Abstract
Patients with Alzheimer’s Disease (AD) and patients with Semantic Dementia (SD) both exhibit deficits on explicit tasks of semantic memory such as picture naming and category fluency. These deficits have been attributed to a degradation of the stored semantic network. An alternative explanation attributes the semantic deficit in AD to an impaired ability to consciously retrieve items from the semantic network. The present study used an implicit lexical-decision priming task to examine the integrity of the underlying semantic network in AD and SD patients matched for degree of impairment on explicit semantic memory tasks. The AD (n=11) and SD (n=11) patient groups were matched for age, education, level of dementia and impairment on four explicit semantic memory tasks. Healthy elderly participants (n=22) were matched for age and education. Semantic priming effects were evaluated for three types of semantic relationships (attributes, category coordinates, and category superordinates) and compared to lexical associative priming. Healthy controls showed significant priming across all conditions. In contrast, AD patients showed normal superordinate priming, and significant (although somewhat reduced) coordinate priming, but no attribute priming. SD patients showed no priming effect for any semantic relationship. All groups showed significant associative priming. The results indicate that SD patients do indeed have substantial degradation of semantic memory, while AD patients have a partially intact network, accounting for priming in superordinate and coordinate conditions. These findings suggest that AD patients’ impairment on explicit semantic tasks is the product of deficient explicit retrieval in combination with a partially degraded semantic network.
Introduction
Semantic memory is a distinct part of the declarative memory system (Tulving, 1972) comprising knowledge of facts, vocabulary, and concepts acquired through everyday life (Squire, 1987). Deficits of semantic memory are prominent in both Alzheimer’s disease (AD; Chertkow & Bub, 1990; Hodges, Salmon, & Butters, 1992; Hodges & Patterson, 1995; Martin & Fedio, 1983) and Semantic Dementia (SD; Hodges, Patterson, Oxbury, & Funnell, 1992; Snowden, Goulding, & Neary, 1989). AD patients often demonstrate a progressive decline in performance on tasks that are dependent upon semantic memory, including word finding and picture naming (Hodges & Patterson, 1995; Hodges et al., 1992; Rogers, Ivanoiu, Patterson, & Hodges, 2006). Patients with SD, the temporal variant of frontotemporal dementia, generally present with a more severe progressive impairment on such tasks (Hodges et al., 1992; Hodges, Patterson, & Tyler, 1994; Rogers et al., 2006).
Over the past two decades, there has been documentation of considerable impairment in performance on semantic memory-dependent tasks in AD patients (Chertkow, Bub, & Seidenberg, 1989; Chertkow & Bub, 1990; Hodges, Salmon, & Butters, 1992). This semantic task impairment can occur with a sparing of other linguistic abilities, such as phonology, prosody, and syntax, and only minor perceptual problems (Chertkow & Bub, 1990; Chertkow et al., 1989). Another neurological condition that impairs explicit semantic memory performance is Semantic Dementia, which presents initially as an isolated loss of semantic knowledge. Like the semantic deficit seen in AD, language functions such as syntax, phonology and prosody are initially unaffected in patients with SD.
Two primary theories have been proposed to explain the semantic deficits observed in patients with AD and patients with SD, on semantic memory tests such as the Hodges Battery (Hodges et al., 1992). Impairment on explicit semantic tests such as these could be linked to either a degradation of the internal semantic network, or to a failure to retrieve the information from that network. That is, it is possible that failure on explicit semantic tests may be the result not of impaired semantic representations per se, but of impairment in the conscious strategic processing needed to access those representations (Nebes, 1992). The integrity of the semantic representations themselves can be better assessed via an implicit processing task, such as priming, which does not require the use of such conscious strategic processing (Chertkow et al., 1989; Glosser & Friedman, 1991; Glosser, Friedman, Grugan, Lee, & Grossman, 1998; Nakamura, Nakanishi, Hamanaka, Nakaaki, & Yoshida, 2000; Ober & Shenaut, 1988). Shallice (1988) states, “On a degraded store deficit, if an item cannot be identified, it should not be possible to prime it.” But, if the semantic representations are not degraded but rather are inaccessible, semantic priming will be intact. Thus, to conclude that a semantic deficit is the result of damage to the semantic representations within the network, one must evaluate both implicit and explicit semantic processing.
Some researchers propose that the semantic deficit in Alzheimer’s disease reflects a degradation of the semantic network itself (Chertkow & Bub, 1990; Hodges & Patterson, 1995; Hodges, Salmon & Butters, 1992; Rogers et al. 2006), while others attribute the deficit to impaired retrieval from the network (Nebes, 1992; Nebes, Martin, & Horn, 1984). The most prevalent argument in support of semantic network damage is the consistency of impairment across a variety of semantic tasks (Chertkow & Bub, 1990; Hodges & Patterson, 1995; Hodges, Salmon & Butters, 1992). The similarity of deficient performance on the same semantic items in multiple tasks is attributed to the degraded representations of those specific objects. Hodges and Patterson (1995) furthered this argument by illustrating the impairment of visual semantic performance as well. The impaired abilities of AD patients on the PPT suggest that the overall task impairment extends beyond verbal semantic tasks. Hodges and Patterson (1995) argue that a retrieval deficit is unlikely to extend across differing modes of input and output. However, it could be argued that a retrieval deficit might cross modalities if it is caused by some impairment in a common mechanism, or mechanisms responsible for the conscious, effortful retrieval of semantic information from the network. For instance, Rich, Park, Dopkins, & Brandt, (2002) discovered that task structure affects the semantic performance of AD patients. AD patients required guidance and explicitly stated categories in order to properly sort pictures. The authors concluded that the “free-sorting” task required a greater degree of strategic processing, which is limited in AD patients. Possibly, AD patients could not strategically limit their search of the semantic system to only retrieve specific information without category information as a guide. This conclusion has been supported by the results of other studies (Moreaud, David, Charnallet, & Pellat, 2001; Nebes, 1992), which suggest that processing limitations could impede semantic performance on explicit tasks independently of any actual network damage. Moreaud et al. (2001) found that a large proportion of seemingly semantic errors in AD resulted from the inability to retrieve the phonological word form. Lastly, Cronin-Golomb, Keane, Kokodis, Corkin, and Growdon (1992) reached a similar conclusion. In that study, AD patients were able to accurately rank the typicality of category exemplars, but were far slower than controls. The results suggested that unspecified factors were limiting the retrievabilty of intact semantic items.
Tests of implicit semantic priming have been used to discriminate between possible causes of impaired semantic ability in AD as well. Nebes et al. (1984), in finding semantic priming effects in AD patients, suggest that the impairment witnessed on explicit semantic tasks must be limited to the conscious retrieval of semantic information from an intact network. Chertkow et al. (1989; Chertkow & Bub, 1990) found intact priming effects in AD patients, as did Ober and colleagues (Ober & Shenaut 1988; Ober, Shenaut, Jagust, & Stillman, 1991). Glosser et al. (1998) evaluated the semantic priming of different categorical semantic relationships and showed that AD patients retained priming for the higher-level, superordinate category labels (“daughter” priming “relative”) even though priming of category coordinates (“cousin” priming “nephew”) was absent. This result demonstrates a loss of priming for concepts with presumably weaker semantic connections and less overlap in attributes within the semantic network, and is suggestive of another issue to be considered in the examination of the semantic networks of AD patients: the nature of attribute storage. The “bottom-up” or “attribute-first” loss of semantic information was originally proposed by Warrington (1975) and has been supported in numerous studies since (Glosser et al., 1998; Martin & Fedio, 1983). The results from Glosser et al. (1998) appear to be an instance of such bottom-up loss, where only the highest-level superordinate connections are still intact. However, the study by Glosser et al. did not indicate the level of explicitly observable semantic impairment of their AD patients. Additionally, Glosser et al. (1998) did not test priming of specific attributes, the theoretical weakest link of the semantic network. Perhaps priming of attributes in demented patients would be reduced compared to either category labels or category coordinates.
