Abstract
β subunits of voltage-gated Ca2+ channels are encoded in four genes and display additional molecular diversity because of alternative splicing. At the functional level, all forms are very similar except for β2a, which differs in that it does not support prepulse facilitation of α1C Ca2+ channels, inhibits voltage-induced inactivation of neuronal α1E Ca2+ channels, and is more effective in blocking inhibition of α1E channels by G protein-coupled receptors. We show that the distinguishing properties of β2a, rather than interaction with a distinct site of α1, are because of the recently described palmitoylation of cysteines in positions three and four, which also occurs in the Xenopus oocyte. Essentially, all of the distinguishing features of β2a were lost in a mutant that could not be palmitoylated [β2a(Cys3,4Ser)]. Because protein palmitoylation is a dynamic process, these findings point to the possibility that regulation of palmitoylation may contribute to activity-dependent neuronal and synaptic plasticity. Evidence is presented that there may exist as many as three β2 splice variants differing only in their N-termini.
Keywords: voltage-gated calcium channels, signal transduction, calcium, Xenopus oocytes, posttranslational modification, protein lipidation, splice variants
Voltage-gated Ca2+ channels are multiprotein complexes made up of at least three distinct types of subunits: an α1, which senses voltage changes and spans the membrane multiple times to form the pore and the β and α2δ subunits, which modulate almost all aspects of α1 function (1). In addition, β and α2δ play structural roles that are important, but not well understood, in channel maturation and accumulation at the cell surface. Voltage-gated Ca2+ channels are molecularly diverse. Six α1 and four β genes are known, and many genes exhibit additional heterogeneity in their translated proteins because of alternative splicing. In contrast, only one gene encoding the α2δ complex has been found so far, but it also yields transcripts that are spliced alternatively to give slightly differing proteins (2, 3)
Although in most cases it has been difficult to ascribe a functional correlate to specific Ca2+ channel splice variants, there is one striking exception: the a-type splice variant of the β2 subunit from rat brain (β2a) acts differently on inactivation of α1E, on prepulse-induced long lasting facilitation of α1C, and also, to some extent, on G protein-mediated inhibition of neuronal Ca2+ channels. In α1E, brain β2a reduces the rate at which α1E inactivates in response to depolarization and causes a right shift in the steady–state inactivation curve. All other β subunits, including the b-type splice variant of β2, accelerate channel inactivation and cause steady–state inactivation curves to be left-shifted along the voltage axis (4, 5). In contrast, β2a is indistinguishable from its homologs in terms of α1E activation (5).
Prepulse facilitation is a phenomenon in which a train of depolarizations, or a long and strong depolarizing pulse, induces a form of the Ca2+ channel that exhibits an increased opening probability in response to a given test potential that persists for several seconds after repolarization (6). There are at least two distinct forms of prepulse-induced facilitation of Ca2+ currents. Both are affected by Ca2+ channel β subunits but in opposite ways. One type, observed in several neuronal cells (7–9), skeletal muscle (10), and mammalian and amphibian cardiac cells (11–15) is displayed by L type Ca2+ channels and has been recapitulated in Xenopus oocytes injected with cDNAs encoding α1C and a β subunit (16). Another type of prepulse facilitation, also referred to as prepulse potentiation, is seen primarily with non-L type Ca2+ channels of neurons and is caused by a reversal or attenuation of the inhibition of channel activity imposed by agonists known to act via Gi/Go-coupled receptors and formation of free Gβγ dimer (17–21).
Long lasting prepulse facilitation of α1C channels does not develop in oocytes injected with α1C alone (16) or with α1C plus β2a (22). In contrast, prepulse relief of agonist-induced inhibition of non-L type Ca2+ channels does not require a β subunit and is attenuated severely by coexpression of β subunits (6, 23–26). Of several β subunits tested (β1b, β2a, and β3), β2a is the most effective suppressor of G protein-mediated Ca2+ channel inhibition (25).
Based on amino acid sequence alignments, Ca2+ channel β subunits have been divided arbitrarily into five sequence similarity domains: D1-D5. The N-terminal D1 and the C-terminal D5 domains are quite diverse and thus share low sequence similarity. The D2 and D4 domains, of ≈150 and 200 aa, respectively, are very similar, being 60–80% identical in amino acid sequence (3). There are two types of D3 domains: one type, found in β1b, β2c, β3, and β4, is only seven aa long and invariant except for its last amino acid. The other type, found in β1 and β2, is either 51 (β1a) or 44 (β2a and β2b) aa long and 60% identical to each other. N-terminal D1 domains are either long (40–58 aa in β1, β2b, and β4) or short (15–18 aa in β2a and β3) (see Fig. 1).
Figure 1.
Amino acid alignment of the N-termini of calcium channel β subunits. –, gap.
In a previous study, we found that one molecular determinant responsible for the effect of β2a to reduce the rate of α1E inactivation resides in its short, 16 aa N-terminal D1 domain: a β1b with the β2a D1 sequence reduced the rate of α1E inactivation and a β2 with any other N-terminal D1 domain (e.g., that of β1b, β3, or the naturally occurring N terminus of β2b) accelerated the rate of α1E inactivation (5, 27). Subsequent studies with β subunits from which the N-terminal D1 domains had been deleted (ΔNβ subunits) showed that although the N-termini are dominant in dictating the effect of β subunits on α1E inactivation, in their absence, the D3 domain also influences the rate at which β subunits promote α1E (27).
