flatten on plastic were not counted; all other cells in the field were counted. A maximum of five cells per high-power field were counted; the field was moved in a discernible pattern to prevent recounting the same cells and to ensure that cells were sampled equally from the center and edge of the cultures. Immunopositive neurite length was determined at ×250 magnification, using a Leitz Ortholux II microscope in conjunction with an Optronix digital video camera, Rasterops frame-grabber application for a MacIntosh Power-PC 8500, and the National Institutes of Health image 1.57 software. Scales were calibrated by using a microscope scale bar at the same magnification.
Measurements included: cell body size (cells that contained MAP-2 immunopositive neurites also contained immunopositive cell bodies), frequency of long neurites (number of cells with neurites longer than two cell body widths/total cell number; this measurement was done at ×100 magnification), neurite length (for both Tau-1 and MAP-2 immunopositive neurites), frequency of growth cones on immunopositive neurites, number of MAP-2+ neurites extending from cell bodies, frequency of immunopositive neurites having varicosities, frequency of immunopositive neurites forming basket-like formations around other cells. The last two criteria were considered to assess the level of neurite–neurite or neurite–cell interactions.
Measurement of Intracellular Calcium.
Neurons were incubated for 40 min at 37°C, with 3 μM of Indo-1 (acetoxymethyl ester) in culture medium containing 0.5% BSA, then harvested, washed and resuspended in the calcium buffer (20 mM Hepes/1.4 mM CaCl2/137 mM NaCl/5 mM KCl/0.6 mM Na2HPO4/5.6 mM glucose/5 mg/ml BSA/0.9 mM MgSO4/10 mM NaHCO3/0.4 mM KH2PO4, pH 7.4). Experiments were carried out with a microfluorometer (Spex Fluorolog II spectrofluorometer, Spex Industries, Edison, NJ), using 3 ml of cell suspension (3.5 × 106 cells/ml) kept at 37°C. Excitation was at 334 nm and emission detection was at 400 nm in the presence or absence of saturating [Ca2+]. Recordings were converted into free intracellular calcium concentration ([Ca2+]i) by the equation: [Ca2+]i = Kβ(R − Rmin)/(R − Rmax), in which R is the ratio of fluorescence measured at 400 nm, K = 250 nM (K = the dissociation constant for Indo-1), and β is the ratio of the blue fluorescence intensity of the calcium-free and -bound dye (β = 4.2 in our experimental conditions). To determine the maximum (Rmax) and minimum (Rmin) ratios, neurons were maintained in a calcium-free calibration buffer containing 1 mM 2-deoxyglucose and recorded in presence of either 10 mM CaCl2 or 10 mM EGTA plus 20 μM ionomycin. The system was recalibrated for each experiment or following any adjustment to the apparatus. Involvement of calcium in the DHEA-response was confirmed by using the cell permeant calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, tetra(acetoxymethyl)ester (BAPTA AM), which clamps intracellular calcium. After BAPTA AM incubation, we were able to abolish the action of DHEA completely.
Statistical Analysis.
Repeated measures ANOVA was applied to the mean of immunopositive neurite lengths to determine differences between control conditions and DHEA or control and DHEAS treatments. To compare each group individually, post hoc analyses were conducted by using Scheffe’s F test with significance set at P < 0.005. This analysis was performed for three independent experiments, and gave the same results. The data from the three experiments were also analyzed by two-way ANOVA, using the dose of DHEA or DHEAS as the first variable and the experimental number as the second variable.
The presence of varicosity on immunopositive neurites and presence of basket-like formations from these neurites were analyzed by χ2 analysis, with subsequent Fisher’s exact test. To determine if there were interactions between DHEA and other agents on [Ca2+]]i concentrations (Fig. 6), we performed a two-way ANOVA using the statistical computer program spss (SPSS, Chicago).
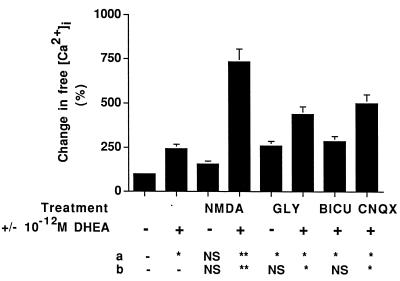
Figure 6.