An important variable that has not been adequately controlled in past priming experiments of AD is the degree of impairment on explicit semantic tasks. Both types of underlying impairments – network degradation and access/retrieval impairment – are expected to affect explicit tasks, while only degradation should impact on implicit tasks. Thus, to determine the contribution of a retrieval deficit, performance on both explicit and implicit tasks must be evaluated.
As is the case with AD, patients with SD demonstrate a high degree of impairment on all explicit semantic tasks (Hodges et al., 1992). Additionally, a consistency of specific item loss has been noted across different explicit tasks (Hodges et al., 1992, 1994; Tyler & Moss, 1998). Hodges et al. (1992) state that if one particular semantic item (e.g. mouse) is consistently impaired across all tasks, then that item’s internal representation within the network is probably lost. Even before SD was clinically defined, Warrington (1975) concluded that her three anomic patients, later identified as having SD, suffered from a semantic network disruption that was responsible for their pervasive loss of semantic knowledge. She reached this conclusion based on the patients’ thorough loss of semantic knowledge as observed through explicit semantic questions. While numerous studies have attributed the semantic memory loss in SD to semantic network degradation (Hodges et al., 1992, 1994; Nakamura et al., 2000; Rogers et al., 2006; Tyler & Moss, 1998; Warrington, 1975), there have not been reports linking the semantic task impairment in SD to a retrieval deficit, similar to what is suggested for AD.
As noted earlier, if the representation of a particular item within the semantic network is degraded, then the activation that can be spread through its connections will be degraded as well. Thus, a priming effect should not be found. Some support for this conclusion comes from the study by Tyler and Moss (1998), who examined one SD patient and found that, even early in the disease’s progression, priming on a lexical decision task was absent for semantically related words with low degrees of lexical association. The absence of semantic priming was shown for superordinate relationships, as well as for priming across category members and attributes. Thus, consistent with the conclusion of a semantic network disruption in SD (Hodges et al., 1992; Warrington, 1975), the impairment on explicit semantic memory tests seen on the Hodges et al. (1992) battery was accompanied by a significant deficit in implicit semantic processing as well. While the trend of these results supports the notion of a degradation of the semantic network in SD, there is a reason to treat this conclusion with caution, as only one patient was evaluated. Therefore, while the Tyler and Moss (1998) study points to the nature of the semantic impairment in SD, further studies are required.
Perhaps the best way to gain insight into the semantic deficits of AD and SD patients is to examine them in parallel. In clinical testing, AD patients typically present with impaired episodic memory and later develop substantial impairments on explicit semantic memory tasks (Hodges, Salmon, & Butters, 1992). In contrast, SD patients initially present with the impaired explicit semantic task performance, while having intact episodic memory (Perry & Hodges, 2000). Considering the substantial variation in temporal atrophy across the patient populations it would seem unlikely that the groups share the same mechanism of semantic memory loss. As stated previously, SD patients’ temporal damage closely coincides with the locus of the semantic network, while the medial temporal atrophy of AD patients is typically tied to their episodic memory loss. This suggests that the deficient impaired performance in SD might be the result of a degraded network, while the problem in AD may be based on retrieval difficulties in addition to a partially degraded semantic system. This would be consistent with prior priming studies, which found greatly reduced priming in SD (Tyler & Moss, 1998) and relatively intact semantic priming in AD (Ober & Shenaut, 1988; Ober et al., 1991).
Nakamura et al. (2000) examined this hypothesis and did find priming in AD but not in SD. Unfortunately, this study had a very small number of subjects, did not examine priming for various semantic relationships, and the AD and SD patients were not matched for level of basic impairment on explicit semantic tasks. Even though the findings of Nakamura et al. (2000) support the current hypothesis, the significantly worse explicit semantic performance in the SD group confounds the results. That study leaves open the possibility that the impairment observed in the two groups was caused by the same underlying deficit, but the SD patients were more advanced in their semantic network degradation, as was concluded by Rogers et al. (2006). Given the individual problems with this and other studies of priming, further investigation is required to further elucidate semantic priming in AD and SD patients.
As seen with the Nakamura et al. (2000) study, comparisons between AD and SD patients are difficult to interpret because SD patients appear to have a far more severe impairment of the semantic memory network than patients with AD. Perry and Hodges (2000) demonstrated a significant difference between the two patient populations, matched for MMSE scores and length of disease duration, on tasks of category fluency, picture naming and PPT. However, MMSE and length of disease duration are not the best variables on which to match the populations when considering the questions at hand. In posing questions about the comparative underlying impairments responsible for the observed explicit semantic deficits, it is crucial that the two groups be matched on degree of impairment on explicit semantic tasks. Only by matching the groups on tests of explicit semantic processing can we determine whether the performance deficits on these tasks stem from the same underlying impairments in AD and SD.
While no comparative studies have yet matched these two groups in this way, finding subsets of the two patient populations with equivalent explicit semantic task deficits is critical. Using the Hodges et al. (1992) battery as a guideline, an overlap can be found in the level of explicit semantic memory performance seen in the AD and SD patients documented in different studies (Bozeat, Lambon Ralph, Patterson, Garrard, & Hodges, 2000; Hodges & Patterson, 1995; Hodges et al., 1992). The goal of this study is to examine AD and SD patients who have matching impairments on explicit semantic tasks, in order to explore the possibility of differences in the mechanisms of their semantic memory loss.
In this study, semantic priming is used to examine the implicit semantic processing of AD and SD patients who are matched for explicit semantic task performance. The priming effects for different levels within the semantic network (Superordinate, Coordinate, and Attributes) are examined in order to support or refute the hypothesis of partial degradation of the semantic network in AD and the complete loss of the semantic network in SD. Finally, the results of the study are analyzed to determine which semantic model best explains the semantic priming results.
Method
Participants
Each of the two patient groups consisted of 11 individuals, one group diagnosed with probable Alzheimer’s disease (AD) and the other with Semantic Dementia (SD). The AD patient group was recruited from the Georgetown University Medical Center’s (GUMC) Memory Disorders Clinic where they were diagnosed with probable Alzheimer’s according to the NINCDS-ADRDA criteria (McKhann et al. 1984). SD patients were diagnosed in the University of Pennsylvania Department of Neurology using the established Neary et al. (1998) criteria. The overall level of cognitive decline was measured in both institutions with the Mini Mental State Examination (Folstein et al. 1975). The mean scores for the AD (21.5) and SD (22.0) patients were not significantly different, and indicated a level of dementia in the mild to moderate range. Prior to beginning the study, all of the patients were demonstrated to have sufficient visual, auditory, and language abilities required to complete the tasks. The control group, recruited from the Washington, DC area, consisted of 22 participants with no known neurological or psychological impairments. The three groups did not differ significantly in age (AD = 74.1, SD = 70.5, C = 69.5) or years of education (AD = 17.8, SD = 15.6, C =17.6). All 44 participants were right-handed native English speakers.
