Abstract
What are the limits and modulators of neural precision? We address this question in the most regular biological oscillator known, the electric organ command nucleus in the brainstem of wave-type gymnotiform fish. These fish produce an oscillating electric field, the electric organ discharge (EOD), used in electrolocation and communication. We show here that the EOD precision, measured by the coefficient of variation (CV = SD/mean period) is as low as 2 × 10−4 in five species representing three families that range widely in species and individual mean EOD frequencies (70–1,250 Hz). Intracellular recording in the pacemaker nucleus (Pn), which commands the EOD cycle by cycle, revealed that individual Pn neurons of the same species also display an extremely low CV (CV = 6 × 10−4, 0.8 μs SD). Although the EOD CV can remain at its minimum for hours, it varies with novel environmental conditions, during communication, and spontaneously. Spontaneous changes occur as abrupt steps (250 ms), oscillations (3–5 Hz), or slow ramps (10–30 s). Several findings suggest that these changes are under active control and depend on behavioral state: mean EOD frequency and CV can change independently; CV often decreases in response to behavioral stimuli; and lesions of one of the two inputs to the Pn had more influence on CV than lesions of the other input.
Recent research concerns the reliability and temporal precision of neural firing times in response to a repeated stimulus (1–4). We chose to study pacemakers where activity repeats without stimulation, and whose precision is hence readily characterized. Precision is quantified by the coefficient of variation (CV) of the period (CV = SD/mean period). With rare exceptions, biological pacemaking systems, including heart, respiratory, and firefly flashing rhythms, have a minimum CV as low as 2–6 × 10−2 (5–7), in the same range as the CV of stimulated neocortical neurons (2). Circadian rhythms have CVs as low as 2–3 × 10−3 (8). However, the electric organ discharge (EOD) can be yet another order of magnitude more regular (9, 10). We chose the EOD and its neural command center, the pacemaker nucleus (Pn), to study the limits and control of neural firing precision. In particular, we consider the EOD of high frequency (60–2,000 Hz) wave-type South American electric fish, which produce an oscillating electric dipole field, continuously throughout the fish’s life, day and night, used in active electrosensing and communication (11). The EOD periods are commanded cycle by cycle by the medullary Pn, a network of ca. 150 synchronously firing neurons (12).
The fish detects its own EOD, distorted by electrically resistive or capacitive objects in the environment, at electroreceptors in the skin. “Electrically visible” objects are detected by comparing changes in the EOD phase and amplitude along the body. In social contexts, the time courses of small EOD frequency changes are compared. Phase differences in the EOD as small as 400 ns will elicit a behavioral response (13). We show that the EOD regularity matches this sensory ability, with temporal precision to 120 ns and that the regularity can vary as though under central control.
MATERIALS AND METHODS
Individuals of both sexes from Apteronotus leptorhynchus (10–15 cm), A. albifrons (13–29 cm), and Eigenmannia virescens (10–19 cm) were obtained from a commercial supplier. Two individuals of A. bonapartii (14 and 16 cm) were collected by W. Heiligenberg and J. Alves-Gomes (National Institute for Research on Amazonia, Manaus, Brazil) in Rio Solimoes, Brazil, and a Sternopygus dariensis (46 cm) was provided by H. Zakon (Univ. of Texas, Austin). Species were kept in separate aquaria, with water maintained in pH (7.0–8.5), resistivity (5–15 kΩ·cm), and temperature (25–28°C).
An inter-species comparison of EOD regularity involved 31 individuals, each isolated for EOD measurement. Another 49 A. leptorhynchus were observed during changes in EOD regularity. The latter fish were immobilized with 2–5 μl of gallamine triethiodide (20 mg/ml intramuscularly) and respirated with a continual stream (3–7 drops/s) of aerated aquarium water flowing into the mouth. The immobilization ensured that the position of the fish’s tail relative to the silver wire recording electrode was constant (0.5–2.0 cm), and thus avoided artificial variation of the signal amplitude. Intracellular recordings in the Pn and lesions of both the prepacemaker nucleus (PPn) and sublemniscal prepacemaker nucleus (SPPn) required exposing the brain under local anesthetic. Glass electrodes were positioned by eye and directed with a micromanipulator into the PPn or SPPn. Focal stimulation of one of these nuclei with l-glutamate elicited maximal EOD frequency changes when the electrode was centered in the nucleus. Once located, the PPn or SPPn was reversibly blocked with a pulse of pressure-injected anesthetic [2% lidocaine·HCl in equal part 1 M NaOAc (14, 15)], or permanently lesioned with high-frequency current (Birtcher Hyfrecator, model 733, Irvine, CA).