Effect of NMDA, glycine and receptor antagonists on the increase of [Ca2+]i mediated by DHEA. Each bar shows the percent mean [Ca2+]i increase over baseline. Error bars are ±SEM of six separate measurements. NMDA (10 μM), glycine (10 μM), or antagonists (10 μM) were added to the cell suspension before addition of DHEA. NMDA increased [Ca2+]i above baseline values, and DHEA synergistically potentiated this response; glycine increased [Ca2+]i, and DHEA increased this response. Bicuculline (BICU) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) did not block the response of the cells to DHEA. These results support the involvement of the NMDA receptor in the DHEA-mediated increase in [Ca2+]i. Statistical differences between (a) control and treatments and between (b) DHEA treatment and other treatments (shown in a table at the bottom of the figure), were determined by using a two-way ANOVA test and post hoc Scheffe’s analysis; ∗∗, P < 0.001; ∗, P < 0.05.
RESULTS
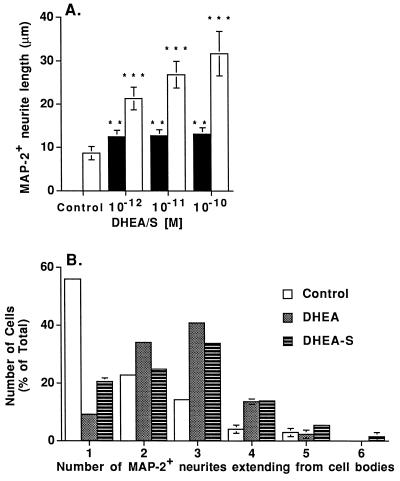
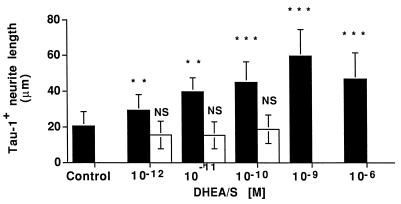
Previous studies using a mixed neuronal and glial culture showed that DHEA and DHEAS stimulated neurite outgrowth (38). This stimulation could have resulted from (i) a direct effect of DHEA on the neuron, (ii) an effect of DHEA on glia, which in turn stimulated neurite outgrowth, or (iii) metabolism of DHEA by the glia, to another steroidal product that would affect neurite growth. We therefore developed a primary culture of embryonic neocortical neurons that were devoid of glial contamination, and studied the effects of (10−9 M) DHEA and DHEAS on these neuronal populations. Our studies with pure neuronal cultures showed that DHEA and DHEAS rapidly stimulated neurite outgrowth observed in phase contrast microscopy, and DHEA also promoted formation of varicosities in elongated neurites. To characterize this steroidally induced neurite outgrowth, we immunostained neocortical neurons for MAP-2 and Tau-1 (Fig. 1 A–F), which are specific for dendrites and axons, respectively (39–44) and measured the length of immunopositive neurites (Figs. 2 and 3). Low concentrations (10−12–10−10 M) of DHEAS increased the length of MAP-2 immunostained (MAP-2+) neurites (Figs. 1F and 2A), and the number of MAP-2+ neurites extending from the cell body (Fig. 2B), whereas low concentrations (10−12–10−9 M) of DHEA increased the length of Tau-1 immunostained (Tau-1+) neurites (Figs. 1B and 3). Low concentrations of DHEA also increased the number of MAP-2+ neurites extending from the cell body (Fig. 2B). Higher doses (10−9–10−6 M) of DHEAS induced cell clustering and extension of Tau-1+ neurites. No MAP-2 staining was seen in neurites of cells receiving high doses (>10−9 M) of DHEAS. Growth of Tau-1+ neurites was maximal in cells receiving 10−9 M DHEA. One way ANOVA analyses showed a very strong effect of treatments on both Tau-1+ and MAP-2+ neurite length and the number of MAP-2+ neurites extending from the cell body. Scheffe’s post hoc analysis of MAP-2+ neurite length (Fig. 2A) indicated that there was no dose-dependent difference with DHEA treatment, although the effect of each concentration of DHEA was significantly different from control. However, DHEAS treatments were highly significantly different from each other as well as from the control and from DHEA treatments (P < 0.0001), indicating a dose-dependent effect of DHEAS on MAP-2+ neurite length. Scheffe’s post hoc analysis of Tau-1+ neurite length (Fig. 3) indicated that DHEAS treatments did not significantly change the Tau-1+ neurite length, with respect to controls, but that DHEA treatments were significantly different from each other (as well as from controls) (P < 0.0001), indicating a dose-dependent effect of DHEA on Tau-1+ neurite length. DHEA and DHEAS treatments yielded the same results in three independent experiments. Two-way ANOVA from three experiments confirmed the dose-dependence of the hormonal treatments on neurite growth (P < 0.0001).