Recently, Hosey and coworkers (20, 28) reported that the rat brain β2a is structurally unique in that its two vicinal cysteines in positions three and four of the D1 domain are palmitoylated. Similar cysteines are not found in any other β subunit N terminus, including that of the β2b, or in either of two other forms also referred to as β2a, the rabbit cardiac β2a, or the mouse heart β2a (Fig. 1). Hosey and coworkers (28) hypothesized that this lipidation at the N terminus of rat brain β2a could play an important regulatory role and contribute to the functional complexity of voltage-gated Ca2+ channels.
Palmitoylation of proteins was first reported in 1979 by Schmidt and coworkers (29, 30) studying the biosynthesis and structure of viral glycoproteins. The derivatized amino acid is cysteine, which is attached to the palmitic acid via a hydroxylamine-sensitive thioester bond (31). This type of posttranslational modification is widespread and occurs on a large variety of membrane proteins that are functionally quite diverse and unrelated, from viral proteins to normal cellular proteins such as some signaling proteins (32). Palmitoylation has been associated with membrane targeting of nontransmembrane proteins to specific areas such as caveolae for endothelial nitric oxide synthase (33) and plasma membrane for src-related tyrosine kinases (34).
Below, we report that palmitoylation of rat β2a confers to it the ability to promote prepulse facilitation of α1C, and to cause a left shift in the voltage-inactivation relationship of α1E. Palmitoylation also has an effect on the ability of β2a to attenuate Gβγ-mediated inhibition of α1E.
METHODS
α1 and β Subunit Constructs and Synthesis of cRNAs.
α1 cDNAs were wild type human α1E (GenBank accession no. L27745; ref. 35); rabbit α1C[DN60], (α1C[60–2171] GenBank accession no. X15539; ref. 36), rat β1b (GenBank accession no. X613940; ref. 37); rat β2a (GenBank accession no. M80545; ref. 38), rabbit β2b (GenBank accession no. X64298; ref. 39), rat β3 (GenBank accession no. M88751; ref. 40), and rat β4 (GenBank accession no. L02315; ref. 41). Mutants [Cys3,4Ser]β2a and chimeras were made by standard recombinant DNA techniques using wild-type cDNAs as donor DNAs. All cDNAs were subcloned into the NcoI site of the transcription competent pAGA2 plasmid (42). cRNAs were synthesized using mMessage mMachine™ reagents and protocols purchased in kit form from Ambion (Austin, TX). The resulting cRNAs were resuspended in diethylpyrocarbonate-treated H2O.
Reverse Transcription-PCR.
Rabbit brain and cardiac total RNA were kindly provided by Dr. S. Ding, which were prepared by the guanidine-based method. Mouse brain and cardiac polyA-enriched RNA were prepared using the Mini RiboSep™ Ultra mRMA Isolation kit (Collaborative Biomedical Products, Bedford, MA). Reverse transcripts were synthesized in a final volume of 20 μl containing 1 μg of polyA RNA or 5 μg of total RNA with hexamer primers and SUPERSCRIPT™ RNaseH− reverse transcriptase (GIBCO/BRL). The RNAs were first denatured 3 min at 68°C and then cooled 5 min on ice. Incubation was for 60 min at 42°C and the reaction was terminated by heating for 10 min at 70°C. Analysis of reverse transcripts by PCR was carried out using 2 μl of the reverse transcript-containing solution in a final volume of 50 μl containing 50 μM dNTP, 2 mM MgCl2, 1 unit of Taq DNA polymerase, and 200 nM each of primers a and b, c and d, or e and f. Primers a and b are predicted to amplify a β2 cDNA fragment of 650 bp encoding to a region that is common to all β2 subunits, and primers c and d are predicted to amplify a β2a cDNA fragment of 468 bp encoding a portion of the N terminus of the type reported for the rabbit heart β2a (39). Primers e and f are predicted to amplify a cDNA fragment of 318 bp encoding a portion of the N terminus of the type reported for the rat brain β2a (38). Primer a: 5′-AAT GAT ATT CCA GCA AAC CAC, encoding amino acids NDIPANH of β2 sequence domain D3. Primer b: (AG)TG (TC)TC (AG)CA NGC (AG)TC (TC)TC corresponds to the antisense sequence encoding EDACEH of the β2 D4 domain. Primer c: ATG CTT GA(CT) (AC)GN CTN GC encodes amino acids MLDRHL of the rabbit heart β2a N terminus. Primer d: 5′-TAT GTC ACC CAA ACT GGA corresponds to the antisense sequence encoding SSLGDI of the β2 D2 domain. Primer e: ATG CAG TGC TGC GGG CT encodes amino acids MQCCGL of the rat brain β2a N terminus. Primer f: CC (TAG)AT CCA CCA (GA)TC (GA)TC corresponds to the antisense sequence encoding NDWWIG of the β2 D2 domain.
Xenopus Oocytes, Expression of Calcium Channels, and Electrophysiological Recordings.
Stage V and VI Xenopus laevis oocytes, isolated as described in Tareilus et al. (43) were injected with 50 nl containing 100 μg/ml each of two cRNAs: one encoding one of the α1 subunits and the other encoding the human M2 muscarinic acetylcholine receptor (M2R) (44), also transcribed from pAGA2. The cut-open vaseline gap voltage clamp method of Taglialatela et al. (45, 46) was used throughout. The external solution had the following composition: 10 mM Ba2+, 96 mM Na+, and 10 mM Hepes titrated to pH 7.0 with methanesulfonic acid (CH3SO3H). The solution in contact with the oocyte interior was 110 mM K-glutamate and 10 mM Hepes titrated to pH 7.0 with KOH. Low access resistance to the oocyte interior was obtained by permeabilizing the oocyte with 0.1% saponin. Currents were recorded 3–5 days after cRNA injection.