Signals were amplified and passed to a Schmitt trigger with independently adjustable hysteresis center and width (Getting Instruments, Iowa City, IA). The trigger marked with a digital pulse the time of each crossing of a voltage threshold, adjusted to the voltage of maximal slope. The time between pulses marked the EOD cycle period, which was measured with a 20-MHz clock (National Instruments ATMIO16E2, Austin, TX). The resolution of the measurement circuit was confirmed to be 0.05 μs. Each period was recorded and stored.
The regularity of the periods was quantified by the CV, calculated over a window, usually chosen to be 200 EOD cycles, overlapping 50% between successive windows. This window length is longer than the duration of significant autocorrelation (ca. five periods, otherwise appearing to be unpatterned and stochastic), but not so long that slow frequency drifts contaminate CV values. The chosen window length also has a physiological basis: it matches the ca. 100-period sensory integration and behavioral response times reported for Eigenmannia (13, 16, 17). For some figures noted in the text, shorter windows were used, or individual periods were analyzed.
RESULTS
Inter-Species Comparison of EOD Regularity.
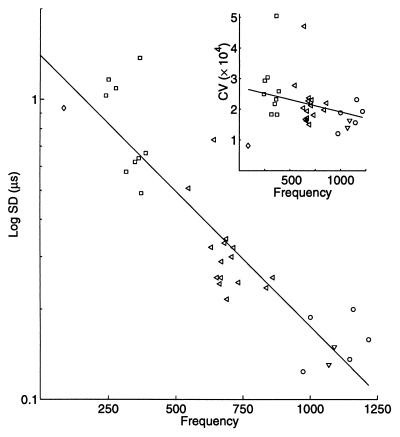
Although one species of electric fish, A. albifrons, previously had been reported (9) to have an EOD CV of 1.2 × 10−4, we confirmed that other individuals of the same and related species also could produce EODs of such extreme precision. Individuals from five species (S. dariensis, E. virescens, A. leptorhynchus, A. albifrons, and A. bonapartii), which represent three families (18) of gymnotiform electric fish, were observed for 10 min each. The 5-s period with the lowest EOD CV was extracted. The rms value of the CV for nonoverlapping data windows during the 5 s is plotted (Fig. 1, Inset) against EOD frequency [temperature corrected for Q10 (19)] for each individual. Individual EOD frequencies fall within the species range, and CV values for all species and individuals fall between 1 and 5 × 10−4. The CV decreases significantly with increasing EOD frequency (Student’s t test of correlation coefficient; P < 0.01) when the CV in each window is considered independently. The EOD CV ranges more widely within the 5 s in individuals from lower-frequency species than in those from higher-frequency species. The rms SD calculated for the same data falls significantly with increasing species frequency (Fig. 1; P < 0.01), yielding a temporal resolution of 0.12–1.0 μs. The SD of the EOD period differed significantly (Mann–Whitney U test, P < 0.05) between E. virescens and each species of Apteronotus: A. leptorhynchus (U = 129), A. albifrons (U = 45), A. bonapartii (U = 18). The EOD SD of A. leptorhynchus is likewise significantly distinguishable from that of A. albifrons (U = 75) and A. bonapartii (U = 30).
Figure 1.
Inter-species comparison of EOD regularity. Log of the minimum observed SD of the EOD period decreases with EOD frequency. Individuals from each species are represented as follows: ◊, S. dariensis; □, E. virescens; ◃, A. leptorhynchus; ○, A. albifrons; and ▵, A. bonapartii. Individuals fall within the species-specific frequency range. E. virescens also differs significantly in SD from all species of Apteronotus. The SD of the EOD differs significantly between A. leptorhynchus and A. albifrons. (Inset) CV decreases with increasing species frequency (same data). Each point is the average CV over 5 s. Significance of the slope of the CV is calculated from successive CV values from nonoverlapping windows, so N is ca. 25 times more than plotted.
Regularity of the EOD and Its Command Signal in A. leptorhynchus.
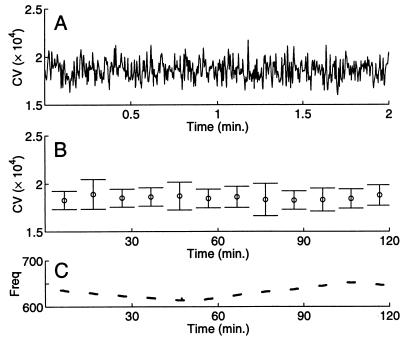
Six A. leptorhynchus were observed for 2 min every 10 min during 2–9 hr to determine whether the EOD CV could remain at its minimum value for extended periods. There was no activity in the room during these observations except periodic checks of experiment progress. The fish’s tank was surrounded with fabric to avoid visual stimulation during these checks. One typical individual maintained a fixed minimum, mean, and range (1.6–2.0 × 10−4) of its EOD CV for minutes (Fig. 2A) and hours (Fig. 2B) even while the frequency drifted slowly (Fig. 2C), primarily because of water temperature changes (19).