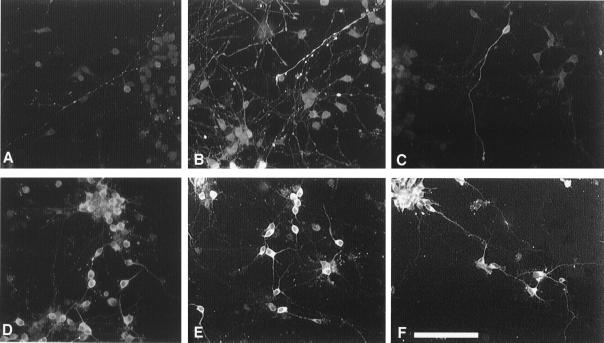
Figure 1.
Immunostaining for Tau-1 (A–C) and MAP-2 (D–F) of E16.5 neocortical neurons cultured for 4 days. DHEA 10−12 M (B, E), DHEAS 10−12 M (C and F), or vehicle (0.1% dimethyl sulfoxide) (A and D) were added to the cultures for the last 20 h. DHEA promotes the abundance of Tau-1+ neurites (B). Vehicle or DHEAS has no effect on Tau-1+ neurites. Very few cell bodies were immunostained with the anti-Tau-1 antibody and no MAP-2+ dendrites were double-stained with the anti-Tau-1 antibody, suggesting that in our cultures, the cellular localization of Tau-1 is restricted to axons. However, the immunostaining with the anti-MAP-2 antibody was cytoplasmic and extended into dendrites. DHEA and DHEAS (E and F) increased MAP-2 staining in the cell bodies, but only DHEAS increased MAP-2+ neurite length (F). There are many faintly MAP-2+ collaterals and clustering of the neurons when cells are cultured with DHEAS. (Bar = 100 μm).
Figure 2.
Dose dependent effect of DHEAS on MAP-2+ neurite outgrowth (A) and effect of steroids on the number of neurites extending from cell bodies (B). (A) Each column shows the mean length of MAP-2+ neurites for at least 100 labeled neurons/condition from three separate wells in one representative experiment. Open bars are DHEAS treatment and solid bars are DHEA treatment. Error bars are ±SD. Neurite length was quantitated by using the nih image 1.57 program. ∗∗∗, P < 0.0001, and ∗∗, P < 0.001 using a one-way ANOVA, with a Scheffe’s post hoc analysis. Three independent experiments gave similar results. (B) Histogram representing the percentage of cells with a specific number (1–6) of MAP-2+ neurites was used to depict data from 10−11 M DHEA and 10−11 M DHEAS treatments. Experiments were performed at 10−12–10−10 M DHEA and DHEAS, and the original data were analyzed by one-way ANOVA, by using Scheffe’s post hoc analysis to determine individual differences at each concentration of steroid. ANOVA showed that DHEA and DHEAS treatments significantly increased the number of MAP-2+ neurites extending from cell bodies compared with untreated control cells. No significant differences were found among the different doses of DHEA and DHEAS, or between DHEA and DHEAS treatments. Open bars are control untreated cells, dark gray bars are DHEA treatment, and hatched bars are DHEAS treatment.
Figure 3.
Dose dependent effect of DHEA on Tau-1+ neurite outgrowth. Each column shows the mean length of Tau-1+ neurites for at least 100 labeled neurons/condition from three separate wells in one representative experiment. Solid bars are DHEA treatment and open bars are DHEAS treatment. Error bars are ±SD. Neurite length was quantitated by using the nih image 1.57 program. Statistical differences were determined by using a one-way ANOVA with a post hoc Scheffe’s analysis; ∗∗∗, P < 0.0001; ∗∗, P < 0.001. Three independent experiments gave similar results.
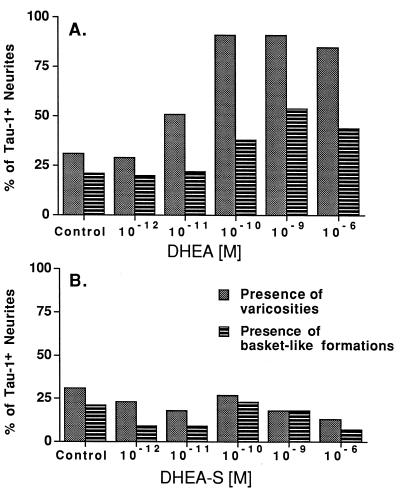
We assessed the effects of DHEA and DHEAS on the presence of varicosities and basket-like formations (Fig. 4). In addition to increasing Tau-1+ neurite length, DHEA, but not DHEAS, increased the presence of varicosities in Tau-1+ neurites. DHEA also increased the presence of basket-like formations of these neurites around other neurons. χ2 analysis showed a high dependency between DHEA treatments and the presence of varicosities (χ2 = 131.4; P < 0.0001) and the presence of basket-like formations (χ2 = 30.3; P < 0.0001). χ2 analysis showed that these criteria were independent of DHEAS treatment (χ2 = 3.8; P < 0.567 for presence of varicosity; χ2 = 4.7; P < 0.446 for presence of basket-like formation). These data suggest that DHEA enhances both the presence of varicosities on Tau-1+ neurites and the formation of baskets around other cell bodies, criteria that assess the potential for contacts within the culture. Thus, low, physiologic concentrations of DHEA and DHEAS respectively elicit specific tropic effects on axonal and dendritic neurite outgrowths.