Palmitoylation and Immunoprecipitation.
Xenopus oocytes were microinjected with α1C, α2δ, and different β subunits (as indicated in Fig. 1) and divided into groups of 20 oocytes that were incubated at room temperature for 24 hr. Fifty μCi/ml of a mixture of [35S]methionine and cysteine (DuPont/NEN, 1,000 Ci/mmol) were added to one group at time zero. The other group was incubated without special additives for 18 hr, at which time it received 2 mCi/ml [3H]palmitic acid (DuPont/NEN, 60 Ci/mmol). Incubations were in 1.0 ml of standard oocyle solution (100 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, and 50 mM Hepes (pH 7.0).
After a total of 24 hr of incubation, the oocytes were washed once with PBS (1.2 mM KH2PO4, 8.1 mM Na2HPO4, 2.7 mM KCl, and 138 mM NaCl), resuspended in 1 ml of RIPA buffer (150 mM NaCl, 50 mM Tris (pH 8.0), 5 mM EDTA, 0.1% Nonidet P-40, 0.25% deoxycholic acid, 0.1% SDS, 100 μg/ml phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 2 μg/ml leupeptins, and 2 μg/ml pepstatin A), and homogenized and sonicated in a bath sonicator (Sonicator XL, Misonix) for 2 min. The homogenates were centrifuged twice at 12,000 × g for 10 min, and the resulting supernatant was transferred to a fresh tube. β subunits were immunoprecipitated by adding 8 μg of either β2 (for β2a and β2a[Cys3,4Ser]) or β3 (for β3 and β2a/3) polyclonal antibodies—raised against β2 and β3 D5 sequences fused to GST (N.Q., unpublished work)—and 25 μl of protein-A Sepharose (Pharmacia) to each sample. After incubation at 4°C overnight, the Sepharose beads with the attached immunecomplexes were washed three times with RIPA buffer, and the immunecomplexes were eluted by adding 30 μl Laemmli’s sample buffer with 50 mM β-mercaptoethanol and then analyzed by 12% SDS/PAGE. Finally, the radiolabeled proteins were visualized by autoradiography (35S, overnight) or fluorography (3H, 12 days) using x-ray film (Kodak, XAR-5) after treating the gels with Amplify (Amersham) for 6 hr.
RESULTS
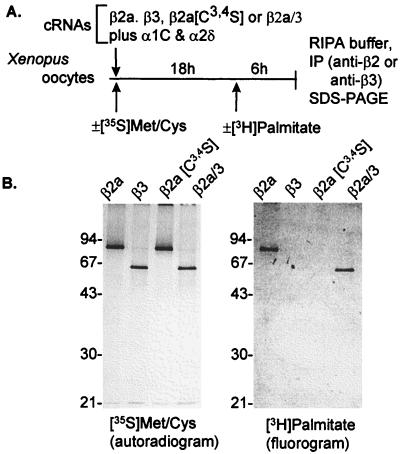
cDNAs encoding β2a subunits were cloned independently from rabbit heart (39), rat brain (38), and mouse heart (GenBank accession no. L20343). Comparison of the published sequences shows that rat brain and rabbit and mouse heart β2a differ in their N-termini. Most of the experiments in the literature have been carried out with the rat brain β2a. Fig. 1 shows the amino acid sequences of the N-termini (D1 domains) of Ca2+ channel β subunits as deduced from the cloned cDNAs. The two vicinal cysteines (Cys3 and Cys4) in the N terminus of the rat brain β2a were shown to be palmitoylated in tsA201 cells by Chien et al. (28). However, it is still unclear whether this β2a is palmitoylated in Xenopus oocytes, although it has been demonstrated that microinjected Ha-Ras can be palmitoylated by Xenopus oocytes (47). Therefore, we injected β2a and β3 into a batch of oocytes, labeled the batch in vivo with either [35S]Met/Cys or [3H]pamitic acid, and immunoprecipitated the β subunits with subunit-specific polyclonal antibodies. As shown in Fig. 2, both β2a and β3 were labeled equally well by [35S]Met/Cys, but only β2a was labeled with [3H]palmitic acid. This result confirmed that as in mammalian cells, the β2a subunit also is palmitoylated in Xenopus oocytes.
Figure 2.
Synthesis and palmitoylation of Ca2+ channel β subunits in Xenopus oocytes. (A) Outline of experiment. (B, Left) synthesis of wild type and mutant β subunit proteins. (B, Right) incorporation of palmitate into β2a and β2a/3 but not into β3 or β2a[C3,4S]. For further details see diagram of protocol A and Methods.
