Abstract
The neuropeptides oxytocin and vasopressin have been implicated in rodent social and affiliative behaviors, including social bonding, parental care, social recognition, social memory, vocalizations, territoriality, and aggression, as well as components of human social behaviors and the etiology of autism. Previous investigations of mice with various manipulations of the oxytocin and vasopressin systems reported unusual levels of ultrasonic vocalizations in social settings. We employed a vasopressin 1b receptor (Avpr1b) knockout mouse to evaluate the role of the vasopressin 1b receptor subtype in the emission of ultrasonic vocalizations in adult and infant mice. Avpr1b null mutant female mice emitted fewer ultrasonic vocalizations, and their vocalizations were generally at lower frequencies, during a resident-intruder test. Avpr1b null mutant pups emitted ultrasonic vocalizations similar to heterozygote and wildtype littermates when separated from the nest on postnatal days 3, 6, 9, and 12. However, maternal potentiation of ultrasonic vocalizations in Avpr1b null and heterozygote mutants was absent, when tested at postnatal day 9. These results indicate that Avpr1b null mutant mice are impaired in the modulation of ultrasonic vocalizations within different social contexts at infant and adult ages.
Keywords: Social recognition, female-female mouse interactions, maternal potentiation, nest odor orientation, autistic spectrum disorders, mouse models
Introduction
Considerable interest has focused on the actions of two hypothalamic neuropeptides, oxytocin and vasopressin, as critical mediators of social affiliative behaviors [2,9,25,26,29]. Targeted gene mutations in mice provide model systems to evaluate the role of a specific neuropeptide or its receptor in a social behavior. Oxytocin knockout mice revealed the role of oxytocin in mediating social recognition, social memory, aggression, maternal behaviors, and ultrasonic vocalizations by pups separated from the nest [5,10,29,39,42,57]. Vasopressin receptor subtype V1a (Avpr1a) knockout mice displayed impaired social recognition and low anxiety-like behaviors [4,5,12], and reduced responses to social olfactory cues [51]. Higher levels of the Avpr1a were detected in the ventral pallidum of prairie voles with monogamous social behaviors [17]. Treatment with Avpr1a antagonist reduced aggression in golden hamsters [14]. Vasopressin receptor subtype V1b (Avpr1b) knockout mice displayed reduced aggression [52,53]. Treatment with Avpr1b antagonists produced anxiolytic and antidepressant effects in rodents [16,18]. A small literature also suggests that oxytocin, vasopressin, and/or their receptors may be aberrant in some cases of autism [2,17,23,25].
Mice deficient in the Avpr1b offer a research tool to test hypotheses about the role of this receptor subtype in mouse behaviors with face validity to some of the symptoms of autism. We hypothesized that ultrasonic vocalizations (USVs) may be a measure of social communication in mice [10], and reduced USVs in mice may be a useful assay relevant to the second diagnostic symptom of autism, impaired communication [27]. To begin to test this notion, we evaluated USVs during social challenges in Avpr1b null mutant, heterozygote, and wildtype mice at two developmental time points, infant and adult. One standard test for vocalizations in mice is the ultrasonic distress call of pups separated from the mother or removed from the nest [19–22,45,46,56]. Avpr1b genotypes were tested during the first two postnatal weeks, at ages 3, 6, 9, and 12 days old, to evaluate developmental patterns [3,7]. An interesting variant is the maternal potentiation response. Maternal potentiation of ultrasonic vocalizations occurs when, after a brief contact with the dam, isolated infant rats produce USVs at a rate significantly higher on the second separation from their mother, as compared to the USVs after the first separation from their mother [19–22,45,46]. The maternal potentiation response, which is robust in rat pups, is thought to assay more cognitive components of the separation response [45], and was therefore attempted in Avpr1b mice at the optimized age of postnatal day 9 [22]. To control for artifacts of potential physical disabilities of the Avpr1b mice, such as inability to detect social olfactory cues or motor dysfunctions, a homing test [36,44] was conducted on postnatal day 11. Youngest pups (pnd 9) in this mouse line tend to be rather still in the homing apparatus. Therefore we chose the latest age in which pups have their eyes still closed, so that they could base their exploration on olfactory rather than visual cues. Vocalizations in adult Avpr1b null mutants, heterozygotes, and wildtype littermates were evaluated in the resident-intruder test, with simultaneous scoring of social interactions. Because adult female mice have been reported to emit more vocalizations than adult male mice during same-sex social interactions [11,32,33], the first experiments with adult Avpr1b mice focused on social interactions by females in a resident-intruder task.
Materials and methods
Targeted disruption of the V1b receptor gene
The generation of this Avpr1b mutation was previously described [52]. Briefly, a 1FIX II mouse 129/SvJ genomic library (Stratagene, La Jolla, CA, USA) was screened with a 32P-labelled PvuII fragment of a rat V1bR cDNA. Two independent clones were identified and the largest (~16.6 kb; GENBANK Accession Nos AF152533 and AF152534) was used to construct the targeting vector. A 1.2-kb PvuII fragment 5′ to the coding region for transmembrane regions I–VI was inserted in the targeting vector pPNT at the XhoI site. The 1.7-kb PstI/SacI piece containing the 3′ end of the exon (TMVI) and most of the following introns were inserted at the HincII site of pPNT (this destroyed the thymidine kinase selection). The targeting construct thus eliminated the V1bR coding region from the initiating methionine just prior to TMVI. The construct was linearized with NotI and electroporated into embryonic stem cells for selection. Two embryonic stem cell clones were identified by PCR and confirmed by Southern analysis. Chimeric mice were generated from one of them and germ line transmission was observed.