Figure 4.
Effect of DHEA (A) and DHEAS (B) on varicosities and basket-like formations on Tau-1+ neurites. The histograms express a single percentage of all neurons sampled. Gray bars are the percentage of Tau-1+ neurites bearing one or more varicosities; hatched bars are the percentage of Tau-1+ neurites making basket-like formations around cultured neurons. Data were analyzed by χ2 analysis. For DHEA treatment, χ2 = 131.4; P < 0.0001 for frequency of varicosities and χ2 = 30.3; P < 0.0001 for frequency of basket-like formations. The presence of varicosities and of basket-like formations is independent of each dose of DHEAS treatment (χ2 = 3.8; P < 0.567 for frequency of varicosities and χ2 = 4.7; P < 0.446 for frequency of basket-like formations).
Neurons expressing P450c17 are present in small numbers among the cultured neocortical neurons. By immunocytochemical analysis, we detected <1% of the cultured cells to be P450c17 immunopositive (data not shown). Because P450c17 activity might produce some 17-OH-progesterone and androstenedione from the low concentrations of progesterone in the culture medium, we tested the effect of androstenedione and testosterone (10−6 M) in our cultures. Androstenedione had no effect on the neocortical neurons, either considering the overall neurite growth or the expression of MAP-2 and Tau-1. Testosterone did not promote neurite growth but resulted in neuronal clusters. These experiments suggest that the effects reported here are due to the DHEA or DHEAS introduced into the culture medium and not to the metabolism of DHEA or progesterone.
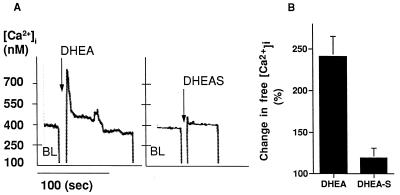
Neurosteroids like DHEA and DHEAS bind GABAA receptors (18–20) and modulate neurosecretion mediated by NMDA receptors (45, 46). To characterize further the neurite outgrowth induced by DHEA and DHEAS, we cultured neocortical neurons with DHEA or DHEAS, plus the noncompetitive NMDA receptor antagonist (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (MK801) or the chloride channel blocker tert-butyl-bicyclo[2.2.2]phosphorothionate (TBPS) and compared the length and occurrence of Tau-1+ and MAP-2+ neurites. MK801 blocked axonal growth stimulated by DHEA, but did not block dendritic growth stimulated by DHEAS (not shown). TBPS did not mimic the effects of either DHEA or DHEAS, suggesting that DHEA and DHEAS did not work by inhibiting chloride channels associated with the GABAA receptor. These morphological results suggest involvement of NMDA receptors in the effects of DHEA. Therefore, we studied the effect of DHEA and DHEAS on modulating [Ca2+]i in neocortical cells by microfluorometry, by using the calcium indicator Indo-1. Low concentrations (10−12–10−9 M) of DHEA increased [Ca2+]i, while DHEAS had no effect (Fig. 5). DHEA rapidly increased [Ca2+]i to 568 ± 207 nM (241 ± 77% of basal) within 10 sec, followed by a sustained, elevated plateau 378 ± 273 nM (148 ± 44% of basal) lasting more than 1 min. Thus DHEA, but not DHEAS, causes a rapid, long lasting increase in intracellular calcium, indicating that these two compounds mediate their effects by different mechanisms.
Figure 5.
DHEA increases [Ca2+]i measured by microfluorometry on a suspension of the cells preloaded with Indo-1. (A) shows a typical response of cells incubated with 10−12 M DHEA. Fluorescence was measured continuously for 3 min immediately following injection of the steroid. (B) Quantitation of peak [Ca2+]i was determined after calibration of the dye. There was a linear correlation between the values of the baseline and the values of the DHEA induced peak and plateau (peak: r2 = 0.879, P = 0.021; plateau: r2 = 0.977, P = 0.008), allowing us to correct peak and plateau values obtained in different experiments with respect to the baseline values. Therefore, we standardized the [Ca2+] for each experiment placing the baseline arbitrarily at 100% and presented the mean quantitative values (solid bars) as percent of variation of the peak value from the baseline. Error bars are ±SEM of the standardized [Ca2+] from at least three experiments, each performed in duplicate.
