Abstract
During development, guidance molecules play a key role in the formation of complex circuits required for neural functions. With the cessation of development, this exuberant growth process slows and stabilizes, and inhibitory molecules expressed by glia prevent initial attempts for axonal regeneration. In this review, we discuss the expression patterns and relative contribution of several guidance molecules on the regenerative process. Injury to the immature CNS or species capable of regenerating exhibit a complete or partial recapitulation of their developmental guidance patterns; whereas, similar injuries to adult mammals results in altered expression that acts to further hinder regeneration. Manipulations of guidance molecules after injury have been used to control detrimental effects of axon sprouting and target regenerating axons within the spinal cord.
I. Introduction
The function of the nervous system is based on complex networks formed by neurons during development, and accordingly, any deficit or alternation in neural circuitry caused by injury leads to impaired function. During normal development, this highly precise network is established by temporal and spatial regulation of guidance molecules that direct the growth of axons along specific pathways to reach the target (Osterfield et al., 2003; Dickson 2002). This process depends on the ability of growth cones (the leading edge of the axon) to sample and respond to multiple guidance cues in the environment (Song and Poo, 2001). In contrast, after injury to the adult mammalian central nervous system (CNS), the ability of axons to regenerate and reestablish specific projections is very limited. Axons fail to regenerate for multiple reasons, including, the expression of myelin inhibitory proteins, reduced intrinsic growth potential of axons, upregulation of inhibitory extracellular matrix at the injury site, and alterations in developmentally important guidance molecules (Silver and Miller 2004). During the past 15 years, major advances in neutralizing glial inhibitory molecules and increasing the intrinsic growth ability of axons has lead to regeneration past the lesion site. Still, little is known about the accuracy in reconstruction of damaged circuits with functional indices being the final arbiter for circuit repair. Likewise, functional recovery is most likely due to the formation of novel circuits from sprouting and regenerating axons that undergo regional as well as supraspinal plasticity and rarely reconstruction of the original circuit (Maier and Schwab, 2006).
During development, axon pathfinding and target recognition are guided by both positive (permissive and attractive) and negative (inhibitory and repulsive) cues, which operate at short- or long-range (Tessier-Lavigne and Goodman, 1996). This gives rise to four different guidance mechanisms: contact attraction, chemoattraction, contact repulsion and chemorepulsion. Specific guidance decisions reflect the combination of all four mechanisms. The growth cone integrates signals of multiple cues presented simultaneously in the environment, and reacts to the relative balance of the attractive and repulsive forces (Tessier-Lavigne and Goodman, 1996). The expression of these guidance molecules is under tight genetic control, orchestrating complex and precise patterning of connections in the CNS. The precision by which the guidance program is maintained within the normal adult CNS or recapitulated after injury has only recently begun to be studied. Many of guidance factors and their receptors persist into adulthood; however, their distribution, particularly after injury, shows incongruities with developmental expression patterns (Koeberle and Bahr, 2003). These variations in patterning could compromise accurate reconstruction of circuits. In addition, the environment in the adult CNS after injury is much more hostile to regenerating axons than during development. Axons navigate through a terrain that is replete with debris, reactive immune cells and cytokines, and contains high levels of inhibitory molecules, reactive astrocytes, extracellular matrix (ECM), and reactive oxygen species - all of which could alter growth cone responsiveness to endogenous cues (Silver and Miller, 2004). Although many factors including neurotrophins, morphogens, and extracellular matrix molecules are involved in axon growth and guidance, we will focus on the best-characterized of these ligand-receptor pairs, that belong to the families of ephrins, netrins, semaphorins, and slits.
II. Guidance cues during development
Ephrins
Ephrins belong to a family of membrane bound guidance molecules that consist of eight mammalian members divided into A- and B-subclasses based on membrane linkage properties. Mammalian ephrinA1-ephrinA5 are attached to the membrane via a glycosylphosphatidylinositol (GPI) anchor, whereas ephrinB1-ephrinB3 are transmembrane proteins (Flanagan and Vanderhaeghen 1998; Goldshmit et al. 2006; Kullander and Klein 2002). Ephrin ligands bind transmembrane receptor tyrosine kinase receptors (Eph). There have been 13 mammalian Eph receptors identified to date; EphA1-EphA-8 and EphB1-EphB4, and EphB-6 (Flanagan and Vanderhaeghen 1998; Goldshmit et al. 2006; Kullander and Klein 2002). The receptors are classified based on sequence homology and the ephrin ligands they bind, although it has recently been shown that receptors EphA4 and EphB2 can bind either A or B class Ephrins (Himanen et al. 2004).
Ephrin/Eph receptor complexes can mediate bi-directional signaling such that propagation of the signal can occur in the cell expressing the receptor (forward signaling) and/or the cell expressing the ligand (reverse signaling). Ephrin/Eph signals can be attractive or repulsive and require clustering of the ligand for initiation (Hattori et al. 2000). Ephrin/Eph signaling is involved in targeting of corticospinal tract axons (Dottori et al. 1998; Kullander et al. 2001a; Kullander et al. 2001b; Yokoyama et al. 2001), retinotectal mapping (Ellsworth et al. 2005; Feldheim et al. 2000; Feldheim et al. 2004), and guidance of axon tracts of the anterior commissure (Cowan et al. 2004; Henkemeyer et al. 1996; Kullander et al. 2001a).
Netrins
Netrins make up a small family of diffusible guidance molecules that mediate long or short range chemoattraction and chemorepulsion in the developing nervous system of worms, flies, and vertebrates(Barallobre et al. 2005; Kennedy 2000). Mammalian members of the netrin family include netrin-1(Metin et al. 1997), netrin-3(Wang et al. 1999a), netrin-4(Yin et al. 2000)(also called β-netrin) (Koch et al. 2000)and netrin-G1 (Nakashiba et al. 2000). Although secreted, netrins can bind ECM and cell membranes and their local affinity for such substrates determines the range of netrin diffusion and signaling (Kennedy 2000). The nature of netrin signaling - attractant or repellant - is determined by activation of different netrin receptor complexes. The deleted in colorectal cancer (DCC) family of netrin receptors primarily mediates netrin attraction, whereas the UNC-5 family of netrin receptors mediates netrin repulsion, either alone or in combination with DCC receptors and as-of-yet unidentified co-receptors (Barallobre et al. 2005; Cooper et al. 1999; Hong et al. 1999; Keleman and Dickson 2001; Stein et al. 2001). Netrins play an important role in attracting axons to the midline of animals with bilateral symmetry. They are also involved in guidance of a wide range of axon populations during development including, but not limited to, spinal commissural neurons (Serafini et al. 1996), olfactory bulb axons (Koch et al. 2000), and retinal ganglion neurons (Deiner et al. 1997).
Semaphorins
The family of semaphorin guidance molecules is made up of more than 30 secreted and membrane-bound members that are divided into 8 sub-classes based on membrane linkage and phylogenetic associations (Semaphorin Nomenclature Committee 1999). Vertebrates express secreted (class III, 3, subgroups 3A, 3B, 3C etc.), transmembrane (classes IV – VI), and GPI-linked (class VII) semaphorin proteins. Semaphorins primarily mediate repulsion of axons via binding receptors from the neuropilin and plexin families (Bagnard et al. 1998; Huber et al. 2003; Raper 2000; Song et al. 1998). Studies, in vitro, have implicated semaphorins in the repulsion of several types of axons including cortical, olfactory, hippocampal, and pontocerebellar neurons (Raper 2000). While knockout mice demonstrated a role for semaphorin signaling in midline guidance (Zou et al. 2000), information regarding the role of specific semaphorins in targeting other axon types during development has been difficult to interpret, presumably due to redundancy of guidance signaling. Alternate, more specific, dominant negative approaches, however, were successful in showing that individual semaphorins are involved in appropriate targeting as well as timing of olfactory axon innervation of the olfactory bulb(Renzi et al. 2000).
