Abstract
Long-term potentiation (LTP) of excitatory transmission is an important candidate cellular mechanism for the storage of memories in the mammalian brain. The subcellular phenomena that underlie the persistent increase in synaptic strength, however, are incompletely understood. A potentially powerful method to detect a presynaptic increase in glutamate release is to examine the effect of LTP induction on the rate at which the use-dependent blocker MK-801 attenuates successive N-methyl-d-aspartic acid (NMDA) receptor-mediated synaptic signals. This method, however, has given apparently contradictory results when applied in hippocampal CA1. The inconsistency could be explained if NMDA receptors were opened by glutamate not only released from local presynaptic terminals, but also diffusing from synapses on neighboring cells where LTP was not induced. Here we examine the effect of pairing-induced LTP on the MK-801 blocking rate in two afferent inputs to dentate granule cells. LTP in the medial perforant path is associated with a significant increase in the MK-801 blocking rate, implying a presynaptic increase in glutamate release probability. An enhanced MK-801 blocking rate is not seen, however, in the lateral perforant path. This result still could be compatible with a presynaptic contribution to LTP in the lateral perforant path if intersynaptic cross-talk occurred. In support of this hypothesis, we show that NMDA receptors consistently sense more quanta of glutamate than do α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. In the medial perforant path, in contrast, there is no significant difference in the number of quanta mediated by the two receptors. These results support a presynaptic contribution to LTP and imply that differences in intersynaptic cross-talk can complicate the interpretation of experiments designed to detect changes in transmitter release.
Keywords: synaptic plasticity, guinea pig, granule cells, glutamate spillover
Long-term potentiation (LTP) is the foremost candidate cellular substrate for memory formation and also may play a major role in the development of neuronal circuits and in neuronal injury (1). In spite of intense effort, the underlying cellular mechanisms of expression remain a matter of some debate (2–4). A potentially sensitive method to detect an increase in presynaptic glutamate release probability is to examine the effect of LTP induction on the rate at which the use-dependent blocker MK-801 attenuates successive excitatory postsynaptic currents (EPSCs) mediated by N-methyl-d-aspartic acid (NMDA) receptors (5, 6). If glutamate is released on a higher proportion of trials after LTP induction, the NMDA receptor-gated channels should be activated more frequently, and MK-801 therefore should produce a faster reduction in the size of the synaptic signal. This method, however, has given conflicting results when applied in the CA1 region of the hippocampus. LTP induced by pairing low-frequency presynaptic stimulation with postsynaptic depolarization was associated with no significant increase in blocking rate (7). Significantly enhanced attenuation, however, was seen when LTP was elicited with tetanic stimulation of presynaptic afferents (8). There are several potential explanations for the discrepancy, including the possibility that tetanization has long-lasting effects on synaptic transmission, separate from the induction of NMDA receptor-dependent LTP. We therefore have examined the effect of pairing-induced LTP on the MK-801 blocking rate in two other pathways in the hippocampal formation: the medial and lateral perforant path inputs to dentate granule cells (MPP and LPP, respectively). Synaptic transmission in these pathways shows profound physiological and pharmacological differences (9–12). We find that pairing-induced LTP in LPP is similar to that in CA1, with no significant increase in the MK-801 blocking rate. LTP in MPP, on the other hand, is associated with an enhanced blocking rate, compatible with an increase in presynaptic transmitter release. Failure to observe an enhanced MK-801 blocking rate in LPP or CA1 still could be compatible with an increase in presynaptic transmitter release probability, if much of the NMDA receptor-mediated signal originated from presynaptic terminals that were not affected by pairing. This could occur if there was appreciable cross-talk between neighboring synapses, mediated by NMDA receptors, but not by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which have a much lower affinity for glutamate (13). We therefore have tested the hypothesis that cross-talk occurs between LPP synapses, but not appreciably between MPP synapses.
MATERIALS AND METHODS
Recordings were obtained in 450-μm thick hippocampal slices obtained from 4- to 5-week-old guinea pigs. The superfusing recording solution contained 119 mM NaCl, 2.5 mM KCl, 1.3 mM MgCl2, 2.5 mM CaCl2, 26.2 mM NaHCO3, 1 mM NaH2PO4, 11 mM glucose, and 0.1 mM picrotoxin, and was gassed with 95% O2/5% CO2. Except where indicated, the experiments were carried out at room temperature (23–25°C).
Stimuli were delivered via bipolar stainless steel electrodes in stratum moleculare (stimulation frequency: 0.05–0.1 Hz). Recordings were made either with extracellular field potential electrodes containing 3 M NaCl or with whole-cell voltage-clamp pipettes containing 117.5 mM Cs gluconate, 17.5 mM CsCl, 10 mM Hepes, 0.2 mM EGTA, 8 mM NaCl, 2 mM MgATP, 0.3 mM GTP, 5 mM QX314 Br (pH 7.2, osmolarity 295 mOsm). In the experiments where EPSC variability was studied, 10 mM 1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid was substituted for EGTA, with a compensatory reduction in Cs gluconate.