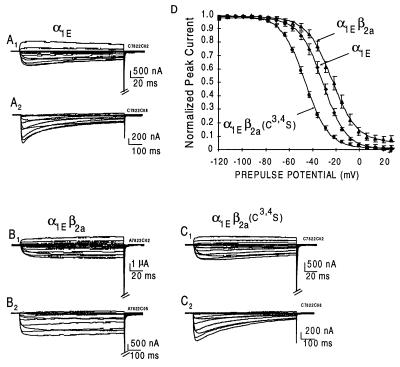
To test whether palmitoylation was a significant factor contributing to properties that separate β2a from other β subunits, we introduced a double mutation into the β2a cDNA changing codons 3 and 4 from TGC and TGC, which encode Cys, to TCC and TCC encoding Ser. In agreement with Chien’s et al. (28) result, the mutant β2a[Cys3,4Ser] is not palmitoylated in Xenopus oocytes (Fig. 2). The mutant cRNA was then injected along with cRNAs encoding α1 subunits and the Gi/Go-coupled M2R to test for the types of effects it would have on inactivation of α1E by voltage G protein activation and on long-lasting prepulse facilitation of α1C. Fig. 3 illustrates the previously reported effect of β2a to retard as a function of time the voltage-induced inactivation of α1E and its effect to cause the voltage-inactivation relationship to be right-shifted (5). In the present set of experiments, the midpoints of steady–state inactivation were (mean ± SEM) −31.6 ± 2.0 (n = 5) and −26.1 ± 2.5 (n = 7) mV for α1E channels expressed in oocytes injected with α1E alone and α1E plus β2a, respectively. Introduction of Ser in positions three and four in place of Cys resulted in a β subunit, β2a[Cys3,4Ser], that shifted the midpoint of steady–state inactivation to −46.6 ± 1.0 mV (n = 9), i.e., to the left of the control voltage-inactivation relationship. An analysis of the time courses of inactivation revealed that although the mutation also caused a loss of its effect to reduce the rate at which α1E inactivates, it did not revert it to the accelerating effect that other β subunits have.
Figure 3.
Comparison of the effects of β2a and β2a[Cys3,4Ser] on inactivation kinetics and steady–state inactivation of Ca2+ channels in Xenopus oocytes injected with α1E cRNA. Steady–state inactivation was recorded after a 10 sec conditioning prepulse as described (5). (A–C) Time courses of activation and inactivation. Note the loss of the effect of β2a to delay inactivation upon preventing palmitoylation in the Cys3,4Ser mutant. (D) Steady–state inactivation as a function of the voltage of the conditioning prepulse. Note the change of the effect of β2a to cause an increase in the voltage required for 50% inactivation to causing a left shift when palmitoylation was prevented by mutating the corresponding cysteines to serines.
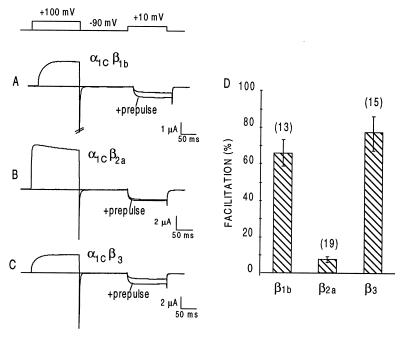
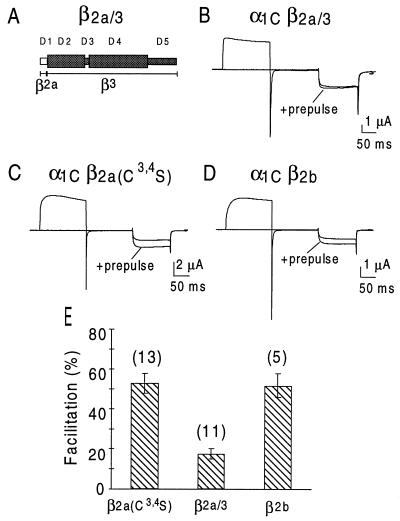
In agreement with previous reports by Bourinet et al. (16) and Cens et al. (22), we found that a 200-ms prepulse to 100 mV increased α1C currents in response to a test pulse by 64 ± 7% (n = 10) and 76 ± 10% (n = 14) (means ± SEM) in oocytes that had been coinjected with β1b and β3, respectively (Fig. 4). α1C facilitation in oocytes coexpressing β2a was only 8 ± 2% (n = 19) (Fig. 4). As shown in Fig. 5, mutating Cys3 and Cys4 in β2a to Ser conferred to β2a the ability to support prepulse facilitation to a similar extent as other β subunits (53 ± 5%, n = 13), including β2b (52 ± 6%, n = 5). Fig. 5 also shows that β2a/3, a β3 subunit with a β2a N terminus (Fig. 5A) has a significantly reduced ability to support prepulse facilitation (17 ± 3%, n = 11, P < 001 when compared with facilitation obtained with β3). As shown in Fig. 2B, the chimera β2a/3 also was subjected to palmitoylation in Xenopus oocytes, further supporting the hypothesis that palmitoylation imparts an unique regulatory properties to a Ca2+ channel β subunit.
Figure 4.
Failure of β2a to support prepulse facilitation of α1C Ca2+ channels expressed in Xenopus oocytes. Top, voltage protocol used to elicit the facilitated state. (A–C) Representative records of prepulse facilitation obtained in oocytes injected with α1C and β1b, β2a, or β3, respectively. (D) Means ± SEM of % facilitation obtained in the indicated number of oocytes. In these and all other experiments reported here, the α1C used is the DN60 variant that lacks amino acids 1–59 (α1C[60–2171]; see ref. 36).
Figure 5.
Development of prepulse facilitation in α1C Ca2+ channels coexpressed with β2b (D) and β2a[Cys3,4Ser] (C) but not with the β2a/3 chimera (B). For details, see Fig. 4 legend and Methods.