Slits
Slits are the most recently identified family of axon guidance molecules. They are large secreted proteins that bind the family of roundabout (robo) transmembrane receptors to mediate signaling. To date three slits, slit1-3, (Brose et al. 1999; Itoh et al. 1998; Li et al. 1999) and four robo receptors, robo1-4, (Huminiecki et al. 2002; Kidd et al. 1998a; Yuan et al. 1999) have been identified in mammals. Slits in cooperation with robos play a major role in regulating guidance of commissural axons at the midline. Several studies combined show that Robo1 and 2 mediate repulsion once axons have crossed the midline, and robo3 serves to attract axons to the midline and allow crossing by inhibiting slit/robo1 signaling (for review see Dickson et. al. 2006) (Dickson and Gilestro 2006; Long et al. 2004; Marillat et al. 2004; Plump et al. 2002; Sabatier et al. 2004; Stein and Tessier-Lavigne 2001). Recently, a mutation in Robo3 identified in humans was shown to be responsible for failed axon crossing within the brainstem and spinal cord, resulting in the disorder of horizontal gaze palsy with progressive scoliosis (HGPPS) (Jen et al., 2004). Slits have also been implicated in the formation of the optic chiasm (Plump et al. 2002), and in mediating repulsion of olfactory axons (Li et al. 1999), forebrain axons (Nguyen Ba-Charvet et al. 1999), and retinal ganglion cell axons (Niclou et al. 2000). In contrast, slits appear to play a positive role in cortical dendrite remodeling (Whitford et al. 2002) and sensory axon elongation and branching (Wang et al. 1999b).
III. Guidance cues in the intact and/or injured adult CNS
CNS circuits are formed during development through intricate and tightly regulated processes. External guidance molecules provide navigational instructions by binding and signaling through receptors on growth cones, which then decide whether to continue on the current path, turn, stall, collapse, or retract. Importantly, several components including the temporal, spatial, and environmental context of these cues must be summated by the growth cone before a molecular signal can be translated into a physiological response. Aberrant expression of critical guidance molecules could lead to premature termination or misguided growth. Given that successful regeneration following injury to the adult CNS requires appropriate axon targeting, it is critical that we understand the context of guidance factor and receptor expression in the injured adult CNS and its potential influence on regenerating axons.
Developmental guidance cues associated with failed regeneration
The immature nervous system has a relatively good regenerative capacity when compared to the adult. For example, transection of the lateral olfactory tract in neonates results in successful regeneration whereas the same injury in adults does not (Pasterkamp et al. 1999). Interestingly, semaphorin III mRNA and its receptor neuropilin-1 are highly expressed in the adult CNS, but absent in the neonate following lateral olfactory tract transaction (Pasterkamp et al. 1999). This relationship is also reflected by the expression profile of inhibitory versus positive netrin receptors in the adult versus embryonic CNS. Inhibitory netrin receptors from the UNC-5 family show increased expression in the adult compared to the embryonic CNS and conversely, positive netrin receptors DCC and neogenin show reduced expression in the adult compared to the embryonic CNS (Manitt et al. 2004). Taken together, these data suggest that some developmental alterations in expression of chemorepulsive signaling molecules and their receptors following injury could contribute to regeneration failure in adults.
Injury to the adult CNS results in widespread changes in gene expression, many of which involve guidance cues and their receptors. Several Eph receptors including, EphA3, A4, A6, A7 and A8 show an increase in mRNA and immunoreactivity compared to controls following spinal cord injury (Willson et al. 2002). Axotomy of intramedullary motoneurons results in persistent up-regulation of semaphorin3A mRNA and protein (Lindholm et al. 2004) and, after spinal cord lesions, semaphorin4D expression is transiently up-regulated in oligodendrocytes, but not astrocytes, microglia, or oligodendrocytes precursors (Moreau-Fauvarque et al. 2003). Furthermore, netrin-1, slit-1, and 3, and their receptors; unc5h-1, 2 and 3, robo-1, 2 and 3, are expressed following injury to the cerebellum and spinal cord (Wehrle et al. 2005). Such changes in expression often correlate with a lack of regeneration. For example, comparison of mRNA levels by in situ hybridization between the intact and transected adult lamprey spinal cord revealed a down-regulation of netrin and up-regulation of semaphorin near the transection site (Shifman and Selzer 2007), possibly accounting for regenerative failure of approximately 50% of the transected spinal axons. Semaphorin 3A was also recently shown to be expressed in terminal Schwann cells preferentially associated with fast-fatigable muscle fibers following injury. These fibers do not demonstrate plasticity during injury induced remodeling of neuromuscular junctions (De Winter et al. 2006). In both of these scenarios, the loss of positive guidance cues and/or gain of negative cues correlated with failed regeneration. Eph/ephrin signaling has recently been correlated with failure of cortical spinal axon regeneration (Fabes et al. 2006). EprhinB2, an EphA4 receptor ligand, is normally expressed flanking the corticospinal tract in adults. Following a dorsal hemisection lesion ephrinB2 is up-regulated in the astrocytes of the glial scar and injured cortical neurons accumulate the EphA4 receptor. It is proposed that retraction of corticospinal axons and inhibition of their regeneration following spinal lesion may be due, in part, to the repulsive Eph/ephrin signaling resulting from the ephrin-rich lesion site and localization of the Eph receptor to injured neurons (Fabes et al. 2006).
In some cases, axon inhibition has been specifically localized to regions expressing repulsive guidance molecules. Following transection of the thoracic dorsal columns, semaphorin3A is persistently up-regulated by fibroblasts in the lesion. Tract tracing and immunohistochemistry demonstrated that dorsal root ganglion neurons expressing semaphorin3A receptor components, neuropilin-1 and plexin-A1, failed to invade glial scar regions occupied by semaphorin3A-positive fibroblasts (Pasterkamp et al. 2001). Similarly, other class 3 semaphorins are expressed by fibroblasts in the glial scar following lesions of the adult rat spinal cord and most neurons of the cortico- and rubrospinal tracts expressing receptor components for class 3 semaphorins are not able to penetrate the semaphorin positive portion of the glial scar formed at the lesion site (De Winter et al. 2002). The high lethality of Sema3A −/− mouse precludes analysis of the contribution of semaphorin 3A inhibition to regenerative failure; however, a small molecule (SM-216289) that inhibits Sema3A induced chemorepulsion and subsequently enhanced axon regeneration beyond a spinal cord lesion (Kaneko et al., 2006). Although axons from several neuronal populations regenerated using this paradigm, regrowth of corticospinal axons was not observed, reinforcing the possibility of redundant inhibitory mechanisms preventing axon regeneration.