To determine whether NMDA, non-NMDA, or GABAA receptors were involved in DHEA modulation of [Ca2+]i, we added specific antagonists of each receptor during the calcium recording of the neocortical neurons. Bicuculline, a GABAA receptor blocker, and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), a kainate/quisqualate antagonist, did not reduce the DHEA-mediated increase of [Ca2+]i (Fig. 6), and CNQX augmented the DHEA-mediated increase of [Ca2+]i, suggesting that blocking GABAA and non-NMDA glutamate receptors does not block the effect of DHEA. However DHEA potentiated the response of neocortical neurons to low concentrations (10 μM) of NMDA. Low concentrations (10 μM) of glycine, which potentiate NMDA receptor activity (47) increased the DHEA response (Fig. 6). This effect was not synergistic, assessed by two-way ANOVA, but rather was additive.
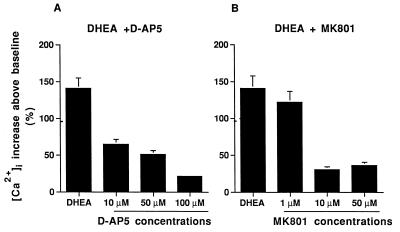
We determined if NMDA receptors were involved in DHEA-mediated increases in [Ca2+]i by blocking these receptors with specific antagonists. When NMDA receptors were blocked by d-2-amino-5-phosphonopentanoic acid (D-AP5) (Fig. 7A) or MK801 (Fig. 7B), the increase in [Ca2+]i mediated by DHEA decreased. D-AP5 and MK801 acted in dose-dependent fashions (Fig. 7 A and B). Thus we have shown NMDA receptor activation in two ways: by DHEA’s potentiation of the NMDA-mediated increase in [Ca2+]i; and by the antagonism of the DHEA-mediated increase in [Ca2+]i by two specific NMDA blockers.
Figure 7.
Dose-dependent effect of NMDA receptor antagonists D-AP5 (A) and MK801 (B) on the stimulation of [Ca2+]i mediated by DHEA. Each bar shows the percent mean [Ca2+]i change over the baseline. Error bars are ±SEM of at least three experiments.
DISCUSSION
DHEA induces morphological changes in primary cultures of embryonic neocortical neurons, increasing axonal length, presence of varicosities, and presence of basket-like formations around other cell bodies. The dose-dependent and saturable effects of both DHEA and DHEAS on Tau-1+ and MAP-2+ neurite growth suggest a receptor-mediated process. Our results also suggest that DHEA acts through NMDA receptors to induce Ca2+ influx into primary cultures of embryonic neocortical neurons. The rapid kinetics of the [Ca2+]i response to DHEA is compatible with the action of a membrane receptor ion channel, but not with the action of a nuclear receptor transcription factor. Thus physiologic concentrations of DHEA that are found in the brain promote axonal growth in vitro whereas physiologic concentrations of brain DHEAS promote branching and dendritic outgrowth in vitro. However, morphological analysis of the DHEAS effect suggested more complex processes that involve both dendrite outgrowth and neuronal motility, resulting in neuronal clusters, possibly mediated by formation of gap junctions. Thus endogenous synthesis of DHEA and DHEAS in the developing rodent neocortex may affect axonal and dendritic growth of neocortical neurons. These changes may ultimately result in the formation of specific contacts and may lead to the formation of appropriate neuronal networks within the neocortex. Because DHEA and DHEAS treatments in vitro lead to a cascade of events that result in diverse neurite outgrowth patterns, conversion of DHEAS to DHEA in vivo by the enzyme steroid sulfohydrolase may be a key regulator of neocortex organization during embryogenesis (48).
The NMDA receptor is involved in cytoarchitectural events including long-term potentiation, neuronal migration, neurite outgrowth, and establishment of complex synaptic networks in the developing cortex (49–51) Activation of NMDA receptors may regulate synapse formation in the developing hippocampus (49) motility in granule cells (50) and circuit-specific alterations during aging (52). Our results suggest that DHEA can act as a positive allosteric modulator at NMDA receptors. Hence, we propose that DHEA, alone or together with NMDA, may induce axonal growth in cortical neurons.