Figure 2.
EOD coefficient of variation can remain constant over short and long time scales. (A) Two-minute continuous recording of EOD intervals (magnified from fourth data segment in B) yields a consistent CV value. (B) Two of every 10 min were recorded for 2 hr (mean ± SD of CV values are plotted). EOD CV remained largely constant over this long time period. Although the CV may briefly increase by 20–100%, it does not drop below a minimum value. The three largest error bars are associated with data segments containing large EOD excursions called chirps. (C) Frequency changes over the 2 hr were independent of CV value, and occurred primarily because of water temperature changes. Window length: 200 cycles.
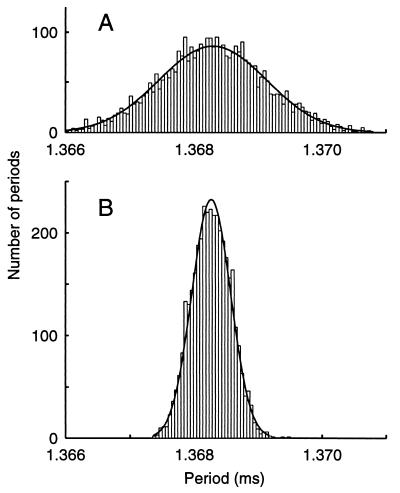
Is this low CV a property only of the EOD or also of its command neurons in the Pn? To answer this question, the EOD and action potentials from a single neuron in the intact population of ca. 150 Pn neurons were simultaneously recorded in vivo. The EOD cycle periods and the intracellularly monitored simultaneous inter-spike intervals of the Pn neuron are plotted in histograms (Fig. 3). The histograms contain the same number of periods from 5 s of recordings, but the Pn neuron’s histogram is wider and shorter, reflecting the somewhat higher CV values of the cell (CV = 6.1 × 10−4) compared with the EOD (CV = 2.2 × 10−4). Both histograms fit Gaussian distributions of widths given by SD = 0.84 μs and SD = 0.30 μs, respectively (cell: χ2 = 173, df = 120; EOD: χ2 = 73, df = 42). These CV and SD values held for at least 74 s. The histogram bin width corresponds to the resolution of the clock: 50 ns. The reported cell CV value is only an upper bound because any noise in the recording increases CV. A Pn neuron’s CV depended strongly on signal-to-noise of the recording, which improved when the resting membrane potential was more negative. Likewise, local field potentials had signal-to-noise levels too low to make accurate measures of a Pn neuron’s CV. Only in the rare case of an irregular EOD did the Pn neuron have a lower CV than the EOD.
Figure 3.
The CV of an impaled Pn neuron’s spike timing can be less than 3 (2.8) times the CV of the EOD in simultaneous recordings. (A) The histogram of a Pn neuron’s inter-spike intervals is Gaussian distributed with measured CV = 6.1 × 10−4 (SD = 0.84 μs). (B) The histogram of EOD cycle periods likewise fits a Gaussian distribution, with measured CV = 2.2 × 10−4 (SD = 0.30 μs). With rare exceptions, a Pn neuron CV was larger than the EOD CV.
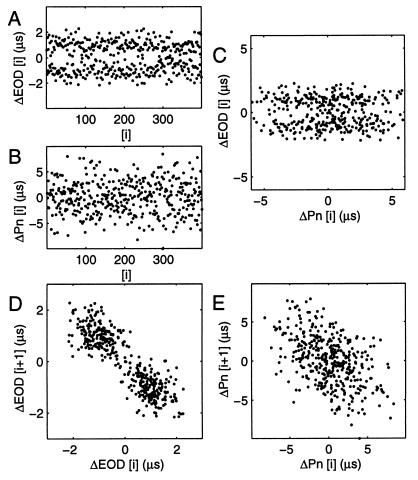
The EOD period histogram usually fit a Gaussian distribution, with a weak tendency, seen in the autocorrelation, for long intervals to be followed by shorter ones. Sometimes EOD cycles could fall bimodally (Fig. 4A). Bimodal cycle periods often were accompanied with a bimodal distribution of EOD amplitude. The sub-distributions of the EOD period were Gaussian and had CVs of the same minimum values described above. A joint interval plot of each EOD period (subtracting the mean EOD period) against the succeeding one (Fig. 4D) shows that a short period tends to follow a longer one and vice versa (negative serial correlation). Whether or not this bimodality occurs naturally, it raised the question: are the cycle-by-cycle fluctuations about the mean EOD period commanded by the Pn or introduced later in the electromotor pathway?