Explicit Semantic Battery
A variation of the Hodges et al. (1992) semantic testing battery was used to match patient groups for explicit semantic impairment. Four semantic tests requiring verbal and nonverbal access and retrieval from the semantic system were included. These tests were Category Fluency, Picture Naming, Word-Picture matching, and the picture version of the Pyramids and Palm Trees test (Howard & Patterson, 1992).
The Picture Naming and Word-Picture Matching tasks each utilized the same 48 items, all taken from the Snodgrass and Vanderwart (1980) corpus of black and white line drawings. These 48 semantic items were chosen from 6 semantic categories and were matched for name agreement and category typicality (Battig & Montague, 1969). During the Word-Picture Matching task, participants were asked to point to the picture named aloud. There were six pictures on the screen for each trial and each of the five foils were from the same superordinate semantic category as the target item. Six separate semantic categories (three living and three non-living) were used for the Category Fluency task. These included Land Animals, Birds, and Water Creatures for the living categories and Household Items, Musical Instruments, and Vehicles, for the non-living.
Inclusion Criteria
To be included in the study, all patients had to demonstrate a degree of semantic impairment on the explicit semantic battery as follows: Impaired performance (Z≤ −2) on the Picture-Naming task was required for all patients. Additionally, patients were to show impairment on at least one other explicit semantic test (Fluency, WPM or PPT).
Table 1 shows the average performance for the three groups on the explicit semantic battery. Using planned comparisons (paired t-test), both patient groups were significantly impaired on all of the semantic tasks as compared to the control participants. The AD and SD patients were well matched for their explicit semantic impairment, except for a small but significant difference on the word picture matching task (t(20) = −2.17, p < .05).
Table 1.
Explicit Semantic Tasks
| Controls (22) | AD (11) | SD (11) | |
|---|---|---|---|
|
|
|||
| Naming (48) | 46.86 (43–48) | 32.55 (12–42) | 32.00 (18–42) |
| WPM (48) | 47.68 (47–48) | 46.09 (40–48)* ↔ | 43.09 (35–48) * |
| PPT (52) | 51.41 (50–52) | 43.82 (33–52) | 42.64 (24–52) |
| Fluency -Living | 46.59 (27–60) | 18.09 (3–29) | 10.45 (0–27) |
| Fluency -Nonliving | 51.95 (31–65) | 19.73 (4–29) | 15.00 (0–31) |
= p < .05
Priming Stimuli
Stimuli for the oral lexical decision task were divided into four distinct priming sets, each containing 20 priming pairs and 20 control pairs. The three semantic priming sets were Superordinate Category Labels (walnut - WOOD), Category Coordinates (cherry - APPLE), and Attributes (couch - FABRIC). A Lexical Associate priming set (needle - HAYSTACK) was included as a control condition (see Glosser & Friedman 1991). Priming pair construction closely followed the methods described in Glosser et al. (1998). Since a high level of lexical association within the prime-target pairs is potentially confounding (Glosser & Friedman 1991), all priming pairs in the three semantic sets were low in association value according to the Postman and Keppel (1980) and Birkbeck (Moss & Older 1996) norms for word association. For further verification, 20 normal adults over the age of 50 were asked to “name five words they associate” with each target. For the category Label (SP) and category coordinate (CP) conditions, a target word was acceptable only if it was neither named by any of the normals nor listed as an associate for the prime word in either published corpus. On the contrary, the pairs in the lexical associate (ASP) set were intentionally chosen to be highly associated on both the published and normed lists. The attribute (AP) pairs were chosen by asking the same 20 normal adults to “Name five features and attributes” for a new set of words. An attribute was chosen for the priming pair if it was provided at least once as an attribute but was not listed as an associate in the two published lists.
The control pairs in each of the four conditions were constructed by rearranging the prime-target pairs. Thus, each target word would be preceded once by an unrelated prime word. For example, the priming pairs “bomb – WEAPON” and “ dill – SPICE” were rearranged to create the control pairs “dill – WEAPON” and “ bomb – SPICE.” This method allowed each target word to serve as its own control. All prime and target words were nouns and were matched for word length (average length of 5.9 letters), frequency (average frequency of 24.9), and category typicality (average typicality of 9.3) across sets. Each set also included 50 pseudoword used as primes and 50 pseudoword used as targets, as well as 20 filler words used as both primes and targets. Pseudowords were chosen to be both pronounceable and similar to real words. Filler words were nouns not associated nor semantically related to any of the other words within the sets. Pseudowords were matched for length (6.1 letters) and filler words were not significantly different in length (5.6 letters) or frequency (Average frequency of 33.8) from the prime and target words.
Each set was divided into five blocks of 20 lexical decision trials. This produced a total of 100 lexical decisions per set. Of the 20 word pairs in a given block, there were four prime-target pairs, four control pairs (unrelated prime-target), eight pseudoword-pseudoword pairs, two pseudoword-filler word pairs, and two filler word-pseudoword pairs. This combination yielded an equal number of “yes” and “no” responses, as well as an equal number of pseudowords and real words used in each block.
Only four of the 20 word pairs per set were related prime-target pairs, yielding a relatedness proportion of 0.2. This low relatedness proportion minimizes predictability, and is necessary to maintain an automatic level of semantic priming. Ober (2002) found that a high level of predictability in priming experiments could induce strategic processing, which is not the goal of this study.
The order of the word pairs in each set was pseudo-randomized with three primary constraints. First, if a specific target word was used in a prime-target pair in the first half of a set, it would then be used in a control pair in the second half of the same set, and vice versa. Second, pseudowords were arranged within the set to minimize any similarity with the prime or target words in their immediate proximity. Third, filler words were placed in a manner to avoid phonologic or orthographic assistance to later target words.
Priming Procedure
The lexical decision task was presented on a computer using the Superlab program. Each lexical decision trial began with a fixation cross located at the middle of the screen. The subject was instructed to press the spacebar to begin the trial. The first, prime word was presented in lower case letters on the screen for 200 ms followed by a visual mask (XXXXXXX) shown for 50 ms. This timing produced a 250 ms SOA for each trial. The target word was then presented in all capital letters and remained on the screen until the verbal lexical decision was recorded. After the target word was removed from the screen, the fixation cross reappeared in preparation for the next trial. Since the lexical decision procedure was based on word pairs, the subjects were instructed to only respond to the second word of each pair, shown in all capital letters.
Each lexical decision was measured by timing the verbal “yes” or “no” response. The responses were recorded using a computer microphone and timed using Superlab software (Abboud, 1991). The exact timing was measured from the initial presentation of the target word to the beginning of the verbal response. The verbal response was also recorded using a digital recorder for backup verification of the response timing. The experimenter manually recorded the response as a yes, no, or error on paper in order to assess accuracy. A response was coded as an error if the subject produced any extraneous word or noise that could affect the timing accuracy.
The priming effect for each participant was determined by subtracting the average response time for the primed target words from the average time for the unprimed target words. For some subjects, a few trials were removed from the calculations if the response time for that individual trial was greater than 2 standard deviations from the mean response time.
The 4 priming sets were given in a random order to each of the subjects. Each subject completed two priming sets, had a break, and then completed the final two priming sets. A practice lexical decision block was given at the beginning of the session.