Subjects
Mice used in the present experiments were bred by heterozygous matings which retained the original mixed background of C57BL/6J and 129/SvJ. All procedures followed NIH guidelines for the care and use of laboratory animals, and were approved by the NIMH Animal Care and Use Committee. Breeding pairs were housed in standard wire-topped Plexiglas cages (42cm × 27cm × 14cm). After ten days, the females were individually housed and subsequently inspected daily at 9:30 a.m. for delivery. The day of birth was considered as postnatal day (pnd) 0. At weaning, pups were tail-clipped for genotyping using PCR analysis of DNA as previously described [52]. Mice were group housed (three or four per cage) after weaning and kept under standard animal housing conditions with free access to food and water. The housing room was maintained at 20 ± 2 °C under a 12:12 reverse light cycle with lights on at 9 PM. All behavioral tests were conducted between 9.30 and 14.00 h, during the dark phase of the circadian cycle. Neonatal ultrasonic vocalizations (USVs) recordings on all pups in each litter were performed before PCR analysis. Pups were tattooed on the paw with animal tattoo ink (Ketchum permanent Tattoo Inks green paste, Ketchum Manufacturing Inc., Brockville ON Canada) by loading the ink into a 30G hypodermic needle and inserting the ink subcutaneously through the needle tip into the center of the paw. The procedure was performed at two days of age, immediately after the USVs test. The procedure causes only minor brief pain and distress and does not require the use of anesthesia.
Adults were identified by implanted microchip transponders (Bio Medic Data Systems, Seaford, Delaware, USA). The identity of each mouse was recorded at the end of its behavioral test, to ensure that the investigator was uninformed of genotypes while scoring behaviors.
Adult Female Resident-Intruder
Four month old female mice, 10 wild-type (Avpr1b +/+); 11 heterozygous (Avpr1b +/−); 11 homozygous null mutant (Avpr1b −/−) were tested in the resident-intruder test [11,33,50]. Behavioral tests were conducted under red light, videotaped using a Panasonic monochrome CCD camera and subsequently analyzed with Noldus Observer 5.0 software (Noldus Information Technology, Leesburg, VA). To ensure uniformity of scoring, one observer rated all videotapes. The observer was unaware of the genotype while scoring the videotapes.
Resident female Avpr1b +/+, +/−, and −/− mice, age four months, were individually housed for three days before the test session. Intruders were C57BL/6J females of comparable age and body weight, bred in the NIMH colony as described above, and maintained in social groups of four per home cage. The resident-intruder test was conducted in a sound attenuating chamber during the dark period between 9:30 and 14:00.
The female intruder was introduced into the home cage of the isolated resident for three min, and then returned to its home cage. During the test, an ultrasonic microphone (Avisoft UltraSoundGate condenser microphone capsule CM16, Avisoft Bioacoustics, Berlin, Germany) sensitive to frequencies between 10–180 kHz was suspended 10 cm above the cage. Vocalizations were recorded using an Avisoft Recorder (Version 3.2). Settings included sampling rate at 250 kHz; format 16 bit. For acoustical analysis, recordings were transferred to Avisoft SASLab Pro (Version 4.40) and a fast Fourier transformation (FFT) was conducted. Spectrograms were generated with an FFT-length of 512 points and a time window overlap of 50% (100% Frame, FlatTop window). The spectrogram was produced at a frequency resolution of 488 Hz and a time resolution of 1 ms. A lower cut-off frequency of 15 kHz was used to reduce background noise outside the relevant frequency band to 0 dB. Call detection was provided by an automatic threshold-based algorithm and a hold-time mechanism (hold time: 0.01 s). An experienced user checked the accuracy of call detection, and obtained a 100% concordance between automated and observational detection. To allow parametric assumptions to be met, a square root transformation was computed on the USVs data [11,33]. Additional qualitative and quantitative analyses included sound frequencies, measured in terms of peak frequencies (frequencies with the highest sound pressure), and peak amplitude at the peak frequency (peak amplitude = maximum of the spectrum). Distribution of USVs were analyzed for three durations (Short = 1–4 ms; Medium = 5–9 ms; Long = 10–20 ms).
Concomitant with the analysis of vocalizations, the frequencies and durations of the following behavioral responses emitted by the resident female were scored from the videotapes: exploring (moving around the cage, sniffing the physical environment, rearing); social investigation (sniffing any part of the partner’s body); and grooming (self-cleaning, licking, combing and stretching any part of its own body).
Pup separation vocalizations and maternal potentiation
A total of 57 pups (9 Avpr1b +/+; 31 Avpr1b +/−; 17 Avpr1b −/−) from seven litters were tested from pnd 3 to 12 in the USVs and maternal potentiation. On pnds 3, 6, 9 and 12, each pup was placed into an empty plastic container (diameter, 5 cm; height 10 cm), located inside a sound-attenuating styrofoam box, and assessed for USVs during a five min test. At the end of the five min recording session, each pup was weighed and its axillary temperature measured by gentle insertion of the thermal probe in the skin pocket between upper foreleg and chest of the animal for about 30 s (Microprobe digital thermometer with mouse probe, Stoelting Co., Illinois, USA). Pups of both sexes were tested. Since no sex-specific patterns of calling were evident, data were collapsed across sex.
Ultrasonic calls were recorded with the same Avisoft equipment described above. The microphone was placed through a hole in the middle of the cover of the styrofoam sound-attenuating chamber, about 20 cm above the plastic container. The temperature of the room was maintained at 22 ± 1 °C. Vocalizations were stored using the Avisoft Recorder with the same settings used for the USVs test, and analyzed for the number of calls and their duration.
Maternal potentiation was examined at pnd 9. Each pup was isolated in an empty container for five min (baseline measurement), then reintroduced into the cage with its mother and the littermates for five min. Maternal behavior measurements included latency to retrieve and time spent in contact with the pups. After five min, the pup was again placed in the clean empty container for five min. Number and duration of pup calls were recorded. At the end of each USVs test, axillary temperature was measured. During the maternal potentiation test, sound frequencies were analyzed automatically in terms of frequency and amplitude at the maximum of the spectrum.
Homing test (pnd 11)
To avoid potential confounds from using handled animals, a separate cohort of pups (14 Avpr1b +/+; 18 Avpr1b +/−; 27 Avpr1b −/−) was used for the homing test, illustrated in Figure 1. On pnd 11, the litter was separated from the dam and kept for 30 min in one holding cage placed on a heating pad set at a temperature of 35°C to maintain normal body temperature of the pups in the nest. Individual pups were then transferred to a Plexiglas arena (36 cm × 22.5 cm, walls 10 cm high). Wood shavings from the home cage were evenly spread under the wire-mesh floor on one side of the arena (14 cm × 22.5 cm, nest area). The pup was placed close to the wall on the opposite side and videorecorded for three min. The floor of the arena was virtually subdivided into three areas (start, middle and nest area) and squares of 7 cm × 7 cm each, to enhance scoring of locomotor activity and exploratory behaviors from the video digital DVDs, using Noldus Observer 5.0 software. Homing performance was scored for latency to reach the area containing nest litter, time spent over the area containing nesting litter, and number of entries into the area containing nest litter. Exploratory and spontaneous behaviors measured included locomotion, wall rearing (standing on hind legs and placing forelimbs on the wall of the arena); grooming (wiping, licking, combing or stretching of any part of the body) and inactivity (no visible movements).