Figure 4.
Simultaneous fluctuations about the mean period (a cycle period minus the mean period, measured in μs) of (A) EOD (EOD [i]) and (B) an impaled Pn neuron (Pn [i]). (C) EOD [i] vs. Pn [i] does not show obvious direct command by this neuron of cycle-by-cycle EOD fluctuations. (D) EOD [i+1] plotted against EOD [i]. Seen in many fish, a short period tended to be followed by a long one and vice versa, producing a bimodal distribution. (E) Pn [i+1] vs. Pn [i] plot also is skewed, with short periods tending to follow long ones and vice versa, although the resulting distribution is not bimodal. The common tendency to the same skewing suggests that the Pn neurons could collectively command cycle-by-cycle fluctuations about the mean period.
A simultaneously recorded Pn neuron (Fig. 4B), showed a similar though weaker tendency to follow a short inter-spike interval with a longer one, resulting in a skewed, but not bimodal, joint interval plot (Fig. 4E). The simultaneous skewing suggests that the Pn neuron could influence cycle-by-cycle EOD fluctuations. On the other hand, plotting the EOD against Pn fluctuations (Fig. 4C) did not show obvious direct command of EOD fluctuations. To allow for synchronization between the digital clocks (0.5 ms) and axonal conduction, the EOD interval plotted was the one following the Pn interval by 8.7 ms (which is the time to the maximum of a broad correlation between the EOD and Pn). This delay was consistent with the time of maximum cross correlation observed during a large frequency modulation in another fish (ca. 6 ms), but is longer than the 3 ms reported (20) for the time from onset of frequency modulation. Because all Pn neurons cannot be monitored at once, it is impossible to show exact mapping of Pn neuron cycle-by-cycle fluctuations to those of the EOD. However, the skew was observed only in the Pn when the EOD period distribution was bimodal, frequently was observed for minutes or more at a time, and is therefore unlikely to be caused by chance. In addition, no other high-frequency center has been found, in decades of research, that could drive both the Pn and EOD. The fluctuations of the EOD period about the mean are thus likely to be driven collectively by the Pn neurons.
Changes in EOD Frequency and CV.
Although the EOD CV can remain near its minimum value for hours, it also can change spontaneously, in either direction. This was studied in curarized A. leptorhynchus, but also observed in freely swimming fish of the same species, and in E. virescens, and S. dariensis. The EOD CV did not generally increase above 10−3.
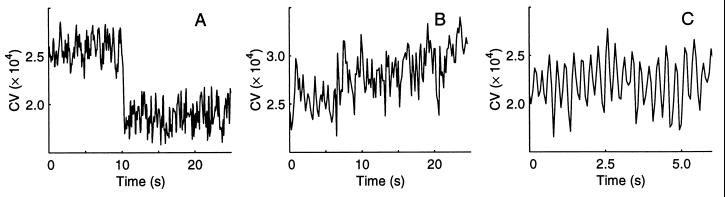
Spontaneous shifts of the EOD CV (Fig. 5) occur rapidly in steps lasting ca. 250 ms (ca. 200 cycles), or gradually over 10–30 s, or the EOD CV oscillates at 3–5 Hz for seconds. Although oscillations occurred alone, they also rode, at lower amplitude, on the step and gradual changes plotted. These CV shifts were observed without concurrent fin, gill operculum, or other body motion. We considered and rejected the possibility that external electronic noise contributed to the effect. Thus the measured shifts in EOD CV appear to be purely changes in electromotor output. The CV values for this figure were calculated over a 100- instead of 200-cycle window to capture rapid CV changes.
Figure 5.
Spontaneous changes in EOD CV. (A) Rapid decreases or increases in CV occurred in ca. 250 ms. (B) Gradual rises occurred over 10 s or more. (C) The CV also could oscillate in the range of 3–5 Hz, sometimes riding on other changes as in B. Though the cause for the oscillations is still unknown, they were not influenced by window length. Window length: 100 cycles.