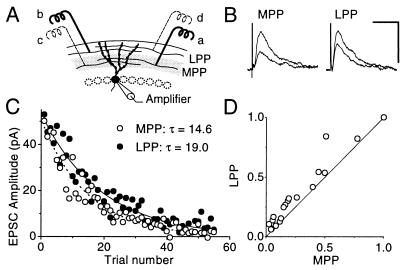
The study depends on reliable identification of MPP and LPP synaptic inputs to granule cells. Before every whole-cell recording, we verified that stimulation in the middle third of stratum moleculare to activate MPP fibers, or in the outer third to activate LPP fibers (Fig. 1A), elicited extracellular field potentials (fEPSPs) with the following features. First, the fEPSPs reversed in polarity as the recording electrode was moved out of the corresponding region of stratum moleculare (12, 14). And second, when two stimuli were delivered in rapid succession (inter-pulse interval 50 or 100 ms), MPP stimulation elicited paired-pulse depression, whereas LPP stimulation elicited facilitation (12, 15). When two MPP or LPP inputs were compared, we positioned the electrodes on either side of the recording pipette and verified that the facilitation or depression ratios in the two pathways were similar.
Figure 1.
(A) Arrangement of stimulating electrodes used to elicit MPP (a and c) and/or LPP (b and d) EPSCs. The two pathways were compared in the same cell by positioning the electrodes at a and b. (B) MPP and LPP EPSCs recorded at a positive holding potential, after addition of MK-801 (in the presence of 5 μM 6-nitro-7-sulfamoylbenzoquinoxaline-2,3-dione to block AMPA receptors). Averages of trials 1–4 and 9–12 are superimposed, showing faster attenuation of MPP than LPP EPSCs. (Calibration bar: 50 ms, 50 pA.) (C) MPP (○) and LPP (•) EPSC peak amplitudes plotted against trial number. Single exponential curves fitted to the two pathways reveal a faster decay in MPP, implying a higher probability of opening of NMDA receptors. (D) MPP EPSC amplitudes plotted against LPP EPSC amplitudes. The data were averaged into groups of four consecutive trials in each pathway, and then normalized by the first average, to reduce the effect of finite sampling. The point at (1.0, 1.0) corresponds to the average of the first four trials. The points fall above the 45° line, indicating that MPP EPSCs decayed faster than LPP EPSCs.
For voltage-clamp recordings, the series resistance was between 15 and 30 MΩ and varied less than 20%. EPSCs were measured by subtracting the average current during a peak window from a baseline window before the stimulus artifact. The windows, determined from the average EPSC time course, were constant within a data set, and also were used to sample the background noise from interleaved traces. The noise variance was subtracted from the EPSC amplitude variance before calculation of 1/CV2, using the formula 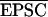 2/(VarEPSC − VarNoise). For the estimation of transmission failure rates, at least 200 trials were recorded at a hyperpolarized potential (−70 or −80 mV) and then at a positive potential (+40 or +50 mV), and in some cases further trials subsequently were collected at a hyperpolarized potential to verify that there was no drift in recording conditions. The stimulation intensity was adjusted in different experiments to obtain a range of initial failure rates (15–85%). No assumption was made about the number of synapses recruited by the stimuli. An oversampled noise distribution was fitted with a sum of two Gaussians. The EPSC amplitude distribution then was fitted with a maximum-likelihood mixture model, each component of which had the same shape as the optimized two-Gaussian noise model. One component of the mixture was constrained to occur at 0 pA, and the probability of this component was taken as an estimate of the failure rate. If, however, the maximum-likelihood solution included other components in the range ±3 pA, the data were rejected from the analysis, to avoid ambiguity about whether these represented failures or small quantal events (16).
2/(VarEPSC − VarNoise). For the estimation of transmission failure rates, at least 200 trials were recorded at a hyperpolarized potential (−70 or −80 mV) and then at a positive potential (+40 or +50 mV), and in some cases further trials subsequently were collected at a hyperpolarized potential to verify that there was no drift in recording conditions. The stimulation intensity was adjusted in different experiments to obtain a range of initial failure rates (15–85%). No assumption was made about the number of synapses recruited by the stimuli. An oversampled noise distribution was fitted with a sum of two Gaussians. The EPSC amplitude distribution then was fitted with a maximum-likelihood mixture model, each component of which had the same shape as the optimized two-Gaussian noise model. One component of the mixture was constrained to occur at 0 pA, and the probability of this component was taken as an estimate of the failure rate. If, however, the maximum-likelihood solution included other components in the range ±3 pA, the data were rejected from the analysis, to avoid ambiguity about whether these represented failures or small quantal events (16).
MK-801 (40 μM) was applied by bath perfusion, with stimulation interrupted for 5 min. We confirmed that MK-801 had reached a stable concentration by the time stimulation was restarted, by verifying that the amplitude-normalized time-course of the first few EPSCs was similar to that of EPSCs recorded later in the course of the application of the drug.