Figure 1.
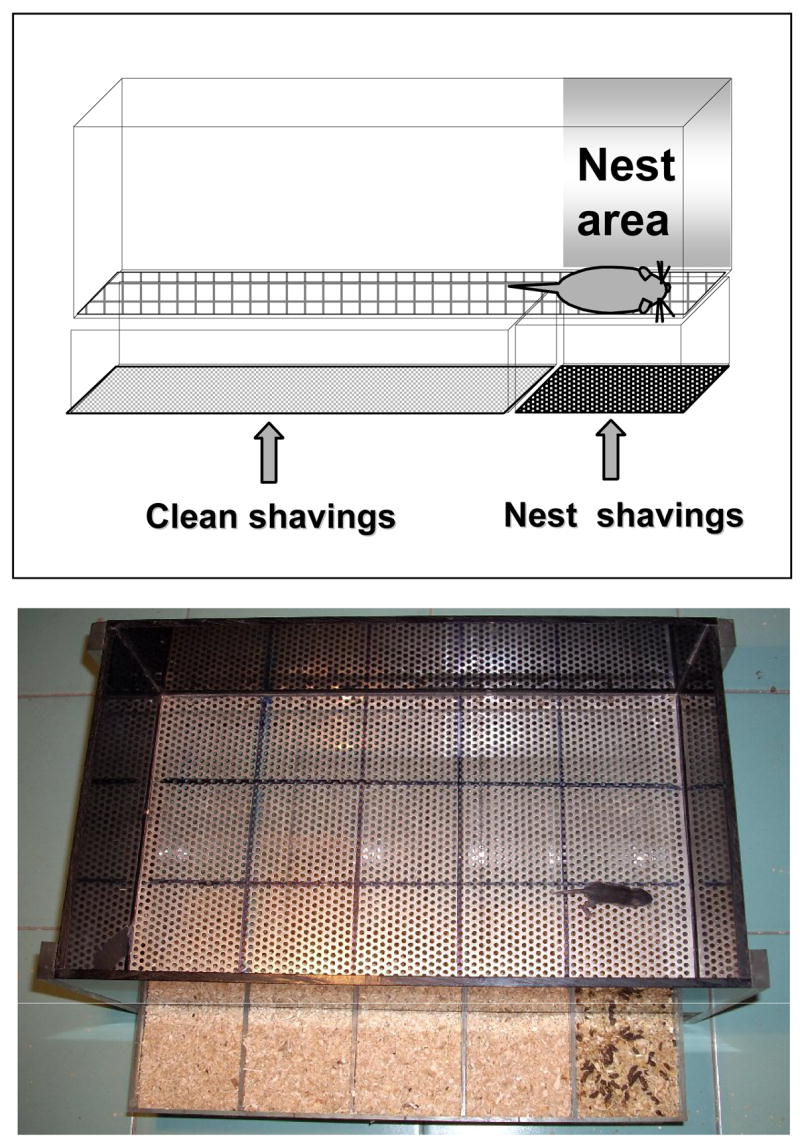
Homing apparatus. Schematic diagram (top) and photograph (bottom) showing a Plexiglas arena (36 cm × 22.5 cm, walls 10 cm high). Wood shavings from the home cage were evenly spread under the wire-mesh floor on one side of the arena (14 cm × 22.5 cm, nest area). The pup was placed close to the wall on the opposite side and videorecorded for three min. The floor of the arena was virtually subdivided into three areas (start, middle and nest area) and squares of 7 cm × 7 cm each, for scoring of locomotor activity and exploratory behaviors.
Statistical analysis
A one-way Analysis of variance (ANOVA) was used to analyze adult social investigation and USVs data in the Resident-Intruder Test. To allow parametric assumptions to be met, a square root transformation was computed on the adult USVs data from the adult females, since their total number of calls was low [11,33].
A mixed-model Analysis of Variance (ANOVA) with Repeated Measures was used to analyze neonatal USVs (for factors of genotype: +/+, +/−, −/−; test day: pnd 3, 6, 9 and 12 in baseline measurements; and separation treatment: before and after the maternal reunion in the maternal potentiation paradigm), as well as to analyze the homing data (frequency and duration of behavioral responses). Nonparametric ANOVA (Kruskal Wallis test) was used to analyze latency data from maternal potentiation, and homing tests. Post-hoc comparisons were performed using Tukey’s Honestly Significant Differences (Tukey’s HSD) test. This post-hoc test can be used in the absence of significant ANOVA results [43,55].
In the neonatal studies, only 9 of the 15 litters included all three genotypes (6 in the USVs experiments and 3 in the homing test). Therefore, statistical analysis based on litters as statistical units and pups as repeated trials within each litter [58] could not be performed.
Results
Adult Female Resident-Intruder
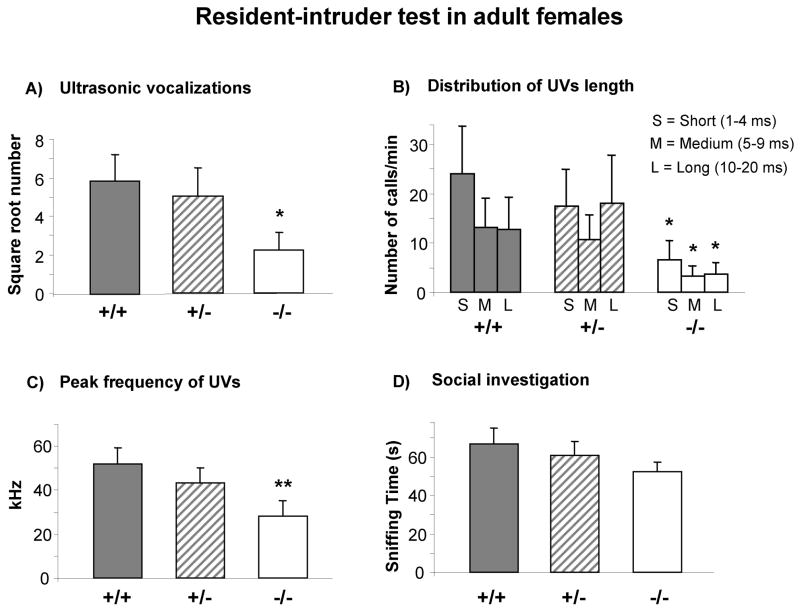
Avpr1b −/− mice emitted significantly fewer USVs during the Resident-Intruder test when compared with Avpr1b +/+ mice (F(2,29)= 2.32, p=0.03, p<0.05 for −/− versus +/+, Figure 2A). When grouped by duration, number of USVs during the test phase revealed that fewer USVs were emitted by Avpr1b −/− at any of the durations analyzed (short = 1–4 ms, medium = 5–9 ms, long = 10–20 ms) (Genotype x duration interaction F(4,58)= 1.32, p=0.03, p<0.05 for −/− versus +/+, Figure 2B).