Molecular or genetic studies showing functional role for cues in regeneration
More recent studies have demonstrated functional roles for Eph/ephrin signaling in either formation of the glial scar (Bundesen et al. 2003) or inhibition of corticospinal tract growth (Benson et al. 2005). Bundesen et. al. showed that ephrin-B2 and EphB2 are expressed by astrocytes and meningeal fibroblasts, respectively, in the intact and injured adult spinal cord (Bundesen et al. 2003). A timeline was established for scar formation that involves bi-directional signaling between ephrin-B2-expressing reactive astrocytes at the lesion surface and infiltrating EphB2-containing fibroblasts in the early days following thoracic transection of the spinal cord. At one week, boundaries begin to form between cell types and by two weeks the astroglial-meningeal fibroblast scar is fully developed with an established segregation of ephrin-B2-expressing astrocytes from EphB2-positive meningeal fibroblasts. Segregation of cell types also corresponds with a decrease in EphB2 and ephrinB2 activation, suggesting that cell contact-mediated bidirectional EphB2/ephrinB2 signaling functions in the formation of the glial scar (Bundesen et al. 2003). Further evidence for a functional role of ephrin guidance molecules in the CNS response to injury comes from studies looking at ephrinB3 (Benson et al. 2005). EphrinB3 is expressed at the midline during development and acts as a repellent for EphA4 positive CST axons. Knockouts of either ephrinB3 or EphA4 induce abnormal midline crossing of CST axons (Dottori et al., 1998) and rhythmic disruption of the central pattern generator resulting in a stereotypic rabbit-like hopping gait (Kullander et al., 2003). In adults, however, ephrinB3 is expressed in the white matter of the spinal cord where it is concentrated around the mature CST. Immunocytochemical co-labeling experiments showed that ephrinB3 was expressed by myelinating oligodendrocytes, but not by neurons or astrocytes (Benson et al. 2005). Furthermore, ephrinB3 was shown to play a role in myelin-based inhibition of axon growth, since growth on myelin from ephrin-B3 knockout mice alleviated the inhibition typically seen when EphA4-positive primary CST axons were grown on myelin from wildtype mice. In agreement with those studies, unilateral hemisection of the dorsal columns in EphA4 knockout mice showed regeneration of CST axons and some return of function (Goldshmit et al., 2004). Co-cultures of neurons and astrocytes isolated from these knockout mice showed increased neurite growth when compared to neurons grown on wildtype astrocytes. Interestingly, these mice also showed a lack of glial scar formation, which further reduced the inhibitory environment of the lesion site; however, it remains uncertain if developmental abnormalities in the EphA4 null mutants contributed to functional recovery. Together these findings indicate that the ephrinB3/EphA4 signaling system functions in inhibition of CST regeneration following SCI.(Benson et al. 2005).
Expression of guidance cues following injury to the visual system
The development and precise topographic wiring of the visual system has been extensively studied and involves guidance by netrins, semaphorins, slits, ephrins, and their respective receptors. Briefly, developing retinal ganglion cells, which express the netrin receptor DCC, are guided out of the eye and into the optic nerve by the presence of netrin in the optic disc. Semaphorins promote axon fasciculation in the optic nerve, whereas ephrin B2 expression at the optic chiasm prevents Eph B1 ipsilateral projections from crossing the midline (Williams et al., 2003). Finally, precisely mapped expression gradients of EphAs in the retina and complimentary gradients of ephrin-As and ephrin-Bs in the superior colliculus regulate topographical synapse formation (for review see McLaughlin and O’Leary 2005). With the cessation of visual development in mammals, many of these molecules are down regulated; however, after optic nerve injury partial re-expression is observed (Wizenmann et al. 1993). Unlike mammals, optic nerve lesions in goldfish result in complete restoration of developmental guidance cues. It is particularly useful to compare the differences seen between lower vertebrates, such as goldfish, that have the capacity to functionally regenerate, and mammals such as rats that do not.
Several studies in adult goldfish have shown a correlation between the maintenance or appropriate re-expression of developmental guidance cues and successful regeneration following optic nerve injury (King et al. 2003; Petrausch et al. 2000; Rodger et al. 2000; Rodger et al. 2004). This is in contrast to the situation observed in adult rats where the optic nerve does not demonstrate functional regeneration (Ellezam et al. 2001; Knoll et al. 2001; Petrausch et al. 2000; Rodger et al. 2001; Symonds et al. 2007). Expression of netrin-1 is maintained at the optic disc in normal and post-lesion adult goldfish (Petrausch et al. 2000). Furthermore, retinal axons express netrin receptors as shown by netrin-1 Fc fusion protein binding to regenerating axons, suggesting the presence of a functional netrin-1 guidance system. In contrast, optic nerve lesion in adult rats results in expression of netrin-1 in retinal ganglion cells and glial cells in the optic nerve(Ellezam et al. 2001), but not in the optic disc (Petrausch et al. 2000), where it is expressed during development. Down regulation of the netrin receptors DCC, UNC5H1 and UNC5H2 was also observed in these animals, leading one to speculate that ineffective or incomplete restoration of the netrin signaling system may contribute to failed regeneration (Ellezam et al. 2001; Petrausch et al. 2000). Application of a peripheral nerve graft to promote cell survival and regeneration did not prevent the down regulation of netrin receptors, even in those axons that regenerated. Thus, it is possible that netrin signaling does not contribute to retinal axon regeneration induced by peripheral nerve graft in adult rats. Further studies examining the presence of factors that are known to regulate netrin signaling such as intracellular levels of cAMP, (Ming et al. 1997; Shen et al. 1999), or the ECM protein laminin (Hopker et al. 1999), are needed to fully interpret role of netrin in peripheral nerve induced regeneration in adult rats. Although netrins function in optic nerve guidance, Eph/Ephrin signaling is a major determinant of appropriate topographic mapping in the developing visual system (McLaughlin and O’Leary 2005).
Similar to the results from studies looking at netrin, the Eph/ephrin signaling system in goldfish appears to be maintained or restored following injury, again correlating with functional regeneration (King et al. 2003; Rodger et al. 2000; Rodger et al. 2004; Symonds et al. 2001). A rostral(high)-caudal(low) gradient of ephrinA2 in the tectum of the developing goldfish visual system plays an important role in retino-tectal mapping. This gradient persists in mature goldfish presumably to maintain appropriate mapping of newly generated axons as retinal and tectal neurogenesis continues beyond development. During optic nerve regeneration, the ephrinA2 rostrocaudal gradient expression throughout the tectum is transiently increased at one month and returns to normal levels by 3 months (Rodger et al. 2000). This upregulation corresponds to restoration of the topographical map and is thought to be initiated by innervation of retinal ganglion cell axons (Rodger et al. 2000). In turn, examination of gradient expression in the retina during nerve regeneration reveals ascending naso-temporal EphA3 and EphA5 gradients and a uniform expression of ephrinA2 (King et al. 2003). Such Eph gradients in the retina are also coincident with restoration of the topographical map. Finally, studies using injections of recombinant EphA3 receptor or phospho-inositol phopholipase-C to disrupt EphA/ephrin-A interactions during optic nerve regeneration demonstrated aberrant mapping along the rostrocaudal axis in the tectum, suggesting that such interactions are required for proper restoration of topographical mapping in goldfish (Rodger et al. 2004).
Studies in adult rats show an incomplete restoration of guidance cues in addition to aberrant expression of inhibitory cues. Restoration and/or maintenance of an ephrin-A gradient in the superior colliculus following injury coincides with a loss of EphA receptor gradient in the RGCs. The net result is an incomplete set of cues for restoration of topographic mapping (Knoll et al. 2001; Rodger et al. 2001). However, the authors suggest that EphA could serve to mask the gradient in normal rats and this decrease in receptor level could uncover ephrin-A gradient for appropriate topographical targeting should retinal ganglion cell (RGC) regeneration occur. Application of a peripheral nerve graft following optic nerve lesions rescued RGC, restored the EphA5 gradient in the retina and subsequently supported regeneration through the graft to the superior colliculus, it failed to support topographic reconstruction in the superior colliculus. Failure, however was due to upregulation of ephrinA2 on astrocyte surrounding the insertion site of the peripheral nerve graft that prevented regenerating axons from leaving the graft (Symonds et al. 2007).