LTP was elicited by pairing 120 pulses at 2 Hz with depolarization to 0 mV, within 10 min of breaking into whole-cell mode. When the same 2-Hz stimulation was delivered in the absence of depolarization, the EPSCs in the test and control pathways, measured 20 min later and normalized by their respective baselines, differed by less than 4% (n = 6). LTP in granule cells is thus pathway specific and depends on the coincidence of presynaptic and postsynaptic activity, as expected from its sensitivity to NMDA receptor antagonists (14). The magnitude of LTP of either the AMPA or the NMDA receptor-mediated component was calculated as the average amplitude in the test pathway EPSCt expressed as a percentage of the AMPA receptor-mediated baseline amplitude BLt. This result then was divided by the control pathway, similarly normalized by its baseline:
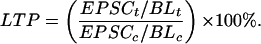 |
Two-tailed paired t tests were applied, and results are given as mean ± SEM. Significance was taken as P < 0.05.
Wherever two inputs were compared, the stimuli were adjusted to obtain EPSCs of similar amplitudes in the test and control (or MPP and LPP) pathways. Posthoc analysis revealed no tendency for differences in MK-801 blocking rate, or differences in the ratio of quantal content mediated by NMDA and AMPA responses, to vary systematically with the initial amplitude of the EPSCs.
Drugs were purchased from Sigma, except for QX314 Br (Alomone Laboratories, Jerusalem), and 6,7-dinitroquinoxaline-2,3-dione and 6-nitro-7-sulfamoylbenzoquinoxaline-2,3-dione (Tocris Cookson, Bristol). MK-801 was a gift from Merck Sharpe & Dohme.
RESULTS
The MK-801 Blocking Rate Is Faster in MPP Than in LPP.
Before using MK-801 to study the loci of expression of LTP, we asked whether the method was sufficiently sensitive to detect differences in release probability in dentate granule cells. The striking difference in paired-pulse facilitation/depression between MPP and LPP generally is taken to indicate a higher baseline release probability in MPP than in LPP. We therefore compared the rate at which MK-801 (40 μM) attenuated MPP and LPP EPSCs simultaneously recorded in the same granule cell. Fig. 1C shows the peak amplitudes of successive NMDA receptor-mediated EPSCs. Single exponential curves fitted to the two pathways revealed a faster decay in MPP than in LPP, in agreement with a higher release probability in MPP. This finding is confirmed in Fig. 1D, where successive EPSCs, normalized by the average of the first four responses, are plotted against one another. This analysis method has the advantage that it avoids fitting a theoretical curve to the data. The points fall above the line of identity, indicating faster attenuation in MPP than in LPP. Similar results were obtained in four cells (P < 0.05; see also Fig. 2E). Although the results are compatible with a higher release probability in MPP, we cannot exclude the possibility that kinetic differences exist between NMDA receptors in MPP and LPP. Such differences, however, would be expected to result in slower NMDA receptor-mediated EPSCs in MPP than in LPP. This expectation was tested for in five cells, by fitting exponentials to the decay of the NMDA receptor-mediated EPSCs recorded in the absence of MK-801. No significant difference was seen (P > 0.8). The simplest explanation for the faster MK-801 blocking rate in MPP therefore is that the release probability is indeed higher than in LPP.
Figure 2.
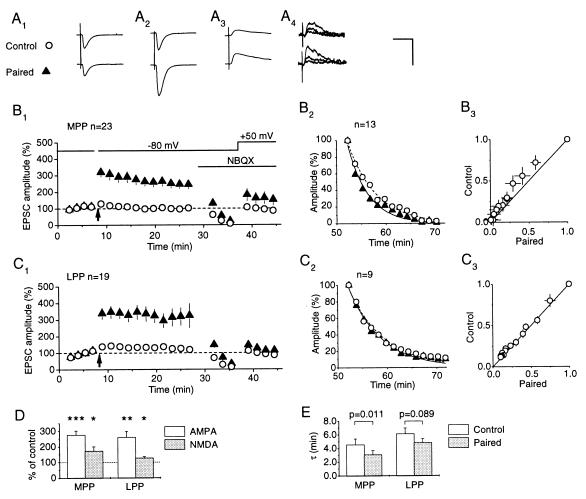
Pairing-induced LTP at MPP synapses is associated with an increase in the MK-801 blocking rate. (A) EPSCs from one cell where two MPP inputs were compared (electrode positions a and c in Fig. 1A). (Upper) Control pathway. (Lower) Test pathway. AMPA receptor-mediated components of the EPSCs before (A1) and after (A2) pairing. (A3) NMDA receptor-mediated components. Expressed as a fraction of the baseline amplitude (A1), the NMDA receptor-mediated component was larger in the test pathway, implying that LTP is not exclusively expressed by AMPA receptors. (A4) NMDA receptor-mediated EPSCs recorded in the presence of MK-801. Averages of trials 1–5, 6–10, and 21–25 are superimposed, showing faster decay in the peak amplitude in the test than in the control pathway. [Calibration bar: 50 ms, 100 pA (A1–A3), 20 pA (A4).] (B) Averaged results obtained in 23 cells where two MPP inputs were studied. (B1) Time course of LTP of the AMPA and NMDA receptor-mediated components (mean ± SEM). EPSC amplitudes were normalized by the baseline before pairing (indicated by the arrow). The NMDA receptor-mediated EPSC amplitudes were rescaled to set the control pathway = 100%. (B2) Averaged EPSC amplitudes recorded in the presence of MK-801 in the 13 cells where complete results were obtained. In 11 of 13 cells the EPSCs in the test pathway decayed faster than in the control pathway. The points in the test and control pathways were calculated by averaging the EPSC amplitudes in groups of five successive trials, and across the 13 cells, and were normalized to set the first average = 100%. The dashed (control) and continuous (test) lines show single exponential