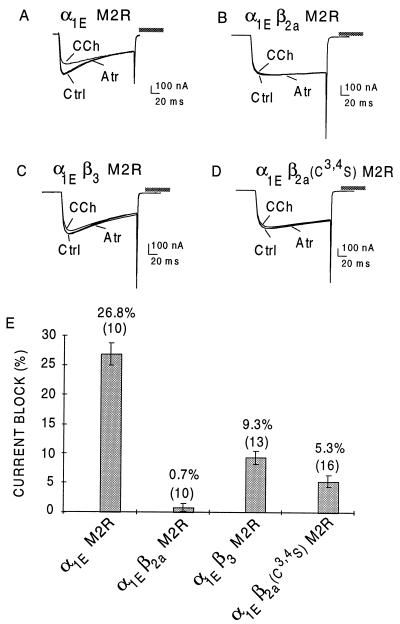
In a recent study, we characterized the ability of β2a, β1b, and β3 to attenuate inhibition of α1E channels by G protein activation and reported that β2a essentially abolished the inhibitory effect of the G protein-coupled pathway, whereas β1b and β3 were less effective preventing the G protein effect by ≈50% (25). Fig. 6 shows representative inhibitions of α1E currents by the M2R agonist carbachol (CCh) in oocytes injected with M2R, α1E, and the indicated β subunits, and the average inhibitions of peak currents that were obtained. Thus, inhibition in oocytes injected with α1E and M2R alone averaged 26.8 ± 1.8%, n = 10 (mean ± SEM). It was reduced to 9.3 ± 1.2% (n = 13) by β3 and to only 0.7 ± 0.6%, (n = 10) by β2a. Upon elimination of palmitoylation by mutating Cys-3 and Cys-4 to Ser, the inhibitory effect was somewhat reduced but still the most marked of the β subunits: CCh inhibition in oocytes injected with M2R, α1E, and β2a[Cys3,4Ser] = 5.3 ± 1.0, n = 16, P < 0.005 when compared with β2a.
Figure 6.
Variable effectiveness of β1b, β2a, and β3 subunits to suppress CCh-induced inhibition of α1E Ca2+ channels. Xenopus oocytes were injected with combinations of cRNAs encoding α1E, the M2R, β1b, β2a, β3, or β2[Cys3,4Ser] as indicated. (A-D) Representative records of Ca2+ channel currents elicited by a depolarizing pulse to 10 mV in individual oocytes before any addition to the bath control (Ctrl), after addition of 50 μM CCh, and after further addition of 50 μM atropine (Atr). (E) Means ± SEM of the CCh-induced reduction of peak inward current calculated as (1-ICCh/Ictrl)x100, in which ICCh is the inward current obtained in the presence of CCh at the time of Ipeak of the current obtained prior to CCh addition, and IAtr is the isochronal inward current obtained in the presence of 50 μM CCh plus 50 μM atropine. These results were obtained from three different batches of oocytes. n = number of oocytes in which the CCh block was assayed for.
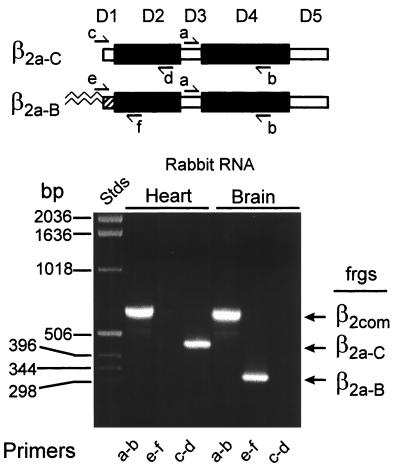
The existence in the database of distinct, short β2 N-termini, cloned respectively from rat brain, human brain, rabbit heart, and mouse heart, led us to investigate to what extent these were because of alternative splicing of independent exons or simply because of species variation. As we previously had cloned the palmitoylated N terminus from rat brain, we investigated whether the same β2a subtype also was present in rabbit brain. If so, this result would indicate the existence in one species of two short β2 N-termini, plus a long one. This was done by PCR analysis of reverse transcripts prepared using rabbit RNA as template (reverse transcription-PCR for predicted amino acid compositions; see Fig. 1). Fig. 7 shows that the rabbit brain does express the palmitoylated version of the β2 subunit. In addition, we confirmed the existence in rabbit heart of the short, nonpalmitoylated cardiac β2 reported by Hullin et al. (39). We conclude that the intron/exon structure of the mammalian β2 gene must include three alternatively spliced exons that each encodes a separate N terminus. We shall refer to the β2 with the short, palmitoylated N terminus as “brain” β2a, to the β2 with the long N terminus as β2b “(cardiac)”, and to the β2 with the short nonpalmitoylated N terminus as “cardiac” β2a.** Brown et al. (11) reported prepulse facilitation in rabbit cardiac cells. Our observation that the palmitoylated form of β2 (brain β2a) is not expressed in cardiac cells (Fig. 7) is consistent with this finding. However, definitive proof that the absence of palmitoylated β2a is permissive for development of prepulse facilitation, presumably supported by any other β subunit, will require the actual determination that the rabbit cardiac β2a is indeed competent to do so and does not interfere with the effects of other β subunits, including that of rabbit cardiac β2b (Fig. 5). The failure of Cens et al. (22) to obtain prepulse facilitation in rat ventricle cells could be either because in this species these cells express its palmitoylated brain β2a instead of the cardiac β2a, or for some other unknown reason. Prepulse facilitation is a complex phenomenon that shows a high degree of species variation (15).
Figure 7.
PCR analysis of rabbit brain and heart RNA for presence of β2a (β2a0B) and β2d (B2a-C) sequences. Top, diagram of β subunit sequence homology domains and relative location of forward (a, c, and e) and reverse (b, d, and f) primers. a and b primers test for presence of β2 sequences regardless of composition of the variable D1 or D5 domians; primers c and d and primers e and f test for presence of sequences encoding the cardiac β2a (β2a-C) or brain β2a (β2a-B) N-termini.