Results
The measure of accuracy was percent correct oral responses (“Yes” or No”) to the target word. Following the standard convention (Ober 2002), the measure of the priming effect was the mean reaction time for the unprimed target words minus the mean reaction time for the primed target words1. The within and between subject comparisons of priming effect were analyzed with planned comparisons (paired t-tests) and a Bonferroni correction was used to account for the number of comparisons performed. This method of analysis was chosen because of the nature of the a priori focused comparisons. The Bonferroni correction was added to ensure that there were no undue false positives in the statistical analysis.
All patients and controls were able to complete the study with minimal difficulty. The overall lexical decision accuracy of the two patient groups (AD = 91.8 %, SD = 90.8 %) was not significantly different, and this high level of accuracy suggested that neither group was impaired in basic lexical processing. While the patients’ overall accuracy was less than controls (95.4%), the majority of the lexical decision errors in the two patient groups were false positives (AD = 86.5% of errors, SD = 80.3% of errors), where pseudowords were incorrectly identified as real words. As such, the priming-effect calculations for the target and control real words were not adversely affected by a high error rate in patients compared to normal controls. Further, the analysis was not hindered by a large number of recording or verbal production errors. On average, only 1–2 responses, deemed outliers for either unregistered responses or long reaction times, were removed from the calculations of any given set. That would account for fewer than 5% of the responses being removed from any group’s analysis. Notably, the average reaction times for the two patient groups were more than 300 ms slower than the control group (Cont =898.1 ms; AD = 1245.4 ms; SD =1243.9 ms). This delayed processing was expected, attributable to the typical cognitive decline present in dementia, and was not factored into the priming effect analysis. Lastly, there was not a significant effect for either word order presentation within a set or set order presentation within the study.
Figures 1–3 illustrate the within group comparisons for the four priming sets. As expected, control subjects (Figure 1) demonstrated a significant priming effect for each condition (ASP: t(21) = 13.33, p < .01; SP: t(21) = 16.27, p < .01; CP: t(21) = 11.56, p < .01; AP: t(21) = 12.9, p < .01). There was no significant difference between the ASP, SP, and CP priming sets, with a small, albeit statistically significant difference seen for the AP condition (CP v AP: t(21) = 3.48, p = .01). These results indicated that the Oral Lexical Decision Task was an effective measure to determine implicit priming for both associative and semantic relationships.
Figure 1.
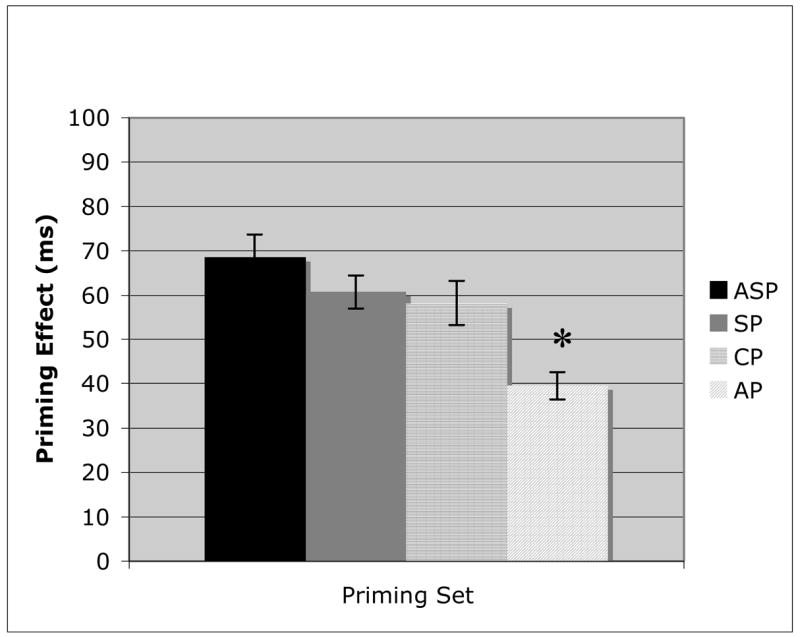
Priming results for the control participants on Associative priming (ASP), Superordinate category label priming (SP), Coordinate category member priming (CP), and Attribute priming (AP). Error bars represent the standard error from the mean. (* = p = .01).
Figure 3.
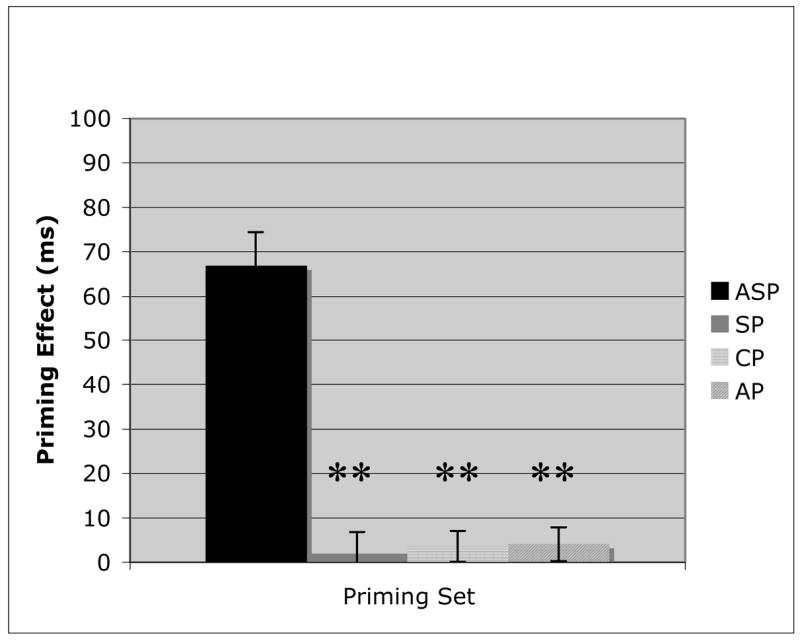
Priming results for the Semantic Dementia patients on Associative priming (ASP), Superordinate category label priming (SP), Coordinate category member priming (CP), and Attribute priming (AP). Error bars represent the standard error from the mean. (** = p < .01).
AD patients (Figure 2) had their largest priming effects for the ASP (t(10) = 10.66, p < .01) and SP (t(10) = 10.88, p < .01) conditions, with no significant difference between them. However, while the CP condition’s priming effect was significantly greater than 0 (t(10) = 9.24, p < .01), indicating the presence of priming, the effect was significantly smaller than either SP (t(10) = 3.9, p = .015) or ASP (t(10) = 5.5, p < .01). Finally, there was no priming effect observed in the AP condition, which was significantly reduced (CP v AP: t(10) = 7.28, p < .01) compared with all other priming conditions. SD patients (Figure 3) demonstrated a unique pattern to their priming results. While there was a significant priming effect (t(10) = 8.72, p < .01) for the lexically associated word pairs (ASP), there was no priming observed for any of the three semantically based priming sets. Each semantic condition was significantly worse (ASP v SP: t(10) = 6.96, p < .01; ASP v CP: t(10) = 7.45, p < .01; ASP v AP: t(10) = 7.33, p < .01) than the non-semantic condition, and none was significantly greater than 0.
Figure 2.