fits. (B3) The same data shown as a plot of test EPSCs against control EPSCs (mean ± SEM). The points lie in a convex distribution above the line of identity, indicating faster block in the test pathway (the error bars overestimate the variability among cells, because the decay rates were correlated in the test and control pathways). (C) Averaged data obtained in 19 cells with two LPP inputs, studied with an identical protocol (electrode positions b and d in Fig. 1A). (C1) Time course of LTP of the AMPA and NMDA receptor-mediated components. The EPSC amplitudes in both test and control pathways continued to increase during the baseline period, but pairing was not delayed to avoid “washout” of LTP induction. This accounts for the small apparent potentiation of the control pathway. (C2 and C3) Data obtained in nine cells in the presence of MK-801, and plotted as in B2 and B3. There was no significant difference between the two pathways. (D) Summary of results on the magnitude of LTP expressed by AMPA and NMDA receptors (∗, P < 0.025; ∗∗, P < 0.0005; ∗∗∗, P < 10−5). (E) Summary of decay time constants fitted to MK-801 data. The blocking rate was significantly faster in the test than in the control pathway in MPP, but not in LPP (P values: paired t test).
LTP in MPP Is Associated with an Increase in MK-801 Blocking Rate.
Fig. 2A shows an example of the results obtained in one cell where two MPP inputs were studied. After recording a stable baseline, LTP was induced by pairing stimulation of one pathway with postsynaptic depolarization. AMPA receptors subsequently were blocked with 5 μM 6-nitro-7-sulfamoylbenzoquinoxaline-2,3-dione, and once the EPSCs had decayed to <5% baseline, the cell was depolarized to +50 mV to uncover the NMDA receptor-mediated component. In agreement with results obtained under identical conditions in CA1 (8), there was a significant potentiation of the NMDA receptor-mediated component, although this was smaller than the increase in the AMPA component (see also Fig. 2B1, for averaged results obtained in 23 cells). MK-801 then was washed in while stimulation was interrupted. When stimulation was restarted, EPSCs in the test pathway decayed faster than in the control pathway. This was the case in 11 of 13 cells where the MK-801 blocking rate was successfully measured (Fig. 2B2). The single exponential time constants fitted to the test and control pathways were different at P = 0.011 (n = 13, Fig. 2E). This result also is indicated in Fig. 2B3, where successive average EPSC amplitudes in the two pathways are plotted against one another, after normalizing by the first average: the points lie in a convex distribution above the line of identity.
Although the results are compatible with an increase in presynaptic release probability secondary to induction of LTP, two other explanations must be considered. First, because the baseline release probability in MPP is higher than that in LPP (Fig. 1), the control pathway could have a slower MK-801 blocking rate simply because it was contaminated by some LPP fibers. This explanation is unlikely because it would imply a systematic bias across the data sample. Nevertheless, if there was a bias of this nature, it should be detected by a smaller degree of paired-pulse depression in the control than in the test pathway, when the fEPSPs were recorded before starting the whole-cell recording. The paired-pulse depression ratios (100% × [1st fEPSP − 2nd fEPSP]/1st fEPSP), however, were 19 ± 2% for the test pathway and 19 ± 2% for the control pathway, lending no support to this interpretation. Second, LTP could be associated with a postsynaptic change in receptor kinetics. If NMDA receptors opened for a larger proportion of the time, the MK-801 blocking rate would be enhanced. Again, this should be detected by a difference in the decay time constants of the NMDA receptor-mediated EPSCs in the test and control pathways, before the addition of MK-801. No such difference was seen (P > 0.9). We cannot exclude a more subtle change in NMDA receptor kinetics, such that the channels were open for a larger proportion of the time, but with no change in the overall burst length determining the EPSC duration.
LTP in LPP Is Associated with No Significant Increase in MK-801 Blocking Rate.
When we repeated the experiments with two LPP inputs, LTP was readily obtained and had a similar amplitude to MPP LTP (Fig. 2C). The NMDA component was also significantly potentiated, although to a smaller degree than in MPP (Fig. 2D). In contrast to MPP, however, the MK-801 blocking rates were similar in the test and control pathways. The normalized EPSC amplitudes in the two pathways fell along the line of origin (Fig. 2C3), and the single exponential time constant was nonsignificantly shorter in the test than in the control pathway (P = 0.089, Fig. 2E). Again, the two pathways were comparable, because the paired-pulse facilitation ratios (100% × [2nd fEPSP − 1st fEPSP]/1st fEPSP) were 29 ± 4% and 29 ± 3% in the test and control pathways, respectively. We thus were unable to obtain evidence for a presynaptic increase in transmitter release with the MK-801 method in LPP.