Figure 2.
Vocalizations during the Resident-Intruder test. Number of ultrasonic vocalizations (USVs) emitted by resident female mice (four months of age) when exposed to a C57BL/6J adult female intruder. A) Avpr1b −/− emitted significantly fewer USVs in comparison to Avpr1b +/+ (*p<.05). B) Avpr1b −/− emitted fewer calls of short, medium and long durations (*p<.05). C) Avpr1b −/− emitted calls with lower peak frequencies than Avpr1b +/+ (**p <.01). D) Genotypes showed similar amounts of time in social investigation by residents toward intruders. Data for number of USVs (panel 2A) are expressed as square root mean ± SEM. In the graphs 2B, C and D, data are expressed as mean ± SEM. Avpr1b +/+ (n = 10); Avpr1b +/− (n = 11); Avpr1b −/− (n = 11).
USV peak frequencies analysis during the Resident-Intruder test revealed that Avpr1b −/− mice emitted calls with lower frequency than Avpr1b +/+ females (F(2,93) = 4.54, p = 0.01, p <0.01 for −/− versus +/+, Figure 2C). Mean USV durations and peak amplitudes showed no genotype differences during the Resident-Intruder test (duration, F(2,29) = 0.94, p = 0.40; peak amplitude, F(2,29) = 0.98, p = 0.38, Table 1).
Table 1.
Additional qualitative analysis of vocalizations during the resident-intruder test
| Call Duration (ms) | Peak Amplitude (dB) | |
|---|---|---|
| Avpr1b +/+ | 9 ±2 | 31.5 ± 4.5 |
| Avpr1b +/− | 12 ± 3 | 30.8 ± 4.0 |
| Avpr1b −/− | 6 ± 2 | 23.3 ± 5.0 |
Data are mean values ± standard errors measurements
n= 10 for Avpr1b +/+ ; n= 11 for Avpr1b +/− ; n= 11 for Avpr1b −/−
No significant genotype differences were detected on time spent sniffing the partner [F(2,29) = 1.02, p = 0.37] (Figure 2D) and exploring the home cage [F(2,29) = 1.06, p = 0.35] (data not shown). There was no significant effect of genotype on grooming frequency [F(2,29) = 0.51, p = 0.61] or duration F(2,29) = 0.24, p = 0.78] (data not shown).
Baseline measurements of pup separation vocalizations
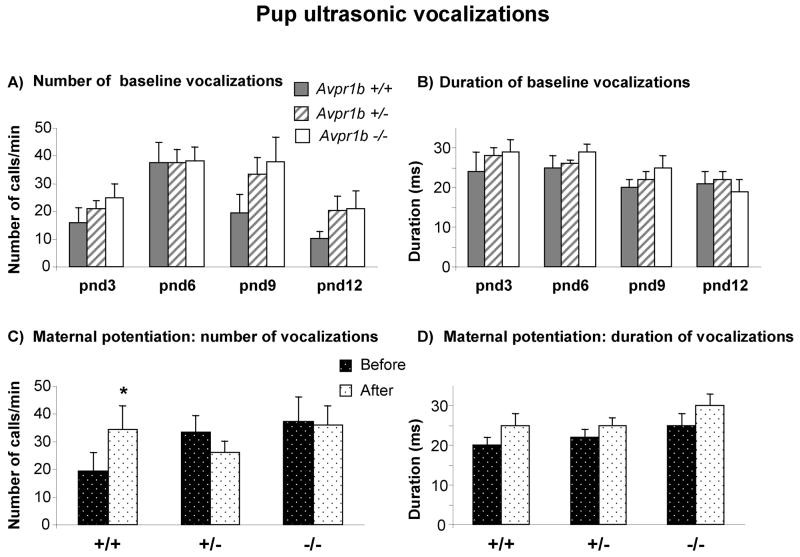
Baseline measurements of ultrasonic vocalizations on postnatal days 3, 6, 9 and 12 did not detect a genotype difference at any postnatal day [Number of USVs, F (2,162) = 0.88, p=0.42; Duration of USVs, F (2,162) = 0.72, p=0.49] (Figure 3A, B). All groups showed a similar ontogenetic profile across pnd days 3–12. No differences were detected on either body temperature [F (2,162) = 1.21, p=0.30] or body weight [F (2,162) = 0.65, p=0.53], as measured after each separation test (Table 2).
Figure 3.
Ultrasonic vocalizations (USVs) in Avpr1b pups. A) Number and B) Duration of vocalizations on postnatal day (pnd) 3, 6, 9 and 12 in response to social separation during a five minute session. No consistent genotype differences were detected across the four ages tested. C) Number and D) Duration of USVs emitted on pnd 9 during the maternal potentiation test by pups during the second five min separation session, following a five min reunion. Before: first period of five min isolation from the mother and siblings. After: second period of isolation, following five min of reunion with the mother and entire litter. Avpr1b +/+ mice emitted more calls (*p< .05) during the second separation after reunion, displaying the expected maternal potentiation. Avpr1b +/− and Avpr1b −/− mice failed to show an effect of the reunion with their mother and siblings on number of calls emitted during the second separation. Data are expressed as mean ± SEM of calls. Avpr1b +/+ (n = 9); Avpr1b +/− (n = 31); Avpr1b −/− (n = 17).