V. Recapitulating developmental cues for repair of the CNS
Manipulation of developmental guidance cues for repair of the injured CNS is a daunting task. Many potential obstacles need to be considered if such an approach is to be successful. Perhaps the most difficult hurdle is organizing the complex spatial and temporal expression gradient patterns required to precisely target regenerating axons, particularly within the overtly growth inhibitory environment of the adult CNS. Furthermore, precise topographic mapping most likely will require cues from multiple guidance factors as occurs during development of the floor plate or visual system. But as observed with the visual system, some of these expression patterns are conserved in the adult or re-established after injury, so it might only be necessary to modify a few factors. Another consideration is whether growth cones of injured axons express the appropriate receptors and signaling mechanisms required for the appropriate response to guidance cues. Whether cues are interpreted as attractive and/or repulsive is regulated by several factors including, receptors expressed by the navigating growth cone (Kidd et al. 1998b; Shirasaki et al. 1998; Stein and Tessier-Lavigne 2001), the ECM molecules with which the cues are associated (Hopker et al. 1999; Nguyen-Ba-Charvet et al. 2001), the presence or activity of proteases known to cleave such cues (Galko and Tessier-Lavigne 2000; Hattori et al. 2000; Janes et al. 2005; Nguyen Ba-Charvet et al. 2001; Webber et al. 2002), and the intracellular cyclic nucleotide levels of the axon (Cai et al. 2001; Ming et al. 1997; Nguyen-Ba-Charvet et al. 2001; Rodger et al. 2005; Shen et al. 1999; Song et al. 1997). It is also clear from the information discussed above that the adult and certainly the injury environment are very different from that which axons encounter during development. In the adult, circuits and tracts are already established resulting in a drastically altered extracellular environment, and the distances required for axons to travel to reach their targets following injury are much greater. Therefore, understanding the effect such differences have on regulation of guidance cue signaling is of paramount importance if such an approach is to be successful.
One region where the exogenous expression of developmental guidance cues has resulted in successful manipulation of sprouting or regenerating axons is the dorsal root entry zone. This region is high amendable because of the short distances axons are required to regenerate prior to establishing functional synapses, and many of the guidance molecules and their receptors have been characterized both during development and in the adult. During development primary peptidergic nociceptive sensory axons grow into the spinal cord through the dorsal root entry zone (DREZ) and terminate primarily in laminae I and II of the dorsal horn (Ozaki and Snider, 1997). Their ingrowth and lamina specific termination are partially determined by a ventral-dorsal gradient of semaphorin 3A, which acts as a chemorepulsive factor to prevent growth of these nociceptive axons into the deeper dorsal lamina (Messersmith et al., 1995; Fu et al., 2000). In the adult, these neurons and their growing axons retain the receptors and responsiveness to semaphorin 3A (Tanelian et al., 1997; Gavazzi 2001), but the semaphorin 3A gradient in the spinal cord has diminished. This condition permits the experimental use of semaphorin 3A as a guidance molecule to control axon sprouting and regeneration after injury to the nervous system. After spinal cord injury, uninjured calcitonin gene-related peptide (CGRP) containing nociceptive axons can sprout into deeper dorsal laminae to aid the development of two detrimental conditions, autonomic dysreflexia and chronic pain (Christensen and Hulsebosch, 1997; Krenz et al., 1998; Tang et al., 2004b; Cameron et al., 2006). Autonomic dysreflexia is the conversion of a nociceptive input from below the injury into a sympathetic response that results in unregulated elevation of blood pressure, with simultaneous decreases in heart rate. Injection of adenovirus-encoding semaphorin 3A into the deep dorsal horn prevented sprouting of CGRP + axons and reduced the severity of both the autonomic response and chronic pain induced by NGF (Tang et al., 2004b; Cameron et al., 2006). In contrast, over-expression of NGF, which is a growth factor for these axons, caused the opposite effect. Thus, strategic placement of a chemorepulsive factors can potentially be used to control injury induced sprouting that leads to detrimental effects of the lesion.
This model has also been used successfully to target regenerating nociceptive axons to their appropriate laminae (Tang et al., 2007). Numerous studies have shown that neurotrophins aid regeneration of many systems, and could be thought of as providing attractive guidance cues (Ramer et al., 2000; Romero et al., 2001; Taylor et al., 2006). After dorsal root rhizotomy, axons regenerate within the peripheral nerve (PN) but stop abruptly at the PN/spinal cord transition zone, known as the DREZ, failing to regenerate into the spinal cord (Liuzzi and Lasek 1987; Kliot et al., 1990). Addition of neurotrophins to the spinal cord enhances regeneration of axons through the DREZ and into the spinal cord where they form functional connections (Ramer et al., 2000, Romero et al., 2001; Tang et al., 2004a). Viral over-expression of NGF in the dorsal horn led to robust regeneration specifically of CGRP+ axons; however, these axons failed to terminate within laminae I and II, and instead grew abnormally throughout the entire dorsal horn (Romero et al., 2001; Tang et al., 2004a), most likely due to the loss of semaphorin 3A expression in the adult. To target regenerating nociceptive axons and recapitulate their developmental lamina specific patterning, slightly overlapping gradients of NGF and semaphorin 3A were established in the dorsal horn (Tang et al., 2007). For these experiments, recombinant adenovirus was used to control both the temporal and spatial aspects of the two gradients, which were important to obtain appropriate termination within laminae I and II. This resulted in targeted regeneration of about 25% of the lesioned nociceptive axons showing a pattern of synaptic terminals very similar to their normal distribution within the uninjured animal and improved the overall functional efficiency of these connections. Thus, faithful reconstruction of regenerating circuits could be important for functional recovery.
Neural replacement strategies could also benefit from establishment of guidance pathways to direct the growth of axons from the transplant site to the target locations. Numerous studies have demonstrated that transplant of exogenous neurons from either fetal or stem cell sources often fail to fully integrate within the host tissue and rarely reconstruct the damaged circuit in adults (Grimaldi et al., 2005). Often transplanted neurons will extend dendrites and receive synaptic connection from host neurons; however, eventhough axons from transplanted neurons grow into the host, most fail to find their appropriate targets (Triarhou et al., 1992). More recently we have investigated the possibility of using guidance molecules not only to target regenerating axons but also axons from transplanted neurons. In this study, a preformed guidance pathway was established along the corpus callosum using adenovirus to overexpress growth promoting factors (Fig. 3). In the contralateral hemisphere the pathway turned toward a target in either the striatum or the cortex. Expression of fibroblast growth factor-2 (FGF2) along the pathway leading to an NGF target produced the best growth, with about 50% of the axons turning. The number of axons turning out of the corpus callosum and into the striatum was enhances when semaphorin 3A was co-expressed distal to the turning point. This study shows the feasibility of directing axon growth for circuit reconstruction with neuronal replacement strategies.
Figure 3.
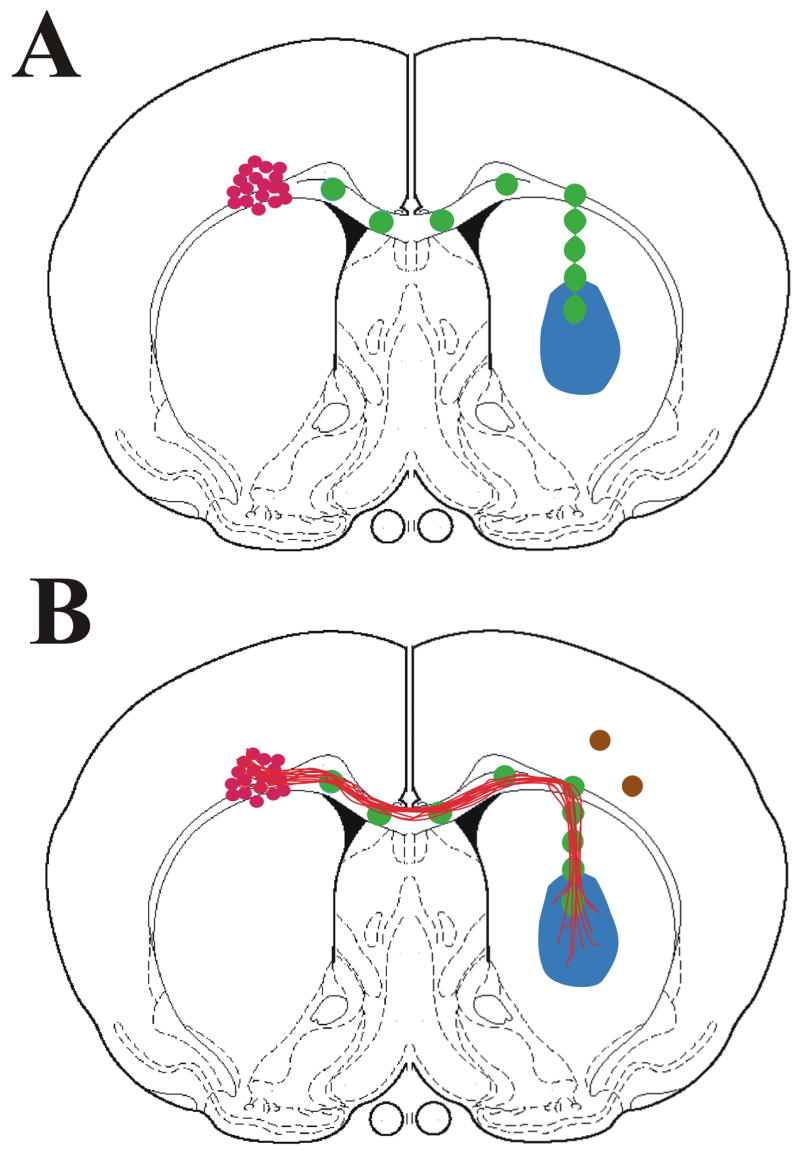
Schematic diagram of coronal cross sections of the rat brain. (A) Adenovirus encoding a target neurotrophic source is injected into the striatum (blue). A pathway of nine smaller injections (green) of a second adenovirus is created from the striatum to the corpus callosum that follows the corpus callosum to the opposite hemisphere. DRG neurons (red) are transplanted above the corpus callosum in the hemisphere contralateral to the target two days post-injection. (B) If the pathway supports growth, axons (red) should grow along the corpus callosum into the contralateral hemisphere and turn toward the target neurotrophic source. To increase the number of axons turning semaphoring (brown circles) are placed at the distal end of the pathway, just after the turning point.