DISCUSSION
Molecular cloning (49) and expression (50) of the skeletal muscle Ca2+ channel α1 subunit showed that this subunit forms the channel proper, including all the elements required to form the ion permeating pore, the selectivity filter, the controlling voltage sensor, and the binding sites for therapeutically active Ca2+ channel blockers. Molecular cloning of skeletal muscle α1 homologs has led to the identification of six nonallelic α1 subunits genes, encoding the S, A, B, C, D, and E α1 proteins. These are responsible for many of the differing qualities of Ca2+ channel currents found in skeletal, smooth, and cardiac muscles and in endocrine cells and, notably, in neurons (1–3, 51, 52). Depending on their location within the central and peripheral nervous system, neurons express all types of α1 subunits except that of the skeletal muscle, α1S (52). Expression of α1S in mouse L cells showed that channels formed by an α1 subunit alone behave abnormally, especially in terms of the rate at which a response to a voltage change develops (50). In several instances, α1 subunits could not be expressed unless they were coexpressed with accessory subunits, i.e., β and α2δ subunits (53). These are proteins that had been identified in biochemical studies as members of the purified skeletal muscle multiprotein complex (1) and later were found also in Ca2+ channels purified from heart and brain. Molecular cloning also revealed that there are four nonallelic β subunit genes and one gene encoding the α2δ complex (41, 54).
Although α2δ modulates α1 currents and expression levels, by far the most striking effects on α1 function are those imparted by β subunits. In their absence, the levels of α1 as a mature and active channel protein on the cell surface are small or nonexistent (20, 43, 50, 55, 56), indicating a role in channel maturation and targeting. Moreover, in the absence of a β subunit, the kinetics of the ionic currents obtained differ from what would be expected from natural cell studies, but can be “normalized” by coexpressing a β, indicating a integral role for this subunit in the moment to moment functioning of the channel. The recorded effects of β subunits include acceleration of the rates of activation and deactivation of Ca2+ channels (5, 57), an improvement in the coupling of voltage sensing to pore opening (5, 46, 48), modulation of voltage-induced inactivation (4, 5) of non-L type α1 subunits, e.g., α1A or α1E, and attenuation of the inhibitory regulation by G protein-coupled receptors (25).
Previously, we reported that regulation of voltage-dependent activation and inactivation of α1 by β are separable events (5), and we also demonstrated that β2a had specific regulatory effects on α1E channel inactivation and G protein inhibition (5, 25). This led us to propose a complex model of α1 regulation by β to account for the unique effects of β2a. The finding that the brain β2a has a unique structural feature, palmitoylation (20), would remove the need for additional α1 binding sites if palmitoylation affected the regulatory actions of this β subunit and, furthermore, if a transfer of the posttranslational modification to another β subunit would make it behave β2a-like. As reported above, we found that of the three β2a-specific functions, two are indeed affected by removal of the palmitoylation sites at its N-terminal cysteines. Notably, palmitoylation affected the ability of the β2a subunit to support or promote prepulse facilitation of α1C because it was absent in α1Cβ2a channels but fully developed in α1Cβ2a[Cys3,4Ser] channels. Transferring the palmitoylated β2a N-terminal to β3 markedly reduced its ability to support prepulse facilitation of α1C.
Removing palmitoylation from β2a had a complex effect on its ability to modulate α1E inactivation. On the one hand, the left shift in the voltage-steady–state inactivation relationship caused by the nonpalmitoylated β2a (−20 mV) was now indistinguishable from that obtained with other β subunits (5, 27), a finding that is consistent with a role of palmitoylation in the way β2a affects α1E inactivation. On the other hand, the time course of inactivation of α1Eβ2a[Cys3,4Ser] channels was very similar to that obtained in oocytes injected with α1E alone, indicating that the mutant lost the ability to reduce the rate of inactivation but did not reverse the effect to an accelerating one, as seen with other β subunits. Additional studies are needed to resolve whether this is because of existence of independent molecular determinants affecting rates and steady–state inactivation or whether lack of palmitoylation resulted in a partial neutralization of the action of the N terminus, causing the effect of the D3 domain to become partly unmasked. We reported (27) that in the absence of an N terminus, the resulting ΔNβ2a is still β2a-like and that this likeness is caused by the D3 domain. Removal of palmitoylation from β2a had only a minor, yet statistically significant effect on the ability of β2a to interfere with the inhibition by a G protein-coupled receptor. Additional studies will be needed to determine whether this small difference relates to the other two changes that occur upon depalmitoylation.
Our finding that the mutant β2a (β2a[C3,4S]) is able to impart long term prepulse facilitation on α1C, whereas the wild-type β2a does not, is of interest. However, the mechanism underlying the failure of the palmitoylated brain β2a to support prepulse facilitation remains a subject for future studies. The same, of course, applies also to the mechanism by which palmitoylated β2a slows voltage-induced inactivation instead of accelerating it as other β subunits do.
Palmitoylation of proteins is not only widespread but also a dynamic process as shown for G protein α subunits (58, 59), for certain nonreceptor tyrosine kinases (60), and for GAP43/neuromodulin (61). In the case of G protein α subunits, the rate of incorporation of radiolabeled palmitate is increased by prolonged stimulation by a receptor (59). In the case of GAP43/neuromodilin (61), inhibition of palmitoylation by tunicamycin in differentiated PC12 or acutely dissociated sensory neurons resulted in an immediate and reversible collapse of the growth cones of extending neurites. It is likely that palmitoylation of calcium channel β2a is also a dynamically active process. Our results would therefore point to the existence of an unrecognized regulation of those neuronal Ca2+ channels that have β2a as their regulatory subunits. Thus, it is conceivable the state of palmitoylation of β2a be activity-dependent and as such a contributor to neuronal and synaptic plasticity.