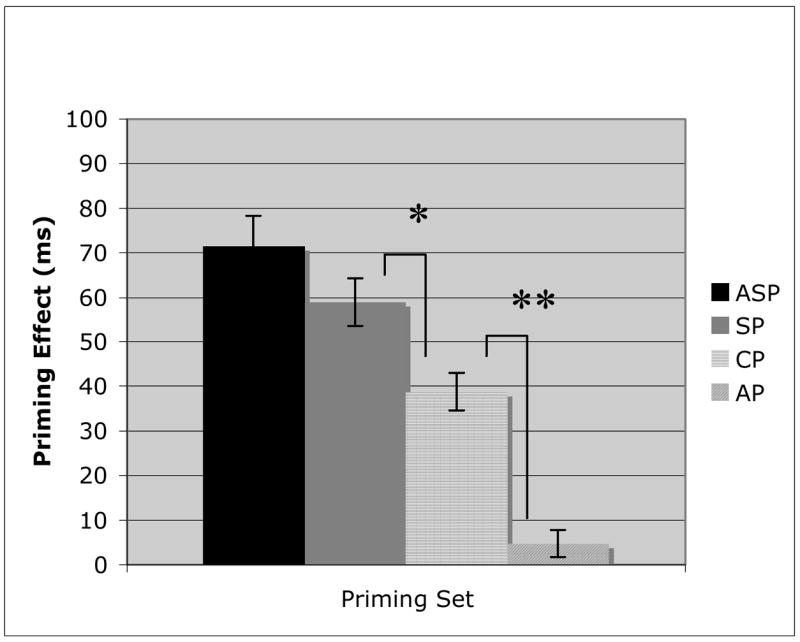
Priming results for the Alzheimer’s Disease patients on Associative priming (ASP), Superordinate category label priming (SP), Coordinate category member priming (CP), and Attribute priming (AP). Error bars represent the standard error from the mean. (* = p =.015; ** = p < .01).
The between group comparison of ASP (Figure 4) demonstrates that all three participant groups had roughly equivalent priming performance for the lexical associates. This control condition illustrates that priming effects can be accurately assessed in dementia populations. The three semantic priming sets (Figures 5–7) indicate markedly different patterns for each of the three groups. While AD patients and controls demonstrate similar priming effects on the highest order semantic labels condition (SP), the SD patients proved to be significantly worse (Figure 5; AD v SD: t(20) = 7.52, p < .01). These findings provide the first evidence that there is a clear distinction between AD and SD semantic processing. In the coordinate category member set (Figure 6), the AD patients demonstrate impaired semantic priming abilities, with a significantly worse effect than control participants (t(31) = 2.5, p = .03), but still significantly better than SD patients (t(20) = 6.31, p < .01). Finally, in the AP condition (Figure 7), there was not a significant difference between the two patient groups, although both were significantly worse than the control group (AD: t(31) = 7.2, p< .01; SD: t(31) = 6.93, p< .01). The decrease in priming for AD patients across the three semantic conditions suggests a continuum of semantic memory loss, dependent on the nature of the semantic relationship. The between group comparisons demonstrated that this gradual semantic loss that occurs in AD, is not characteristic of SD.
Figure 4.
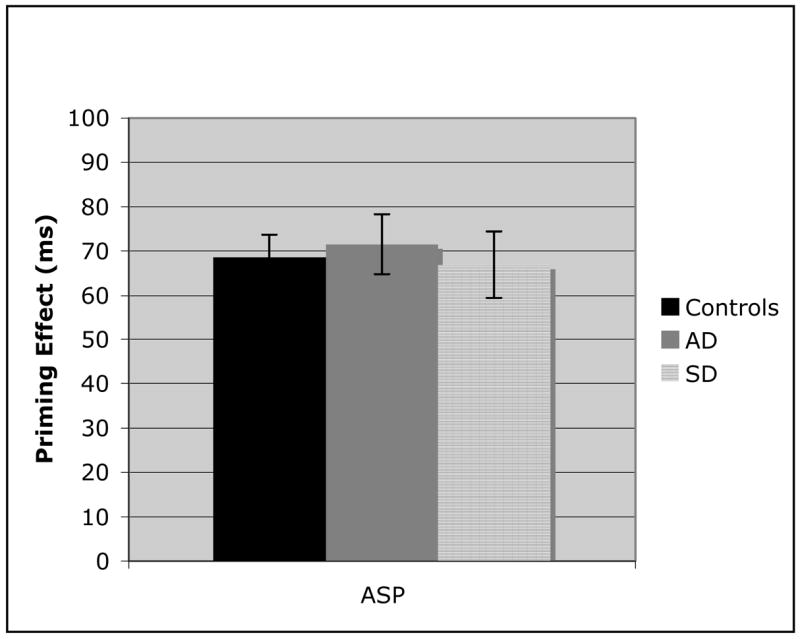
Analysis of the associative priming effects for control, Alzheimer’s disease (AD), and Semantic dementia (SD) populations. Error bars represent standard error from the mean.
Figure 5.
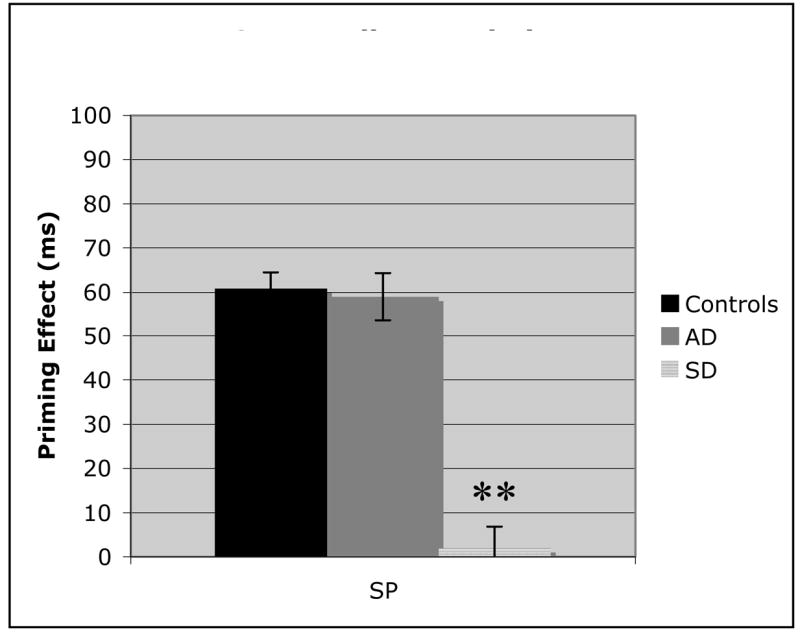
Analysis of the superordinate category label priming effects for control, Alzheimer’s disease (AD), and Semantic dementia (SD) populations. Error bars represent standard error from the mean. (** = p < .01).
Figure 7.

Analysis of the attribute priming effects for control, Alzheimer’s disease (AD), and Semantic dementia (SD) populations. Error bars represent standard error from the mean. (** = p < .01).
Figure 6.