VI. Conclusions
During development, guidance cues within the CNS play an important role in shaping the formation of neural circuits. With the cessation of development, some of the developmental guidance cues are lost, reduced, or altered. Injury to the CNS can further augment the expression patterns, leading to increased inhibition at the lesion site. While it is tempting to assume that recapitulation of the developmental guidance process in the injured adult CNS would enhance functional recovery, it must be taken within the context of the adult environment. Alterations in the developmental expression patterns, receptor repertoire on growth cones, and the generally inhospitable environment after injury need to be taken into consideration. Since axonal guidance required multiple factors acting in concert, it seems important that the approaches enhance and not interfere with any endogenous attempts at reforming appropriate circuits. Strategic replacement of key guidance molecules in the appropriate spatial and temporal conformation could, thus, be exploited to reduce inappropriate or detrimental sprouting, while inducing more appropriately targeted growth.
Figure 1.
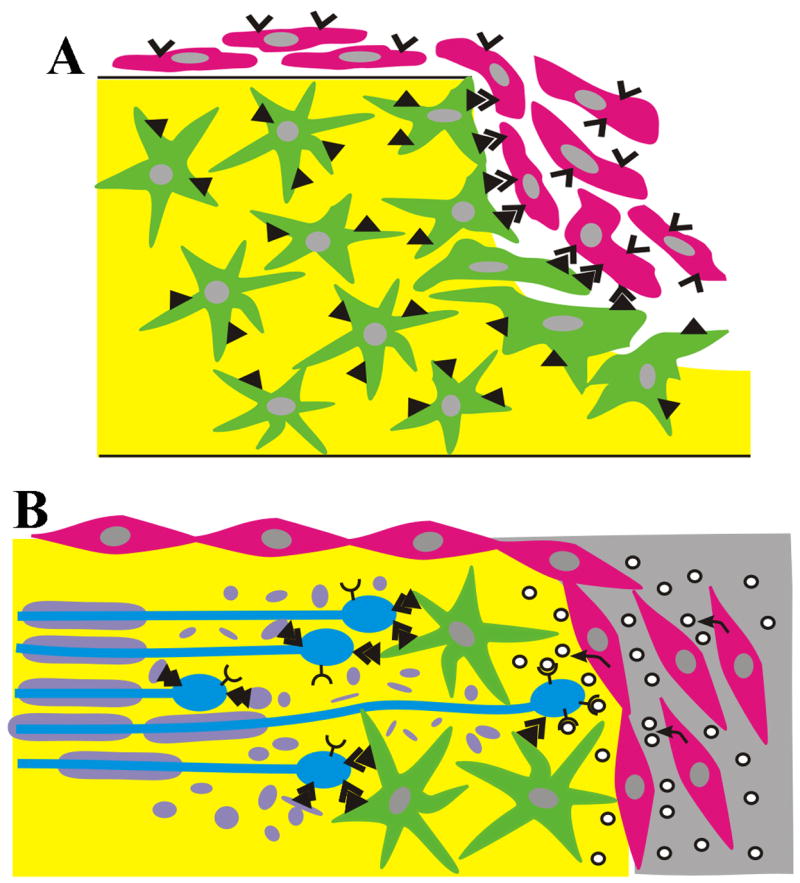
Spinal cord injury increases guidance factor expression that effects glial scar formation and axon regeneration. A) Fibroblasts (red) migrating into the wound cavity express EphB2 receptors (v) on fibroblasts and ephrin B2 ligand (▲) on astrocytes (green). The binding of EphB2 to ephrinB2 restricts cell migration and results in segregation and boundary formation between these two cell types. B) Fibroblasts migrating into the wound cavity secrete semaphorins (o) that act as chemorepulsive agents inducing growth cone collapse and stopping regenerating axons (blue). In addition, Eph/ephrin interactions between growing axons and either astrocytes or myelin (purple) prevent axons from regenerating.
Figure 2.