Differences in Intersynaptic Cross-Talk Can Explain the Effects of LTP on the MK-801 Blocking Rate in MPP and LPP.
It has been argued that the sensitivity of the MK-801 method would be compromised if much of the NMDA receptor-mediated signal originated from terminals that were not influenced by the LTP induction protocol (8). If, for instance, some of the terminals were presynaptic to neighboring cells, and were unaffected by the putative retrograde message, they should contribute an NMDA receptor-mediated signal with an unchanged sensitivity to MK-801. We have reported evidence for extrasynaptic spillover in the CA1 region (8) and have argued that it could account for the observation that NMDA receptors generally mediate a larger number of quanta of transmitter (quantal content) than do AMPA receptors, whose affinity for glutamate is much lower (13). The different effects of pairing-induced LTP in MPP and LPP on the MK-801 blocking rate thus could be explained if cross-talk also occurred between LPP synapses, but not (or to a lesser extent) between MPP synapses. We therefore asked whether MPP and LPP synapses show the same discrepancy in quantal content mediated by AMPA and NMDA receptors as seen in CA1.
We examined the trial-to-trial amplitude variability of population EPSCs, under conditions designed to isolate either the AMPA or the NMDA receptor-mediated component. By keeping the stimulus frequency and intensity constant, we compared the statistic 1/CV2 for the two components (17). Assuming a binomial model of transmission, 1/CV2 varies linearly with quantal content if the number of release sites is varied, but is independent of mean quantal amplitude. It therefore should be larger for the NMDA than for the AMPA receptor-mediated component if the same discrepancy in quantal content occurs as in CA1.
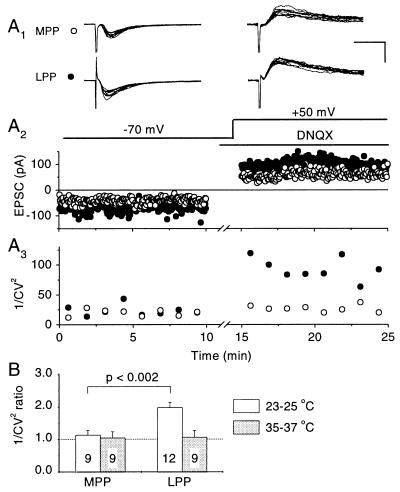
Fig. 3A shows an example of an experiment where MPP and LPP inputs were studied simultaneously. In the LPP input, 1/CV2 was considerably larger for the NMDA than for the AMPA receptor-mediated component (Fig. 3A3), implying that NMDA receptors do indeed sense a larger number of quanta than AMPA receptors. In the MPP input, on the other hand, there was little difference in 1/CV2. When estimated in 12 LPP inputs, the ratio of 1/CV2 (NMDA/AMPA) was 1.98 ± 0.17 (P < 0.0005), similar to that seen in CA1 (Fig. 3B). In nine MPP inputs, in contrast, the ratio was only 1.14 ± 0.14 (not significant). Assuming that the discrepancy in 1/CV2 indeed reflects intersynaptic spillover of glutamate, these results are in agreement with the hypothesis that the phenomenon occurs between LPP synapses, but not appreciably between MPP synapses.
Figure 3.
A comparison of EPSC variability reveals a discrepancy in quantal content mediated by AMPA and NMDA receptors at LPP synapses, but not at MPP synapses at room temperature. (A) Results obtained in one cell where both MPP and LPP synapses were studied simultaneously. (A1) Ten consecutive trials recorded initially at −70 mV (Left), and subsequently at +50 mV in the presence of 10 μM 6,7-dinitroquinoxaline-2,3-dione to block AMPA receptors (Right). (Calibration bar: 25 ms, 100 pA.) (A2) Peak amplitudes of successive MPP (○) and LPP (•) EPSCs. Although the mean amplitude ratio (NMDA/AMPA) in LPP was larger than in MPP, this does not necessarily indicate different ratios of quantal contents, because it also could be explained by systematic differences in quantal amplitudes. (A3) 1/CV2 calculated for successive epochs of 25 trials. The mean value of 1/CV2 was significantly larger for the NMDA than the AMPA receptor-mediated component in LPP, implying a larger quantal content mediated by NMDA receptors. In MPP, in contrast, 1/CV2 was similar for the two components, implying no significant difference in quantal content. (B) Summary of 1/CV2 ratios (NMDA/AMPA) from several cells where either or both pathways were studied with an identical protocol, either at room temperature or at physiological temperature (number of cells indicated). The ratio of 1/CV2 for LPP at room temperature is similar to that seen in Schaffer collateral-CA1 synapses.