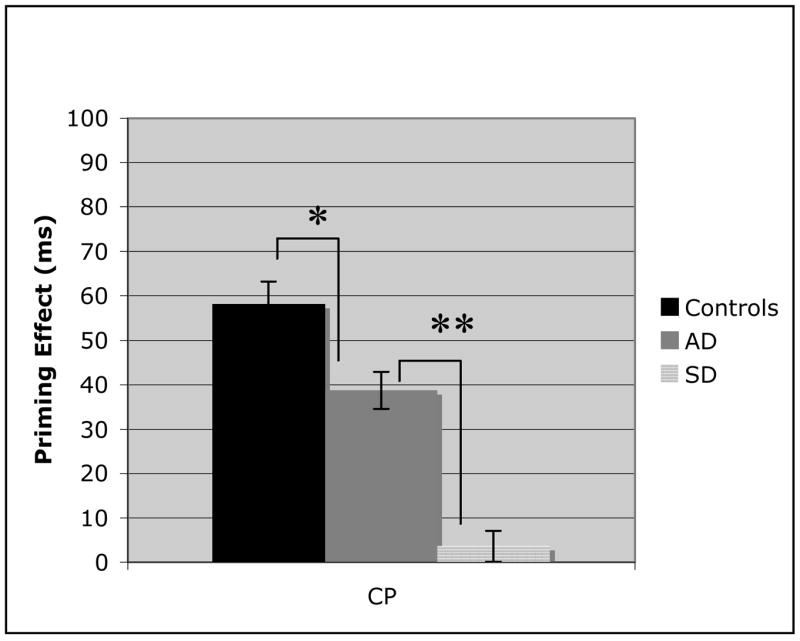
Analysis of the coordinate category member priming effects for control, Alzheimer’s disease (AD), and Semantic dementia (SD) populations. Error bars represent standard error from the mean. (* = P <=.03; ** = p < .01).
As noted previously, comparing the semantic priming effects of AD and SD patients would not be valid if performance on explicit tasks were not equated. The current study matched the patients groups on picture naming, while maintaining roughly equivalent performances on Category Fluency, WPM, and the PPT (Table 1). The patient groups were significantly different only on WPM. There was no significant difference between the patient groups on Fluency or PPT. However, there was a trend towards better performance by the AD patients, and it is conceivable that with increased N this trend would be significant. Thus, there is a possibility that the SD patients had a greater overall semantic impairment resulting in their apparent decreased semantic priming effect.
To evaluate this possibility, a subset of the patient groups were identified for whom scores on the explicit tests were nearly identical, or the SD patients’ scores were actually better than the AD patients’ scores. The results for this subset are shown in Table 2 and these subset comparisons of explicit tests were analyzed with planned comparisons (t-tests). There were no statistically significant differences between the AD and SD patients on any of the explicit tasks and thus there was no need for a Bonferroni correction for this comparison.
Table 2.
Mean Experimental Performance for Closely Matched Alzheimer’s Disease (AD) and Semantic Dementia (SD) Subsets.
| Task | AD (6) | SD (6) | |
|---|---|---|---|
| Explicit Semantic Battery | Naming | 29.2 | 35.3 |
| Fluency – Living | 13.7 | 13.5 | |
| Fluency – Nonliving | 16.7 | 18.0 | |
| PPT | 41.8 | 43.2 | |
| WPM | 45.0 | 44.8 | |
| Priming Sets | ASP (ms) | 69.1 | 60.6 |
| SP (ms) | 57.0 ** ↔ | 0.2 ** | |
| CP (ms) | 43.1 ** ↔ | 7.2 ** | |
| AP (ms) | 1.6 | 5.4 |
= p < .001
Analysis of these subsets illustrated the reliability of the overall priming results. An analysis of variance (ANOVA) was used to evaluate the priming results for the two subsets. An ANOVA was used at this stage as no specific planned comparisons had been set out for this post-hoc analysis. The ANOVA demonstrated a significant group effect between the AD and SD subsets for the SP (F(3, 18) = 17.28, p < .001) and CP (F(3, 18) = 16.32, p < .001) conditions only. Additionally, the pattern of priming effects in the subsets were nearly identical to the overall experimental results, with no semantic priming for SD patients and varying levels of semantic priming in AD. These findings demonstrate a consistent maintenance of semantic priming in AD, even in the patients with the worst explicit semantic performance. This sharply contrasts with the loss of semantic priming in SD patients, independent of explicit semantic task ability.
Discussion
The healthy control participants demonstrated priming effects in all four experimental conditions, affirming the implicit connections between words at both lexical and semantic levels. These findings are consistent with previously published results (Glosser & Friedman 1991; Glosser et al. 1998) and further validate the need to examine semantic priming independently of lexical associative priming. Moreover, the control participants confirm that semantic priming effects can be obtained using varied semantic relationships. Extending the results of Glosser et al. (1998), superordinate category label and category member conditions yield the strongest priming effects. In addition, the healthy control participants also provided insight into the priming of attributes. Data from the control subjects in this study illustrate that semantic attributes – even those with a low level of lexical association as were used in the present study - can elicit a significant priming effect, albeit at a reduced level compared to other semantic relationships.
The SD patients showed no priming effect for any of the three semantic conditions. These findings closely match the results obtained in earlier studies of semantic priming (e.g. Nakamura 2000; Tyler & Moss 1998). The implicit semantic deficit seen in this experiment, paired with the impairment on the explicit semantic memory tasks, supports the conclusion that SD patients have a severe disruption of the central semantic network. Indeed, the consistent semantic memory deficit demonstrated across all semantic memory tasks confirms a generalized semantic loss, which impairs semantic performance equally, regardless of task constraints.
The pattern of performance for the AD patients partially replicates the findings of Glosser et al. (1998). As with the SD patients, lexical associative priming was maintained at normal levels in the AD group. This result confirms intact lexical representations in the AD population consistent with previous published results (Friedman, Ferguson, Robinson, & Sunderland, 1992; Glosser & Friedman, 1991; Glosser et al., 1998; Hodges & Patterson 1995; Nebes 1992). Also, in accordance with the results of Glosser et al. (1998), the semantic priming effect for AD patients was dependent on the nature of the semantic relationships used in the priming pairs. AD patients show a normal level of priming for category labels. This result matches with the consistent finding that superordinate information is retained even on explicit semantic tasks. The intact priming in the superordinate category condition illustrates a degree of implicit connectivity within the semantic network. According to the Collins and Loftus (1975) model, successful semantic priming illustrates spreading activation through the semantic memory network, which requires intact connections within the system. Taken alone, this finding of semantic priming without the assistance of lexical association would suggest a functional semantic network in the AD patients as concluded in previous studies (Nakamura et al. 2000; Nebes 1992; Nebes & Brady 1990; Nebes, Brady, & Huff 1989; Nebes et al. 1984). However, the significant decline in priming for coordinate category members and attribute conditions is indicative of at least a partial disruption of the implicit spreading activation in the central semantic storage. Unlike the results of Glosser et al. (1998), AD patients did have a significant priming effect for category coordinates, although at a significantly reduced level compared to healthy control participants, suggesting at least a mild semantic disturbance. The complete lack of attribute priming in AD patients was comparable to the SD group, demonstrating a complete disconnect at the semantic attribute level.
This study improves on previous studies with regard to task design and patient selection. The threshold oral reading task of Glosser et al. (1998) may not have been sensitive enough to pick up a priming effect for category coordinates. In fact, AD patients did show a trend towards a priming effect in the coordinate condition, but it did not reach significance in that study. However, the ratio of superordinate to coordinate priming in AD is not dissimilar to the results of this study. Perhaps, if Glosser et al. (1998) had used more subjects, resulting in greater power, a priming effect would have been observed for category coordinates.
Also, while we found reduced priming for AD patients in two of the three semantic priming conditions, other studies have found increased priming, or “hyperpriming,” in AD patients compared normal controls (Giffard et al. 2001; Nebes et al. 1989). Giffard et al. (2001) attributed this result to the general cognitive slowing that occurs during dementia. Specifically, the AD patients who took much longer on the unprimed trials than the healthy aged participants could theoretically show a greater drop when they receive assistance in the primed trials. However, in the current study, the cognitive slowing was not as much of a factor because the button press used by Giffard et al. (2001) was eliminated. Therefore, a large disadvantage to the patient groups was removed.