The EOD CV was calculated during two well-studied behaviors that involve changing mean frequency: the jamming avoidance response (JAR) and chirping. A JAR is a slow EOD frequency shift away from that of a “jamming stimulus”: another real or simulated EOD of nearly the same frequency (11, 21). With removal of the jamming stimulus, the fish’s EOD relaxes to its original frequency. The EOD CV was constant during a JAR (Fig. 6A), both its rising and its falling frequency phase. Contamination of the fish’s own EOD by the jamming stimulus was avoided by presenting the jamming stimulus only for long enough (ca. 8 s) to obtain a frequency rise that was briefly sustained after the stimulus was removed. The second behavior involving changing mean frequency is called chirping. Chirps in A. leptorhynchus are ca. 15 ms (22) increases of the EOD frequency used for conspecific communication (23). Preceding a spontaneous chirp by 5–15 s, the CV rose dramatically, often doubling in value, in 5/10 cases. The CV returned to its original level only many seconds after the end of the chirp.
Figure 6.
Changes in the CV of the EOD during behaviors known to affect EOD frequency. (A) Neither during the rising nor the falling phase of the JAR, in response to a jamming stimulus (Stim), does the EOD CV change. (B) A spontaneous rise in EOD frequency, called a chirp, often is associated with a prolonged increase in CV, lasting many seconds on either side of the chirp. Window length: 200 cycles.
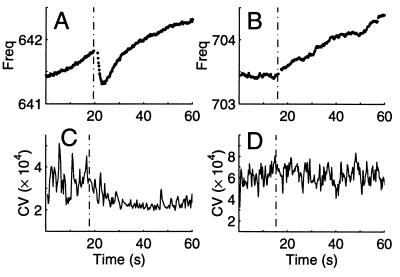
Concomitant changes in EOD frequency and in CV can be induced by a startling stimulus. A gentle hammer tap to the fish’s tank led to a small, rapid, and temporary frequency drop of up to 0.5 Hz (510 mHz) within 4.0 s in 18/21 trials (86%) in five fish (Fig. 7A). As the frequency dropped, the CV decreased (increase in regularity, Fig. 7C) in 6/9 trials (67%). Trials in which the CV was at its minimum (see Fig. 1) or maximum (ca. 10−3) before stimulus presentation were not included in the tally. The CV response was highly variable, depending on the initial CV value, and could range from a 20–75% decrease in CV, either as multiple rapid steps or as a gradual decrease. The average time to maximum decrease in CV was 6.4 s. Another stimulus, visual looming, induced EOD CV decreases (6/7 trials) in one fish, without frequency changes.
Figure 7.
Stimulus-dependent changes in EOD frequency (stimulus onset marked by dashed vertical line). (A) Typical frequency response to a tap on the fish’s tank. This temporary drop in frequency and rapid recovery also was observed after other stimuli (water drop). (B) Typical frequency response to light touch is a reversal of frequency slope, in this case switching from constant to rising EOD frequency. (C) Characteristic decrease in the CV of the EOD after a tap to the tank. Example is from the same stimulus type as in A but a different trial. (D) After touching the tail, the CV of the EOD did not change. Example is same stimulus type as for B but a different trial. Window length: 200 cycles.
EOD Frequency and CV Changes Are Independent.
In contrast, some stimuli caused a change in frequency without altering the CV. Gently touching the fish’s tail with a styrofoam rod elicited a change in the EOD frequency (Fig. 7B) in 4/5 (80%) trials. If the frequency was dropping before the stimulus, it gradually rose after the stimulus, and vice versa. This type of stimulus did not cause the CV to change (Fig. 7D), even in the same fish that showed CV changes with other stimuli. EOD frequency increased gradually by over 30 Hz with increased tank water flow over the gills, whereas the CV remained constant at its minimum value (Fig. 8A). The EOD frequency also changed when a water drop fell on the water surface just above the head of an immobilized fish. The CV did not change for any fish with this stimulus.
Figure 8.
Changes in the EOD’s frequency and CV occur independently. (A) Large frequency change in response to increased respirator flow rate does not alter CV values. (B) The CV spontaneously stepped by large values, whereas frequency varied within 1 Hz. Window length: 100 cycles to avoid contamination by the large frequency rise.
Other stimuli tested, but without effect on EOD CV, include acoustic tone bursts, light flashes, electrical simulation of a conspecific at frequencies that did or did not initiate a JAR, and food odors. Each stimulus was presented at an intensity the fish could easily detect, although some did not cause an EOD frequency shift.
The EOD frequency did not necessarily change with EOD CV changes. For example, the EOD CV makes multiple steps to 60% of its maximum while the frequency slowly drifts (Fig. 8B), most likely with temperature, by a few tenths of one Hz over tens of seconds. In 1,000 s of data, the frequency and CV from one individual were found not to be significantly correlated.
Lesions of Pn Inputs.