NMDA receptor subunit expression may play a role in DHEA action. The identities of specific NMDA receptor subunits are largely unknown in the regions containing P450c17 (7); however, rat NMDAR1, NMDAR2A, and NMDAR2B subunits are present in the neocortex during late gestation (53, 54), and NMDAR-L is developmentally expressed in layer V of the neocortex during late gestation and early neonatal life in the rat (55). Different combinations of subunits exhibit different electrophysiological properties and different sensitivity to phosphorylation, and may result in different NMDA responses during development (56–58). The actions of DHEA thus may be developmentally regulated by the appearance of specific forms of NMDA receptor that promote axonal growth and formation of contacts, events that are needed to initiate neuronal circuits that will subsequently be refined in the adult brain. Thus, DHEA may participate in processes that lead to the organization of the brain that is necessary during embryogenesis and early neonatal life. During adulthood there is a refinement of axons reaching inappropriate targets and of circuits that are not activated by learning. Neurosteroids other than DHEA (59) may play a role in eliminating axonal or dendritic branches that are directed toward inappropriate targets, thereby remodeling the pattern of connections initiated from specific neocortical regions.
DHEA and DHEAS may also modulate neurosecretion mediated by NMDA receptors via sigma (σ) receptors (45, 46, 60–62). Whereas progesterone has high affinity (nanomolar) for the σ receptor (61, 62), it does not alter NMDA responses, while DHEA, which has a much lower affinity for σ receptors, enchances neuronal responses to NMDA (45, 46). Selective competition studies indicate that DHEA may mediate its effects via the σ1 receptor subtype. It is currently unknown how σ receptors modulate NMDA responses, or how NMDA receptors modulate sigma receptor function (reviewed in ref. 63), but the recent purification, molecular cloning, and expression of mammalian σ1 receptors (64) should enable investigators to determine how this receptor’s function is modified by NMDA and by neurosteroids.
A thorough understanding of the role of DHEA and DHEAS in neural development would be aided the creation of mice in which the P450c17 gene has been ablated. Although a mouse lacking this gene currently does not exist, human beings with severe mutations in P450c17 have not been reported to have neurological defects. Therefore it is unclear whether DHEA and DHEAS serve similar functions in human beings and in mice. However, there are redundant systems operational in neural development, often permitting relatively normal development in the absence of any one system; thus a lack of human brain DHEA may increase the production of other factors serving similar functions. Alternatively, absence of DHEA or DHEAS may result in subtle neurological defects that may be reflected in behavioral or emotional abnormalities that may not have been noted in the hormonal descriptions of these patients. While such effects have not been studied in human beings with P450c17 deficiency, studies in human beings supplemented with DHEA/S suggest that these compounds do indeed play a considerable role in modulating human behavior (65).
Acknowledgments
This work was supported by National Institutes of Health Grants HD27970 and HD11979, by the Alzheimer’s Association, by the National Niemann–Pick Foundation, and by the Obstetrics and Gynecology Research and Education Foundation. N.A.C. was supported in part by funds from the Phillipe Foundation and from the Singer Polignac Foundation.
ABBREVIATIONS
- DHEA
dehydroepiandrosterone
- DHEAS
sulfated derivative of DHEA
- En
embryonic day n
- GABA
γ-aminobutyric acid
- MAP-2
microtubule-associated protein-2
- NMDA
N-methyl-d-aspartate
- MK801
(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate
Note Added in Proof
Our recent experiments with primary cultures of human fetal neocortical cells and with a human neuroral cell line give results similar to those presented in rodent cells.
Footnotes
A commentary on this article begins on page 4089.