While our study does partially replicate the findings of Nakamura et al. (2000), the criteria for patient inclusion was far better controlled in the current study. Nakamura et al. found a priming effect equivalent to normal controls using primarily category coordinates. However, as stated previously, the AD patients had a far milder explicit semantic impairment than the SD patients. It is therefore understandable that the patients would not produce the drop-off in coordinate priming. Finally, as the current study included some patients with moderate dementia, a better understanding of the progression of semantic loss was possible.
The three semantic priming conditions in the current study offer a unique perspective of the mechanism of semantic memory loss in AD. It is apparent from the combination of explicit and implicit task impairment that SD patients have severe damage throughout their semantic system. The nature of the semantic deficit in AD patients is less clear-cut. On the surface, all of the AD patients appear to have a severe semantic system deficit, as measured by performance on the Hodges et al. (1992) battery. In fact, on almost every explicit semantic test, the AD patients were nearly identical to the patients with SD. This finding alone would typically lead to the assumption that both groups have equivalent underlying mechanisms of semantic loss. However, the dissociation in implicit semantic performance tells a different story. The complete maintenance of superordinate priming and partial maintenance of coordinate priming point to intact concepts and connections at the heart of the semantic system. These findings were confirmed by the subset analyses, which demonstrated consistent semantic priming in AD that was not affected by the level of explicit semantic task performance. Therefore, we conclude that AD patients do not have semantic system damage to the same extent as SD patients. Nevertheless, we conclude that the semantic network is not completely intact in AD. The lack of attribute priming, coupled with the mild reduction of coordinate priming, indicates that some amount of semantic damage has occurred, acting to impede the implicit spreading activation.
If the AD patients have a mild disruption of their semantic system, and the SD patients have a more severe disruption, then how are we to account for the equivalent impairment of explicit semantic processing for these two groups? The most logical interpretation is that AD patients suffer from a dual impairment: a partially damaged semantic network, and deficient retrieval from that network.
Consider the pattern of explicit task results in the context of this hypothesis. The centralized semantic system damage in SD affects all of the explicit semantic tasks in a consistent manner. It is proposed that in AD, the same explicit semantic tasks are affected not only by a mild semantic system impairment (Hodges & Patterson, 1995; Hodges, Salmon, & Butters, 1992), but also by impaired conscious, effortful retrieval (Nebes & Brady, 1990; Nebes et al., 1984, 1989). Therefore, the nature of each task will affect AD patients’ performance. Verbal production tasks such as Picture Naming and Category Fluency would be far more effortful, and subsequently more difficult, than a recognition task such as Word-Picture matching. In fact, Word-Picture matching is the only task where AD patients performed significantly better than SD patients. The underlying semantic network damage in AD was sufficient to produce Word-Picture matching results that were significantly worse than controls, but the task itself did not tax the conscious retrieval process enough to produce results equivalent to SD. In the future, it would prove useful to determine what tasks, or aspects of tasks, are most impaired in AD patient performance. Such data would aid in the understanding of the specific nature of the cognitive impairment in AD that impedes the conscious, effortful retrieval of semantic information from the network.
In evaluating these conclusions, it is important to consider alternative interpretations of the findings. For example, it is possible that the words selected for the priming task could have factored into the poor performance of the patient groups. While the stimuli were matched for length, frequency, and typicality, it is still possible that certain words were more affected by the semantic deficits in either AD or SD. There is no reason to believe that this was the case, as none of the stimuli were inherently more or less difficult to comprehend. In fact, there were no individual items that were missed consistently or had significantly longer reaction times for any of the three groups. Nevertheless, it might be beneficial in future studies to determine if any of the stimuli are not well comprehended by the patient groups as a whole after the priming task is completed.
From a neuroanatomical perspective, this dual mechanism of semantic loss could be explained based on the initial locus and eventual spread of cortical degradation in the two patient groups. The location of cortical damage in SD overlaps with the areas said to be most important for semantic memory, namely in the anterior and inferolateral portions of the temporal lobe (Garrard & Hodges 2000; Hodges 2001; Lambon Ralph et al. 2001; Mummery et al. 2000; Warrington 1975). In fact, damage in this temporal region is considered a supportive characteristic to the diagnosis of SD (Neary et al. 1998). As such, it is only logical that SD patients would suffer from a deficit within their semantic network. In contrast, the earliest cortical damage present in AD is typically located in the medial temporal lobe, producing the impairment to anterograde episodic memory (Hodges & Patterson, 1995; Hodges, Salmon, & Butters, 1992, McKhann et al., 1984). As the cortical damage spreads outward from the medial temporal lobe, numerous cognitive functions can be affected (McKhann et al., 1984). Specifically, as the damage draws closer to the temporal pole, the semantic memory system will be disrupted. Retrieval from the semantic system can be damaged during this spread of cortical atrophy as well, perhaps as white matter connections along the temporal lobe are lost (Harasty et al., 2001). That is, while SD atrophy starts from within the areas of semantic memory function and works outward, the AD semantic memory system is damaged from the outside in, yielding a milder semantic impairment until well into the time-course of AD. Further imaging studies of semantic processing in dementia would be required to determine what pathways may be involved in the impaired conscious retrieval present in AD.
Acknowledgments
We would like to thank Paul Aisen, Ph.D., Murray Grossman, Ph.D., and every member of their labs, for assisting with patient testing, as well as Larry Muenz, Ph.D., for his statistical knowledge. This research was made possible by an NIDCD Fellowship 1F31DC05885 and an NIH Grant AG17586, awarded to Dr. Grossman.