We hypothesized that one of the two known inputs to the Pn could modulate EOD CV. PPn activity elicits chirps and gradual EOD frequency rises; the SPPn is involved in the JAR in A. leptorhynchus (24). The EOD modulations can be experimentally initiated with glutamate injection into the appropriate nuclei. PPn lesions might have been expected to have more effect on EOD CV than lesions of the SPPn because the EOD CV often changed with chirps but not necessarily during the JAR.
Focal injection of lidocaine temporarily blocked each nucleus, as shown by the lack of response to glutamate for 10–15 min after injection. Results from five of six fish with lidocaine blocks were consistent with the following progression. The EOD CV first increased (1–5 min), then slowly decreased slightly (5–15 min), sometimes becoming more regular (ca. 50%) than before the block. In late recovery (> 15 min), the CV increased again and some fish began spontaneously chirping more than before the block. One temporary bilateral PPn block had no effect. After a permanent bilateral PPn lesion, made by massive current injection, no chirps were observed during the 9-day survival time; day-to-day CV trends were not clear.
Neither permanent nor temporary (n = 4 each) bilateral SPPn lesions caused any noticeable, consistent change in the EOD CV. Even aspirating parts of the brain, including the large cerebellum, in crude sections from forebrain toward the brainstem had little effect on the EOD CV until the mesencephalic PPn and SPPn were lesioned. This caused a brief frequency rise, and the EOD CV subsequently doubled. Aspiration near the brainstem, presumably damaging the Pn, caused a major breakdown of the EOD synchrony.
DISCUSSION
Regularity of Oscillators.
The electromotor system of these gymnotiforms is unmatched in regularity. We observed the EOD of dozens of fish from five species representing three families, confirming and extending Bullock’s results for A. albifrons (9). All showed EODs that are regular to approximately CV = 2 × 10−4. The individual- and species-specific EOD frequencies range widely among species (70–1,250 Hz), with EODs of higher frequency tending to have lower minimum EOD CVs (increased regularity). This agrees with many other systems, including the frog stretch receptor (25) and snake infrared receptor (26). The EOD SD decreases significantly with increased EOD frequency: from 1.0 μs at an EOD frequency of ca. 250 Hz to 0.12 μs at ca. 1,000 Hz.
The regularity of the EOD, considered a hallmark of healthy fish, has been exploited as a biological monitor of water quality (27). Sick or dying fish often have high EOD CV [as in Sternopygus (9)], to the point of an asynchronous EOD.
Not only the EOD, but also single neurons of the Pn, which commands the EOD, produce extraordinarily regular signals. We showed that a Pn neuron in A. leptorhynchus can have a CV = 6.1 × 10−4 (SD = 0.84 μs) while the EOD CV at the same time is 2.2 × 10−4 (SD = 0.30 μs). Thus the SD of the time between a Pn neuron’s action potentials is three to four orders of magnitude smaller than its action potential width (1–2 ms). Neocortical neuron spike timing in response to electrical stimulation, in contrast, varies from trial to trial with millisecond SD (2), only slightly less than the action potential width. Neural firing times thus can be far more reliable than previously thought.
The higher precision of the EOD compared with a single Pn neuron may result from convergence along the electromotor command pathway. Assuming that an isolated Pn neuron oscillates slightly more regularly (SD = 10−3) than neurons of other systems, the law of large numbers predicts a convergence of hundreds of thousands of independent neurons to achieve the regularity of a Pn neuron measured in the intact nucleus. In fact, the Pn is made up of only ca. 150 neurons. The converging inputs onto any one neuron are not independent: the Pn cells, the source of the oscillation, are largely electrotonically coupled (12, 20, 28). Thus, the mechanism of CV reduction in this motor pathway is not yet understood.
Changing Regularity.
During long-term observation, the CV of the EOD changed spontaneously in what we term steps, ramps, and oscillations, each with its own time scale. Although we knew that the EOD changes frequency in social contexts (23, 29), it was not previously known whether or how the EOD CV might change. Thus, the present work starts to answer Kramer’s question: “[Do] disturbances of various kinds affect the regularity of EOD frequency, if measured at sufficient accuracy?” (30). Sufficient accuracy (±0.05 μs) was attained here for simultaneously measuring two signals: the Pn intracellular action potentials and the EOD, over more than 100 s.
We showed that EOD regularity improves in association with some external stimuli. The responsiveness of the CV (as a measure of regularity) to some but not all stimuli, the delay of the maximal response of the CV (seconds = thousands of cycles), and the spontaneous changes all suggest central control of regularity. Effective stimuli for changing the CV were some of the same stimuli that initiate Mauthner cell-mediated startle responses in fish (31), including tapping the aquarium and visual looming. Mauthner cell activation induces an EOD frequency rise, called the novelty response, in a related pulse-type fish (32), and is used in Eigenmannia during prey capture (33). A role of the Mauthner cell in control of EOD CV remains to be investigated.
