Abstract
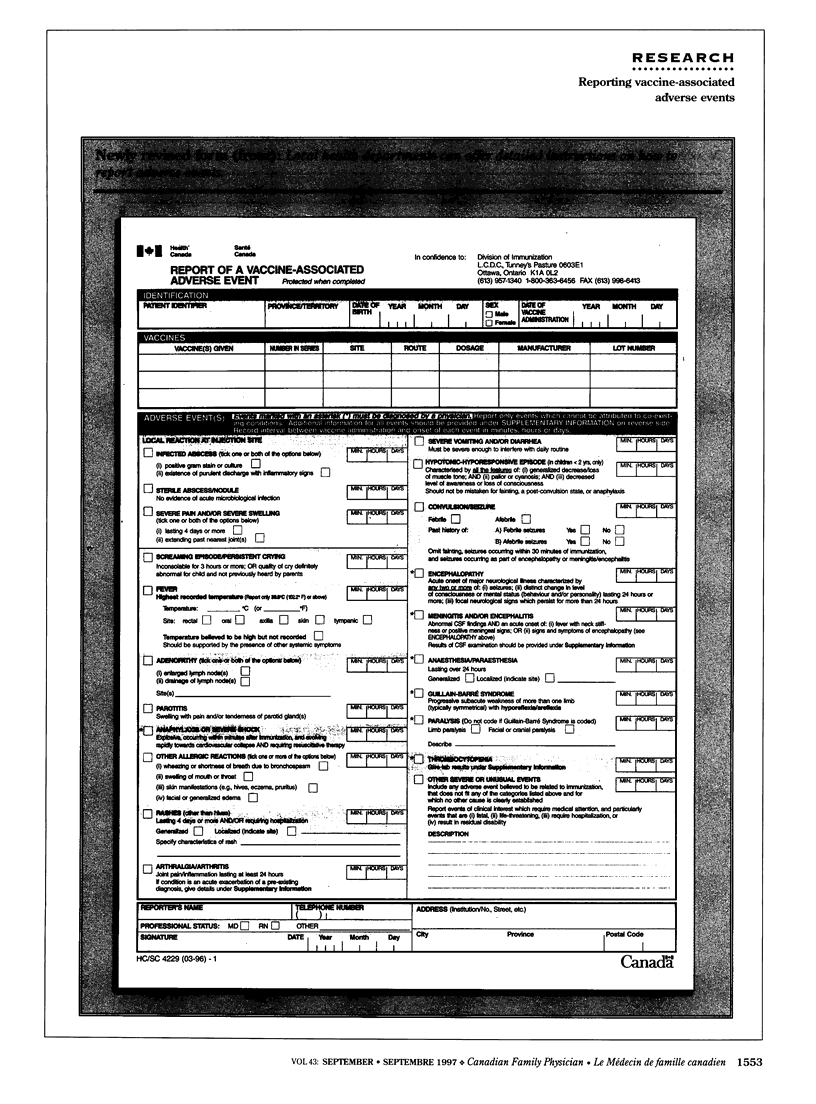
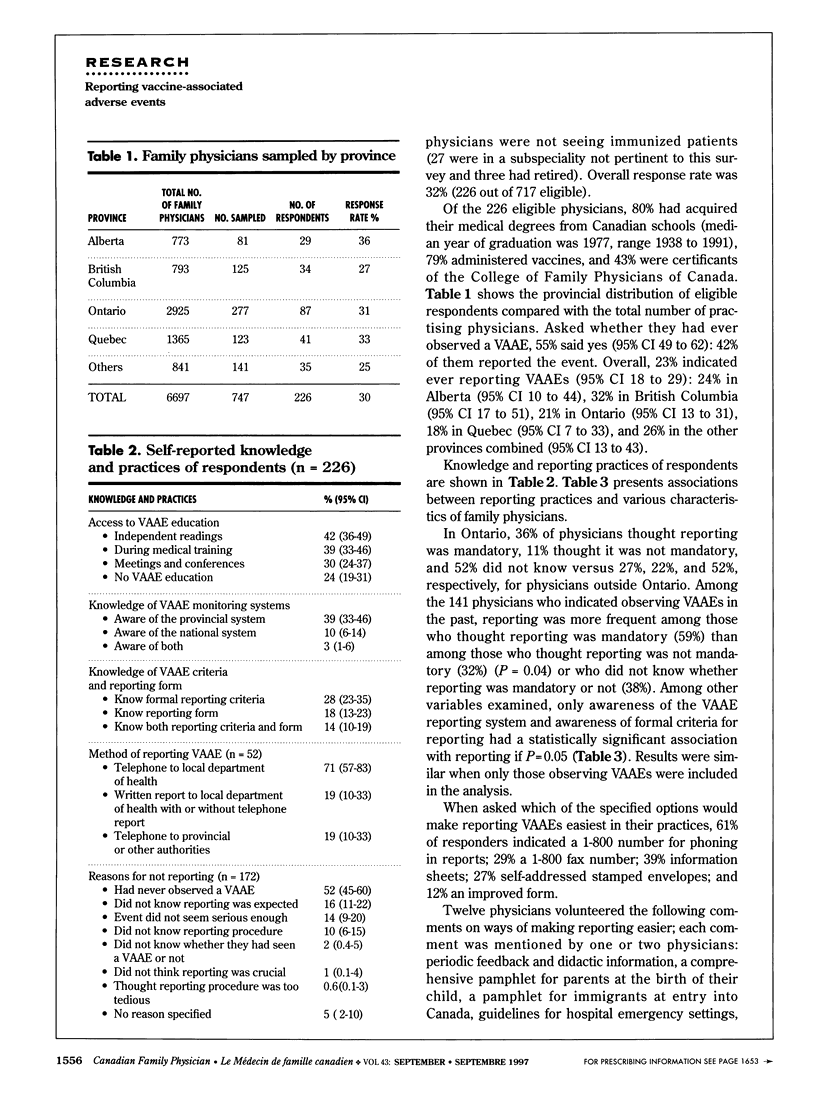
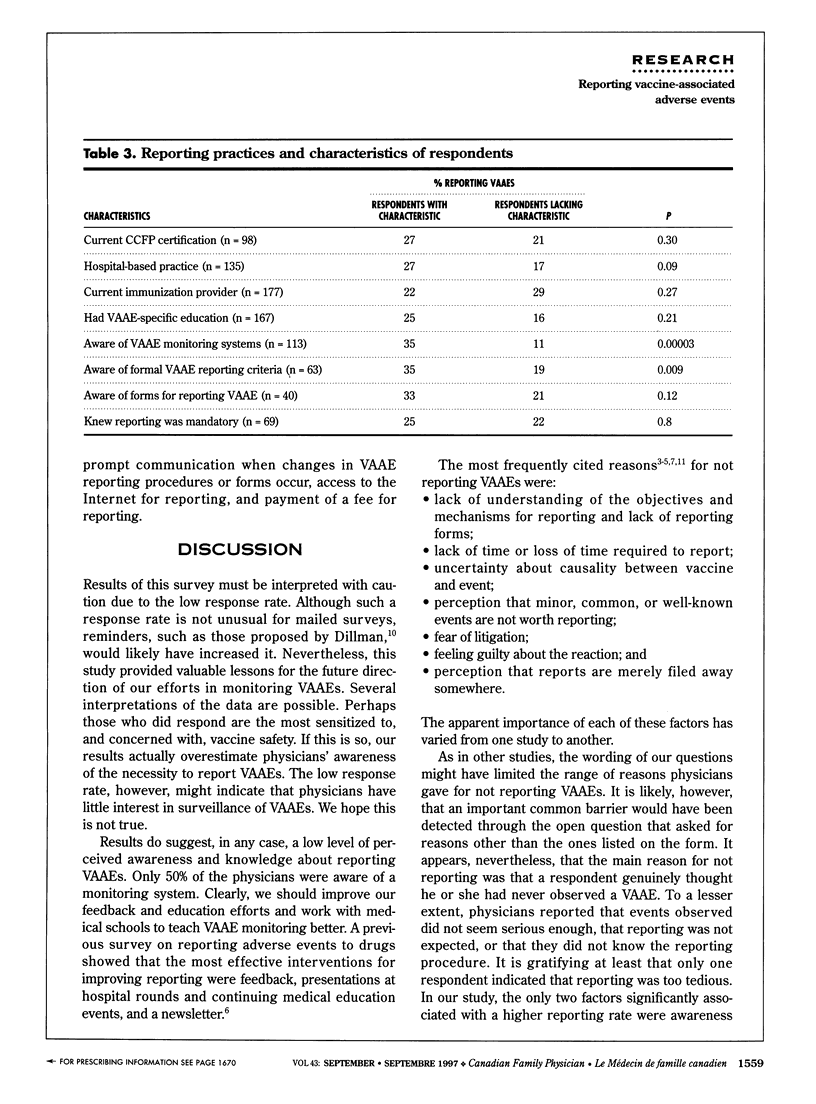
OBJECTIVE: To determine family physicians' awareness of the need to monitor and report vaccine-associated adverse events (VAAE) in Canada and to identify mechanisms that could facilitate reporting. DESIGN: Mailed survey. SETTING: Canadian family practices. PARTICIPANTS: Random sample of 747 family physicians. Overall response rate was 32% (226 of 717 eligible physicians). MAIN OUTCOME MEASURES: Access to education on VAAE; knowledge about VAAE monitoring systems, reporting criteria, and reporting forms; method of reporting VAAEs and reasons for not reporting them; and current experience with VAAEs. RESULTS: Of 226 respondents, 55% reported observing VAAEs, and 42% reported the event. Fewer than 50% were aware of a monitoring system for VAAE, and only 39% had had VAAE-related education during medical training. Only 28% knew