Table 2.
Body temperature and body weight of Avpr1b mice through the first two postnatal weeks
| Body temperature (°C) | Body weight (g) | |||||
|---|---|---|---|---|---|---|
| Avpr1b +/+ | Avpr1b +/− | Avpr1b −/− | Avpr1b +/+ | Avpr1b +/− | Avpr1b −/− | |
| pnd 3 | 35.5 ± 0.5 | 35.1 ± 0.3 | 35.0 ± 0.3 | 2.3 ± 0.1 | 2.5 ± 0.1 | 2.3 ± 0.1 |
| pnd 6 | 36.4 ± 0.3 | 36.7 ± 0.1 | 36.4 ± 0.2 | 3.8 ± 0.2 | 3.9 ± 0.1 | 3.8 ± 0.1 |
| pnd 9 | 37.9 ± 0.2 | 38.1 ± 0.1 | 37.7 ± 0.1 | 5.3 ± 0.1 | 5.3 ± 0.1 | 5.3 ± 0.1 |
| pnd 9 after MP | 37.7 ± 0.3 | 38.0 ± 0.1 | 37.7 ± 0.2 | |||
| pnd 12 | 39.1 ± 0.3 | 39.5 ± 0.1 | 39.3 ± 0.2 | 6.4 ± 0.1 | 6.4 ± 0.1 | 6.3 ± 0.1 |
MP= Maternal potentiation
Data shown are mean values ± standard errors of measurements.
n= 9 for Avpr1b +/+; n= 31 for Avpr1b +/−; n= 17 for Avpr1b −/−
Maternal potentiation of pup separation vocalizations
Maternal potentiation in nine-day-old pups showed a trend for higher numbers of calls during the second separation than during the first separation, only in the Avpr1b +/+ (Genotype x maternal reunion interaction, F(2,54) = 2.85, p = 0.06) (Figure 3C). Because the interaction P value was so close to statistical significance, a Tukey’s HSD test was conducted to compare first and second separations within each genotype. Tukey’s HSD test is designed for individual comparisons in such cases [55]. For the Avpr1 +/+, Tukey’s HSD = 14.68, p<0.05 first versus second separation. Number of calls during the second separation were not higher than number of calls during the first separation for Avpr1b +/− (Tukey’s HSD = 7.43, ns) or Avpr1b −/− (Tukey’s HSD = 1.27, ns). Duration of USVs significantly increased after maternal reunion [maternal reunion, F(1, 54) = 9.06, p < 0.01]. In all three genotypes, the brief exposure to the mother increased USVs duration (Figure 3D). Peak frequency and peak amplitude did not show any significant effects of genotype, maternal reunion, or their interactions [peak frequency F(2,54) = 0.06, p = 0.94; peak amplitude F(2, 54) = 0.20, p = 0.81].
Maternal responsiveness did not vary according to pup genotype, as measured by time spent in contact with pups during the maternal reunion (Avpr1b +/+ = 121.1 ± 27; Avpr1b +/− = 99.2 ± 14; Avpr1b −/− = 104.8 ± 25) [F(2, 54) = 0.22, p= 0.80] or latency to retrieval (Avpr1b +/+ = 2.8 ± 0.7; Avpr1b +/− = 6.1 ± 1.0; Avpr1b −/− = 3.9 ± 1.2) [Kruskal Wallis, F(2,3) = 4.83, p= 0.09].
No significant genotype effect was detected on body temperature measured after each separation test [first separation, F(2, 54) = 2.73, p = 0.74; second separation, F(2, 54) = 1.79, p = 0.18] (Table 2).
Homing test (pnd 11)
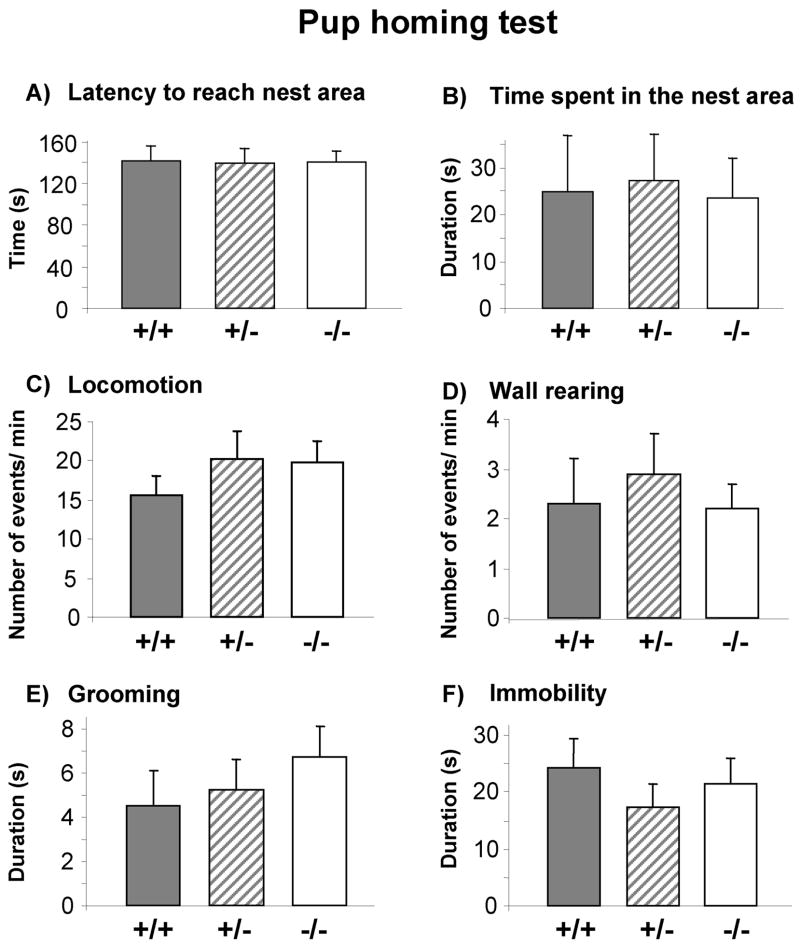
In the homing test, no significant effect of genotype was found on latency to reach the area containing the nest litter [F(2,3) = 0.02, p = 0.99], or the time spent over the area containing the nest litter [F(2,56) = 0.03, p = 0.96]. Moreover, ANOVA did not reveal any significant effect of genotype on general locomotor activity, [F(2,56) = 0.53, p = 0.59]; immobility, [F(2,56) = 0.42, p = 0.66]; grooming [F(2,56) = 0.57, p = 0.56]; or wall rearing responses [F(2,56) = 0.15, p = 0.85] (Figure 4).