The electrosensory acuity matches the electromotor performance in the only species tested, Eigenmannia. Although one individual could behaviorally discriminate phase differences of ca. 0.400 μs (13) over a 200-ms integration time, the EOD of another individual was regular to 0.394 μs (SD) over the same integration time. Carr (34) has summarized the thinking of many researchers on the value of detecting fractional microsecond changes in the timing of the EOD locally on parts of the body. We know that weakly electric fish make sensory discriminations by detecting distortions in their electric field, apparently integrating information over ca. 200 ms (16). They also require a similar decision time (21) before raising or lowering their EOD frequency in the context of the JAR. One hypothesis is that the more regular the EOD signal during this integration or decision time, the better the fish can attribute phase and amplitude deviations at electroceptors to surroundings. Thus, the response of CV decrease could be a form of novelty response for wave-type fish. That is, wave-type fish may temporarily improve their electrosensory acuity by briefly decreasing their EOD CV in a manner analogous to the pulse-type fish’s increased electrosensory sampling rate with increased EOD frequency (35). A regular EOD signal thus could improve a fish’s electrosensory ability.
Neural Pathways Controlling Regularity.
The neural pathways that could be involved in determining EOD CV were investigated in lesion studies. We separately lesioned the only two known inputs to the Pn: the PPn and the SPPn. Their activity modulates (24, 36, 37) the Pn and hence EOD frequencies. Our data support the hypothesis that the PPn is the main input influencing EOD CV, whereas the SPPn did not appear to affect EOD CV. These results are consistent with the observation that the EOD CV can increase surrounding a chirp, a PPn-mediated electrical behavior, but is not altered during the SPPn-mediated JAR.
We did not strictly confirm that CV changes are commanded by the Pn neurons, and therefore cannot rule out the possibility that more direct inputs, particularly in the spinal cord, to the electromotor pathway also alter EOD CV. Even increased blood flow could indirectly alter EOD CV. Aside from the incoming relay axons, there are no known inputs to the electromotor neurons. However, Schaeffer and Zakon (38) have reported dendrites on the electromotor neurons that could receive modulatory signals, and thereby influence EOD CV.
Acknowledgments
We thank B. Nielsen for electronics expertise, and M. Deweese, J. Enright, M. van der Heyden, M. Illouz, B. Keeley, J. E. Lewis, L. Maler, P. Rowat, D. Ruderman, and C. J. H. Wong for helpful discussions and comments. Research was conducted in accordance with the University of California at San Diego Animal Care protocol. This work was supported by a National Institutes of Health Predoctoral Fellowship to K.T.M., National Institutes of Health and National Science Foundation grants to W. Heiligenberg, and the Howard Hughes Medical Institute to T.J.S.
ABBREVIATIONS
- CV
coefficient of variation
- EOD
electric organ discharge
- JAR
jamming avoidance response
- Pn
pacemaker nucleus
- PPn
prepacemaker nucleus
- SPPn
sublemniscal prepacemaker nucleus
References
- 1.Softky W R, Koch C. J Neurosci. 1993;13:334–350. doi: 10.1523/JNEUROSCI.13-01-00334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mainen Z F, Sejnowski T J. Science. 1995;268:1503–1506. doi: 10.1126/science.7770778. [DOI] [PubMed] [Google Scholar]
- 3.de Ruyter van Steveninck R R, Lewen G D, Strong S P, Koberle R, Bialek W. Science. 1997;275:1805–1808. doi: 10.1126/science.275.5307.1805. [DOI] [PubMed] [Google Scholar]
- 4.Berry M J, Warland D K, Meister M. Proc Natl Acad Sci USA. 1997;94:5411–5416. doi: 10.1073/pnas.94.10.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustafson D, Willsky A S, Wang J Y, Lancaster M C, Triebwasser J H. IEEE Trans Biomed Eng. 1978;25:344–353. doi: 10.1109/tbme.1978.326260. [DOI] [PubMed] [Google Scholar]