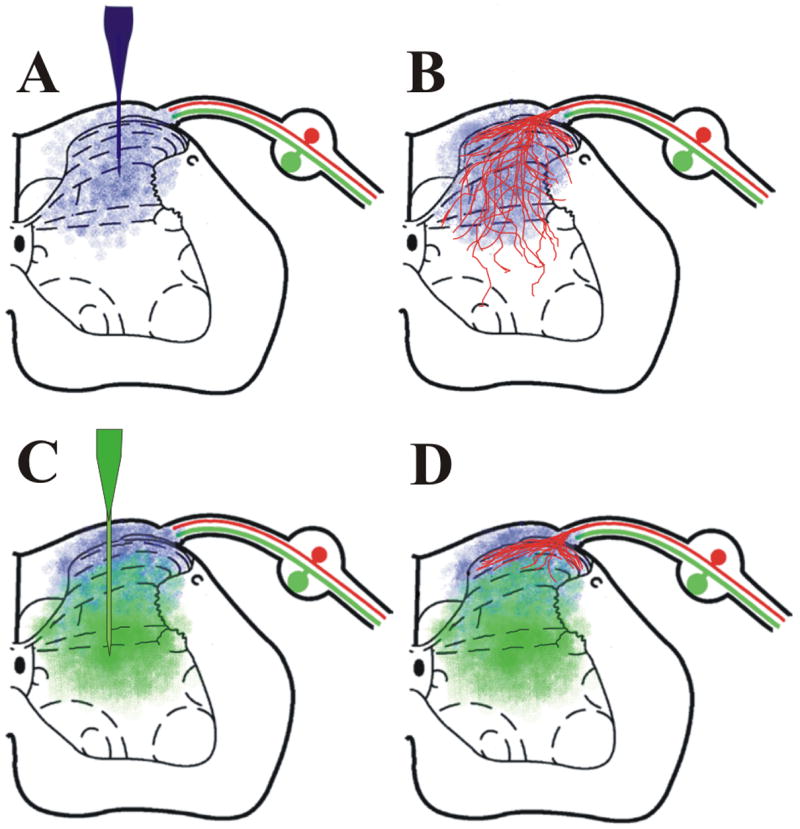
Overlapping gradients of NGF and semaphorin 3A restrict the regeneration of CGRP+ nociceptive axons to laminae I and II. A) Injection of NGF adenovirus into the dorsal horn of the spinal cord induces expression of a neurotrophin gradient (blue). B) This gradient supports the regeneration of peptidergic (CGRP+) nociceptive axons (red) throughout the entire dorsal horn, generating an abnormal innervation pattern. C) Co-expression of NGF (dorsally) and semaphorin 3A (ventrally, green) establishes slightly overlapping gradients that supports axon regeneration into the dorsal horn. D) As axons regenerate into the dorsal horn they encounter increasing amounts of semaphorin 3A that prevents their growth into deeper dorsal laminae to produce a reinnervation pattern similar to normal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Semaphorin Nomenclature Committee. Unified nomenclature for the semaphorins/collapsins. Cell. 1999;97(5):551–2. doi: 10.1016/s0092-8674(00)80766-7. [DOI] [PubMed] [Google Scholar]
- Bagnard D, Lohrum M, Uziel D, Puschel AW, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125(24):5043–53. doi: 10.1242/dev.125.24.5043. [DOI] [PubMed] [Google Scholar]
- Barallobre MJ, Pascual M, Del Rio JA, Soriano E. The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain Res Brain Res Rev. 2005;49(1):22–47. doi: 10.1016/j.brainresrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci U S A. 2005;102(30):10694–9. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96(6):795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Bundesen LQ, Scheel TA, Bregman BS, Kromer LF. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci. 2003;23(21):7789–800. doi: 10.1523/JNEUROSCI.23-21-07789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21(13):4731–9. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AA, Smith GM, Randall DC, Brown DR, Rabchevsky AG. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J Neurosci. 2006;26:2923–2932. doi: 10.1523/JNEUROSCI.4390-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MD, Hulsebosch CE. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Exp Neurol. 1997;147:463– 475. doi: 10.1006/exnr.1997.6608. [DOI] [PubMed] [Google Scholar]
- Cooper HM, Gad JM, Keeling SL. The Deleted in Colorectal Cancer netrin guidance system: a molecular strategy for neuronal navigation. Clin Exp Pharmacol Physiol. 1999;26(9):749–51. doi: 10.1046/j.1440-1681.1999.03106.x. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Saxena A, Chumley MJ, Silvany RE, Baker LA, Srivastava D, Henkemeyer M. Ephrin-B2 reverse signaling is required for axon pathfinding and cardiac valve formation but not early vascular development. Dev Biol. 2004;271(2):263–71. doi: 10.1016/j.ydbio.2004.03.026. [DOI] [PubMed] [Google Scholar]
- De Winter F, Oudega M, Lankhorst AJ, Hamers FP, Blits B, Ruitenberg MJ, Pasterkamp RJ, Gispen WH, Verhaagen J. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol. 2002;175(1):61–75. doi: 10.1006/exnr.2002.7884. [DOI] [PubMed] [Google Scholar]
- De Winter F, Vo T, Stam FJ, Wisman LA, Bar PR, Niclou SP, van Muiswinkel FL, Verhaagen J. The expression of the chemorepellent Semaphorin 3A is selectively induced in terminal Schwann cells of a subset of neuromuscular synapses that display limited anatomical plasticity and enhanced vulnerability in motor neuron disease. Mol Cell Neurosci. 2006;32(1–2):102–17. doi: 10.1016/j.mcn.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19(3):575–89. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006;22:651–75. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- Dottori M, Hartley L, Galea M, Paxinos G, Polizzotto M, Kilpatrick T, Bartlett PF, Murphy M, Kontgen F, Boyd AW. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc Natl Acad Sci U S A. 1998;95(22):13248–53. doi: 10.1073/pnas.95.22.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellezam B, Selles-Navarro I, Manitt C, Kennedy TE, McKerracher L. Expression of netrin-1 and its receptors DCC and UNC-5H2 after axotomy and during regeneration of adult rat retinal ganglion cells. Exp Neurol. 2001;168(1):105–15. doi: 10.1006/exnr.2000.7589. [DOI] [PubMed] [Google Scholar]
- Ellsworth CA, Lyckman AW, Feldheim DA, Flanagan JG, Sur M. Ephrin-A2 and -A5 influence patterning of normal and novel retinal projections to the thalamus: conserved mapping mechanisms in visual and auditory thalamic targets. J Comp Neurol. 2005;488(2):140–51. doi: 10.1002/cne.20602. [DOI] [PubMed] [Google Scholar]
- Fabes J, Anderson P, Yanez-Munoz RJ, Thrasher A, Brennan C, Bolsover S. Accumulation of the inhibitory receptor EphA4 may prevent regeneration of corticospinal tract axons following lesion. Eur J Neurosci. 2006;23(7):1721–30. doi: 10.1111/j.1460-9568.2006.04704.x. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, Kim YI, Bergemann AD, Frisen J, Barbacid M, Flanagan JG. Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron. 2000;25(3):563–74. doi: 10.1016/s0896-6273(00)81060-0. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, Nakamoto M, Osterfield M, Gale NW, DeChiara TM, Rohatgi R, Yancopoulos GD, Flanagan JG. Loss-of-function analysis of EphA receptors in retinotectal mapping. J Neurosci. 2004;24(10):2542–50. doi: 10.1523/JNEUROSCI.0239-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–45. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- Fu SY, Sharma K, Luo Y, Raper JA, Frank E. SEMA3A regulates developing sensory projections in the chicken spinal cord. J Neurobiol. 2000;45:227–236. [PubMed] [Google Scholar]
- Galko MJ, Tessier-Lavigne M. Function of an axonal chemoattractant modulated by metalloprotease activity. Science. 2000;289(5483):1365–7. doi: 10.1126/science.289.5483.1365. [DOI] [PubMed] [Google Scholar]
- Gavazzi I. Semaphorin-neuropilin-1 interactions in plasticity and regeneration of adult neurons. Cell Tissue Res. 2001;305:275–284. doi: 10.1007/s004410100365. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Galea MP, Wise G, Bartlett PF, Turnley AM. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci. 2004;24:10064–10073. doi: 10.1523/JNEUROSCI.2981-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, McLenachan S, Turnley A. Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res Rev. 2006;52(2):327–45. doi: 10.1016/j.brainresrev.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Grimaldi P, Carletti B, Roosi F. Neuronal replacement and integration in the rewiring of cerebellar circuits. Brain Research Reviews. 2005;49:330–342. doi: 10.1016/j.brainresrev.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289(5483):1360–5. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder J, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86(1):35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, et al. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7(5):501–9. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97(7):927–41. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401(6748):69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–63. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Huminiecki L, Gorn M, Suchting S, Poulsom R, Bicknell R. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics. 2002;79(4):547–52. doi: 10.1006/geno.2002.6745. [DOI] [PubMed] [Google Scholar]
- Itoh A, Miyabayashi T, Ohno M, Sakano S. Cloning and expressions of three mammalian homologues of Drosophila slit suggest possible roles for Slit in the formation and maintenance of the nervous system. Brain Res Mol Brain Res. 1998;62(2):175–86. doi: 10.1016/s0169-328x(98)00224-1. [DOI] [PubMed] [Google Scholar]
- Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, Blobel CP, Himanen JP, Lackmann M, Nikolov DB. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123(2):291–304. doi: 10.1016/j.cell.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Jen JC, Chan WM, Bosley TM, Wan J, Carr JR, Rub U, Shattuck D, Salamon G, Kudo LC, Ou J, Lin DD, Salih MA, Kansu T, Al Dhalaan H, Al Zayed Z, MacDonald DB, Stigsby B, Plaitakis A, Dretakis EK, Gottlob I, Pieh C, Traboulsi EI, Wang Q, Wang L, Andrews C, Yamada K, Demer JL, Karim S, Alger JR, Geschwind DH, Deller T, Sicotte NL, Nelson SF, Baloh RW, Engle EC. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science. 2004;304:1509–1513. doi: 10.1126/science.1096437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, Okano HJ, Ikegami T, Moriya A, Konishi O, Nakayama C, Kumagai K, Kimura T, Sato Y, Goshima Y, Taniguchi M, Ito M, He Z, Toyama Y, Okano H. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- Keleman K, Dickson BJ. Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron. 2001;32(4):605–17. doi: 10.1016/s0896-6273(01)00505-0. [DOI] [PubMed] [Google Scholar]
- Kennedy TE. Cellular mechanisms of netrin function: long-range and short-range actions. Biochem Cell Biol. 2000;78(5):569–75. [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998a;92(2):205–15. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Kidd T, Russell C, Goodman CS, Tear G. Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron. 1998b;20(1):25–33. doi: 10.1016/s0896-6273(00)80431-6. [DOI] [PubMed] [Google Scholar]
- King CE, Wallace A, Rodger J, Bartlett C, Beazley LD, Dunlop SA. Transient up-regulation of retinal EphA3 and EphA5, but not ephrin-A2, coincides with re-establishment of a topographic map during optic nerve regeneration in goldfish. Exp Neurol. 2003;183(2):593–9. doi: 10.1016/s0014-4886(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Kliot M, Smith GM, Siegal JD, Silver J. Astrocyte-Polymer implants promote regeneration of dorsal root fibers into the adult mammalian spinal cord. Exper Neurol. 1990;109:57–69. doi: 10.1016/s0014-4886(05)80008-1. [DOI] [PubMed] [Google Scholar]
- Knoll B, Isenmann S, Kilic E, Walkenhorst J, Engel S, Wehinger J, Bahr M, Drescher U. Graded expression patterns of ephrin-As in the superior colliculus after lesion of the adult mouse optic nerve. Mech Dev. 2001;106(1–2):119–27. doi: 10.1016/s0925-4773(01)00431-2. [DOI] [PubMed] [Google Scholar]
- Koch M, Murrell JR, Hunter DD, Olson PF, Jin W, Keene DR, Brunken WJ, Burgeson RE. A novel member of the netrin family, beta-netrin, shares homology with the beta chain of laminin: identification, expression, and functional characterization. J Cell Biol. 2000;151(2):221–34. doi: 10.1083/jcb.151.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeberle PD, Bahr M. Growth and guidance cues for regenerating axons: where have they gone. J Neurobiol. 2004;59:162–180. doi: 10.1002/neu.10345. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience. 1998;85:443– 458. doi: 10.1016/s0306-4522(97)00622-2. [DOI] [PubMed] [Google Scholar]
- Kullander K, Croll SD, Zimmer M, Pan L, McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD, et al. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 2001a;15(7):877–88. doi: 10.1101/gad.868901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3(7):475–86. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Kullander K, Mather NK, Diella F, Dottori M, Boyd AW, Klein R. Kinase-dependent and kinase-independent functions of EphA4 receptors in major axon tract formation in vivo. Neuron. 2001b;29(1):73–84. doi: 10.1016/s0896-6273(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, Rydstrom A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, et al. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999;96(6):807–18. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Lindholm T, Skold MK, Suneson A, Carlstedt T, Cullheim S, Risling M. Semaphorin and neuropilin expression in motoneurons after intraspinal motoneuron axotomy. Neuroreport. 2004;15(4):649–54. doi: 10.1097/00001756-200403220-00015. [DOI] [PubMed] [Google Scholar]
- Liuzzi FJ, Lasek RJ. Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science. 1987;237:642–645. doi: 10.1126/science.3603044. [DOI] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42(2):213–23. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- Maier IC, Schwab ME. Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity. Phil Trans R Soc B. 2006;361:1611–1634. doi: 10.1098/rstb.2006.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manitt C, Thompson KM, Kennedy TE. Developmental shift in expression of netrin receptors in the rat spinal cord: predominance of UNC-5 homologues in adulthood. J Neurosci Res. 2004;77(5):690–700. doi: 10.1002/jnr.20199. [DOI] [PubMed] [Google Scholar]
- Marillat V, Sabatier C, Failli V, Matsunaga E, Sotelo C, Tessier-Lavigne M, Chedotal A. The slit receptor Rig-1/Robo3 controls midline crossing by hindbrain precerebellar neurons and axons. Neuron. 2004;43(1):69–79. doi: 10.1016/j.neuron.2004.06.018. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, O’Leary DD. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–55. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron. 1995;14:949–959. doi: 10.1016/0896-6273(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Metin C, Deleglise D, Serafini T, Kennedy TE, Tessier-Lavigne M. A role for netrin-1 in the guidance of cortical efferents. Development. 1997;124(24):5063–74. doi: 10.1242/dev.124.24.5063. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19(6):1225–35. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, Love C, Jones EY, Kikutani H, Lubetzki C, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23(27):9229–39. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Ikeda T, Nishimura S, Tashiro K, Honjo T, Culotti JG, Itohara S. Netrin-G1: a novel glycosyl phosphatidylinositol-linked mammalian netrin that is functionally divergent from classical netrins. J Neurosci. 2000;20(17):6540–50. doi: 10.1523/JNEUROSCI.20-17-06540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Ma L, Wang KH, Marillat V, Sotelo C, Tessier-Lavigne M, Chedotal A. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J Neurosci. 2001;21(12):4281–9. doi: 10.1523/JNEUROSCI.21-12-04281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Marillat V, Kidd T, Goodman CS, Tessier-Lavigne M, Sotelo C, Chedotal A. Slit2-Mediated chemorepulsion and collapse of developing forebrain axons. Neuron. 1999;22(3):463–73. doi: 10.1016/s0896-6273(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet KT, Brose K, Marillat V, Sotelo C, Tessier-Lavigne M, Chedotal A. Sensory axon response to substrate-bound Slit2 is modulated by laminin and cyclic GMP. Mol Cell Neurosci. 2001;17(6):1048–58. doi: 10.1006/mcne.2001.0994. [DOI] [PubMed] [Google Scholar]
- Niclou SP, Jia L, Raper JA. Slit2 is a repellent for retinal ganglion cell axons. J Neurosci. 2000;20(13):4962–74. doi: 10.1523/JNEUROSCI.20-13-04962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterfield M, Kirschner MW, Flanagan JG. Graded positional information: interpretation for both fate and guidance. Cell. 2003;113:425– 428. doi: 10.1016/s0092-8674(03)00359-3. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Snider WD. Initial trajectories of sensory axons toward laminar targets in the developing mouse spinal cord. J Comp Neurol. 1997;380:215–229. [PubMed] [Google Scholar]
- Pasterkamp RJ, Anderson PN, Verhaagen J. Peripheral nerve injury fails to induce growth of lesioned ascending dorsal column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur J Neurosci. 2001;13(3):457–71. doi: 10.1046/j.0953-816x.2000.01398.x. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ, Ruitenberg MJ, Holtmaat AJ, De Wit J, De Winter F, Verhaagen J. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci. 1999;13(2):143–66. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]
- Petrausch B, Jung M, Leppert CA, Stuermer CA. Lesion-induced regulation of netrin receptors and modification of netrin-1 expression in the retina of fish and grafted rats. Mol Cell Neurosci. 2000;16(4):350–64. doi: 10.1006/mcne.2000.0877. [DOI] [PubMed] [Google Scholar]
- Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, Tessier-Lavigne M. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron. 2002;33(2):219–32. doi: 10.1016/s0896-6273(01)00586-4. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403:312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Raper JA. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol. 2000;10(1):88–94. doi: 10.1016/s0959-4388(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Renzi MJ, Wexler TL, Raper JA. Olfactory sensory axons expressing a dominant-negative semaphorin receptor enter the CNS early and overshoot their target. Neuron. 2000;28(2):437–47. doi: 10.1016/s0896-6273(00)00123-9. [DOI] [PubMed] [Google Scholar]
- Rodger J, Bartlett CA, Beazley LD, Dunlop SA. Transient up-regulation of the rostrocaudal gradient of ephrin A2 in the tectum coincides with reestablishment of orderly projections during optic nerve regeneration in goldfish. Exp Neurol. 2000;166(1):196–200. doi: 10.1006/exnr.2000.7486. [DOI] [PubMed] [Google Scholar]
- Rodger J, Goto H, Cui Q, Chen PB, Harvey AR. cAMP regulates axon outgrowth and guidance during optic nerve regeneration in goldfish. Mol Cell Neurosci. 2005;30(3):452–64. doi: 10.1016/j.mcn.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Rodger J, Lindsey KA, Leaver SG, King CE, Dunlop SA, Beazley LD. Expression of ephrin-A2 in the superior colliculus and EphA5 in the retina following optic nerve section in adult rat. Eur J Neurosci. 2001;14(12):1929–36. doi: 10.1046/j.0953-816x.2001.01822.x. [DOI] [PubMed] [Google Scholar]
- Rodger J, Vitale PN, Tee LB, King CE, Bartlett CA, Fall A, Brennan C, O’Shea JE, Dunlop SA, Beazley LD. EphA/ephrin-A interactions during optic nerve regeneration: restoration of topography and regulation of ephrin-A2 expression. Mol Cell Neurosci. 2004;25(1):56–68. doi: 10.1016/j.mcn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Romero MI, Rangappa N, Li L, Lightfoot E, Garry MG, Smith GM. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J Neurosci. 2000;20:4435– 4445. doi: 10.1523/JNEUROSCI.20-12-04435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MI, Rangappa N, Garry MG, Smith GM. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J Neurosci. 2001;21:8408–8416. doi: 10.1523/JNEUROSCI.21-21-08408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117(2):157–69. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87(6):1001–14. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Shen S, Wiemelt AP, McMorris FA, Barres BA. Retinal ganglion cells lose trophic responsiveness after axotomy. Neuron. 1999;23(2):285–95. doi: 10.1016/s0896-6273(00)80780-1. [DOI] [PubMed] [Google Scholar]
- Shifman MI, Selzer ME. Differential expression of class 3 and 4 semaphorins and netrin in the lamprey spinal cord during regeneration. J Comp Neurol. 2007;501(4):631–46. doi: 10.1002/cne.21283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki R, Katsumata R, Murakami F. Change in chemoattractant responsiveness of developing axons at an intermediate target. Science. 1998;279(5347):105–7. doi: 10.1126/science.279.5347.105. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281(5382):1515–8. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388(6639):275–9. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Song H, Poo M. The cell biology of neuronal navigation. Nat Cell Biol. 2001;3:E81–88. doi: 10.1038/35060164. [DOI] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–38. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- Stein E, Zou Y, Poo M, Tessier-Lavigne M. Binding of DCC by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science. 2001;291:1976–82. doi: 10.1126/science.1059391. [DOI] [PubMed] [Google Scholar]
- Symonds AC, King CE, Bartlett CA, Sauve Y, Lund RD, Beazley LD, Dunlop SA, Rodger J. EphA5 and ephrin-A2 expression during optic nerve regeneration: a ‘two-edged sword’. Eur J Neurosci. 2007;25(3):744–52. doi: 10.1111/j.1460-9568.2007.05321.x. [DOI] [PubMed] [Google Scholar]
- Symonds AC, Rodger J, Tan MM, Dunlop SA, Beazley LD, Harvey AR. Reinnervation of the superior colliculus delays down-regulation of ephrin A2 in neonatal rat. Exp Neurol. 2001;170(2):364–70. doi: 10.1006/exnr.2001.7722. [DOI] [PubMed] [Google Scholar]
- Tanelian DL, Barry MA, Johnston SA, Le T, Smith GM. Semaphorin III can repulse and inhibit adult sensory afferents in vivo. Nat Med. 1997;3:1398–1401. doi: 10.1038/nm1297-1398. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Cai J, Nelson KD, Peng XJ, Smith GM. Functional repair after dorsal root rhizotomy using nerve conduits and neurotrophic molecules. Eur J Neurosci. 2004a;20:1211–1218. doi: 10.1111/j.1460-9568.2004.03595.x. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Tanelian DL, Smith GM. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci. 2004;24:819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XQ, Heron P, Mashburn C, Smith GM. Targeting sensory axon regeneration in adult spinal cord. J Neurosci. 2007;27:6068–6078. doi: 10.1523/JNEUROSCI.1442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Triarhou LC, Low WC, Ghetti B. Intraparenchymal grafting of cerebellar cell suspensions to the deep cerebellar nuclei of pcd mutant mice, with particular emphasis on the re-establishment of a Purkinje cell cortico-nuclear projection. Anat Embryol. 1992;185:409–420. doi: 10.1007/BF00174079. [DOI] [PubMed] [Google Scholar]
- Wang H, Copeland NG, Gilbert DJ, Jenkins NA, Tessier-Lavigne M. Netrin-3, a mouse homolog of human NTN2L, is highly expressed in sensory ganglia and shows differential binding to netrin receptors. J Neurosci. 1999a;19(12):4938–47. doi: 10.1523/JNEUROSCI.19-12-04938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999b;96(6):771–84. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Webber CA, Hocking JC, Yong VW, Stange CL, McFarlane S. Metalloproteases and guidance of retinal axons in the developing visual system. J Neurosci. 2002;22(18):8091–100. doi: 10.1523/JNEUROSCI.22-18-08091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle R, Camand E, Chedotal A, Sotelo C, Dusart I. Expression of netrin-1, slit-1 and slit-3 but not of slit-2 after cerebellar and spinal cord lesions. Eur J Neurosci. 2005;22(9):2134–44. doi: 10.1111/j.1460-9568.2005.04419.x. [DOI] [PubMed] [Google Scholar]
- Whitford KL, Marillat V, Stein E, Goodman CS, Tessier-Lavigne M, Chedotal A, Ghosh A. Regulation of cortical dendrite development by Slit-Robo interactions. Neuron. 2002;33(1):47–61. doi: 10.1016/s0896-6273(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39:919–935. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Willson CA, Irizarry-Ramirez M, Gaskins HE, Cruz-Orengo L, Figueroa JD, Whittemore SR, Miranda JD. Upregulation of EphA receptor expression in the injured adult rat spinal cord. Cell Transplant. 2002;11(3):229–39. [PubMed] [Google Scholar]
- Wizenmann A, Thies E, Klostermann S, Bonhoeffer F, Bahr M. Appearance of target-specific guidance information for regenerating axons after CNS lesions. Neuron. 1993;11(5):975–83. doi: 10.1016/0896-6273(93)90126-c. [DOI] [PubMed] [Google Scholar]
- Yin Y, Sanes JR, Miner JH. Identification and expression of mouse netrin-4. Mech Dev. 2000;96(1):115–9. doi: 10.1016/s0925-4773(00)00369-5. [DOI] [PubMed] [Google Scholar]
- Yokoyama N, Romero MI, Cowan CA, Galvan P, Helmbacher F, Charnay P, Parada LF, Henkemeyer M. Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron. 2001;29(1):85–97. doi: 10.1016/s0896-6273(01)00182-9. [DOI] [PubMed] [Google Scholar]
- Yuan SS, Cox LA, Dasika GK, Lee EY. Cloning and functional studies of a novel gene aberrantly expressed in RB-deficient embryos. Dev Biol. 1999;207(1):62–75. doi: 10.1006/dbio.1998.9141. [DOI] [PubMed] [Google Scholar]
- Ziemba KS, Chaudhry N, Ngo TB, Smith GM. Targetting of axon growth from neurons transplanted into the central nervous system. Abstract submitted to American Society of Neural Transplantation and repair (ASNTR) 2004 [Google Scholar]
- Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102(3):363–75. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]