A potential weakness of comparing ratios of 1/CV2 for MPP and LPP is that the membrane voltage may be clamped better for synapses close to the soma (MPP) than further out on the dendritic tree (LPP). This phenomenon somehow might introduce a systematic error in estimating the variability arising from quantal fluctuations in the different populations. Because granule cells are electrically compact (18), and even more so with the Cs-based pipette solution used here, this is an unlikely explanation for the results. We nevertheless addressed a possible systematic voltage-clamp error by comparing the ratios of 1/CV2 in CA1 cells, with two stimulating electrodes positioned in stratum radiatum, either within 100 μm of stratum pyramidale, or further out in the dendritic region, approximately 100 μm from stratum lacunosum-moleculare. By positioning the electrodes directly over the main axis of the recorded CA1 cells, we recruited two comparable populations of Schaffer collateral synapses, which differed only in their relative distance from the cell body. The ratio of 1/CV2 (NMDA/AMPA) was 1.99 ± 0.36 for proximal synapses, and 1.95 ± 0.43 for distal synapses (P = 0.87, n = 8), both values in close agreement with previous estimates (16). This test thus lends no support to the hypothesis that the difference between MPP and LPP synapses arises as a consequence of voltage-clamp errors.
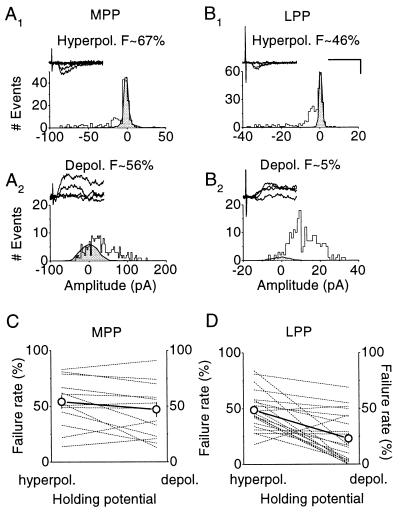
Because the variance method relies on the assumption that the intrinsic quantal variability does not differ appreciably between AMPA and NMDA receptor-mediated components, we applied a second method to compare the quantal contents mediated by the two receptor types. We compared transmission failure rates with minimal stimulation, initially holding the postsynaptic cell at −70 or −80 mV, where NMDA receptors are blocked by Mg2+ ions, and then at +40 or +50 mV, where both AMPA and NMDA receptors sense transmitter release (19, 20) (Fig. 4). At negative potentials the failure rate estimate F− was unambiguous, because of a clear peak in the EPSC amplitude distribution at 0 pA. At positive potentials, this peak was not always seen. We therefore applied a numerical optimization method to estimate the proportion of trials F+ that failed to generate a postsynaptic signal (see Materials and Methods). This approach revealed a much smaller failure rate at positive than at negative voltages for LPP synapses (F+/F− = 0.47 ± 0.09, n = 21, P < 0.001), again implying that NMDA receptors sense a larger quantal content than AMPA receptors. For MPP synapses, however, the failure rates estimated in an identical manner were much more similar (F+/F− = 0.85 ± 0.08, n = 13, P = 0.085; different from LPP at P < 0.02). The difference between MPP and LPP is again unlikely to result from a greater voltage-clamp error for the more distally located synapses. If a proportion of small EPSCs arising from distal synapses was missed at positive holding potentials, this would give rise to a reduced discrepancy in failure rates for LPP, opposite to the actual result.
Figure 4.
A comparison of failure rates at positive and negative potentials reveals a discrepancy in quantal content mediated by AMPA and NMDA receptors at LPP, but not MPP, synapses. (A) EPSC amplitude histograms obtained in one cell where MPP EPSCs were elicited, initially at a negative potential (A1), and subsequently at a positive potential (A2). (Insets) Sample traces recorded under each condition. The estimated failure rates (F− and F+ at negative and positive holding potentials, respectively) were obtained from the probability of a component at 0 pA in a maximum-likelihood mixture model fitted to the data. The shaded histogram shows the noise distribution, scaled by F− or F+. The estimated failure rate was 67% at the negative holding potential and 56% at the positive potential. (B1 and B2) Failure rates in another cell where LPP EPSCs were elicited. The failure rate fell from 46% (B1) to 5% (B2), implying that NMDA receptors sense a larger quantal content than AMPA receptors. [Calibration bar: 25 ms, 50 pA (A) and 25 pA (B).] (C) Failure rates plotted at the two holding potentials for 13 MPP inputs, shown together with the means indicated by ○ (±SEM). (D) Failure rates plotted for 21 LPP inputs. The difference in failure rates was highly significant for LPP (P < 0.001) but not for MPP (P = 0.085). These results imply that, at LPP synapses, but not MPP synapses, NMDA receptors sense a larger average number of quanta released than do AMPA receptors.
Assuming that transmitter release is described by a binomial model, the ratio of the number of release sites sensed by NMDA and AMPA receptors is given by log(F+)/log(F−). This ratio was 2.04 for LPP synapses and 1.23 for MPP synapses, in good agreement with the results obtained from the comparison of 1/CV2 for the two components (1.98 and 1.14, respectively). The variance method and the failure method thus converge on the same conclusion, both in CA1 (16) and in the present experiments: LPP (and CA1), but not MPP, synapses show a large discrepancy in quantal content mediated by AMPA and NMDA receptors. This finding is compatible with the hypothesis that intersynaptic glutamate spillover occurs between LPP (or CA1) synapses, but not appreciably between MPP synapses. This could explain the lower sensitivity of the MK-801 method for detecting changes in transmitter release probability in CA1 and LPP.