Figure 4.
Pup homing test performed at pnd 11. No significant effect of genotype was found on A) latency to reach the area containing nest litter; B) time in area containing nest litter; C) general locomotor activity; D) wall rearing; E) grooming; or F) immobility responses. Avpr1b +/+ (n = 14); Avpr1b +/− (n = 18); Avpr1b −/− (n = 27).
Discussion
Vasopressin systems have been implicated in aspects of rodent social interaction, including aggression, social recognition, and interest in social olfactory cues [5,6,13,14,16,18,48,51–53]. The present experiments addressed the question of whether vocalizations emitted within social contexts in adult and infant mice require the vasopressin 1b receptor subtype. Null mutation of the Avpr1b gene was previously reported to reduce aggression in male mice in the resident-intruder task [51–53]. In contrast to male residents, female resident mice usually display more social investigation and less attack behavior toward a female intruder mouse [11,32]. In the present experiments with female Avpr1b null mutants, heterozygotes, and wildtype littermates, there were no genotype differences in amount of social sniffing during the resident-intruder test. The main finding was that fewer ultrasonic vocalizations (USVs) were detected when the residents were Avpr1b −/− than when the residents were Avpr1b +/− or +/+. USVs emitted by resident Avpr1b −/− females were not only fewer but of qualitatively different frequencies from those emitted by Avpr1b +/+ and +/− females (30 kHz vs 50 kHz, approximately). The biological meaning of variation of USVs frequency in the adult mouse has been only sporadically investigated [30,38,54]. In rats, USV frequency has been related to aversive and appetitive states [8,28]. Further, rat “alarm calls” at 22 kHz are recorded immediately after or before aversive experiences, whereas mouse 50-kHz USVs are typically emitted concomitantly with positive social interactions [37]. It can therefore be hypothesized that the difference in frequency of the calls emitted by Avpr1b −/− females during the resident intruder test could be associated with a different social motivational state, as compared to their heterozygote and wildtype littermates.
The present results indicate that the absence of the vasopressin receptor subtype 1b may reduce the tendency of female mice to vocalize during a social encounter, without affecting other aspects of social interactions. However, it remains possible that some of the vocalizations recorded during the resident-intruder test session were emitted by the intruder. Previous studies using outbred albino NMRI and BALB/c female mice have provided evidence that USVs are emitted mainly by the resident mouse [11,15]. These studies demonstrated that when the resident of the pair was anesthetized, few [15] or no USVs [11] were detected during the resident-intruder test. In contrast, when the intruder was anesthetized the rate of calling was comparable to the situation when both the mice were awake. In addition, observations by the first author (MLS) during the present experiments suggest that the USVs were emitted primarily when the resident was exhibiting social investigation while the intruder exploring the environment. Even if some authors have questioned the role of the female mouse vocalization in naturalistic conditions [35], during resident-intruder interactions it has been interpreted as a main factor contributing to the establishment of female social dominance hierarchies [32]. These calls may serve as communication signals, enhancing physical proximity and enabling social information gathering [11,32,33,41]. Since the Avpr1b −/− showed social sniffing responses (i.e. physical proximity with the intruder) that were not significantly different from Avpr1b +/+ in the present experiments, our data lend support to the role of USVs emitted by the resident female in other forms of communication, e.g. the establishment of hierarchical ranks.
Some studies have shown that the estrus cycle may modulate the rate of calling in a same-sex resident/intruder paradigm [33]. We cannot exclude that the estrus phase of the cycle could be a possible source of variability in our data. However, when working on knockout mice, it is difficult to obtain enough mice to create groups at the identical estrus stage. Since the standard errors of the mean did not differ across genotype groups, all three genotypes are likely to have been equally affected by difference in stages in the estrus cycle.
Fewer vocalizations to social stimuli emitted by Avpr1b −/− virgin females in the present study is consistent with recent data on maternal aggression to a male intruder in the same line of mutant mice [51]. Avpr1b −/− lactating females displayed significantly fewer attacks on a male intruder, suggestive of a reduced response to social cues, leading to a reduced nest defense.
Absence of the vasopressin 1b receptor subtype did not significantly affect the number of ultrasonic vocalization emissions during the conventional pup separation test, conducted at postnatal days 3, 6, 9, and 12. Baseline USVs emissions in pups did not differ among the three genotypes during a five minute test session in which the pup was removed from the nest and placed in a Styrofoam recording chamber. A trend toward more separation vocalizations by Avpr1b +/− and Avpr1b −/− than by +/+ littermates may be seen at pnd 9 and pnd 12. In contrast, while Avpr1b +/+ pups appeared to display maternal potentiation of USVs, Avpr1b +/− and −/− pups failed to display maternal potentiation in the test conducted on pnd 9. The trend toward genotype differences was unlikely to be caused by different experiences during the maternal reunion, since maternal care levels were comparable for pups of the three genotypes on measures of time spent in contact with pups and latency to retrieval. Further, similar scores across genotypes on control measures obtained in the homing test indicate that sensory and motor abilities were generally normal in all three genotypes. Lack of maternal potentiation in the null mutants and heterozygotes could be interpreted as a mild impairment in the USVs response to maternal cues. We consider this impairment as a mild one, since a different picture emerges from USVs duration data. All genotypes showed a significant increase in USVs duration during the second separation after maternal reunion, consistent with detailed sonographic analysis reported for maternally-potentiated USVs calls of neonatal rats [34]. The discrepancy between number and duration of USVs calls in our Avpr1b +/− and Avpr1b −/− mouse lines suggests that a complete description of vocalization properties will be of greater benefit than the simple measure of numbers of calls in separated mouse pups.