References
- 1.Baulieu E E. Biol Cell. 1991;71:3–10. doi: 10.1016/0248-4900(91)90045-o. [DOI] [PubMed] [Google Scholar]
- 2.Mellon S H. J Clin Endocrinol Metab. 1994;78:1003–1008. doi: 10.1210/jcem.78.5.8175951. [DOI] [PubMed] [Google Scholar]
- 3.Le Goascogne C, Robel P, Gouezou M, Sananes N, Baulieu E E, Waterman M. Science. 1987;237:1212–1215. doi: 10.1126/science.3306919. [DOI] [PubMed] [Google Scholar]
- 4.Mellon S H, Deschepper C F. Brain Res. 1993;629:283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- 5.Dupont E, Simard J, Luu-The V, Labrie F, Pelletier G. Mol Cell Neurosci. 1994;5:119–123. doi: 10.1006/mcne.1994.1014. [DOI] [PubMed] [Google Scholar]
- 6.Compagnone N A, Bulfone A, Rubenstein J L R, Mellon S H. Endocrinology. 1995;136:2689–2696. doi: 10.1210/endo.136.6.7750493. [DOI] [PubMed] [Google Scholar]
- 7.Compagnone N A, Bulfone A, Rubenstein J L R, Mellon S H. Endocrinology. 1995;136:5212–5223. doi: 10.1210/endo.136.11.7588260. [DOI] [PubMed] [Google Scholar]
- 8.Lauber M E, Lichtensteiger W. Endocrinology. 1996;137:2718–2730. doi: 10.1210/endo.137.7.8770891. [DOI] [PubMed] [Google Scholar]
- 9.Mellon S H, Compagnone N A. In: Neurosteroids: A New Regulatory Function in the Nervous System. Baulieu E E, Robel P, Schumacher M, editors. Totowa, NJ: Humana; 1998. [Google Scholar]
- 10.Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu E E. Proc Natl Acad Sci USA. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corpechot C, Synguelakis M, Talha S, Axelson M, Sjovall J, Vihko R, Baulieu E E, Robel P. Brain Res. 1983;270:119–125. doi: 10.1016/0006-8993(83)90797-7. [DOI] [PubMed] [Google Scholar]
- 12.Robel P, Bourreau E, Corpechot C, Dang D C, Halberg F, Clarke C, Haug M, Schlegel M L, Synguelakis M, Vourch C, Baulieu E E. J Steroid Biochem. 1987;27:649–655. doi: 10.1016/0022-4731(87)90133-6. [DOI] [PubMed] [Google Scholar]
- 13.Jo D H, Abdallah M A, Young J, Baulieu E E, Robel P. Steroids. 1989;54:287–297. doi: 10.1016/0039-128x(89)90003-2. [DOI] [PubMed] [Google Scholar]
- 14.Mathur C, Prasad V V, Raju V S, Welch M, Lieberman S. Proc Natl Acad Sci USA. 1993;90:85–88. doi: 10.1073/pnas.90.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakajin S, Shively J E, Yuan P M, Hall P F. Biochemistry. 1981;20:4037–4042. doi: 10.1021/bi00517a014. [DOI] [PubMed] [Google Scholar]
- 16.Zuber M X, Simpson E R, Waterman M R. Science. 1986;234:1258–1261. doi: 10.1126/science.3535074. [DOI] [PubMed] [Google Scholar]
- 17.Chung B C, Picado-Leonard J, Haniu M, Bienkowski M, Hall P F, Shively J E, Miller W L. Proc Natl Acad Sci USA. 1987;84:407–411. doi: 10.1073/pnas.84.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison N L, Simmonds M A. Brain Res. 1984;323:284–293. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- 19.Majewska M D, Harrison N L, Schwartz R D. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 20.Puia G, Santi M R, Vicini S, Pritchett D B, Purdy R H, Paul S M, Seeburg P H, Costa E. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 21.Wu F S, Gibbs T T, Farb D H. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- 22.Fahey J M, Lindquist D G, Pritchard G A, Miller L G. Brain Res. 1995;669:183–188. doi: 10.1016/0006-8993(94)01223-5. [DOI] [PubMed] [Google Scholar]
- 23.Purdy R H, Morrow A L, Moore P H, Jr, Paul S M. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bitran D, Shiekh M, McLeod M. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 25.Maione S, Berrino L, Vitagliano S, Leyva J, Rossi F. Eur J Pharmacol. 1992;219:477–479. doi: 10.1016/0014-2999(92)90493-n. [DOI] [PubMed] [Google Scholar]
- 26.Flood J F, Smith G E, Roberts E. Brain Res. 1988;447:269–278. doi: 10.1016/0006-8993(88)91129-8. [DOI] [PubMed] [Google Scholar]
- 27.Flood J F, Roberts E. Brain Res. 1988;448:178–181. doi: 10.1016/0006-8993(88)91116-x. [DOI] [PubMed] [Google Scholar]
- 28.Flood J F, Morley J E, Roberts E. Proc Natl Acad Sci USA. 1992;89:1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathis C, Paul S M, Crawley J N. Psychopharmacology (Berlin) 1994;116:201–206. doi: 10.1007/BF02245063. [DOI] [PubMed] [Google Scholar]
- 30.Bellino F L, Daynes R A, Hornsby P J, Lavrin D H, Nestler J E, editors. Dehydroepiandrosterone (DHEA) and Aging. Vol. 774. New York: N.Y. Acad. Sci.; 1995. [Google Scholar]