The Discrepancy in Quantal Content in LPP Is Reduced at Physiological Temperatures.
In the CA1 region the discrepancy in quantal contents mediated by AMPA and NMDA receptors is much smaller at physiological than at room temperature, which can be explained by a critical role for temperature-sensitive glutamate uptake in limiting the phenomenon (16). We therefore asked whether the discrepancy in 1/CV2 in LPP persisted at physiological temperature. In nine cells we recorded both LPP and MPP inputs simultaneously. The ratio of 1/CV2 (NMDA/AMPA) was 1.06 ± 0.21 for LPP and 1.05 ± 0.18 for MPP (Fig. 3B). The discrepancy in quantal contents for LPP synapses thus disappears under more physiological conditions, implying that it is, at least in part, an artifact of low temperature. This finding can be explained by steep temperature sensitivity for active glutamate uptake from the extracellular space (21).
DISCUSSION
We have described (i) enhancement of the MK-801 blocking rate with pairing-induced LTP in MPP, but not in LPP; (ii) a larger quantal content mediated by NMDA than AMPA receptors in LPP, but not in MPP; and (iii) disappearance of the quantal content discrepancy in LPP at physiological temperature. We suggest that LTP induced in granule cells is accompanied by an increase in presynaptic transmitter release probability, and that the MK-801 method fails to detect this in LPP because of dilution of the NMDA receptor-mediated signal by glutamate released from neighboring, unpotentiated synapses (22). We previously have argued that LTP in CA1 also is accompanied by an increase in presynaptic transmitter release probability (8). In that system, an enhanced MK-801 blocking rate was revealed by tetanizing the presynaptic afferents, a manipulation that induces LTP not only at synapses on the postsynaptic cell, but also at neighboring synapses, which also contribute to the measured NMDA receptor-mediated signal. LPP and CA1 synapses thus share in common, not only a similar degree of facilitation in response to closely spaced stimuli, but also a similar discrepancy in quantal content mediated by AMPA and NMDA receptors, which is greatly reduced at physiological temperatures.
An alternative explanation for the discrepancy in quantal content mediated by the two receptor types, seen in both CA1 and LPP, is that there exists a subset of synapses normally devoid of functional AMPA receptors (3, 17, 19, 20). The increase in quantal content seen in LTP then could be explained by NMDA receptor-dependent recruitment of latent clusters of AMPA receptors at these synapses. On its own, this model fails to account for the disappearance of the discrepancy in quantal content as the temperature is raised or for the MK-801 results. On the other hand, an exclusively presynaptic locus of LTP does not explain the present results either: because there is no evidence for intersynaptic cross-talk in MPP, simply increasing the quantal content should give equivalent increases in AMPA and NMDA receptor-mediated signals. The relatively larger increase in the AMPA receptor-mediated signal (Fig. 2D), however, could be explained by an additional postsynaptic increase in the quantal amplitude for this component. Among other explanations for the smaller potentiation of the NMDA receptor-mediated component is use-dependent desensitization (23), which would be expected to occur during the induction stimulus in the test pathway.
The increase in the NMDA receptor-mediated component is even more modest in LPP than in MPP (Fig. 2D). This finding is again compatible with the hypothesis that much of the NMDA receptor-mediated signals in LPP, but not in MPP, arises from spillover of glutamate from neighboring synapses that do not undergo LTP, because they are unaffected by the putative retrograde messenger arising from the recorded cell. Previous reports on the expression of LTP by AMPA and NMDA receptors in granule cells are difficult to relate to the present results, because the two afferent inputs have not been compared in the same way. Two recent reports, however, concluded that tetanus-induced LTP in MPP synapses is expressed either entirely by AMPA receptors (24) or equally by AMPA and NMDA receptors (25). Our results fall between these extremes, but we are unable to account for the disagreement between these previous reports (see also ref. 14 for a description of LTP of isolated NMDA receptor-mediated signals).
The discrepancy in quantal content mediated by AMPA and NMDA receptors in LPP and CA1, but not in MPP, is unlikely to be causally related to the different release probabilities in the different systems. In CA1 deliberately raising or lowering the release probability has no effect on the ratio of quantal contents mediated by the two receptor types (16). Moreover, raising the temperature causes the discrepancy in quantal content in LPP to disappear, while the striking difference in paired-pulse facilitation/depression in the two pathways persists (data not shown), again illustrating that the two phenomena can be dissociated.
Little is known about the ultrastructural differences between the termination zones of MPP and LPP afferents, except that in rats there are more “thin” and fewer “stubby” spines in the outer third (LPP) than in the middle third (MPP) of stratum moleculare (26). If thin spines are taken as being at a more advanced stage of development, then the results are paradoxical, because a large discrepancy in quantal content mediated by AMPA and NMDA receptors is more prominent at earlier stages of development in the CA1 region (27) and at thalamocortical synapses (28). If, however, the different patterns of quantal signaling in MPP and LPP reflect differences in intersynaptic glutamate spillover, then a number of other parameters play a critical role, including extrasynaptic diffusional barriers, glutamate transporter properties, and intersynaptic distances. Several of these parameters indeed have been found to vary steeply with age (29, 30), possibly explaining some of the developmental changes in quantal signaling.