Acknowledgments
We thank Dr. S. Ding (Department of Pediatrics, University of California Los Angeles, School of Medicine) for providing rabbit brain and heart total RNA. This work was supported by National Institutes of Health Grants AR43411 (to L.B.) and AR38970 (to E.S.) and by the American Heart Association (National) Scientist Development grant (to N.Q.) and the American Heart Association (Greater Los Angeles) Grant-in-Aid (to R.O.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: M2R, M2 muscarinic receptor; CCh, carbachol.
In this nomenclature, cardiac β2c is a splice variant of unknown N terminus that has the short D3 domain of the β1c subunit (39).
References
- 1.Catterall W A. Science. 1991;253:1499–1500. doi: 10.1126/science.1654596. [DOI] [PubMed] [Google Scholar]
- 2.Tsien R W, Ellinor P T, Horne W A. Trends Pharmacol Sci. 1991;12:349–354. doi: 10.1016/0165-6147(91)90595-j. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Reyes E, Schneider T. Kidney Int. 1995;48:1111–1124. doi: 10.1038/ki.1995.395. [DOI] [PubMed] [Google Scholar]
- 4.Ellinor P T, Zhang J F, Randall A D, Zhou M, Schwarz T L, Tsien R W, Horne W A. Nature (London) 1993;363:455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- 5.Olcese R, Qin N, Schneider T, Neely A, Wei X, Stefani E, Birnbaumer L. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 6.Dolphin A C. Trends Neurosci. 1996;19:35–43. doi: 10.1016/0166-2236(96)81865-0. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda S R. J Physiol (London) 1991;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artalejo C R, Mogul D J, Perlman R L, Fox A P. J Physiol (London) 1991;444:213–240. doi: 10.1113/jphysiol.1991.sp018874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavalali E T, Plummer M R. J Neurosci. 1996;16:1072–1082. doi: 10.1523/JNEUROSCI.16-03-01072.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson B D, Brousal J P, Peterson B Z, Gallombardo P A, Hockerman G H, Lai Y, Scheuer T, Catterall W A. J Neurosci. 1997;17:1243–1255. doi: 10.1523/JNEUROSCI.17-04-01243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown H F, Kimura J, Noble D, Noble S J, Taupignon A. Proc R Soc Lond Ser B. 1984;222:329–347. doi: 10.1098/rspb.1984.0067. [DOI] [PubMed] [Google Scholar]
- 12.Lee K S. Proc Natl Acad Sci USA. 1987;84:3941–3945. doi: 10.1073/pnas.84.11.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedida D, Noble D, Spindler A J. J Physiol (London) 1988;405:461–475. doi: 10.1113/jphysiol.1988.sp017342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zygmunt A C, Maylie J. J Physiol (London) 1990;428:653–671. doi: 10.1113/jphysiol.1990.sp018233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble S, Shimoni Y. J Physiol (London) 1981;310:77–95. doi: 10.1113/jphysiol.1981.sp013538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourinet E, Charnet P, Tomlinson W J, Stea A, Snutch T P, Nargeot J. EMBO J. 1994;13:5032–5039. doi: 10.1002/j.1460-2075.1994.tb06832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunlap K, Fischbach G D. J Physiol (London) 1981;371:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsunoo A, Yoshii M, Narahashi T. Proc Natl Acad Sci USA. 1986;83:9832–9836. doi: 10.1073/pnas.83.24.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bean B P. Nature (London) 1997;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 20.Chien A J, Zhao X, Shirokov R M, Puri T S, Chang C F, Sun D, Rios E, Hosey M M. J Biol Chem. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- 21.Herlitze S, Garcia D E, Mackie K, Hille B, Scheuer T, Catterall W A. Nature (London) 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 22.Cens T, Mangoni M E, Richard S, Nargeot J, Charnet P. Pflügers Arch. 1996;431:771–774. [PubMed] [Google Scholar]
- 23.Campbell V, Berrow N S, Fitzgerald E M, Brickley K, Dolphin A C. J Physiol (London) 1995;485:365–372. doi: 10.1113/jphysiol.1995.sp020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roche J P, Anantharam V, Treistman S N. FEBS Lett. 1995;371:43–46. doi: 10.1016/0014-5793(95)00860-c. [DOI] [PubMed] [Google Scholar]
- 25.Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Proc Natl Acad Sci USA. 1997;94:8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourinet E, Soong T W, Stea A, Snutch T P. Proc Natl Acad Sci USA. 1996;93:1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin N, Olcese R, Zhou J, Cabello O A, Birnbaumer L, Stefani Am J Physiol. 1996;271:C1539–C1545. doi: 10.1152/ajpcell.1996.271.5.C1539. [DOI] [PubMed] [Google Scholar]
- 28.Chien A J, Carr K M, Shirokov R E, Rios E, Hosey M M. J Biol Chem. 1996;271:26465–26468. doi: 10.1074/jbc.271.43.26465. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt M F, Schlesinger M J. Cell. 1979;17:813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt M F, Bracha M, Schlesinger M J. Proc Natl Acad Sci USA. 1979;76:18635–18639. [Google Scholar]