- 6.Sammon M, Romaniuk J R, Bruce E N. J Appl Physiol. 1993;75:887–901. doi: 10.1152/jappl.1993.75.2.887. [DOI] [PubMed] [Google Scholar]
- 7.Buck J, Buck E. Sci Am. 1976;234:74–85. doi: 10.1038/scientificamerican0576-74. [DOI] [PubMed] [Google Scholar]
- 8.Enright J T. Science. 1980;209:1542–1545. doi: 10.1126/science.7433976. [DOI] [PubMed] [Google Scholar]
- 9.Bullock T H. J Gen Physiol. 1970;55:565–584. doi: 10.1085/jgp.55.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moortgat K T, Bullock T H, Sejnowski T J. Soc Neurosci Abstr. 1996;22:179.10. [Google Scholar]
- 11.Heiligenberg W. Neural Nets in Electric Fish. Cambridge, MA: MIT Press; 1991. [Google Scholar]
- 12.Elekes K, Szabo T. Exp Brain Res. 1985;60:509–520. doi: 10.1007/BF00236936. [DOI] [PubMed] [Google Scholar]
- 13.Carr C E, Heiligenberg W, Rose G J. J Neurosci. 1986;6:107–119. doi: 10.1523/JNEUROSCI.06-01-00107.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi T, Keller C H. J Comp Physiol A. 1992;170:161–170. doi: 10.1007/BF00196898. [DOI] [PubMed] [Google Scholar]
- 15.Bastian J. J Neurosci. 1986;6:553–562. doi: 10.1523/JNEUROSCI.06-02-00553.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabbiani F, Metzner W, Wessel R, Koch C. Nature (London) 1996;384:564–567. doi: 10.1038/384564a0. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki M. Curr Opin Neurobiol. 1997;7:473–479. doi: 10.1016/s0959-4388(97)80025-6. [DOI] [PubMed] [Google Scholar]
- 18.Alves-Gomes J A, Orti G, Haygood M, Heiligenberg W, Meyer A. Mol Biol Evol. 1995;12:298–318. doi: 10.1093/oxfordjournals.molbev.a040204. [DOI] [PubMed] [Google Scholar]
- 19.Enger P S, Szabo T. Comp Biochem Physiol. 1968;27:625–627. doi: 10.1016/0010-406x(68)90263-6. [DOI] [PubMed] [Google Scholar]
- 20.Dye J, Heiligenberg W. J Comp Physiol A. 1987;161:187–200. doi: 10.1007/BF00615240. [DOI] [PubMed] [Google Scholar]
- 21.Bullock T H, Hamstra R H, Scheich H. J Comp Physiol. 1972;77:1–48. [Google Scholar]
- 22.Zupanc G K H, Maler L. Can J Zool. 1993;71:2301–2310. [Google Scholar]
- 23.Hagedorn M, Heiligenberg W. Anim Behav. 1985;33:254–265. [Google Scholar]
- 24.Heiligenberg W, Metzner W, Wong C J, Keller C H. J Comp Physiol A. 1996;179:653–674. doi: 10.1007/BF00216130. [DOI] [PubMed] [Google Scholar]
- 25.Hagiwara S. Jpn J Physiol. 1954;4:234–240. doi: 10.2170/jjphysiol.4.234. [DOI] [PubMed] [Google Scholar]
- 26.Bullock T H, Diecke F P J. J Physiol. 1956;134:47–87. doi: 10.1113/jphysiol.1956.sp005624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas M, Florion A, Chrétien D, Terver D. Water Res. 1996;30:3083–3091. [Google Scholar]
- 28.Moortgat K T, Keller C H. Soc Neurosci Abstr. 1995;21:79.21. [Google Scholar]
- 29.Hopkins C D. Am Sci. 1974;62:426–437. [PubMed] [Google Scholar]
- 30.Kramer B. Electroreception and Communication in Fishes. Jena, Germany: Fischer; 1996. [Google Scholar]
- 31.Eaton R C. Neural Mechanisms of Startle Behavior. New York: Plenum; 1982. [Google Scholar]
- 32.Falconi A, Borde M, Henández-Cruz A, Morales F R. J Comp Physiol A. 1995;176:679–689. [Google Scholar]
- 33.Canfield J G, Rose G J. J Comp Physiol A. 1993;172:611–618. [Google Scholar]
- 34.Carr C E. Annu Rev Neurosci. 1993;16:223–243. doi: 10.1146/annurev.ne.16.030193.001255. [DOI] [PubMed] [Google Scholar]
- 35.Moller P. Electric Fishes: History and Behavior. New York: Chapman & Hall; 1995. [Google Scholar]
- 36.Kawasaki M, Heiligenberg W. J Comp Physiol A. 1989;165:731–741. doi: 10.1007/BF00610872. [DOI] [PubMed] [Google Scholar]
- 37.Keller C, Kawasaki M, Heiligenberg W. J Comp Physiol A. 1991;169:441–450. doi: 10.1007/BF00197656. [DOI] [PubMed] [Google Scholar]
- 38.Schaeffer J E, Zakon H H. J Neurosci. 1996;16:2860–2868. doi: 10.1523/JNEUROSCI.16-08-02860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]