We have demonstrated differences between MPP and LPP, in both the effect of LTP induction on the MK-801 blocking rate, and in quantal signaling by AMPA and NMDA receptors. These differences can be explained by proposing that LTP is partly expressed presynaptically, and that the degree of intersynaptic glutamate spillover differs between the two pathways. It remains to be determined what ultrastructural or pharmacological phenomena underlie the differences between the two areas of stratum moleculare.
Acknowledgments
We are grateful to Merck Sharpe & Dohme for the gift of MK-801. This work was supported by the Medical Research Council. F.A. was a Wellcome Swedish Travelling Fellow.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: LTP, long-term potentiation; EPSC, excitatory postsynaptic current; MPP, medial perforant path; LPP, lateral perforant path; NMDA, N-methyl-d-aspartic acid; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; fEPSP, extracellular field potential.
References
- 1.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 2.Stevens, C. F. (1993) Neuron 10, Suppl., 55–63.
- 3.Kullmann D M, Siegelbaum S A. Neuron. 1995;15:997–1002. doi: 10.1016/0896-6273(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 4.Nicoll R A, Malenka R C. Trends Neurosci. 1995;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- 5.Rosenmund C, Clements J D, Westbrook G L. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 6.Hessler N A, Shirke A M, Malinow R. Nature (London) 1993;366:568–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- 7.Manabe T, Nicoll R A. Science. 1994;265:1888–1892. doi: 10.1126/science.7916483. [DOI] [PubMed] [Google Scholar]
- 8.Kullmann D M, Erdemli G, Asztely F. Neuron. 1996;17:461–175. doi: 10.1016/s0896-6273(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 9.Abraham W C, McNaughton N. Brain Res. 1984;303:251–260. doi: 10.1016/0006-8993(84)91211-3. [DOI] [PubMed] [Google Scholar]
- 10.Kahle J S, Cotman C W. Brain Res. 1989;482:159–163. doi: 10.1016/0006-8993(89)90554-4. [DOI] [PubMed] [Google Scholar]
- 11.Tielen A M, Lopes da Silva F H, Mollvanger W J. Exp Brain Res. 1981;42:231–233. doi: 10.1007/BF00236913. [DOI] [PubMed] [Google Scholar]
- 12.McNaughton B L. Brain Res. 1980;199:1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
- 13.Patneau D K, Mayer M L. J Neurosci. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanse E, Gustafsson B. Eur J Neurosci. 1992;4:1191–1201. doi: 10.1111/j.1460-9568.1992.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 15.Colino A, Malenka R C. J Neurophysiol. 1993;69:1150–1159. doi: 10.1152/jn.1993.69.4.1150. [DOI] [PubMed] [Google Scholar]
- 16.Asztely F, Erdemli G, Kullmann D M. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 17.Kullmann D M. Neuron. 1994;12:1111–1120. doi: 10.1016/0896-6273(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 18.Staley K J, Otis T, Mody I. J Neurophysiol. 1992;67:1346–1358. doi: 10.1152/jn.1992.67.5.1346. [DOI] [PubMed] [Google Scholar]
- 19.Isaac J T, Nicoll R A, Malenka R C. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Liao D, Hessler N A, Malinow R. Nature (London) 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 21.Tong G, Jahr C E. Neuron. 1994;13:1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 22.Kullmann D M, Asztely F. Trends Neurosci. 1998;21:8–14. doi: 10.1016/s0166-2236(97)01150-8. [DOI] [PubMed] [Google Scholar]
- 23.Tong G, Shepherd D, Jahr C. Science. 1995;267:1510–1512. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Wojtowicz J M, Atwood H L. Synapse. 1996;22:78–86. doi: 10.1002/(SICI)1098-2396(199601)22:1<78::AID-SYN9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor J J, Rowan M J, Anwyl R. J Neurosci. 1995;15:2013–2020. doi: 10.1523/JNEUROSCI.15-03-02013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desmond N L, Levy W B. Neurosci Lett. 1985;54:219–224. doi: 10.1016/s0304-3940(85)80082-3. [DOI] [PubMed] [Google Scholar]
- 27.Durand G M, Kovalchuk Y, Konnerth A. Nature (London) 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 28.Isaac J T R, Crair M C, Nicoll R A, Malenka R C. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- 29.Lehmenkühler A, Syková E, Svoboda J, Zilles K, Nicholson C. Neuroscience. 1993;55:339–351. doi: 10.1016/0306-4522(93)90503-8. [DOI] [PubMed] [Google Scholar]
- 30.Shibata T, Watanabe M, Tanaka K, Wada K, Inoue Y. NeuroReport. 1996;7:705–709. doi: 10.1097/00001756-199602290-00006. [DOI] [PubMed] [Google Scholar]