It is noteworthy that the absence of maternal potentiation of USVs in Avpr1b mutant pups is more selective than deficits previously reported for other lines of mice with mutations relevant to autism spectrum disorders, such as oxytocin [49,56,57], Foxp2 [47] or MeCP2 knockouts [40], in which genotype differences were detected in the conventional separation paradigm. The baseline separation vocalization response is usually interpreted as a distress call, and has been considered as a possible reflex response to variations in temperature or tactile cues in the isolation environment [1]. In contrast, maternal potentiation of USVs is suggested to have its basis in a learning process whereby pups associate their calling with positive maternal reinforcement [20,22]. Maternal potentiation may therefore incorporate a cognitive component that is less prominent or absent during the first separation.
Since the USV are considered early markers of “emotional” disturbances in rodent models we cannot exclude that a reduced USV response to the second maternal separation might reflect abnormal (i.e. reduced) anxiety/fear/distress rather than a deficit in communication. More recently, there has been increasing evidence to support V1b antagonists in the treatment of anxiety and depression also using the pup vocalization as a sensitive behavioral endpoint [18,24].
Taken together, our data indicate that the deletion of the Avpr1b gene reduces vocalizations by adult females during the resident-intruder test but does not significantly change social sniffing and exploration. Deletion of the Avpr1b gene did not affect standard pup separation vocalizations, but appeared to prevent the expected increase in pup vocalizations during the second separation from the mother and siblings. The present results are indicative of an Avpr1b −/− impairment in the modulation of ultrasonic vocalizations within different social contexts throughout the lifespan. It is interesting to speculate that the tendency of Avpr1b null mutant adult females to vocalize less during a social encounter, and the possible tendency of Avpr1b null and heterozygous mutant infants to vocalize less when repeatedly separated from their mother and siblings, may offer an additional behavioral response (related to intraspecific social communication) to a mouse model of autism, in which the second diagnostic symptom is delayed and/or deficient communication [31].
Acknowledgments
We are grateful to Igor Branchi for his advice and guidance with the ultrasound vocalization recording. This research was supported by the National Institute of Mental Health Intramural Research program (Z01-MH-002179-22, JNC), and ISS-NIH 0F14 “Neurobehavioral phenotyping of genetically modified mouse models of mental retardation” (LR).
References
- 1.Allin JT, Banks EM. Effects of temperature on ultrasound production by infant albino rats. Dev Psychobiol. 1971;4:149–156. doi: 10.1002/dev.420040206. [DOI] [PubMed] [Google Scholar]
- 2.Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Horm Behav. 2006;50:518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Bell RW, Nitschke W, Zachman TA. Ultra-sounds in three inbred strains of young mice. Behav Biol. 1972;7:805–814. doi: 10.1016/s0091-6773(72)80172-x. [DOI] [PubMed] [Google Scholar]
- 4.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard RJ, Griebel G, Farrokhi C, Markham C, Yang M, Blanchard DC. AVP V1b selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacol Biochem Behav. 2005;80:189–194. doi: 10.1016/j.pbb.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res. 2001;125:49–56. doi: 10.1016/s0166-4328(01)00277-7. [DOI] [PubMed] [Google Scholar]
- 8.Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behav Genet. 2005;35:85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- 9.Carter CS, Altemus M. Integrative functions of lactational hormones in social behavior and stress management. Ann N Y Acad Sci. 1997;807:164–174. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- 10.Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- 11.D’Amato FR, Moles A. Ultrasonic vocalizations as an index of social memory in female mice. Behav Neurosci. 2001;115:834–840. doi: 10.1037//0735-7044.115.4.834. [DOI] [PubMed] [Google Scholar]
- 12.Egashira N, Tanoue A, Matsuda T, Koushi E, Harada S, Takano Y, Tsujimoto G, Mishima K, Iwasaki K, Fujiwara M. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav Brain Res. 2007;178:123–127. doi: 10.1016/j.bbr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Engelmann M, Ludwig M, Landgraft R. Simultaneous monitoring of intracerebral release and behavior: endogenous vasopressin improves social recognition. J Neuroendocrinol. 1994;6:391–395. doi: 10.1111/j.1365-2826.1994.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferris CF, Lu SF, Messenger T, Guillon CD, Heindel N, Miller M, Koppel G, Robert Bruns F, Simon NG. Orally active vasopressin V1a receptor antagonist, SRX251, selectively blocks aggressive behavior. Pharmacol Biochem Behav. 2006;83:169–174. doi: 10.1016/j.pbb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Gourbal BE, Barthelemy M, Petit G, Gabrion C. Spectrographic analysis of the ultrasonic vocalisations of adult male and female BALB/c mice. Naturwissenschaften. 2004;91:381–385. doi: 10.1007/s00114-004-0543-7. [DOI] [PubMed] [Google Scholar]
- 16.Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodgson RA, Higgins GA, Guthrie DH, Lu SX, Pond AJ, Mullins DE, Guzzi MF, Parker EM, Varty GB. Comparison of the V1b antagonist, SSR149415, and the CRF1 antagonist, CP-154,526, in rodent models of anxiety and depression. Pharmacol Biochem Behav. 2007;86:431–440. doi: 10.1016/j.pbb.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Hofer MA, Brunelli SA, Masmela J, Shair HN. Maternal interactions prior to separation potentiate isolation-induced calling in rat pups. Behav Neurosci. 1996;110:1158–1167. doi: 10.1037//0735-7044.110.5.1158. [DOI] [PubMed] [Google Scholar]
- 20.Hofer MA, Brunelli SA, Shair HN. Potentiation of isolation-induced vocalization by brief exposure of rat pups to maternal cues. Dev Psychobiol. 1994;27:503–517. doi: 10.1002/dev.420270804. [DOI] [PubMed] [Google Scholar]
- 21.Hofer MA, Masmela JR, Brunelli SA, Shair HN. Behavioral mechanisms for active maternal potentiation of isolation calling in rat pups. Behav Neurosci. 1999;113:51–61. doi: 10.1037//0735-7044.113.1.51. [DOI] [PubMed] [Google Scholar]