- 31.Schmidt M F, Rott R. J Biol Chem. 1988;263:18635–18639. [PubMed] [Google Scholar]
- 32.Mumby S M. Curr Opin Cell Biol. 1997;9:148–154. doi: 10.1016/s0955-0674(97)80056-7. [DOI] [PubMed] [Google Scholar]
- 33.Shaul P W, Smart E J, Robinson L J, German Z, Yuhanna I S, Ying Y, Anderson R G, Michel T. J Biol Chem. 1996;271:6518–5522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 34.Shenoy-Scaria A M, Dietzen D J, Kwong J, Link D C, Lublin D M. J Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider T, Wei X, Olcese R, Costantin J L, Neely A, Palade P, Perez-Reyes E, Qin N, Zhou J, Crawford G D. Recept Channels. 1994;2:255–270. [PubMed] [Google Scholar]
- 36.Wei X Y, Neely A, Olcese R, Stefani E, Birnbaumer L. Recept Channels. 1996;4:205–215. [PubMed] [Google Scholar]
- 37.Pragnell M, Sakamoto J, Jay S D, Campbell K P. FEBS Lett. 1991;291:253–258. doi: 10.1016/0014-5793(91)81296-k. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Reyes E, Castellano A, Kim H S, Bertrand P, Baggstrom E, Lacerda A E, Wei X, Birnbaumer L. J Biol Chem. 1992;267:1792–1797. [PubMed] [Google Scholar]
- 39.Hullin R, Singer-Lahat D, Freichel M, Biel M, Dascal N, Hofmann F, Flockerzi V. EMBO J. 1992;11:885–890. doi: 10.1002/j.1460-2075.1992.tb05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castellano A, Wei X Y, Birnbaumer L, Perez-Reyes E. J Biol Chem. 1993;268:3450–3455. [PubMed] [Google Scholar]
- 41.Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. J Biol Chem. 1993;268:12359–12366. [PubMed] [Google Scholar]
- 42.Sanford J, Codina J, Birnbaumer L. J Biol Chem. 1991;266:9570–9579. [PubMed] [Google Scholar]
- 43.Tareilus E, Roux M, Qin N, Olcese R, Zhou J, Stefani E, Birnbaumer L. Proc Natl Acad Sci USA. 1997;94:1703–1708. doi: 10.1073/pnas.94.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peralta E G, Ashkenazi A, Winslow J W, Smith D H, Ramachandran J, Capon D J. EMBO J. 1987;6:3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neely A, Olcese R, Wei X Y, Birnbaumer L, Stefani E. Biophys J. 1994;66:1895–1903. doi: 10.1016/S0006-3495(94)80983-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noceti F, Baldelli P, Wei X Y, Qin N, Toro L, Birnbaumer L, Stefani E. J Gen Physiol. 1996;108:143–155. doi: 10.1085/jgp.108.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudler T, Gelb M H. J Biol Chem. 1996;271:11541–11547. doi: 10.1074/jbc.271.19.11541. [DOI] [PubMed] [Google Scholar]
- 48.Neely A, Wei X, Olcese R, Birnbaumer L, Stefani E. Science. 1993;262:575–578. doi: 10.1126/science.8211185. [DOI] [PubMed] [Google Scholar]
- 49.Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S. Nature (London) 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- 50.Perez Reyes E, Kim H S, Lacerda A E, Horne W, Wei X Y, Rampe D, Campbell K P, Brown A M, Birnbaumer L. Nature (London) 1989;340:233–236. doi: 10.1038/340233a0. [DOI] [PubMed] [Google Scholar]
- 51.Snutch T P, Reiner P B. Curr Opin Neurobiol. 1992;2:247–253. doi: 10.1016/0959-4388(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 52.Birnbaumer L, Campbell K P, Catterall W A, Harpold M M, Hofmann F, Horne W A, Mori Y, Schwartz A, Snutch T P, Tanabe T, Tsien R W. Neuron. 1994;13:505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 53.Soong T W, Stea A, Hodson C D, Dubel S J, Vincent S R, Snutch T P. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- 54.Williams M E, Feldman D H, McCue A F, Brenner R, Velicelebi G, Ellis S B, Harpold M M. Neuron. 1992;8:71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- 55.Berrow N S, Campbell V, Fitzgerald E M, Brickley K, Dolphin A C. J Physiol (London) 1995;482:481–491. doi: 10.1113/jphysiol.1995.sp020534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gregg R G, Messing A, Strube C, Beurg M, Moss R, Behan M, Sukhareva M, Haynes S, Powell J A, Coronado R, Powers P A. Proc Natl Acad Sci USA. 1996;93:13961–13966. doi: 10.1073/pnas.93.24.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lacerda A E, Kim H S, Ruth P, Perez Reyes E, Flockerzi V, Hofmann F, Birnbaumer L, Brown A M. Nature (London) 1991;352:527–530. doi: 10.1038/352527a0. [DOI] [PubMed] [Google Scholar]
- 58.Linder M E, Middleton J P, Hepler J R, Tssig R, Gilman A G, Mumby S M. Proc Natl Acad Sci USA. 1993;90:3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Degtyarev M Y, Spiegel A M, Jones T L. J Biol Chem. 1993;268:23769–23772. [PubMed] [Google Scholar]
- 60.Paige L A, Nadler M J, Harrison M L, Cassady J M, Geahlen R L. J Biol Chem. 1993;268:8669–8674. [PubMed] [Google Scholar]
- 61.Patterson S I, Skene J H. J Cell Biol. 1994;124:521–526. doi: 10.1083/jcb.124.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]