- 31.McConnell S K, Ghosh A, Shatz C J. Science. 1989;245:978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- 32.McConnell S K, Ghosh A, Shatz C J. J Neurosci. 1994;14:1892–1907. doi: 10.1523/JNEUROSCI.14-04-01892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh A, Shatz C J. Development (Cambridge, UK) 1993;117:1031–1047. doi: 10.1242/dev.117.3.1031. [DOI] [PubMed] [Google Scholar]
- 34.Herrmann K, Antonini A, Shatz C J. Eur J Neurosci. 1994;6:1729–1742. doi: 10.1111/j.1460-9568.1994.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 35.Bottenstein J E, Sato G H. Proc Natl Acad Sci USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucius R, Mentlein R. Anat Anz. 1995;177:447–454. doi: 10.1016/S0940-9602(11)80152-4. [DOI] [PubMed] [Google Scholar]
- 37.Lustig R H, Hua P, Yu W, Ahmad F J, Baas P W. J Neurosci. 1994;14:3945–3957. doi: 10.1523/JNEUROSCI.14-06-03945.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bologa L, Sharma J, Roberts E. J Neurosci Res. 1987;17:225–234. doi: 10.1002/jnr.490170305. [DOI] [PubMed] [Google Scholar]
- 39.Binder L I, Frankfurter A, Rebhun L I. Ann NY Acad Sci. 1986;466:145–166. doi: 10.1111/j.1749-6632.1986.tb38392.x. [DOI] [PubMed] [Google Scholar]
- 40.Caceres A, Banker G, Steward O, Binder L, Payne M. Brain Res. 1984;315:314–318. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Kanai Y, Cowan N J, Hirokawa N. Nature (London) 1992;360:674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- 42.Kanai Y, Hirokawa N. Neuron. 1995;14:421–432. doi: 10.1016/0896-6273(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 43.Black M M, Slaughter T, Moshiach S, Obrocka M, Fischer I. J Neurosci. 1996;16:3601–3619. doi: 10.1523/JNEUROSCI.16-11-03601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kempf M, Clement A, Faissner A, Lee G, Brandt R. J Neurosci. 1996;16:5583–5592. doi: 10.1523/JNEUROSCI.16-18-05583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monnet F P, Mahe V, Robel P, Baulieu E E. Proc Natl Acad Sci USA. 1995;92:3774–3778. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergeron R, de Montigny C, Debonnel G. J Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson J W, Ascher P. Nature (London) 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 48.Compagnone N A, Salido E, Shapiro L J, Mellon S H. Endocrinology. 1997;138:4768–4773. doi: 10.1210/endo.138.11.5504. [DOI] [PubMed] [Google Scholar]
- 49.Durand G M, Kovalchuk Y, Konnerth A. Nature (London) 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 50.Komuro H, Rakic P. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- 51.Shatz C J. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- 52.Gazzaley A H, Siegel S J, Kordower J H, Mufson E J, Morrison J H. Proc Natl Acad Sci USA. 1996;93:3121–3125. doi: 10.1073/pnas.93.7.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheng M, Cummings J, Roldan L A, Jan Y N, Jan L Y. Nature (London) 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 54.Monyer H, Burnashev N, Laurie D J, Sakmann B, Seeburg P H. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 55.Sucher N J, Akbarian S, Chi C L, Leclerc C L, Awobuluyi M, Deitcher D L, Wu M K, Yuan J P, Jones E G, Lipton S A. J Neurosci. 1995;15:6509–6520. doi: 10.1523/JNEUROSCI.15-10-06509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg P H. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 57.Durand G M, Gregor P, Zheng X, Bennett M V, Uhl G R, Zukin R S. Proc Natl Acad Sci USA. 1992;89:9359–9363. doi: 10.1073/pnas.89.19.9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brady R J, Gorter J A, Monroe M T, Swann J W. Brain Res Dev Brain Res. 1994;83:190–196. doi: 10.1016/0165-3806(94)00136-7. [DOI] [PubMed] [Google Scholar]
- 59.Brinton R D. J Neurosci. 1994;14:2763–2774. doi: 10.1523/JNEUROSCI.14-05-02763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Debonnel, G., Bergeron, R. & de Montigny, C. (1996) J. Endocrinol. 150 Suppl, S33–S42. [PubMed]
- 61.Su T P, London E D, Jaffe J H. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- 62.McCann D J, Su T P. J Pharmacol Exp Ther. 1991;257:547–554. [PubMed] [Google Scholar]
- 63.Itzhak Y, editor. Sigma Receptors. San Diego: Academic; 1994. [Google Scholar]
- 64.Hanner M, Moebius F F, Flandorfer A, Knaus H G, Striessnig J, Kempner E, Glossmann H. Proc Natl Acad Sci USA. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morales A J, Nolan J J, Nelson J C, Yen S S. J Clin Endocrinol Metab. 1994;78:1360–1367. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]