- 22.Hofer MA, Masmela JR, Brunelli SA, Shair HN. The ontogeny of maternal potentiation of the infant rats’ isolation call. Dev Psychobiol. 1998;33:189–201. doi: 10.1002/(sici)1098-2302(199811)33:3<189::aid-dev1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Iijima M, Chaki S. Separation-induced ultrasonic vocalization in rat pups: further pharmacological characterization. Pharmacol Biochem Behav. 2005;82:652–657. doi: 10.1016/j.pbb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Insel TR, O’Brien DJ, Leckman JF. Oxytocin, vasopressin, and autism: is there a connection? Biol Psychiatry. 1999;45:145–157. doi: 10.1016/s0006-3223(98)00142-5. [DOI] [PubMed] [Google Scholar]
- 26.Kendrick KM. The neurobiology of social bonds. J Neuroendocrinol. 2004;16:1007–1008. doi: 10.1111/j.1365-2826.2004.01262.x. [DOI] [PubMed] [Google Scholar]
- 27.Klin A, Saulnier CA, Sparrow SS, Cicchetti DV, Volkmar FR, Lord C. Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: the Vineland and the ADOS. J Autism Dev Disord. 2007;37:748–759. doi: 10.1007/s10803-006-0229-4. [DOI] [PubMed] [Google Scholar]
- 28.Knutson B, Burgdorf J, Panksepp J. High-frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiol Behav. 1999;66:639–643. doi: 10.1016/s0031-9384(98)00337-0. [DOI] [PubMed] [Google Scholar]
- 29.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Liu RC, Miller KD, Merzenich MM, Schreiner CE. Acoustic variability and distinguishability among mouse ultrasound vocalizations. J Acoust Soc Am. 2003;114:3412–3422. doi: 10.1121/1.1623787. [DOI] [PubMed] [Google Scholar]
- 31.Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- 32.Maggio JC, Whitney G. Ultrasonic vocalizing by adult female mice (Mus musculus) J Comp Psychol. 1985;99:420–436. [PubMed] [Google Scholar]
- 33.Moles A, Costantini F, Garbugino L, Zanettini C, D’Amato FR. Ultrasonic vocalizations emitted during dyadic interactions in female mice: A possible index of sociability? Behav Brain Res. 2007 doi: 10.1016/j.bbr.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Myers MM, Ali N, Weller A, Brunelli SA, Tu AY, Hofer MA, Shair HN. Brief maternal interaction increases number, amplitude, and bout size of isolation-induced ultrasonic vocalizations in infant rats (Rattus norvegicus) J Comp Psychol. 2004;118:95–102. doi: 10.1037/0735-7036.118.1.95. [DOI] [PubMed] [Google Scholar]
- 35.Nyby JG. Auditory communication among adults. In: Willott JF, editor. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. New York: CRC; 2001. pp. 3–18. [Google Scholar]
- 36.Ognibene E, Adriani W, Macri S, Laviola G. Neurobehavioural disorders in the infant reeler mouse model: interaction of genetic vulnerability and consequences of maternal separation. Behav Brain Res. 2007;177:142–149. doi: 10.1016/j.bbr.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 37.Panksepp J. Neuroevolutionary sources of laughter and social joy: Modeling primal human laughter in laboratory rats. Behav Brain Res. 2007 doi: 10.1016/j.bbr.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav. 2006;5:274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- 40.Picker JD, Yang R, Ricceri L, Berger-Sweeney J. An altered neonatal behavioral phenotype in Mecp2 mutant mice. Neuroreport. 2006;17:541–544. doi: 10.1097/01.wnr.0000208995.38695.2f. [DOI] [PubMed] [Google Scholar]
- 41.Pomerantz SM, Nunez AA, Bean NJ. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol Behav. 1983;31:91–96. doi: 10.1016/0031-9384(83)90101-4. [DOI] [PubMed] [Google Scholar]
- 42.Ragnauth AK, Devidze N, Moy V, Finley K, Goodwillie A, Kow LM, Muglia LJ, Pfaff DW. Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes Brain Behav. 2005;4:229–239. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 43.Ricceri L, Minghetti L, Moles A, Popoli P, Confaloni A, De Simone R, Piscopo P, Scattoni ML, di Luca M, Calamandrei G. Cognitive and neurological deficits induced by early and prolonged basal forebrain cholinergic hypofunction in rats. Exp Neurol. 2004;189:162–172. doi: 10.1016/j.expneurol.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 44.Scattoni ML, Puopolo M, Calamandrei G, Ricceri L. Basal forebrain cholinergic lesions in 7-day-old rats alter ultrasound vocalisations and homing behaviour. Behav Brain Res. 2005;161:169–172. doi: 10.1016/j.bbr.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Shair HN. Acquisition and expression of a socially mediated separation response. Behav Brain Res. 2007 doi: 10.1016/j.bbr.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shair HN, Brunelli SA, Masmela JR, Boone E, Hofer MA. Social, thermal, and temporal influences on isolation-induced and maternally potentiated ultrasonic vocalizations of rat pups. Dev Psychobiol. 2003;42:206–222. doi: 10.1002/dev.10087. [DOI] [PubMed] [Google Scholar]
- 47.Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Sosa MA, Rabidou D, Santucci AC, Perl D, Morrisey E, Buxbaum JD. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci U S A. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stemmelin J, Lukovic L, Salome N, Griebel G. Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V1b receptor antagonist, SSR149415. Neuropsychopharmacology. 2005;30:35–42. doi: 10.1038/sj.npp.1300562. [DOI] [PubMed] [Google Scholar]
- 49.Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venerosi A, Calamandrei G, Ricceri L. A social recognition test for female mice reveals behavioral effects of developmental chlorpyrifos exposure. Neurotoxicol Teratol. 2006;28:466–471. doi: 10.1016/j.ntt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Wersinger SR, Caldwell HK, Christiansen M, Young WS., 3rd Disruption of the vasopressin 1b receptor gene impairs the attack component of aggressive behavior in mice. Genes Brain Behav. 2006 doi: 10.1111/j.1601-183X.2006.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 53.Wersinger SR, Kelliher KR, Zufall F, Lolait SJ, O’Carroll AM, Young WS., 3rd Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Horm Behav. 2004;46:638–645. doi: 10.1016/j.yhbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 54.White NR, Prasad M, Barfield RJ, Nyby JG. 40- and 70-kHz vocalizations of mice (Mus musculus) during copulation. Physiol Behav. 1998;63:467–473. doi: 10.1016/s0031-9384(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 55.Wilcox RG. New statistical procedures for the social sciences. Hillsdale NJ: Erlbaum; 1987. Pages. [Google Scholar]
- 56.Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- 57.Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36:221–229. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- 58.Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]