Footnotes
An additional analysis was done using ratios instead of raw scores. The same pattern of results emerged, with the exception that AD superordinate priming was slightly lower than control superordinate priming, but still significantly higher than SD superordinate priming.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abboud H. Superlab. Cedrus. 1991 [Computer Software]
- Battig WF, Montague WE. Category norms for verbal items in 56 categories. Journal of Experimental Psychology. 1969;80:1–45. [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–15. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bub D. Semantic memory loss in dementia of Alzheimer type: what do various measures measure? Brain. 1990;113:397–419. doi: 10.1093/brain/113.2.397. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bub D, Seidenberg M. Priming and semantic memory in Alzheimer’s disease. Brain and Language. 1989;36:420–46. doi: 10.1016/0093-934x(89)90078-3. [DOI] [PubMed] [Google Scholar]
- Collins AM, Loftus EF. A spreading-activation theory of semantic processing. Psychological Review. 1975;82(6):407–428. [Google Scholar]
- Cronin-Golomb A, Keane MM, Kokodis A, Corkin S, Growdon JH. Category knowledge in Alzheimer’s disease: Normal Organization and a general retrieval deficit. Psychology and Aging. 1992;7(3):359–366. doi: 10.1037//0882-7974.7.3.359. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, Mchugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forstl H, Sattel H, Besthorn C, Daniel S, Geiger-Kabisch C, Hentschel F, Sarochan M, Zerfass R. Longitudinal cognitive, electroencephalographic and morphological brain changes in ageing and Alzheimer’s disease. British Journal of Psychiatry. 1999;168(3):280–6. doi: 10.1192/bjp.168.3.280. [DOI] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency Analysis of English Usage. Boston: Houghton Mifflin; 1982. [Google Scholar]
- Friedman RB, Ferguson S, Robinson S, Sunderland T. Mechanisms of reading in Alzheimer’s disease. Brain and Language. 1992;43:400–413. doi: 10.1016/0093-934x(92)90109-r. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon Ralph MA, Williams G, Antoun N, Sahakian BJ, Hodges JR. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;57:216–25. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Garrard P, Hodges JR. Semantic dementia: clinical, radiological, and pathological perspectives. Journal of Neurology. 2000;247:409–22. doi: 10.1007/s004150070169. [DOI] [PubMed] [Google Scholar]
- Giffard B, Desgranges B, Nore-Mary F, Lalevee C, De la Sayette V, Pasquier &, Eustache F. The Nature of Semantic Memory Deficits in Alzheimer’s Disease. New Insights from Hyperpriming Effects. Brain. 2001;124(8):1522–1532. doi: 10.1093/brain/124.8.1522. [DOI] [PubMed] [Google Scholar]
- Glosser G, Friedman RB. Lexical but not semantic priming in Alzheimer’s disease. Psychology and Aging. 1991;6(4):522–27. doi: 10.1037//0882-7974.6.4.522. [DOI] [PubMed] [Google Scholar]
- Glosser G, Friedman RB, Grugan PK, Lee JH, Grossman M. Lexical semantic and associative priming in Alzheimer’s disease. Neuropsychology. 1998;12(2):218–24. doi: 10.1037//0894-4105.12.2.218. [DOI] [PubMed] [Google Scholar]
- Grober E, Buschke H, Kawas C, Fuld P. Impaired ranking of semantic attributes in dementia. Brain and Language. 1985;26:276–286. doi: 10.1016/0093-934x(85)90043-4. [DOI] [PubMed] [Google Scholar]
- Harasty JA, Halliday GM, Xuereb J, Croot K, Bennet H, Hodges JR. Cortical degeneration associated with phonologic and semantic language impairments in AD. Neurology. 2001;56:944–50. doi: 10.1212/wnl.56.7.944. [DOI] [PubMed] [Google Scholar]
- Hodges JR. Frontotemporal dementia (Pick’s disease): clinical features and assessment. Neurology. 2001;56:S6–10. doi: 10.1212/wnl.56.suppl_4.s6. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer’s disease: failure of access or degraded knowledge? Neuropsychologia. 1992;30:301–14. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Tyler LK. Loss of semantic memory: implications for the modularity of mind. Cognitive Neuropsychology. 1994;11(5):505–42. [Google Scholar]
- Hodges JR, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer’s disease? Neuroanatomical and diagnostic implications. Neuropsychologia. 1995;33(4):441–59. doi: 10.1016/0028-3932(94)00127-b. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and Palm trees: A Test of Semantic Access from Pictures and Words. Thames Valley Publishing; 1992. [Google Scholar]
- Huff FJ, Becker JT, Belle SH, Nebes RD, Holland AL, Boller F. Cognitive deficits and clinical diagnosis of Alzheimer’s disease. Neurology. 1987;37:1119–24. doi: 10.1212/wnl.37.7.1119. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13(3):341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Martin A, Fedio P. Word production and comprehension in Alzheimer’s Disease: The breakdown of semantic knowledge. Brain and language. 1983;19:124–141. doi: 10.1016/0093-934x(83)90059-7. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Moreaud O, David D, Charnallet A, Pellat J. Are semantic errors actually semantic?: Evidence from Alzheimer’s Disease? Brain and Language. 2001;77:176–186. doi: 10.1006/brln.2000.2427. [DOI] [PubMed] [Google Scholar]
- Moss H, Older L. Birkbeck Word Association Norms. East Sussex, UK: Psychology Press; 1996. [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR. A Voxel Based Morphometry Study of Semantic Dementia: The Relation of Temporal Lobe Atrophy to Cognitive Deficit. Annals of Neurology. 2000;47:36–45. [PubMed] [Google Scholar]
- Nakamura H, Nakanishi M, Hamanaka T, Nakaaki S, Yoshida S. Semantic priming in patients with Alzheimer and semantic dementia. Cortex. 2000;36:151–62. doi: 10.1016/s0010-9452(08)70521-5. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nebes RD. Cognitive dysfunction in Alzheimer’s disease. In: Craik FI, Salthouse TA, editors. The Handbook of Aging and Cognition. Hillsdale, NJ: Lawrence Erlbaum Assoc; 1992. [Google Scholar]
- Nebes RD, Brady CB. Integrity of semantic fields in Alzheimer’s Disease. Cortex. 1988;24:291–299. doi: 10.1016/s0010-9452(88)80037-6. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Brady CB. Preserved organization of semantic attributes in Alzheimer’s disease. Cortex. 1990;25:305–315. doi: 10.1037//0882-7974.5.4.574. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Brady CB, Huff FJ. Automatic and attentional mechanisms of semantic priming in Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology. 1989;11:219–230. doi: 10.1080/01688638908400884. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Martin DC, Horn LC. Sparing of semantic memory in Alzheimer’s disease. Journal of Abnormal Psychology. 1984;93:321–330. doi: 10.1037//0021-843x.93.3.321. [DOI] [PubMed] [Google Scholar]
- Ober BA. RT and Non-RT Methodology for Semantic Priming Research with Alzheimer’s Disease Patients: A Critical Review. Journal of Clinical and Experimental Neuropsychology. 2002;24(7):883–911. doi: 10.1076/jcen.24.7.883.8384. [DOI] [PubMed] [Google Scholar]
- Ober BA, Shenaut GK. Lexical decision and priming in Alzheimer’s disease. Neuropsychologia. 1988;26(2):273–86. doi: 10.1016/0028-3932(88)90080-2. [DOI] [PubMed] [Google Scholar]
- Ober BA, Shenaut GK, Jagust WJ, Stillman RC. Automatic semantic priming with various category relations in Alzheimer’s disease and normal aging. Psychology and Aging. 1991;6(4):647–60. doi: 10.1037//0882-7974.6.4.647. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer’s disease. Neurology. 2000;54:2277–84. doi: 10.1212/wnl.54.12.2277. [DOI] [PubMed] [Google Scholar]
- Postman L, Keppel G. Norms of Word Association. San Diego, CA: Academic Press; 1980. [Google Scholar]
- Rich JB, Park NW, Dopkins S, Brandt J. What do Alzheimer’s disease patients know about animals? It depends on task structures and presentation format. Journal of the International Neuropsychological Society. 2002;8:83–84. [PubMed] [Google Scholar]
- Rogers TT, Ivanoiu A, Patterson K, Hodges JR. Semantic Memory in Alzheimer’s Disease and the Frontotemporal Dementias: A Longitudinal Study of 236 Patients. Neuropsychology. 2006;20(3):319–335. doi: 10.1037/0894-4105.20.3.319. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. Structure and Deterioration of Semantic Memory: A Neuropsychological and Computational Investigation. Psychological Review. 2004;111(1):205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Shallice T. From Neuropsychology to Mental Structure. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behavioral Neurology. 1989;2:167–82. [Google Scholar]
- Squire LR. Memory and Brain. New York: Oxford University Press; 1987. [Google Scholar]
- Tulving E. Organization of Memory. New York and London: Academic Press; 1972. Episodic and semantic memory; pp. 381–403. [Google Scholar]
- Tyler LK, Moss HE. Going, going, gone..? Implicit and explicit tests of conceptual knowledge in a longitudinal study of semantic dementia. Neuropsychologia. 1998;36(12):1313–23. doi: 10.1016/s0028-3932(98)00029-3. [DOI] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Quarterly Journal of Experimental Psychology. 1975;27:635–57. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]