the reporting criteria. Reporting was significantly associated with knowledge of VAAE monitoring systems and reporting criteria (P < 0.01). CONCLUSION: Physicians need more feedback and education on VAAE reporting and more information about the importance of reporting and about reporting criteria and methods.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belton K. J., Lewis S. C., Payne S., Rawlins M. D., Wood S. M. Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom. Br J Clin Pharmacol. 1995 Mar;39(3):223–226. doi: 10.1111/j.1365-2125.1995.tb04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos P., Bentsi-Enchill A. Current thoughts on the risks and benefits of immunisation. Drug Saf. 1993 Jun;8(6):404–413. doi: 10.2165/00002018-199308060-00002. [DOI] [PubMed] [Google Scholar]
- Duclos P. La surveillance des effets secondaires des vaccins après leur commercialisation. Rev Epidemiol Sante Publique. 1994;42(5):425–433. [PubMed] [Google Scholar]
- Duclos P., Pless R., Koch J., Hardy M. Adverse events temporally associated with immunizing agents. Can Fam Physician. 1993 Sep;39:1907–1913. [PMC free article] [PubMed] [Google Scholar]
- Rogers A. S., Israel E., Smith C. R., Levine D., McBean A. M., Valente C., Faich G. Physician knowledge, attitudes, and behavior related to reporting adverse drug events. Arch Intern Med. 1988 Jul;148(7):1596–1600. [PubMed] [Google Scholar]
- Scott H. D., Rosenbaum S. E., Waters W. J., Colt A. M., Andrews L. G., Juergens J. P., Faich G. A. Rhode Island physicians' recognition and reporting of adverse drug reactions. R I Med J. 1987 Jul;70(7):311–316. [PubMed] [Google Scholar]
- Scott H. D., Thacher-Renshaw A., Rosenbaum S. E., Waters W. J., Jr, Green M., Andrews L. G., Faich G. A. Physician reporting of adverse drug reactions. Results of the Rhode Island Adverse Drug Reaction Reporting Project. JAMA. 1990 Apr 4;263(13):1785–1788. [PubMed] [Google Scholar]



